Abstract
1. Cutaneous reflex responses have been recorded in human first dorsal interosseous and extensor digitorum brevis muscles following electrical stimulation of the digital nerves of the index finger and second toe respectively.
2. Recordings have been made in normal subjects and in patients with central nervous lesions.
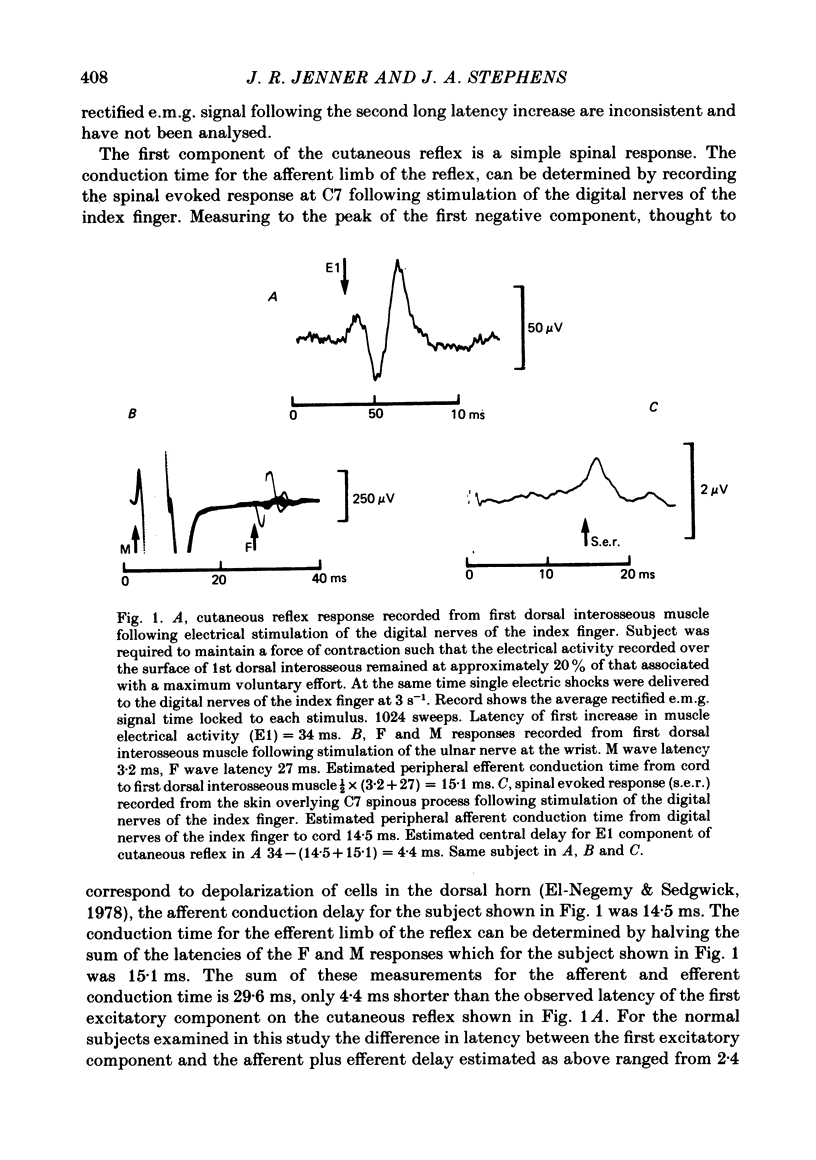
3. Cutaneous reflex responses in first dorsal interosseous were triphasic, consisting of initial short latency excitation, followed by inhibition, followed by prominent long latency excitation. Cutaneous reflex responses in extensor digitorum brevis were biphasic, consisting of short and long latency periods of excitation.
4. Estimated central delay for the initial excitatory components of the cutaneous reflex in first dorsal interosseous and extensor digitorum brevis muscles ranged from 2·4 to 6·2 ms (mean 4·6 ms) and 0·6 to 4·1 ms (mean 2·3 ms) respectively.
5. Differences in latency between short and long latency excitatory components of the cutaneous reflexes recorded in first dorsal interosseous and extensor digitorum brevis muscles ranged from 16 to 18 ms (mean 17·3 ms) and 27 to 32 ms (mean 29·3 ms) respectively.
6. Differences in time delay between short and long latency excitation in first dorsal interosseous and extensor digitorum brevis muscles when compared in individual subjects ranged from 9 to 14 ms (mean 12 ms). These values lay within 0-7 ms (mean 4 ms) of estimates in each subject of conduction time along central pathways between T12 and C7 spinal segments.
7. Differences in latency between short and long latency excitatory components of the cutaneous reflex recorded in first dorsal interosseous were 3·5-8·5 ms longer than the estimated minimum time for impulse conduction along a pathway travelling through the dorsal columns to cerebral cortex and returning by way of the corticospinal tract.
8. The long latency excitatory component of the cutaneous reflex in first dorsal interosseous muscle is reduced and often delayed in patients with dorsal column lesions.
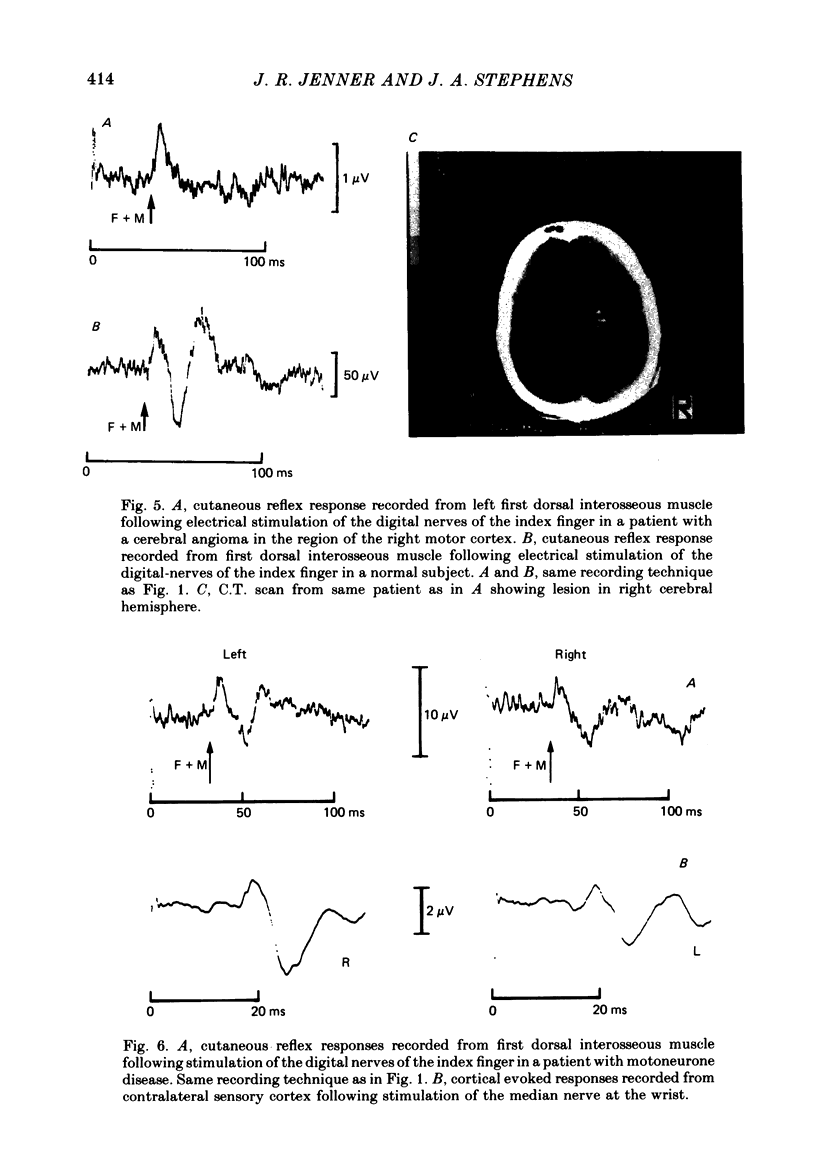
9. The long latency excitatory and short latency inhibitory components of the cutaneous reflex in first dorsal interosseous muscle are absent in patients with damage to motor cortex.
10. The long latency excitatory component of the cutaneous reflex in first dorsal interosseous muscle is reduced in amplitude and often delayed in patients with motoneurone disease causing damage to the corticospinal tract. The timing of short latency excitatory and inhibitory components is unchanged.
11. It is concluded that the short latency excitatory and inhibitory components of the cutaneous reflex response of first dorsal interosseous muscle have a spinal pathway and that the interneurones mediating the inhibitory component are under descending extrapyramidal control from systems whose inputs are deranged by damage to motor cortex.
12. It is concluded that the long latency excitatory component of the cutaneous reflex response of first dorsal interosseous muscle is of supraspinal origin requiring transmission of afferent impulses through the dorsal columns, a relay in the sensori-motor cortex and then descending transmission to the lower motoneurone pool by way of the corticospinal tract.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Hagan P. J., Phillips C. G., Powell T. P. Mapping by microstimulation of overlapping projections from area 4 to motor units of the baboon's hand. Proc R Soc Lond B Biol Sci. 1975 Jan 21;188(1090):31–36. doi: 10.1098/rspb.1975.0002. [DOI] [PubMed] [Google Scholar]
- Asanuma H., Larsen K. D., Yumiya H. Receptive fields of thalamic neurons projecting to the motor cortex in the cat. Brain Res. 1979 Aug 24;172(2):217–228. doi: 10.1016/0006-8993(79)90534-1. [DOI] [PubMed] [Google Scholar]
- Brinkman J., Bush B. M., Porter R. Deficient influence of peripheral stimuli on precentral neurones in monkeys with dorsal column lesions. J Physiol. 1978 Mar;276:27–48. doi: 10.1113/jphysiol.1978.sp012218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller N. P., Garnett R., Stephens J. A. The reflex responses of single motor units in human hand muscles following muscle afferent stimulation. J Physiol. 1980 Jun;303:337–349. doi: 10.1113/jphysiol.1980.sp013289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Jankowska E., ten Bruggencate G. A comparison of peripheral and rubrospinal synaptic input to slow and fast twitch motor units of triceps surae. J Physiol. 1970 May;207(3):709–732. doi: 10.1113/jphysiol.1970.sp009090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccia M. R., McComas A. J., Upton A. R., Blogg T. Cutaneous reflexes in small muscles of the hand. J Neurol Neurosurg Psychiatry. 1973 Dec;36(6):960–977. doi: 10.1136/jnnp.36.6.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough J. F., Kernell D., Phillips C. G. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol. 1968 Sep;198(1):145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Watanabe S., Lux H. D. Relations between EEG phenomena and potentials of single cortical cells. I. Evoked responses after thalamic and erpicortical stimulation. Electroencephalogr Clin Neurophysiol. 1966 Jan;20(1):1–18. doi: 10.1016/0013-4694(66)90136-2. [DOI] [PubMed] [Google Scholar]
- Desmedt J. E., Cheron G. Central somatosensory conduction in man: neural generators and interpeak latencies of the far-field components recorded from neck and right or left scalp and earlobes. Electroencephalogr Clin Neurophysiol. 1980 Dec;50(5-6):382–403. doi: 10.1016/0013-4694(80)90006-1. [DOI] [PubMed] [Google Scholar]
- Desmedt J. E., Cheron G. Somatosensory evoked potentials to finger stimulation in healthy octogenarians and in young adults: wave forms, scalp topography and transit times of parietal and frontal components. Electroencephalogr Clin Neurophysiol. 1980 Dec;50(5-6):404–425. doi: 10.1016/0013-4694(80)90007-3. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D., German D. C. Corticomotoneuronal connections of precentral cells detected by postspike averages of EMG activity in behaving monkeys. Brain Res. 1976 Sep 24;114(3):505–510. doi: 10.1016/0006-8993(76)90973-2. [DOI] [PubMed] [Google Scholar]
- GIBLIN D. R. SOMATOSENSORY EVOKED POTENTIALS IN HEALTHY SUBJECTS AND IN PATIENTS WITH LESIONS OF THE NERVOUS SYSTEM. Ann N Y Acad Sci. 1964 May 8;112:93–142. doi: 10.1111/j.1749-6632.1964.tb26744.x. [DOI] [PubMed] [Google Scholar]
- Garnett R., Stephens J. A. The reflex responses of single motor units in human first dorsal interosseous muscle following cutaneous afferent stimulation. J Physiol. 1980 Jun;303:351–364. doi: 10.1113/jphysiol.1980.sp013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett R., Stephens J. A., Usherwood T. P. Changes in the probability of firing of human motor units following cutaneous stimulation [proceedings]. J Physiol. 1976 Sep;260(2):69P–70P. [PubMed] [Google Scholar]
- Hagbarth K. E., Hägglund J. V., Wallin E. U., Young R. R. Grouped spindle and electromyographic responses to abrupt wrist extension movements in man. J Physiol. 1981 Mar;312:81–96. doi: 10.1113/jphysiol.1981.sp013617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne M. K., Tracey D. J. The afferents and projections of the ventroposterolateral thalamus in the monkey. Exp Brain Res. 1979 Jun 1;36(1):129–141. doi: 10.1007/BF00238473. [DOI] [PubMed] [Google Scholar]
- Jenner J. R., Stephens J. A. Evidence for a transcortical cutaneous reflex response in man [proceedings]. J Physiol. 1979 Aug;293:39P–40P. [PubMed] [Google Scholar]
- Kievit J., Kuypers H. G. Organization of the thalamo-cortical connexions to the frontal lobe in the rhesus monkey. Exp Brain Res. 1977 Sep 28;29(3-4):299–322. doi: 10.1007/BF00236173. [DOI] [PubMed] [Google Scholar]
- Lemon R. N. Functional properties of monkey motor cortex neurones receiving afferent input from the hand and fingers. J Physiol. 1981 Feb;311:497–519. doi: 10.1113/jphysiol.1981.sp013601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon R. N., Porter R. Afferent input to movement-related precentral neurones in conscious monkeys. Proc R Soc Lond B Biol Sci. 1976 Oct 29;194(1116):313–339. doi: 10.1098/rspb.1976.0082. [DOI] [PubMed] [Google Scholar]
- Lemon R. N. Variety of functional organization within the monkey motor cortex. J Physiol. 1981 Feb;311:521–540. doi: 10.1113/jphysiol.1981.sp013602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon R. N., van der Burg J. Short-latency peripheral inputs to thalamic neurones projecting to the motor cortex in the monkey. Exp Brain Res. 1979 Aug 1;36(3):445–462. doi: 10.1007/BF00238515. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B., Adam J. The effect of lesions of the sensorimotor cortex and the capsular pathways on servo responses from the human long thumb flexor. Brain. 1977 Sep;100(3):503–526. doi: 10.1093/brain/100.3.503. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B., Adam J. The effect of posterior column lesions on servo responses from the human long thumb flexor. Brain. 1977 Mar;100(Pt 1):185–200. doi: 10.1093/brain/100.1.185. [DOI] [PubMed] [Google Scholar]
- Milner-Brown S. H., Girvin J. P., Brown W. F. The effects of motor cortical stimulation on the excitability of spinal motoneurons in man. Can J Neurol Sci. 1975 Aug;2(3):245–253. doi: 10.1017/s0317167100020345. [DOI] [PubMed] [Google Scholar]
- Stephens J. A., Usherwood T. P., Garnett R. Technique for studying synaptic connections of single motoneurones in man. Nature. 1976 Sep 23;263(5575):343–344. doi: 10.1038/263343a0. [DOI] [PubMed] [Google Scholar]
- Stephens J. A., Usherwood T. P. Proceedings: Changes in the probability of firing of human motor units following cutaneous nerve stimulation. J Physiol. 1976 Jun;258(2):49P–51P. [PubMed] [Google Scholar]
- Tracey D. J., Asanuma C., Jones E. G., Porter R. Thalamic relay to motor cortex: afferent pathways from brain stem, cerebellum, and spinal cord in monkeys. J Neurophysiol. 1980 Sep;44(3):532–554. doi: 10.1152/jn.1980.44.3.532. [DOI] [PubMed] [Google Scholar]
- Wong Y. C., Kwan H. C., MacKay W. A., Murphy J. T. Spatial organization of precentral cortex in awake primates. I. Somatosensory inputs. J Neurophysiol. 1978 Sep;41(5):1107–1119. doi: 10.1152/jn.1978.41.5.1107. [DOI] [PubMed] [Google Scholar]
- el-Negamy E., Sedgwick E. M. Properties of a spinal somatosensory evoked potential recorded in man. J Neurol Neurosurg Psychiatry. 1978 Aug;41(8):762–768. doi: 10.1136/jnnp.41.8.762. [DOI] [PMC free article] [PubMed] [Google Scholar]



