Abstract
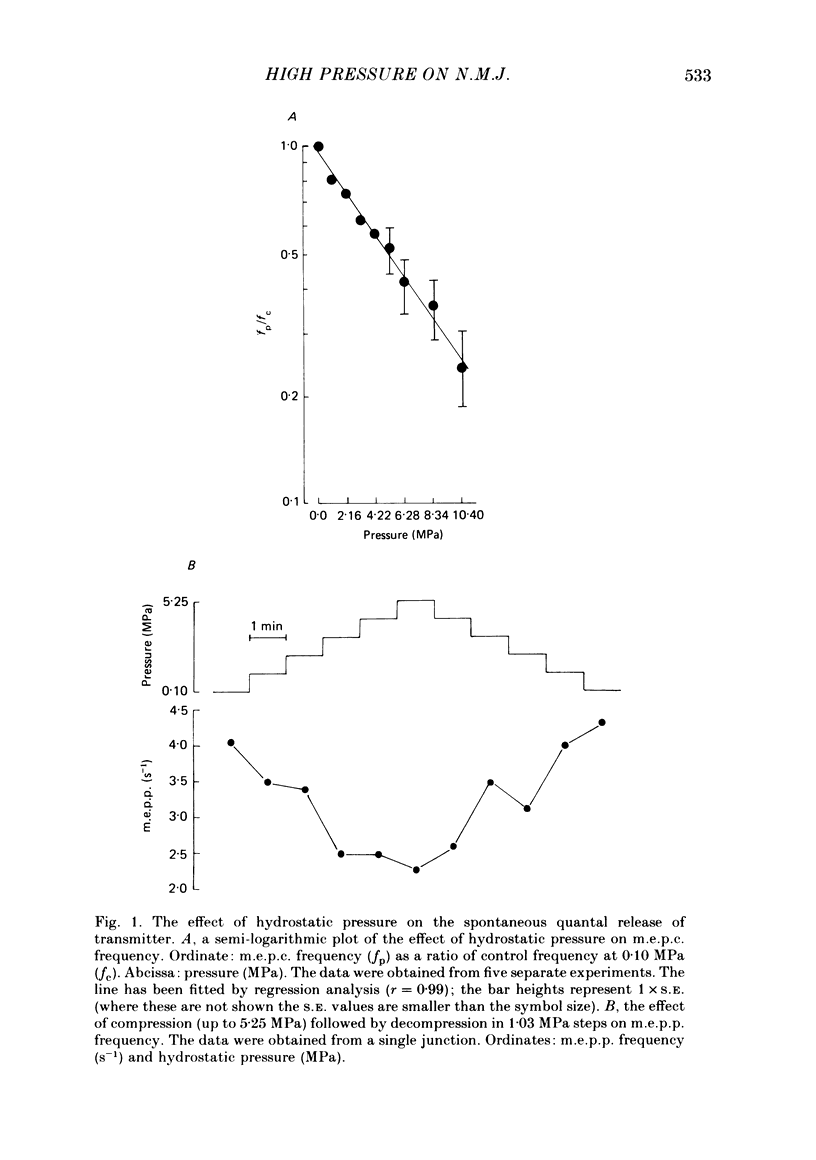
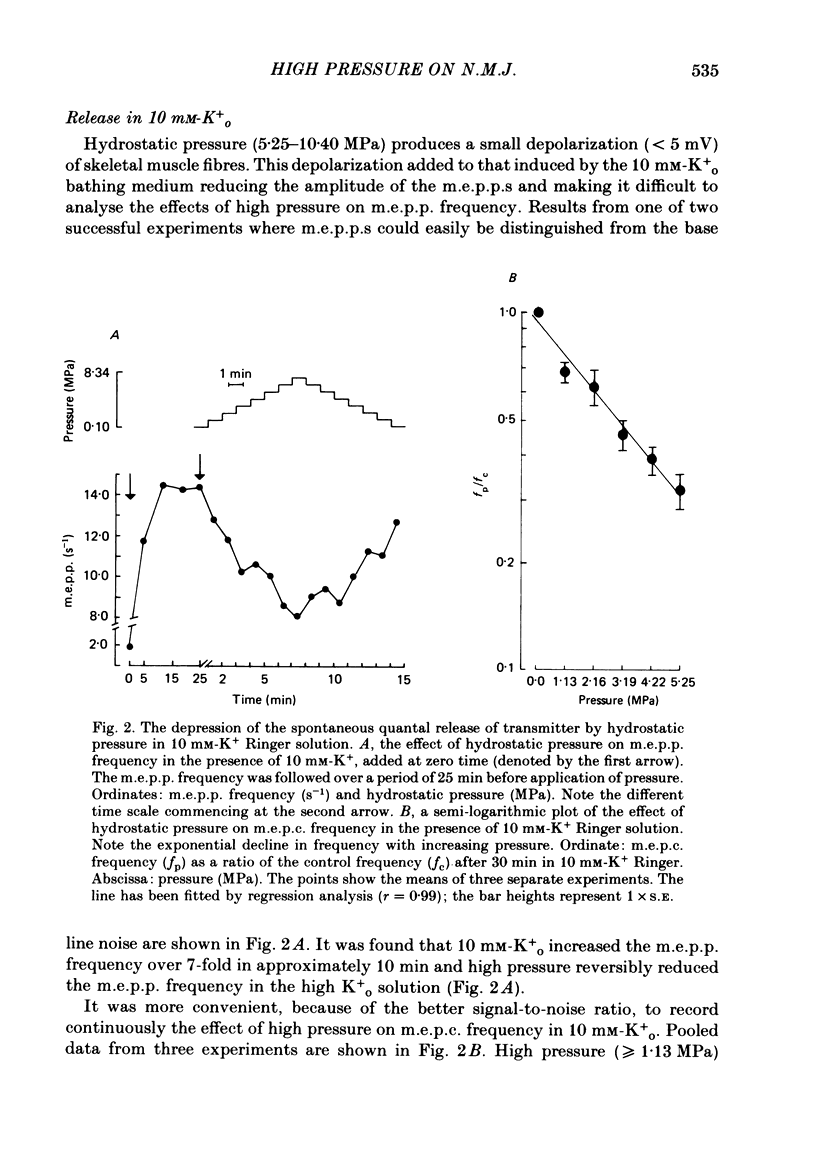
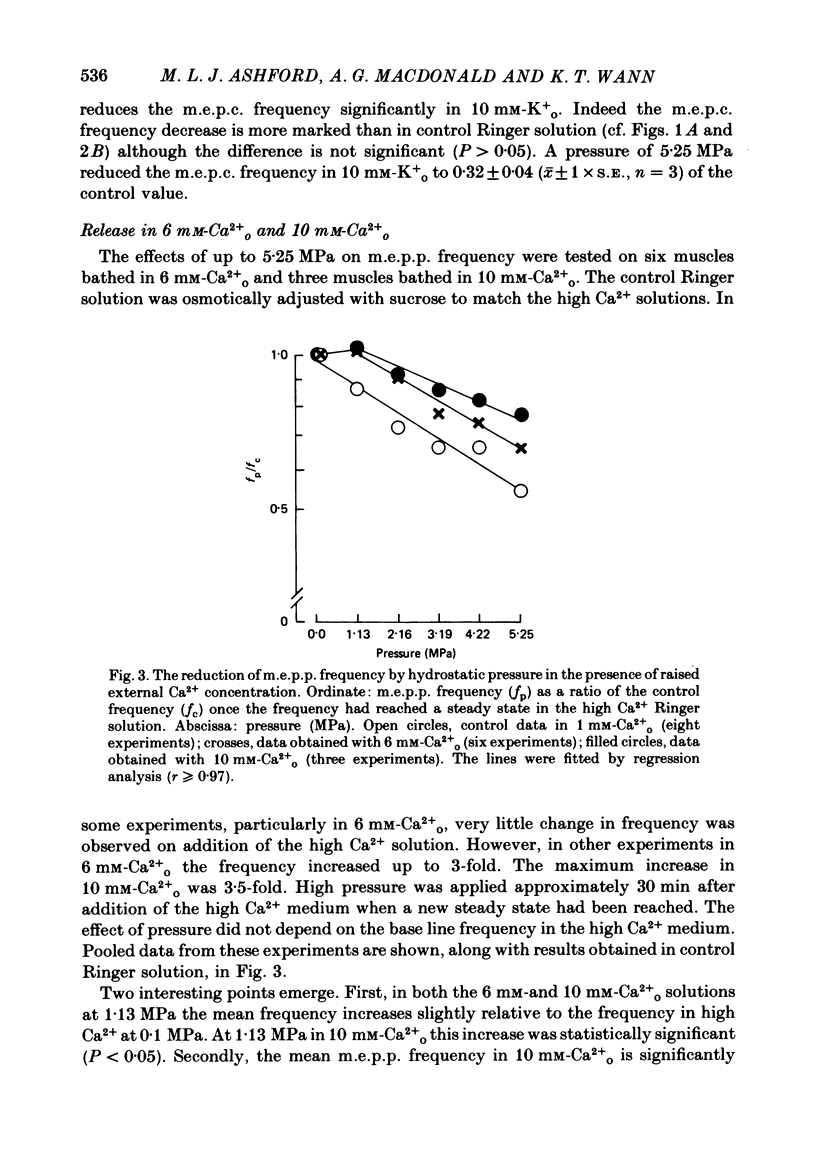
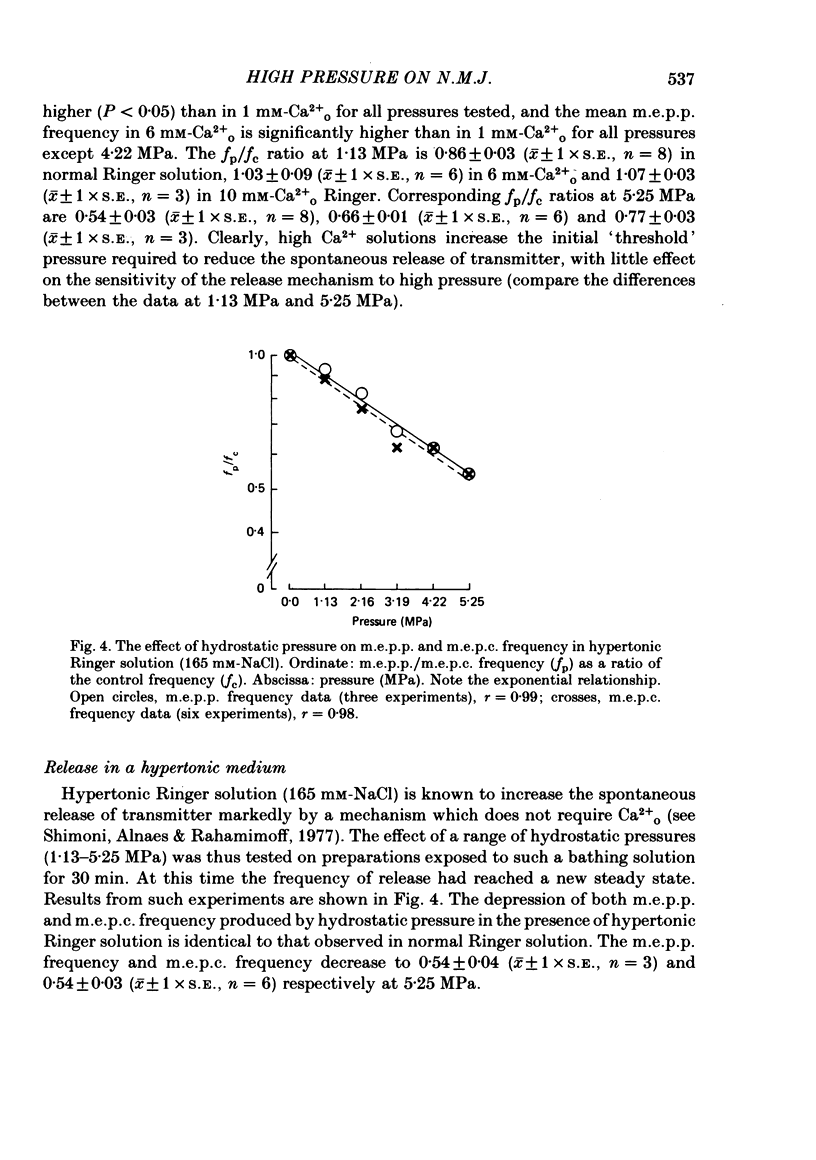
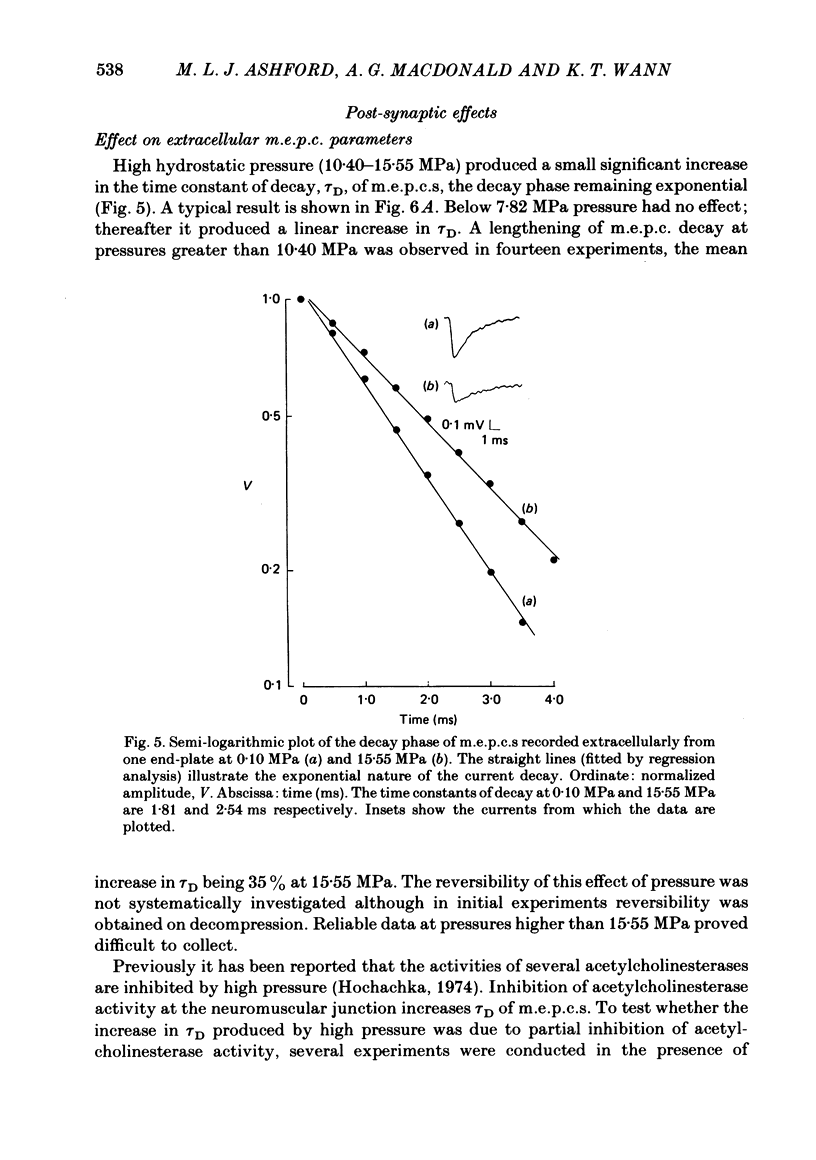
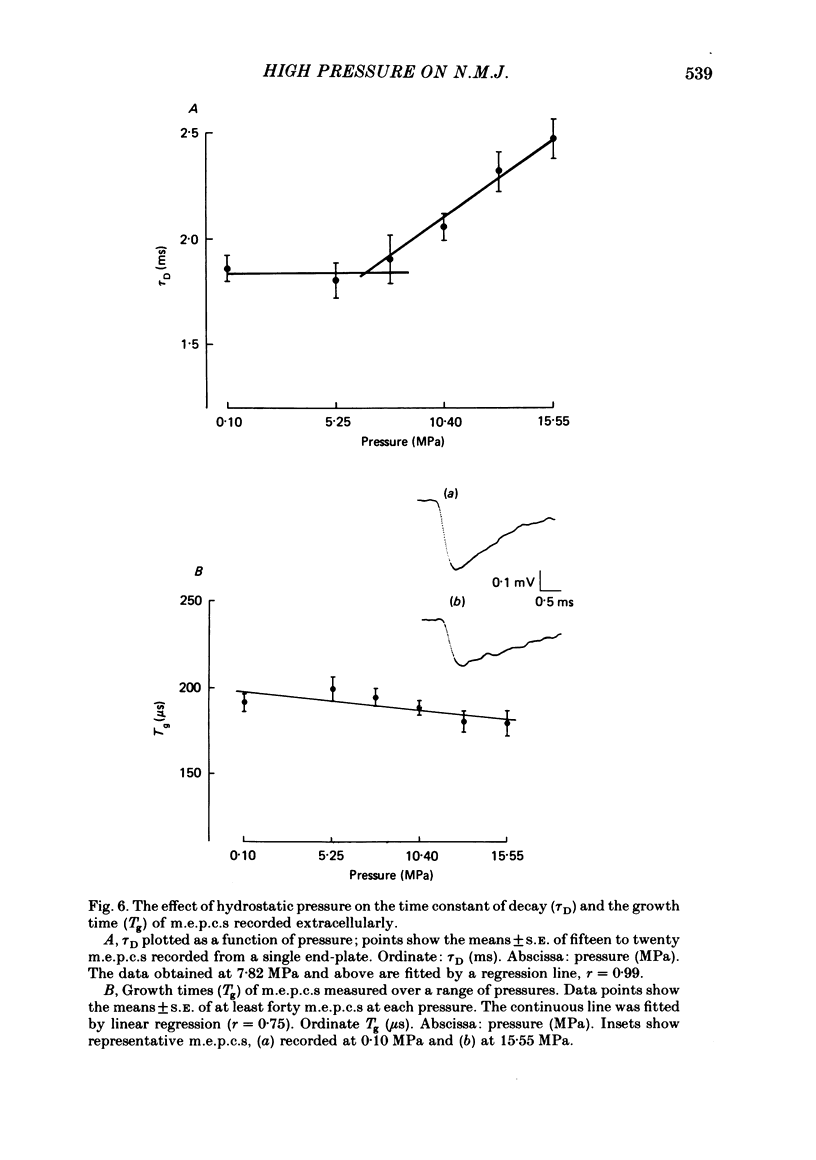
1. The effects of hydrostatic pressure (0.1-15.55 MPa) on the spontaneous release of transmitter at the frog neuromuscular junction were investigated. 2. The major effect of high pressure is on the release mechanism, pressure (0.1-10.40 MPa) producing an exponential decrease in frequency of the miniature end-plate currents in normal Ringer solution. The frequency decreases to 0.52 and 0.24 of the control value at 5.25 and 10.40 MPa respectively. This effect is reversible on decompression. 3. The sensitivity of the release process to high pressure is unaltered in 10 mM-K+, 6 mM- and 10 mM-Ca2+ and hypertonic (165 mM-NaCl) Ringer solution, although the high Ca2+ media shift the threshold for the pressure effect to higher pressures. 4. Higher pressure (10.40-15.55 MPa) produces a small increase in the time constant of decay (tau D) of m.e.p.c.s with no effect on the growth phase. A pressure of 15.55 MPa increases tau D to 1.35 of the control value. 5. The possible actions of high pressure on both the pre- and post-synaptic processes are briefly discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford M. L., Macdonald A. G., Wann K. T. Moderate hydrostatic pressures reduce the spontaneous release of transmitter in the frog [proceedings]. J Physiol. 1979 Jul;292:44P–44P. [PubMed] [Google Scholar]
- Campenot R. B. The effects of high hydrostatic pressure on transmission at the crustacean neuromuscular junction. Comp Biochem Physiol B. 1975 Sep 15;52(1):133–140. doi: 10.1016/0305-0491(75)90128-5. [DOI] [PubMed] [Google Scholar]
- Duncan C. J., Statham H. E. Interacting effects of temperature and extracellular calcium on the spontaneous release of transmitter at the frog neuromuscular junction. J Physiol. 1977 Jun;268(2):319–333. doi: 10.1113/jphysiol.1977.sp011859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hamill O. P. Effects of anesthetics on ion channels in synapses. Int Rev Physiol. 1981;25:1–45. [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N., Schneider G. T. Effects of some aliphatic alcohols on the conductance change caused by a quantum of acetylcholine at the toad end-plate. J Physiol. 1975 Jan;244(2):409–429. doi: 10.1113/jphysiol.1975.sp010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gountis-Bonikos C., Kendig J. J., Cohen E. N. The actions of neuromuscular relaxants at hyperbaric pressures. Anesthesiology. 1977 Jul;47(1):11–15. doi: 10.1097/00000542-197707000-00003. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Macdonald A. G., Wann K. T. Proceedings: Apparatus for intracellular recording from excitable cells subjected to high hydrostatic pressure. J Physiol. 1975 Sep;251(1):7P–8P. [PubMed] [Google Scholar]
- Harris D. J. Observations on the knee-jerk reflex in oxygen-helium at 31 and 43 bars. Undersea Biomed Res. 1979 Mar;6(1):55–74. [PubMed] [Google Scholar]
- Hochachka P. W. Temperature and pressure adaptation of the binding site of acetylcholinesterase. Biochem J. 1974 Dec;143(3):535–539. doi: 10.1042/bj1430535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B., Deutsch J. W., Carlson S. S., Wagner J. A. Biochemistry of neurotransmitter release. Annu Rev Neurosci. 1979;2:399–446. doi: 10.1146/annurev.ne.02.030179.002151. [DOI] [PubMed] [Google Scholar]
- Kendig J. J., Cohen E. N. Neuromuscular function at hyperbaric pressures: pressure-anesthetic interactions. Am J Physiol. 1976 May;230(5):1244–1249. doi: 10.1152/ajplegacy.1976.230.5.1244. [DOI] [PubMed] [Google Scholar]
- Marsh D., Radda G. K., Ritchie G. A. A spin-label study of the chromaffin granule membrane. Eur J Biochem. 1976 Dec;71(1):53–61. doi: 10.1111/j.1432-1033.1976.tb11089.x. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Newton C., Nir S., Jacobson K., Poste G., Lazo R. Studies on membrane fusion. III. The role of calcium-induced phase changes. Biochim Biophys Acta. 1977 Mar 17;465(3):579–598. doi: 10.1016/0005-2736(77)90275-9. [DOI] [PubMed] [Google Scholar]
- Stefani E., Schmidt H. A convenient method for repeated intracellular recording of action potentials from the same muscle fibre without membrane damage. Pflugers Arch. 1972;334(3):276–278. doi: 10.1007/BF00626229. [DOI] [PubMed] [Google Scholar]


