Abstract
Background
Craniofacial mucormycosis is a highly lethal infectious disease. This study aims to assess and analyze multiple variables, including clinical, socioeconomic, and biochemical markers, to identify and examine risk factors for mortality associated with this mycotic infection.
Material and Methods
A retrospective analysis was conducted on 38 patients who sought medical attention at the Otolaryngology and Head and Neck Surgery Division of a tertiary-level hospital in Monterrey, Mexico. A broad range of variables was analyzed: clinical features, including the extent of mucormycosis infection; socioeconomic factors such as monthly income, marital status, geographical residence, educational level, and insurance status; as well as biochemical markers, including glucose levels, lactate dehydrogenase (LDH), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and immune cell counts, specifically neutrophils (NEU) and lymphocytes (LYM). Statistical analysis was conducted using SPSS v26. Risk factors for mortality were evaluated using Cox regression. Overall survival (OS) was assessed with the Kaplan-Meier method. The Fisher's exact test and the Chi-square test were used for categorical variables. For median comparisons, the Student’s t-test and Mann-Whitney U test were applied; with normality assessed using the Shapiro-Wilk test. A p-value <0.05 was considered statistically significant.
Results
Mucormycosis was associated with higher mortality in men (p=0.032). The disease primarily affected the paranasal sinuses (p=0.021) and was associated with increased mortality when involving the orbit (p=0.035). Additionally, compromised lymphocyte counts (LYM) (p=0.034) and lower educational levels (p=0.009) were associated with higher mortality. Individuals residing in rural areas also exhibited an elevated risk of mortality (p =0.023).
Conclusions
Prevention strategies should focus on high-risk groups to reduce the mortality rate of craniofacial mucormycosis, particularly targeting men and individuals residing in rural areas. Special emphasis should be placed on those without education or health insurance. Early diagnosis and appropriate management are crucial for improving outcomes.
Key words:Mucormycosis, socioeconomic, mortality, rural residency.
Introduction
Mucormycosis encompasses a broad spectrum of opportunistic infections primarily characterized by angioinvasion, leading to rapid and extensive tissue necrosis (Fig. 1), which may result in significant mortality. Mortality rates are alarmingly high, with only 40% of infected individuals surviving three months after being diagnosed (1). This infection is primarily caused by fungi from the genera Rhizopus spp. and Mucor spp., which are the most prevalent (2) (Fig. 2). The virulence factors of these microorganisms and the host's immune response are crucial determinants of prognosis, with the condition predominantly affecting immunocompromised individuals (3). Although less common, immunocompetent individuals can also be affected. Globally, the incidence of mucormycosis ranges from 0.005 to 1.7 cases per million people, with an estimated incidence of 0.12 per 100,000 inhabitants in Mexico (4).
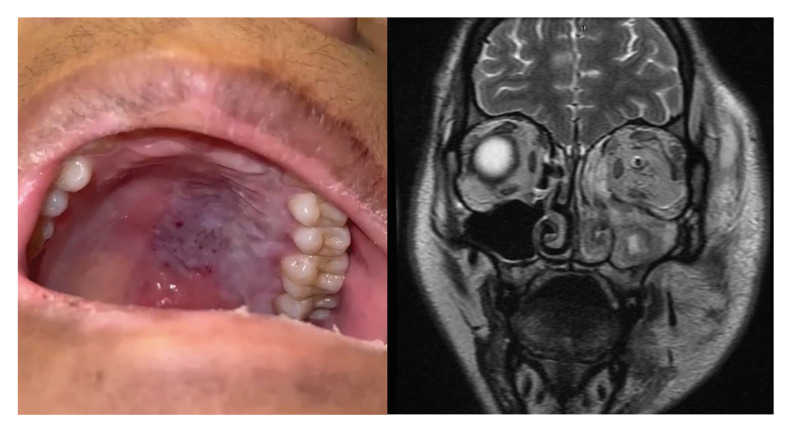
Figure 1.
(Left) Purplish mucous lesion of left hemipalate with necrosis; (Right) Coronal section of MRI demonstrating involvement of mucormycosis infection of left ethmoid sinus, lateral wall of nose, maxillary sinus, floor and medial wall of the left orbit.
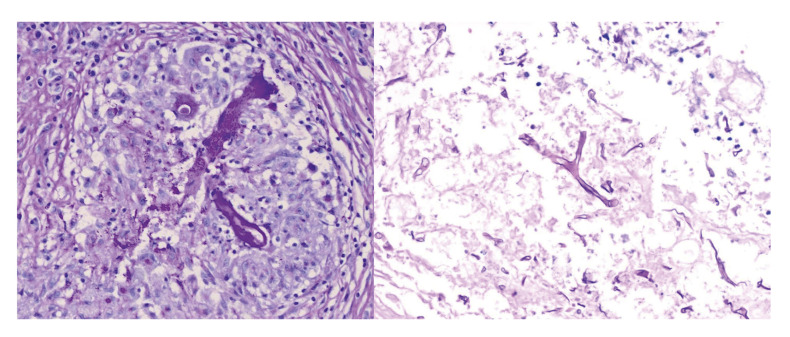
Figure 2.
(Left) Histopathological image showing lymphocytes and epithelioid cells conforming a granuloma with a non-septate hypha in its center. PAS 40x. (Right) Necrotic tissue associated to a non-septate, irregular, thick hyphae. PAS 40x.
Prompt diagnosis and accurate treatment are essential to improving patient survival. Mucormycosis can present as a cutaneous infection or as more severe forms, such as rhino-orbital-cerebral mucormycosis (ROCM), which is the most common clinical presentation (5). Other forms of the disease include sino-pulmonary, gastrointestinal, and disseminated infections.
This study aims to describe and analyze the clinical, socioeconomical and biochemical markers of Hispanic patients with ROCM at a tertiary - level hospital. The main goal encompasses identifying risk factors for mortality and to raise awareness and encourage both medical and non-medical community to implement preventive measures in high - risk population in order to improve survival rates.
Materials and Methods
A retrospective study including 38 hospitalized patients who were diagnosed with mucormycosis by the Otolaryngology and Head and Neck Surgery Division from 2018 to 2023 at “Hospital Universitario Dr. José Eleuterio González” in the northeast of México was performed.
Registered patients had different comorbidities such as diabetes mellitus, high blood pressure, hematological diseases, or were otherwise immunocompetent. Diagnosis was confirmed through histopathological examination.
Demographic data, including age, sex, personal medical history (e.g., Charlson comorbidity index), treatment, and socioeconomic factors such as monthly income (USD), marital status, and residency (categorized as rural or urban) were collected. Educational level and medical insurance status were dichotomized for Cox regression analysis. Blood counts, including median glucose levels, lactate dehydrogenase (LDH), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and immune cell counts (white blood cells [WBC] and lymphocytes [LYM]), were recorded. Additionally, disease characteristics of rhino-orbital-cerebral mucormycosis (ROCM), such as disease extension, were analyzed to determine risk factors and prognosis for mortality.
Statistical analysis was performed using SPSS v26 (IBM SPSS Statistics, IBM Corp., Armonk, NY). Cox regression was used to study risk factors for mortality, while Kaplan-Meier methods assessed overall survival. Fisher’s exact test or Chi-square test was used for categorical variables, and T-student test and Mann-Whitney U test were employed for median comparisons, with normality assessed by the Shapiro-Wilk test. A p-value <0.05 was considered statistically significant. The study was conducted in compliance with STROBE reporting guidelines and received approval from the local ethics committee.
Results
- Overall clinical characteristics
The median age of the evaluated patients was 49 years, with a range from 5 to 78 years. 76.3% of them were adults, and 63.2% of the entire cohort were male. The most prevalent comorbidity was Diabetes Mellitus type 2 (DMT2), present in 60.5% of the patients, with a median of disease duration of 13 years, ranging from 5 to 20. Only 28.9 of the patients presented hematological diseases. Further baseline characteristics are descripted in Table 1.
Table 1.
Baseline characteristics of 38 patients with mucormycosis and P differences for mortality.
| Baseline characteristics | n (%) | P value | |
|---|---|---|---|
| Age, median (range) | 49 years (5 - 74) | - | |
| Sex | Men | 24 (63.2%) | 0.032° |
| Women | 14 (36.8%) | ||
| Recent history of COVID-19 | Present | 7 (18.4%) | 0.676° |
| Absent | 31 (81.6%) | ||
| Comorbidities | None | 3 (7.9%) | 0.001° |
| Diabetes mellitus | 23 (60.5%) | ||
| Arterial hypertension and DM | 13 (34.2%) | ||
| Hematological diseases | 11 (28.9%) | ||
| Chronic kidney disease | 5 (13.2%) | ||
| Overweight/Obesity | 7 (18.4%) | ||
| Alcohol history | Yes | 7 (18.4%) | 0.767° |
| No | 18 (47.4%) | ||
| Unknown | 13 (34.2%) | ||
| Charlson index | 2 (0 to 5) | 0.004° | |
Diabetes mellitus (DM); Fisher or Chi-square test °.
ROCM primarily involved the paranasal sinuses (31.6%) (p=0.021). Functional Endoscopic Sinus Surgery (FESS) was the treatment of choice in 60.5% of cases; additionally, all patients received antifungal therapy with amphotericin B (dose adjusted based on age, comorbidities, and the severity of the disease).
The median time from symptom onset to diagnosis was 4 weeks, ranging from 1 to 39. The overall survival (OS), defined as the time from either diagnosis or treatment initiation until death from any cause, was 30.53 weeks (CI 12.24 - 48.81). A total of 12 patients (31.5%) died as a result of mucormycosis infection. Mortality was predominantly observed in men (26.3%, n=10) (p=0.032), patients with DMT2 (23.6%) (p=0.498), and those with orbital involvement (p=0.035).
- Socioeconomic features
Addressing socioeconomic aspects, 60.5% of the cohort patients were married and 68.4% of them lived within an urban residency. 73.5% had either no formal education or only basic education. 52.6% lacked medical insurance. Further information can be found in Table 2.
Table 2.
Socioeconomic and biochemical markers of 38 patients with mucormycosis and P differences for mortality.
| Baseline characteristics | n (%) | P value | |
|---|---|---|---|
| Socioeconomic features | Monthly Income (USD) | 243.7 (111.7 to 314.8) | - |
| Marital status | Married | 23 (60.5%) | 0.761° |
| Single | 13 (34.2%) | ||
| Divorced | 2 (5.3%) | ||
| Residence | Rural | 9 (23.7%) | 0.063° |
| Urban | 26 (68.4%) | ||
| Unknown | 3 (7.9%) | ||
| Education | Unschooled and Basic | 29 (76.5%) | 0.009° |
| Middle and Higher | 9 (23.5%) | ||
| Medical Insurance | Present | 12 (31.6% ) | 0.204° |
| Absent | 20 (52.6 %) | ||
| Unknown | 6 (15.8%) | ||
| Biochemical markers | Glucose, mg/dL | 189 (111.25 to 340.25) | 0.096" |
| LDH, IU/L | 162 (139.25 to 244) | 0.260" | |
| WBC, x109/L | 9.59 (0.61 to 15.77) | 0.286" | |
| CRP, mg/dL | 7.7 (7.7 to 17.9) | 0.003" | |
| ESR, mm | 34 (0 to 50) | 0.279" | |
United States Dollar (USD); Lactate dehydrogenase (LDH); White Blood Cells (WBC); C-Reactive Protein (CRP); Fisher or Chi-square test°, Mann-Whitney U test".
Among deceased patients, educational level was found to significantly impact mortality (p=0.009) (Table 2). Univariate analysis indicated that rural residency was associated with a sevenfold increase in mortality risk due to mucormycosis (p=0.023). Patients from rural areas exhibited noTable socioeconomic disparities compared to their urban counterparts, including differences in education (p=0.005) and insurance status (p<0.001).
- Biochemical markers
Laboratory tests revealed altered levels of several parameters. Most patients (60.5%) had elevated glucose levels, with a median of 189 mg/dL (range: 111.25 - 340.25 mg/dL). Inflammatory markers such as the Erythrocyte Sedimentation Rate (ESR) and C-reactive protein (CRP) were also elevated (see Table 2). In deceased patients, CRP levels were significantly higher, with a median of 21.17 mg/dL (range: 6.7 - 44.1 mg/dL) (p=0.003).
Discussion
Mucormycosis is a severe and life-threatening infectious disease with a high mortality rate, ranging from 40% to 80% (6). Recognizing mortality predictors is essential for improving patient care and establishing effective preventive measures for high-risk populations. Research on this topic has been conducted in countries such as Turkey, Pakistan, Iran, and India, primarily focusing on Middle Eastern and South Asian populations (7-10). To our knowledge, this is the first retrospective cohort study addressing the Hispanic population. This gap emphasizes the need for further research into mortality predictors to improve patient-centered outcomes.
In this study, male patients were predominantly affected (63.2%) and exhibited a poorer prognosis (26.3%). Literature reports indicate a 55% mortality rate among men (11). Numerous studies from South Asia and the Middle East have also reported a higher prevalence of this disease among male patients, with low survival outcomes (12-14).
Diabetes mellitus was the most prevalent underlying condition in this retrospective cohort population (60.5%), consistent with findings from an Indian study reporting a prevalence of 97% (12). This comorbidity may facilitate the local dissemination of the infection due to microvascular complications in the sensitive structures of the sinuses (11). Complications associated with DMT2, such as ketoacidosis, are linked to increased mortality in individuals diagnosed with mucormycosis (8). The elevated free iron levels resulting from ketoacidosis offers an ideal environment for fungal proliferation (15).
Mucormycosis predominantly affects the paranasal sinuses. Nair et al. reported that 94.2% of patients with rhino-orbital-cerebral mucormycosis (ROCM) had frontal sinus involvement (12). In our study, the paranasal sinuses were the most frequently affected region (31.6%), with no intracranial involvement observed. Patients with orbital involvement experienced a mortality rate of 66.5%. In comparison, a retrospective cohort study conducted by Harun et al. reported a fatality rate of 46.1% (16). According to the global guidelines from the European Confederation of Medical Mycology, the mortality rate for central nervous system involvement is 80%, whereas the mortality rate for isolated paranasal sinus infections remains unspecified but is generally lower (6).
The socioeconomic environment significantly impacts the accessibility of medical services, thereby influencing disease prognosis. For patients residing in rural areas, a sevenfold increased risk of mortality was observed. Poswal et al. indicated that rural populations have a higher prevalence of mucormycosis, which may be attributed to occupations involving dust or soil, such as farming or labor (17). Our findings revealed that low educational attainment and lack of medical insurance contributed to higher mortality rates, with only 25% of deceased rural patients having insurance.
The DEFEAT Mucor study identified laboratory abnormalities such as neutropenia, elevated serum iron or ferritin levels, and malignancies, which are associated with mortality rates of 100%, 80%, 80%, and 80%, respectively (18). Mojtahedi et al. found that lymphopenia was the most common abnormal laboratory finding in patients with craniofacial mucormycosis (19). In this study, elevated levels of ESR and CRP were observed; however, only CRP was statistically significant (p < 0.05), consistent with findings previously reported by Cho et al. in Asian patients (20).
This study has several limitations, the foremost being the small and heterogeneous sample of patients. The population varied widely, encompassing individuals as young as 5 years with malignancies to those up to 60 years old with elevated glucose levels. Variations in treatment among patients could impact survival outcomes. Despite these limitations, the findings should be interpreted in the context of the Hispanic population studied.
To reduce mortality rates associated with craniofacial mucormycosis in the Hispanic population, prevention strategies should focus on high-risk groups, particularly men and those residing in rural areas. Special attention should be given to individuals without formal education or health insurance, with the goal of facilitating early diagnosis and timely medical-surgical intervention. Given the distinct socioeconomic and demographic characteristics of this population, further research is essential to identify specific mortality predictors and develop effective intervention strategies.
Acknowledgement
Declared none.
Institutional Review Board Statement
The research protocol was approved by the local Research and Institutional Ethics Committee with number OT16-00005. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Author Contributions
Josefina A. Morales-del Angel MD MSc: Conception and design of study, Acquisition of data, Drafting of article and/or critical revision, Final approval, and guarantor of manuscript. Andrea Sarahi Guerra-Garza MD: Conception and design of study, Acquisition of data: laboratory or clinical, Drafting of article and/or critical revision, Analysis and interpretation of data collected. Silvia Merari Macias-Alfaro MD: Conception and design of study, Acquisition of data, Drafting of article and/or critical revision. Jorge Eduardo Juárez Silva MD: Conception and design of study, Acquisition of data, Drafting of article and/or critical revision. Analysis and interpretation of data collected. Baltazar Gonzalez-Andrade PhD: Conception and design of study, Acquisition of data, Drafting of article and/or critical revision. Marco Antonio Sanchez-Corella MD: Conception and design of study, Acquisition of data, Drafting of article and/or critical revision. José Luis Treviño-González MD PhD: Conception and design of study, Acquisition of data: laboratory or clinical, Drafting of article and/or critical revision, Final approval and guarantor of manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Ben-Ami R. Experimental Models to Study the Pathogenesis and Treatment of Mucormycosis. J Fungi (Basel) 2024;10(1):85. doi: 10.3390/jof10010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nucci M, Engelhardt M, Hamed K. Mucormycosis in South America: A review of 143 reported cases. Mycoses. 2019;62(9):730–738. doi: 10.1111/myc.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corzo-León DE, Chora-Hernández LD, Rodríguez-Zulueta AP, Walsh TJ. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: Epidemiology, diagnosis, and outcomes of reported cases. Med Mycol. 2018;56(1):29–43. doi: 10.1093/mmy/myx017. [DOI] [PubMed] [Google Scholar]
- 5.Gutiérrez-Delgado EM, Treviño-González JL, Montemayor-Alatorre A, Ceceñas-Falcón LA, Ruiz-Holguín E, Andrade-Vázquez CJ. Chronic rhino-orbito-cerebral mucormycosis: A case report and review of the literature. Ann Med Surg (Lond) 2016;6:87–91. doi: 10.1016/j.amsu.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aksoy M, Ozcan AA, Ulas B. Prognostic factors and clinical features of rhino-orbital-mucormycosis cases: an update for patient and visual survivals. Int J Ophthalmol. 2024;17(5):916–923. doi: 10.18240/ijo.2024.05.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalid R, Khanum I, Habib K, Ali AS, Farooqi J, Iqbal N. Clinical characteristics, outcome, and factors associated with mortality of pulmonary mucormycosis: a retrospective single-center study from Pakistan. Ther Adv Infect Dis. 2024;11:20499361241251744. doi: 10.1177/20499361241251744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afhami S, Adibimehr A, Mousavi SA, Vaezi M, Montazeri M, Salehi M. Rate, Risk Factors, and Outcomes of Invasive Fungal Infections in Patients with Hematologic Malignancies. Int J Hematol Oncol Stem Cell Res. 2024;18(1):75–82. doi: 10.18502/ijhoscr.v18i1.14746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thanjavur Sethuraman K, Athimanjeri Thiruvengadam J, Ravichandran A, Thoppappatty Sengottaiyan S. Prevalence, predictors, and outcome of pulmonary mucormycosis in COVID-19 associated rhino orbital mucormycosis in a tertiary care center in South India. Curr Med Mycol. 2023;9(3):33–37. doi: 10.22034/cmm.2023.345154.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 12.Nair KS, Alagesan M, Jose D, Yoganathan C, Saravanan R, Karthikeyan K. Clinical Profile and Factors Associated with Adverse Outcomes in Coronavirus Disease 2019-associated Mucormycosis: A Single-centre Study. touchREV Endocrinol. 2023;19(2):73–79. doi: 10.17925/EE.2023.19.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allaw F, Zakhour J, Nahhal SB, Koussa K, Bitar ER, Ghanem A. Mucormycosis: A 14-Year Retrospective Study from a Tertiary Care Center in Lebanon. J Fungi (Basel) 2023;9(8):824. doi: 10.3390/jof9080824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajabi MT, Aghajani A, Rafizadeh SM, Jamshidian Tehrani M, Poursayed Lazarjani SZ, Keshmirshekan MM. COVID-19 associated rhino-orbito-cerebral mucormycosis, risk factors and outcome predictors; a multicentric study. Int Ophthalmol. 2023;43(4):1375–1386. doi: 10.1007/s10792-022-02536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhansali A, Bhadada S, Sharma A, Suresh V, Gupta A, Singh P. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J. 2004;80(949):670–674. doi: 10.1136/pgmj.2003.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gür H, İsmi O, Vayısoğlu Y, Görür K, Arpacı RB, Horasan EŞ. Clinical and surgical factors affecting the prognosis and survival rates in patients with mucormycosis. Eur Arch Otorhinolaryngol. 2022;279(3):1363–1369. doi: 10.1007/s00405-021-06910-6. [DOI] [PubMed] [Google Scholar]
- 17.Poswal L, Bunkar GL, Sharma R, Jain A, Samar N, Mathur N. A Cross-Sectional Study of First Hundred Cases of Rhino-Orbital-Cerebral Mucormycosis Admitted at a Tertiary Care Hospital. Indian J Community Med. 2022;47(3):433–436. doi: 10.4103/ijcm.ijcm_948_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spellberg B, Kontoyiannis DP, Fredricks D, Morris MI, Perfect JR, Chin-Hong PV. Risk factors for mortality in patients with mucormycosis. Med Mycol. 2012;50(6):611–618. doi: 10.3109/13693786.2012.669502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mojtahedi SS, Zarrinfar H, Bakhshaee M. Hematological Indices in COVID-19 Patients with Rhinosinusitis Mucormycosis. Iran J Otorhinolaryngol. 2024;36(2):399–405. doi: 10.22038/IJORL.2024.75276.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho HJ, Jang MS, Hong SD, Chung SK, Kim HY, Dhong HJ. Prognostic factors for survival in patients with acute invasive fungal rhinosinusitis. Am J Rhinol Allergy. 2015;29(1):48–53. doi: 10.2500/ajra.2015.29.4115. [DOI] [PubMed] [Google Scholar]