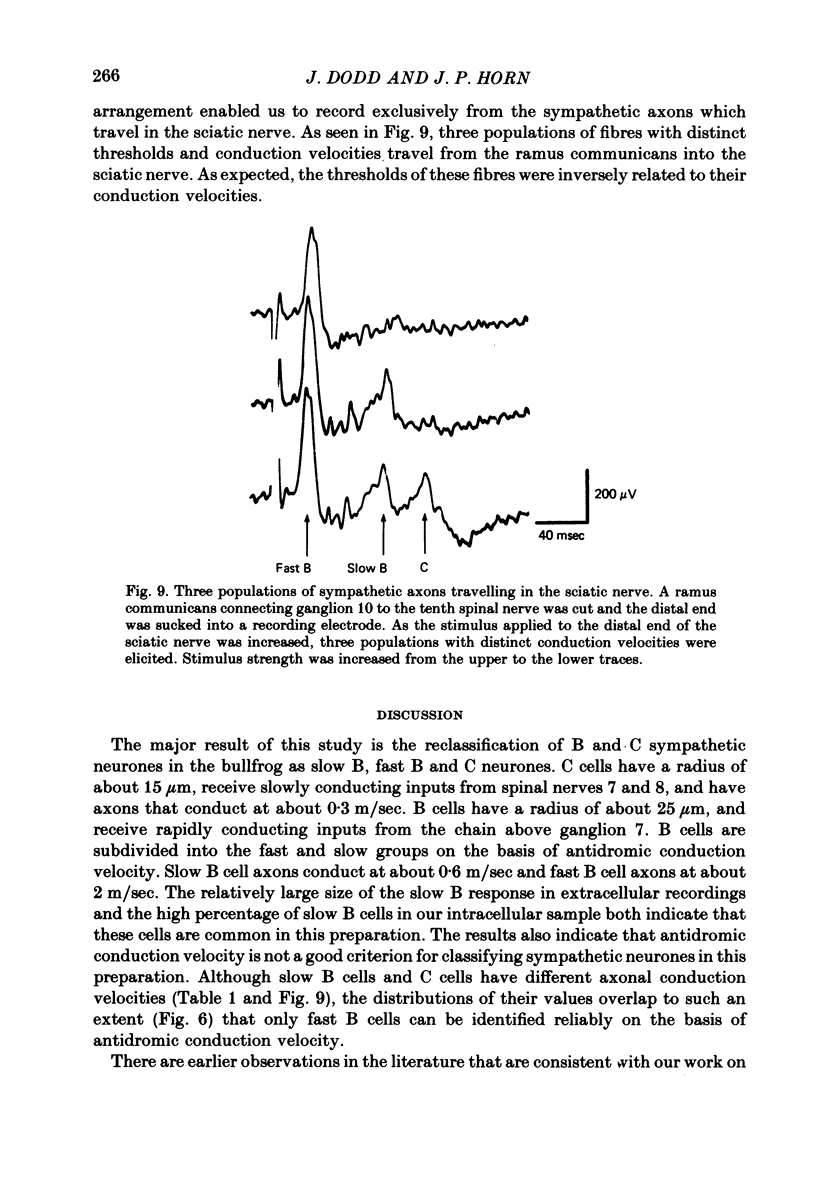
Abstract
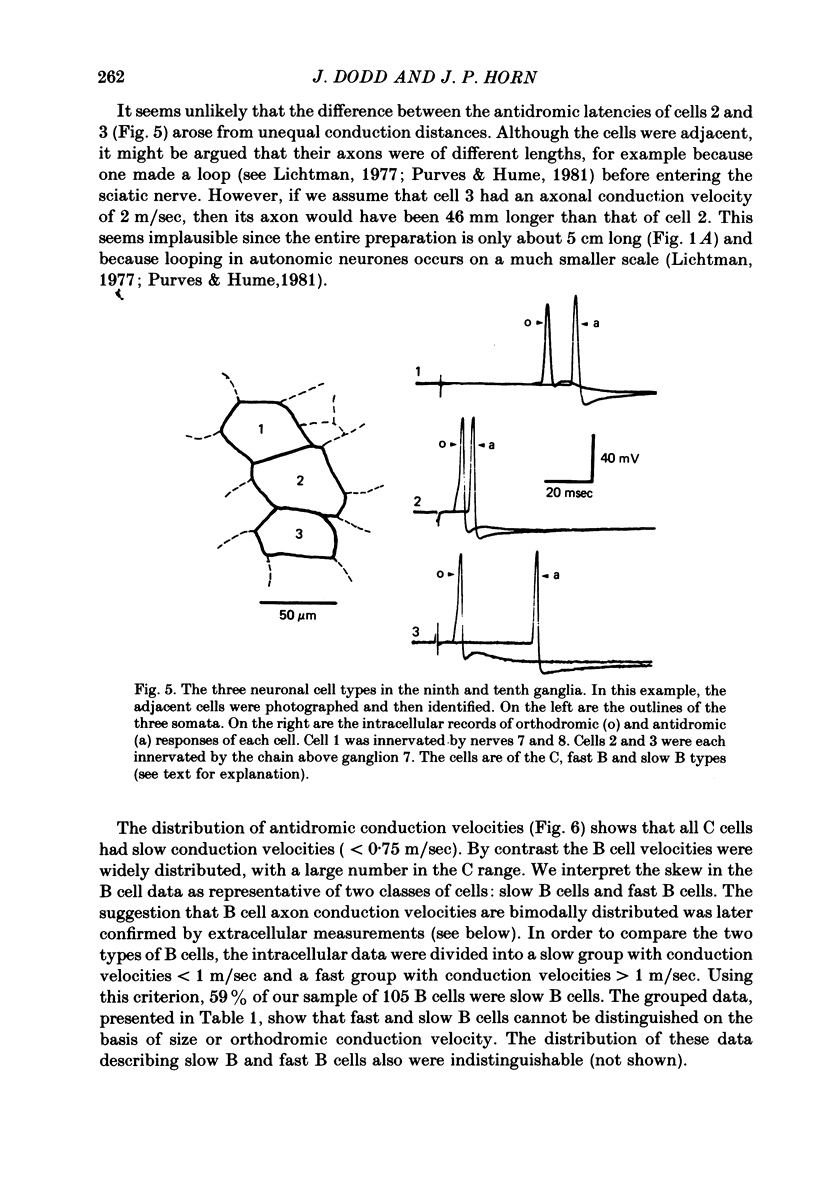
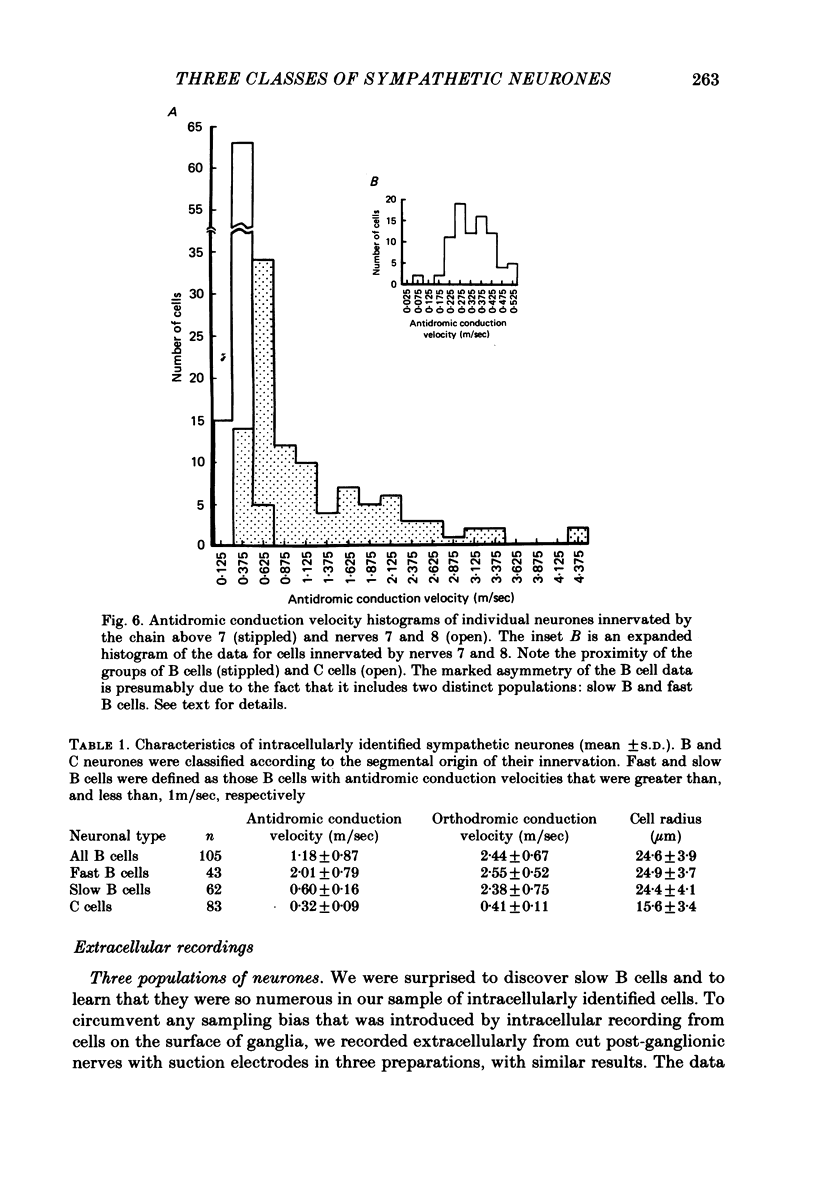
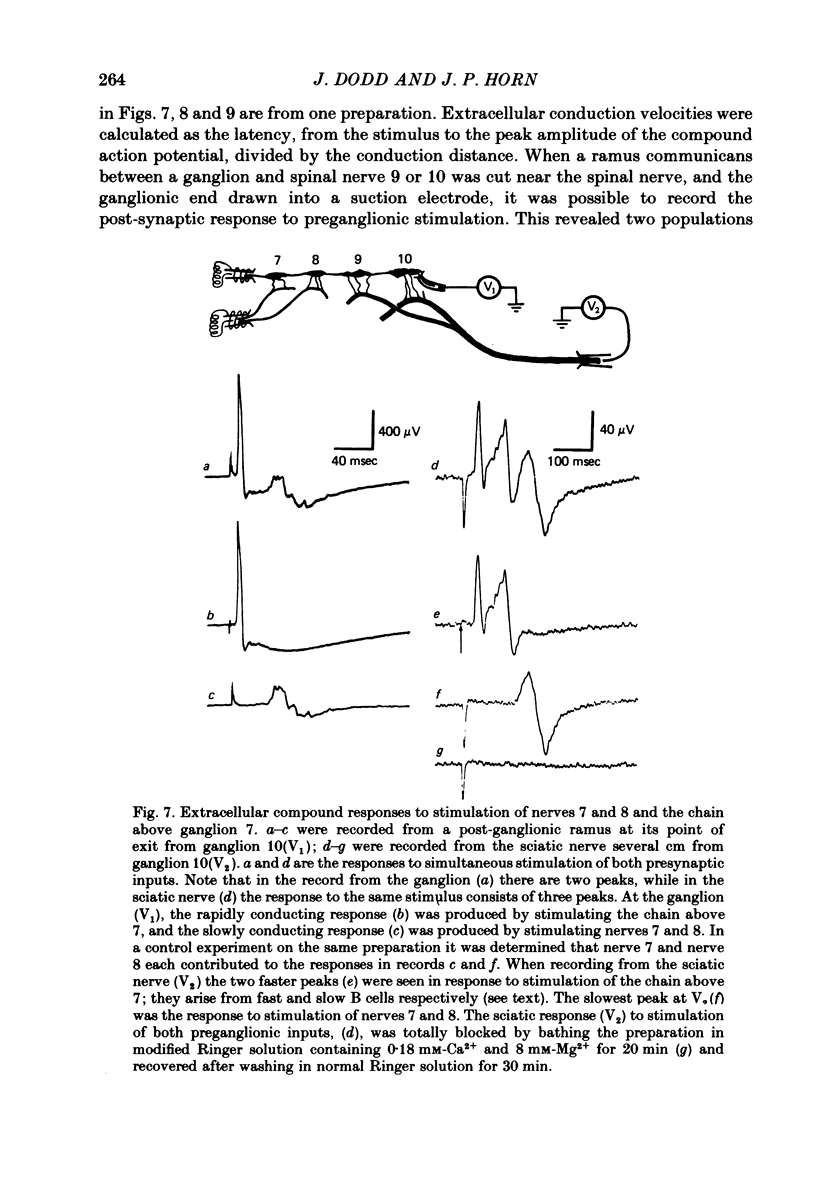
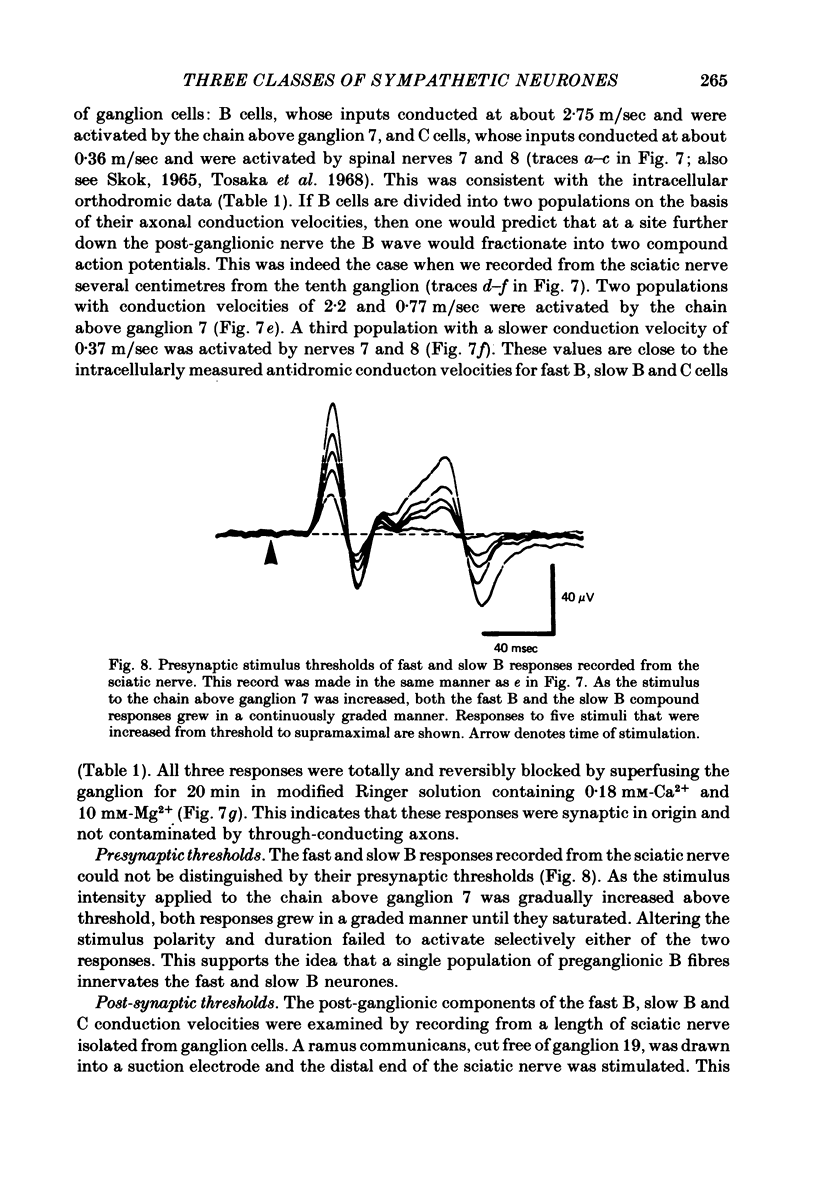
1. The cellular organization of the ninth and tenth paravertebral sympathetic ganglia in the bullfrog was studied with intracellular and extracellular recording methods. An isolated preparation was used in which anatomical details of individual cells could be resolved while making physiological measurements. This permitted the characterization of neurones in terms of their size, the segmental origin of their cholinergic innervation, and their orthodromic and antidromic conduction velocities. With these criteria, three classes of sympathetic neurones were identified. 2. As in previous studies, C cells were distinguished from B cells by the origin of their innervation. C cells are innervated by slowly conducting axons (0.4 m/sec) from spinal nerves 7 and 8 and B cells are innervated by rapidly conducting axons (2.4 m/sec) from the sympathetic chain above ganglion 7. 3. In earlier work it has been suggested that the conduction velocity of a preganglionic axon generally matches that of its target neurone. In this study we have characterized a large group of B cells for which this is not true. The axons of B cells fall into a rapidly conducting group (2.0 m/sec) and a slowly conducting group (0.6 m/sec). In contrast, C neurones, like their preganglionic inputs, have only slowly conducting axons (0.3 m/sec). Consequently, neurones have been classified as C type, fast B type, and slow B type. Fifty-nine percent of the B cells that we studied were slow B cells. These findings were corroborated by measurements of compound extracellular responses in post-ganglionic nerves. 4. Some neurones can be identified also by the size of their cell bodies. C cells are about 30 microns in diameter while B cells are about 50 microns in diameter. In our sample, 96% of the cells with radius less than 16 microns were C cells and 94% of the cells with radius greater than 21 microns were B cells. However, fast B cells could not be distinguished from slow B cells by size.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dodd J., Horn J. P. Muscarinic inhibition of sympathetic C neurones in the bullfrog. J Physiol. 1983 Jan;334:271–291. doi: 10.1113/jphysiol.1983.sp014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. On the presynaptic acetylcholine receptors in sympathetic ganglia of the frog. J Physiol. 1971 Jul;216(1):237–246. doi: 10.1113/jphysiol.1971.sp009521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., LOEWENSTEIN W. R. Nature of neuromuscular facilitation by sympathetic stimulation in the frog. J Physiol. 1955 Dec 29;130(3):559–571. doi: 10.1113/jphysiol.1955.sp005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma S. Functional differentiation in sB and sC neurons of toad sympathetic ganglia. Jpn J Physiol. 1970 Jun 15;20(3):281–295. doi: 10.2170/jjphysiol.20.281. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Dodd J. Monosynaptic muscarinic activation of K+ conductance underlies the slow inhibitory postsynaptic potential in sympathetic ganglia. Nature. 1981 Aug 13;292(5824):625–627. doi: 10.1038/292625a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N., Brownfield M. S. Peptidergic transmitters in synaptic boutons of sympathetic ganglia. Nature. 1980 Nov 27;288(5789):380–382. doi: 10.1038/288380a0. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1501–1505. doi: 10.1073/pnas.76.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Analysis of the slow excitatory postsynaptic potential in bullfrog sympathetic ganglion cells. Jpn J Physiol. 1976;26(6):651–669. doi: 10.2170/jjphysiol.26.651. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W. Slow synaptic responses in autonomic ganglia and the pursuit of a peptidergic transmitter. J Exp Biol. 1980 Dec;89:257–286. doi: 10.1242/jeb.89.1.257. [DOI] [PubMed] [Google Scholar]
- Libet B., Chichibu S., Tosaka T. Slow synaptic responses and excitability in sympathetic ganglia of the bullfrog. J Neurophysiol. 1968 May;31(3):383–395. doi: 10.1152/jn.1968.31.3.383. [DOI] [PubMed] [Google Scholar]
- Lichtman J. W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol. 1977 Dec;273(1):155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott A. B., Connor E. A., Dionne V. E., Parsons R. L. Voltage clamp study of fast excitatory synaptic currents in bullfrog sympathetic ganglion cells. J Gen Physiol. 1980 Jan;75(1):39–60. doi: 10.1085/jgp.75.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L. M. Synaptic localization of alpha-bungarotoxin binding which blocks nicotinic transmission at frog sympathetic neurons. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1948–1952. doi: 10.1073/pnas.78.3.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., Soeda H., Koketsu K. Studies on sympathetic B and C neurons and patterns of pregnaglionic innervation. J Cell Physiol. 1965 Aug;66(1):19–32. doi: 10.1002/jcp.1030660103. [DOI] [PubMed] [Google Scholar]
- Purves D., Hume R. I. The relation of postsynaptic geometry to the number of presynaptic axons that innervate autonomic ganglion cells. J Neurosci. 1981 May;1(5):441–452. doi: 10.1523/JNEUROSCI.01-05-00441.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOK V. I. CONDUCTION IN TENTH GANGLION OF THE FROG SYMPATHETIC TRUNK. Fed Proc Transl Suppl. 1965 Mar-Apr;24:363–367. [PubMed] [Google Scholar]
- Tosaka T., Chichibu S., Libet B. Intracellular analysis of slow inhibitors and excitatory postsynaptic potentials in sympathetic ganglia of the frog. J Neurophysiol. 1968 May;31(3):396–409. doi: 10.1152/jn.1968.31.3.396. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Padjen A. Acetylcholine and slow synaptic inhibition in frog sympathetic ganglion cells. Brain Res. 1973 May 30;55(1):225–228. doi: 10.1016/0006-8993(73)90506-4. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Padjen A. Slow synaptic inhibition: evidence for synaptic inactivation of sodium conductance in sympathetic ganglion cells. Brain Res. 1973 May 30;55(1):219–224. doi: 10.1016/0006-8993(73)90505-2. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Weitsen H. A. Identification of small intensely fluorescent (SIF) cells as chromaffin cells in bullfrog sympathetic ganglia. Brain Res. 1977 Jun 10;128(2):213–226. doi: 10.1016/0006-8993(77)90989-1. [DOI] [PubMed] [Google Scholar]
- Weitsen H. A., Weight F. F. Synaptic innervation of sympathetic ganglion cells in the bullfrog. Brain Res. 1977 Jun 10;128(2):197–211. doi: 10.1016/0006-8993(77)90988-x. [DOI] [PubMed] [Google Scholar]