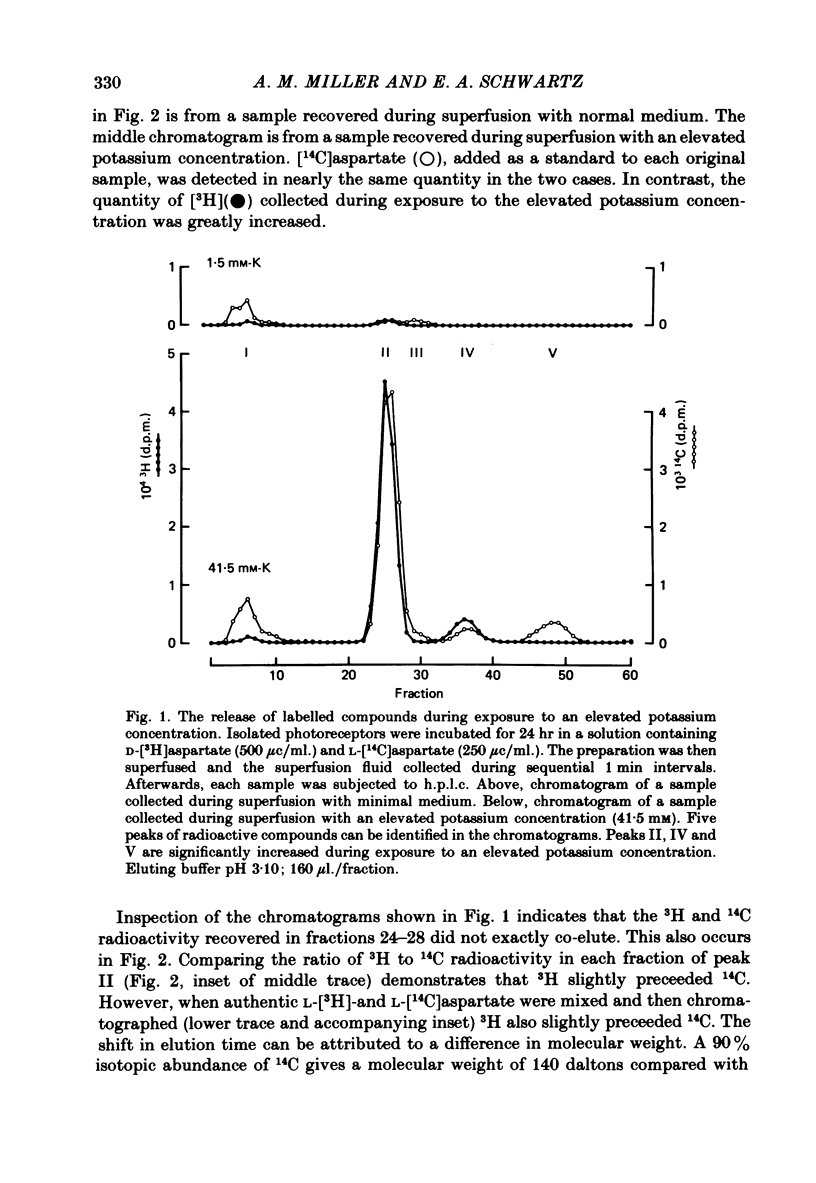
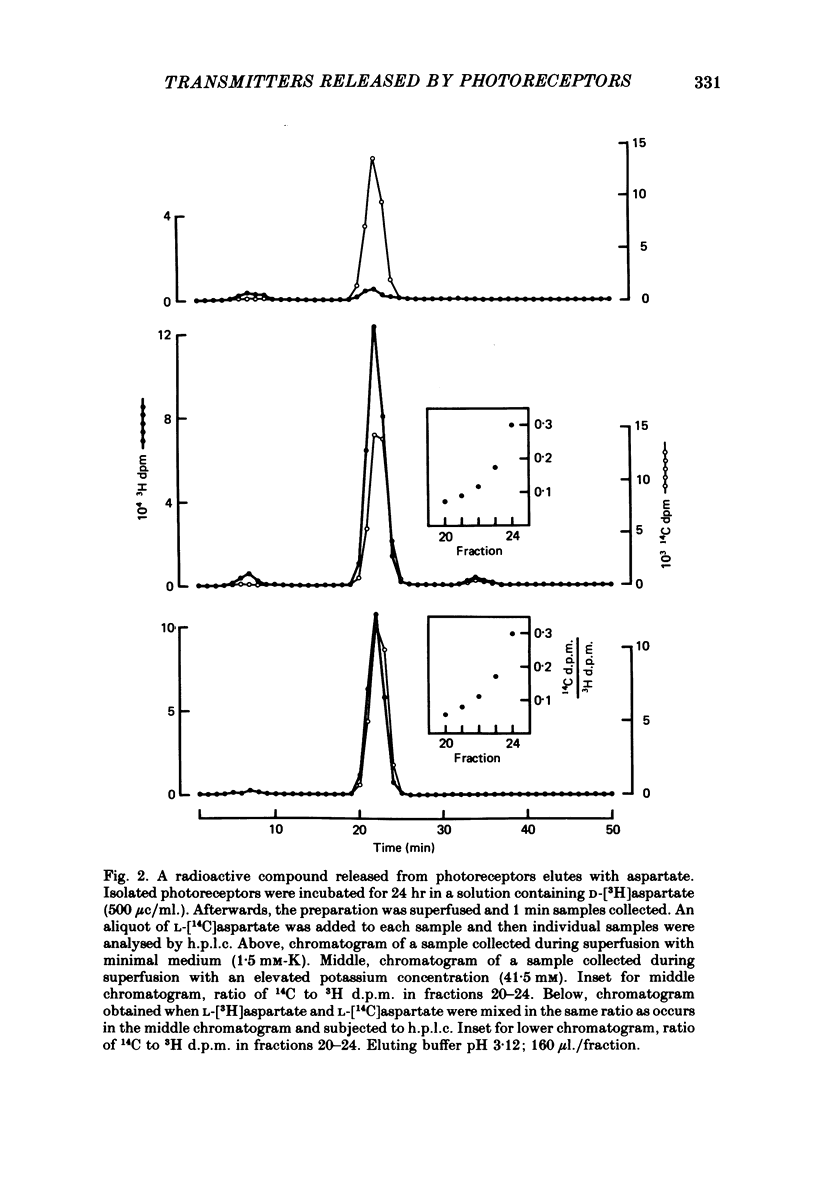
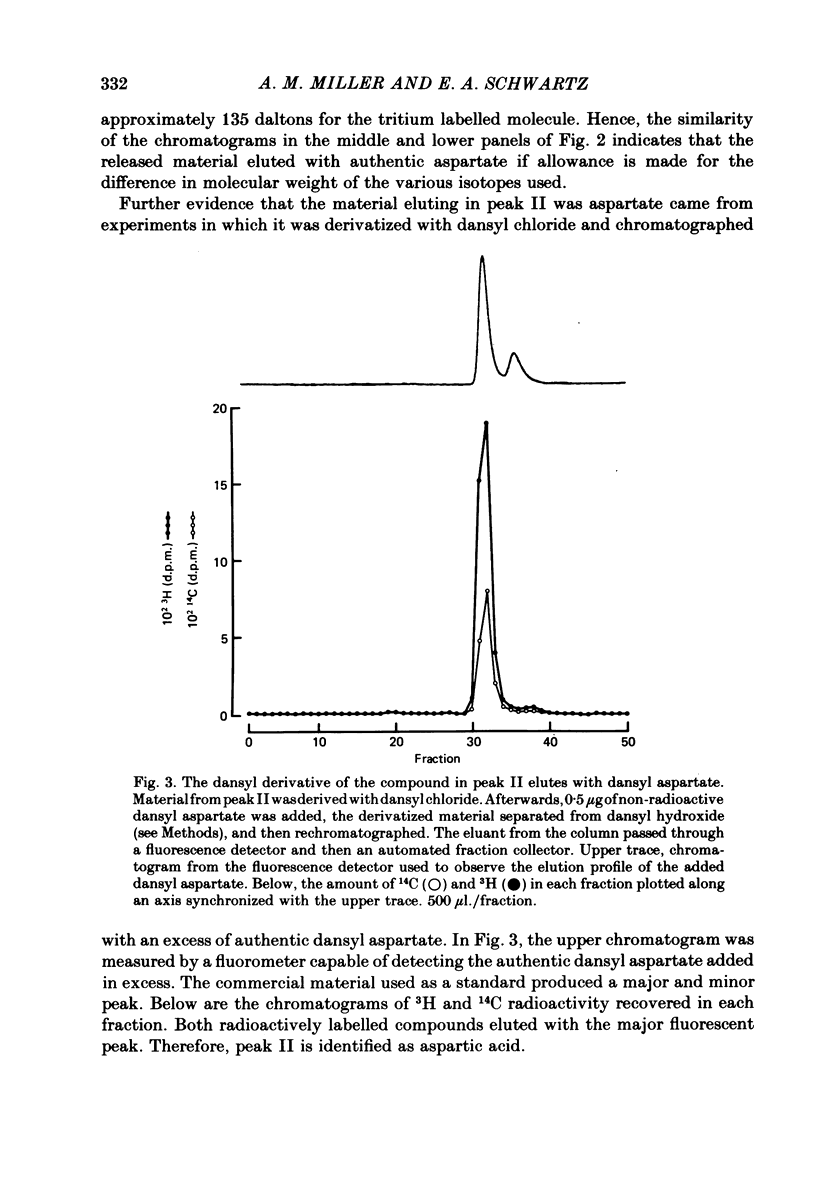
Abstract
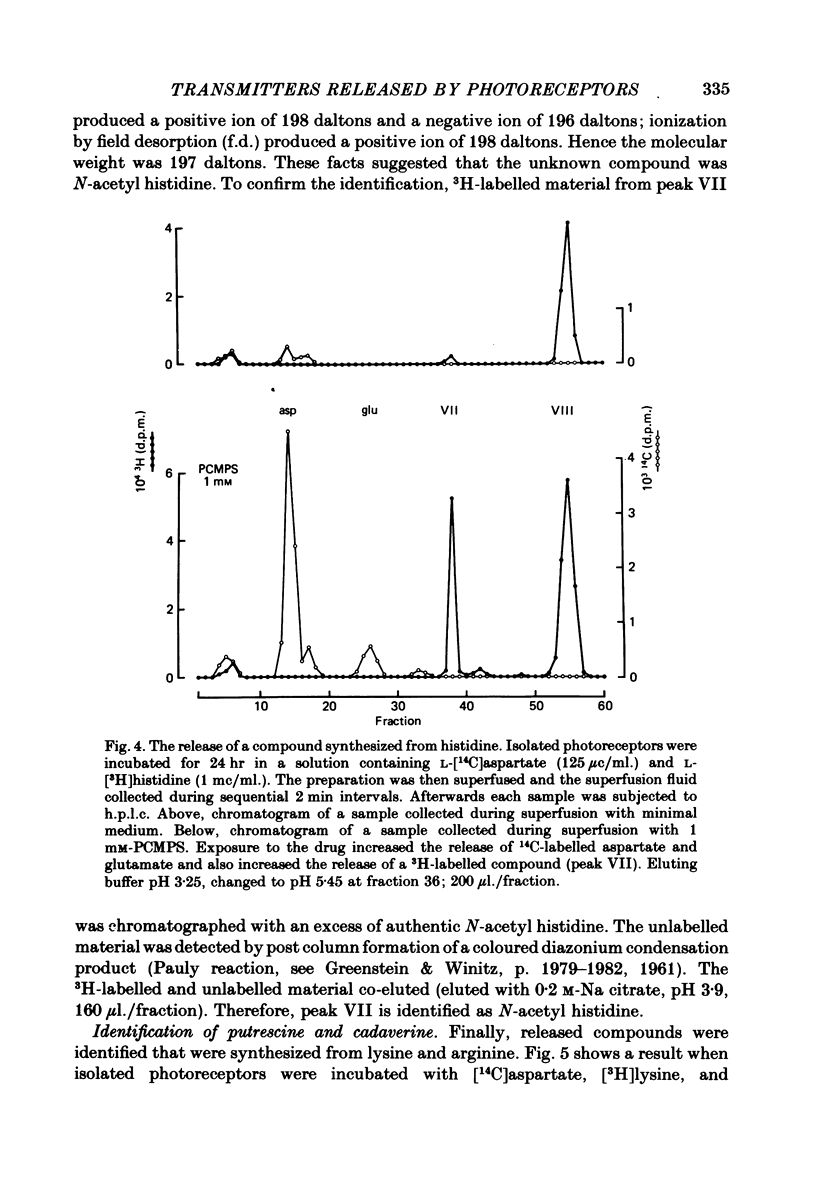
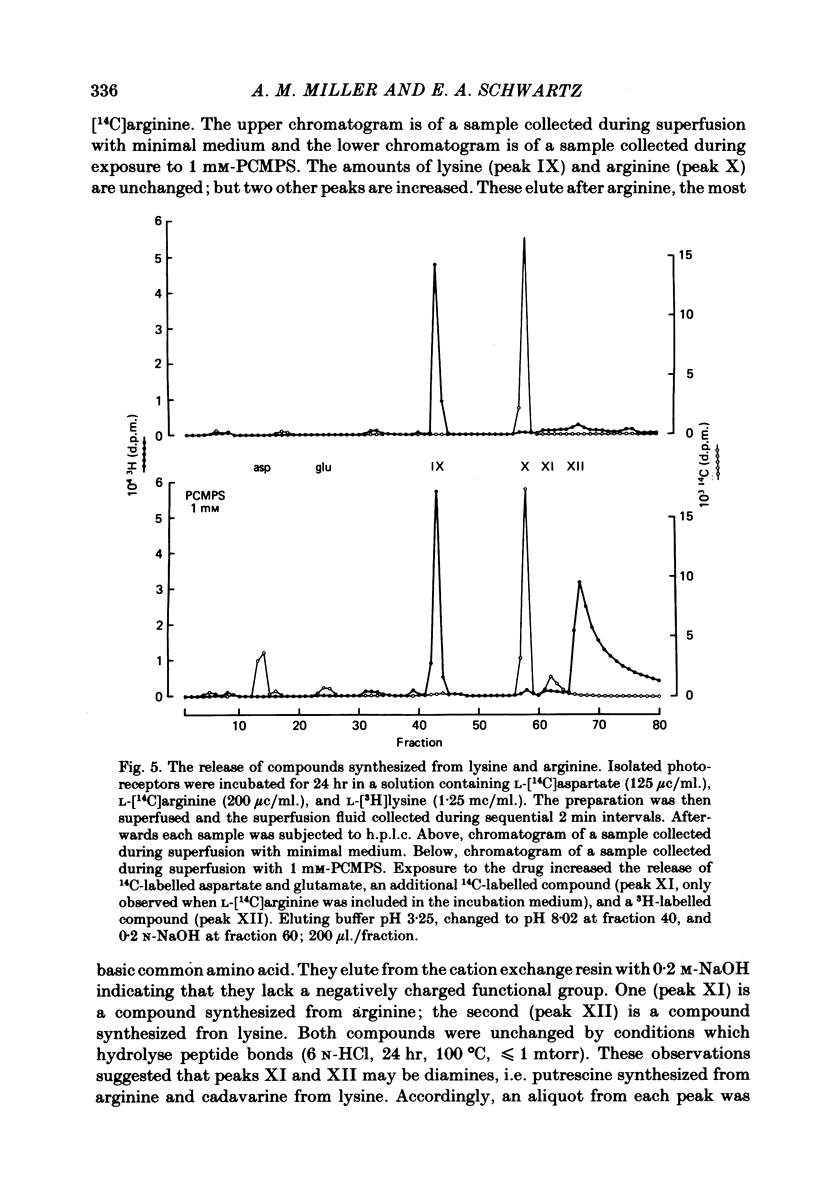
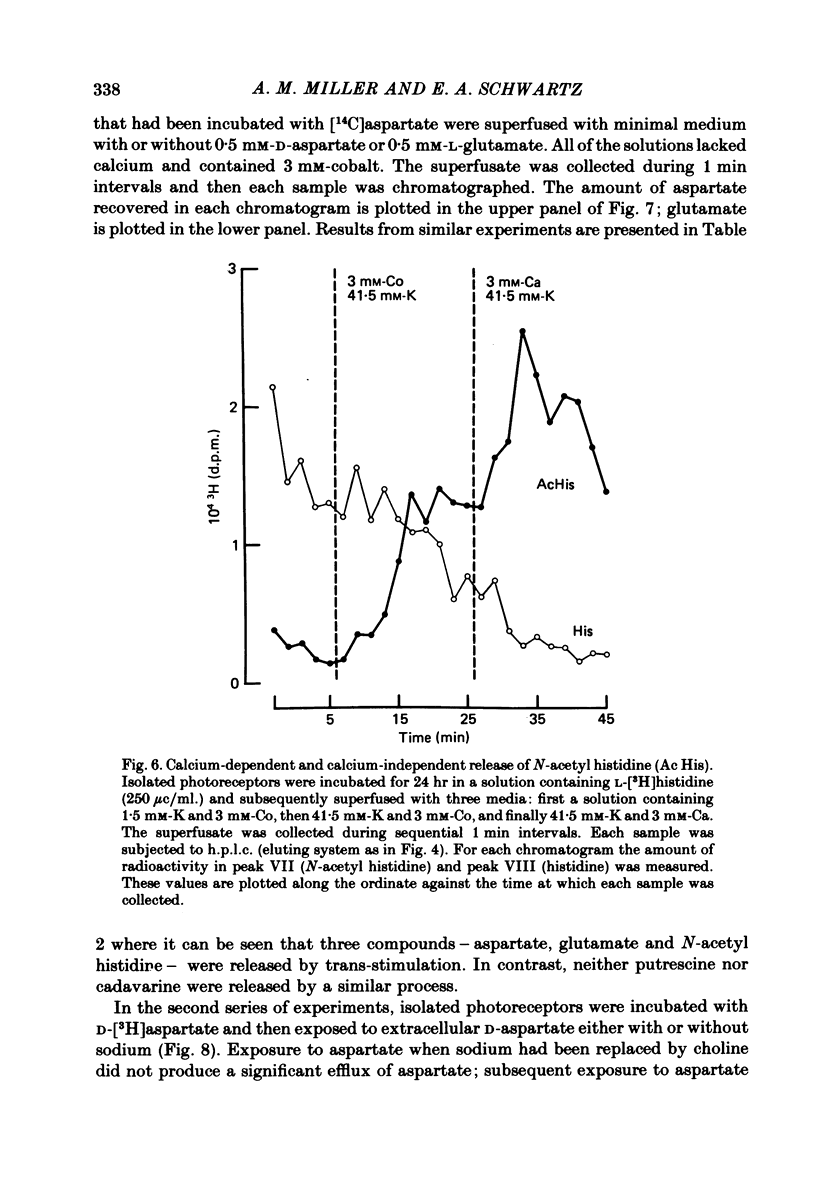
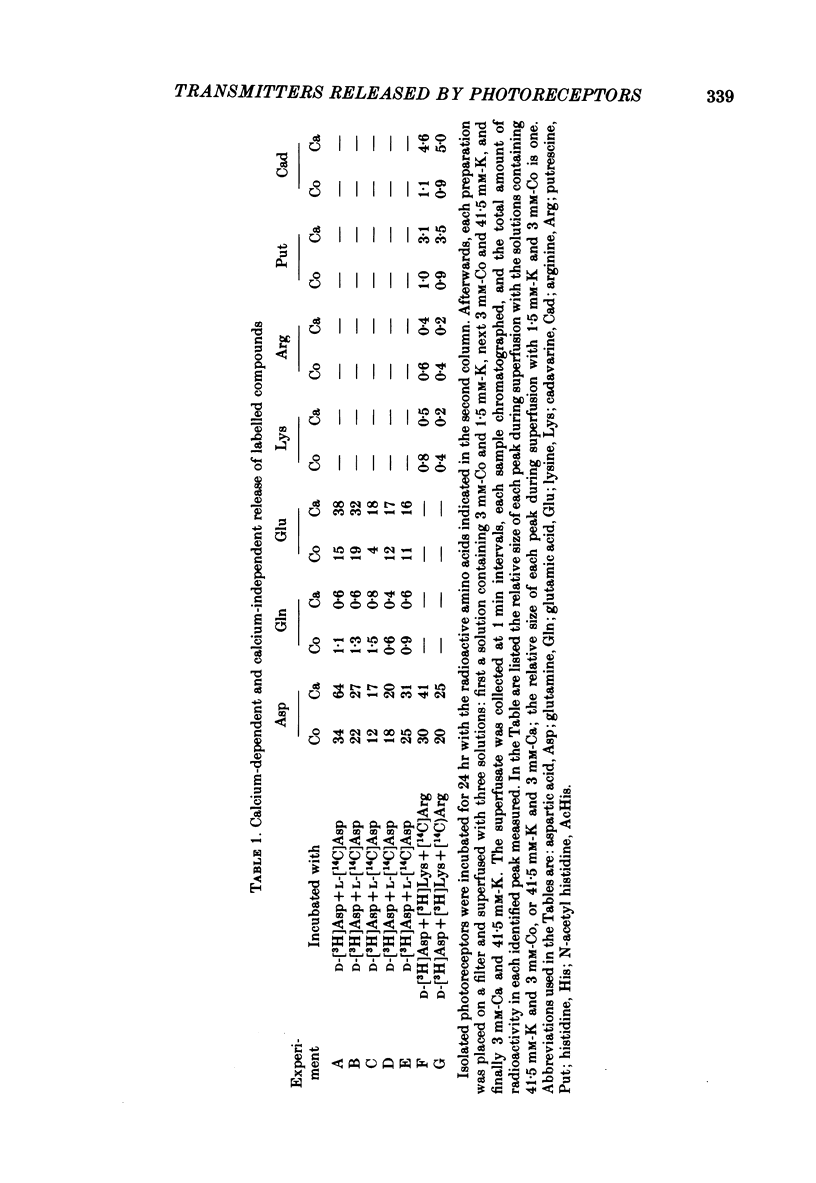
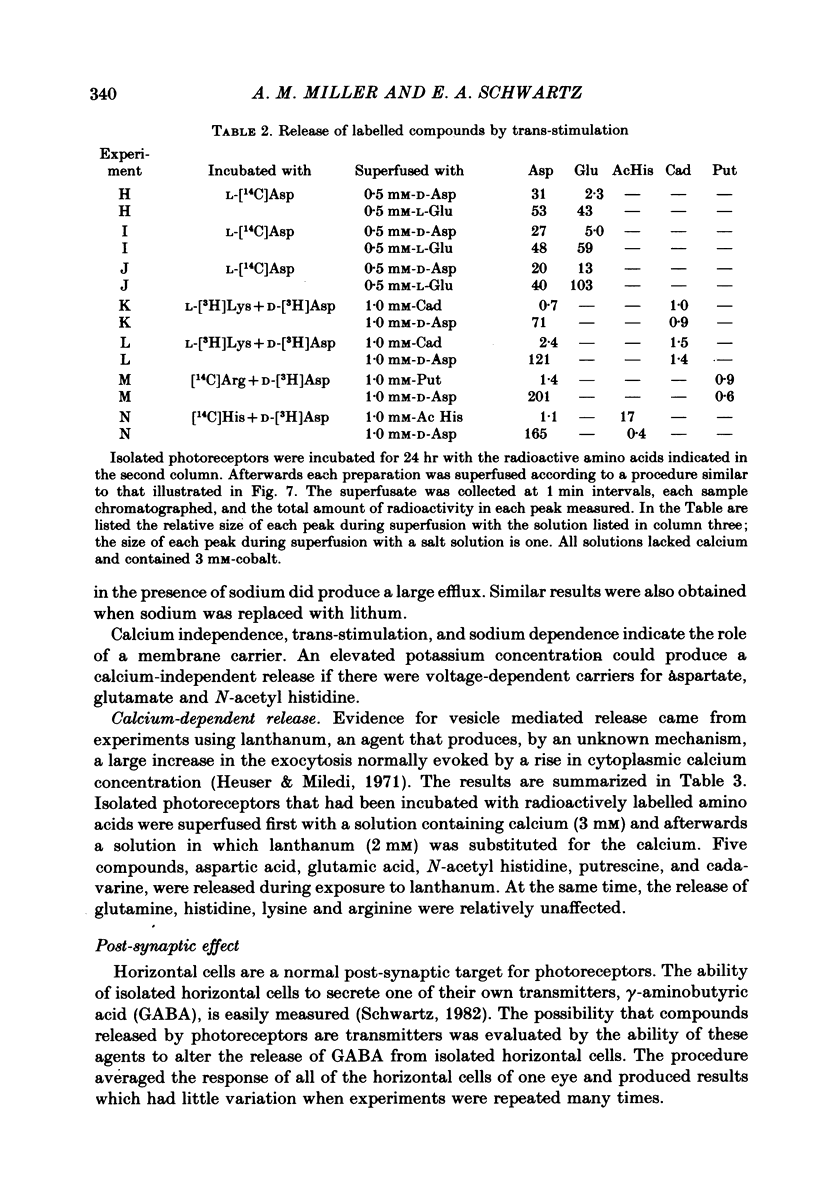
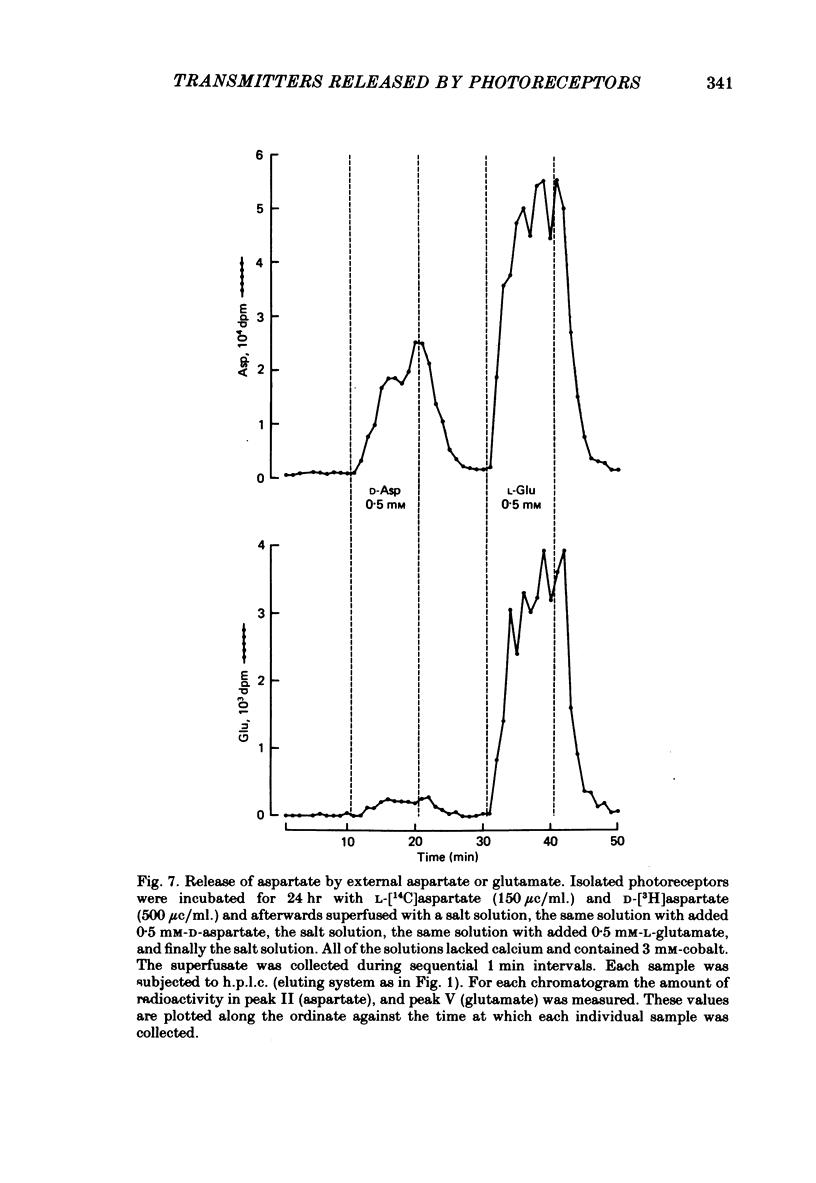
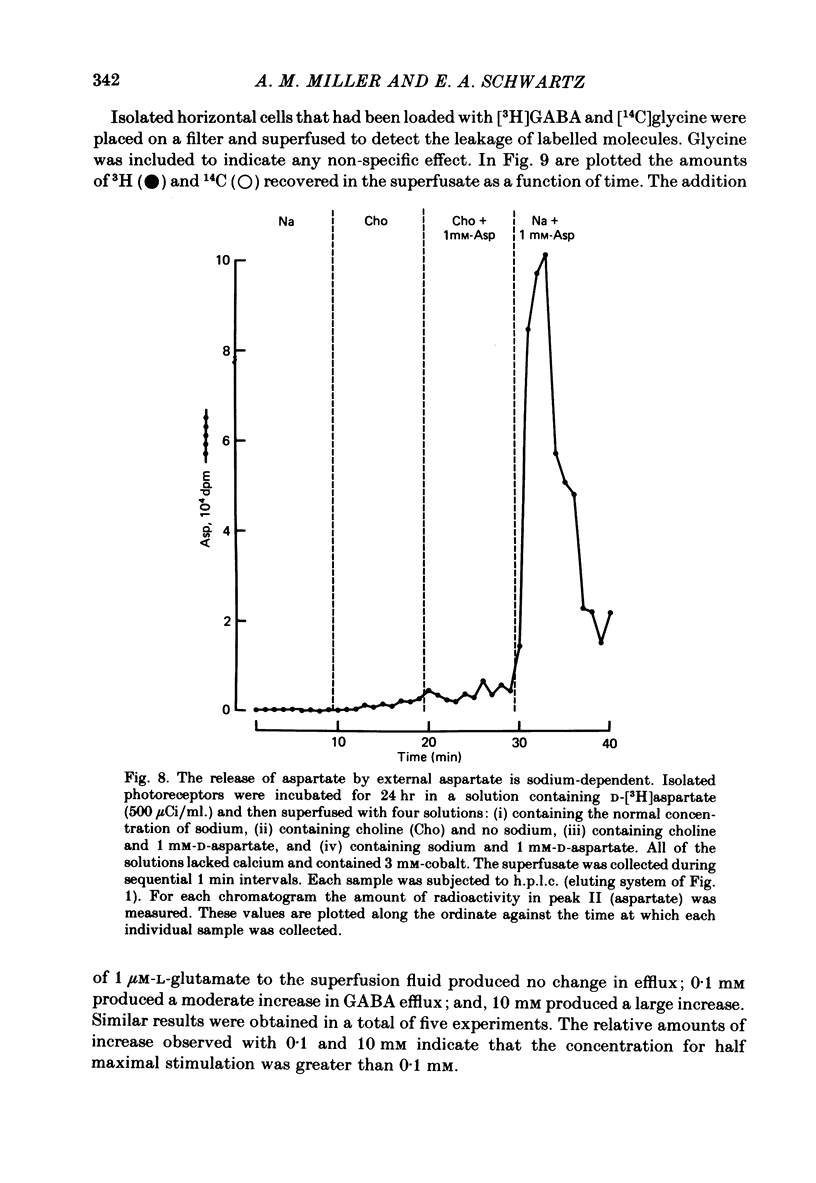
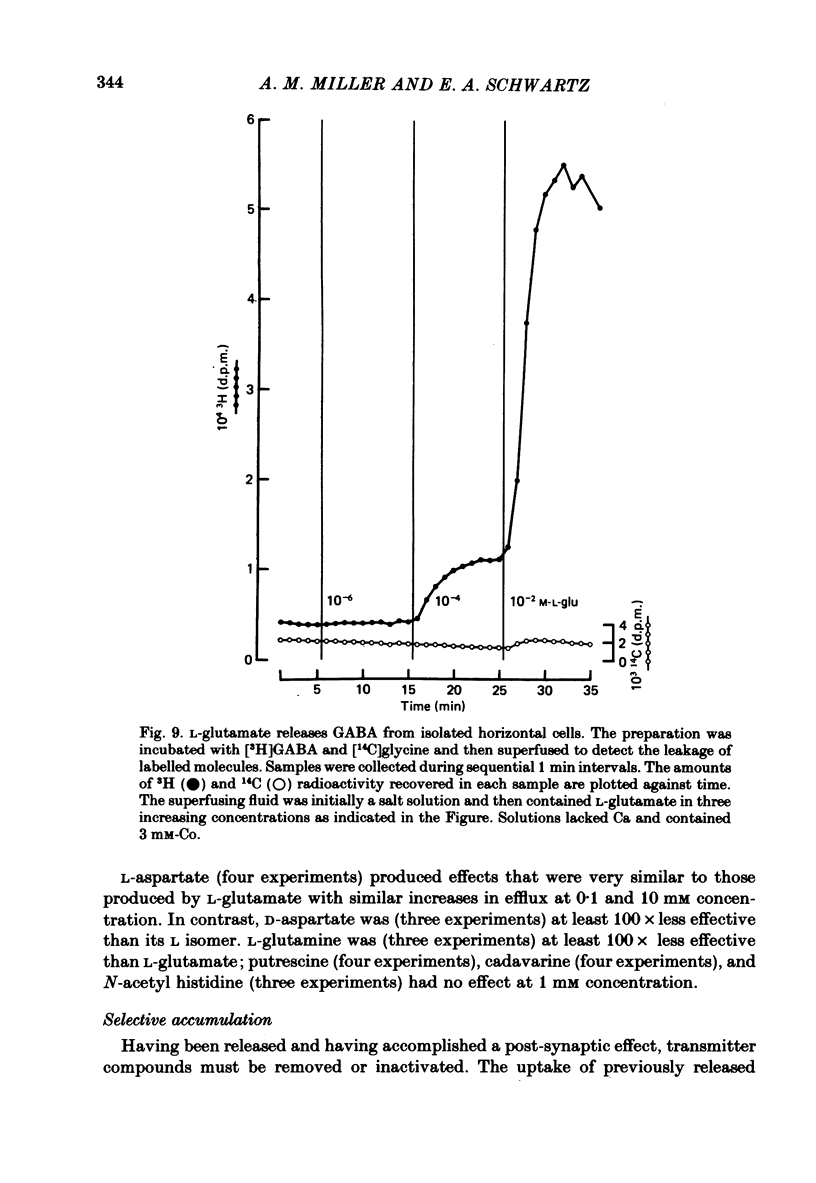
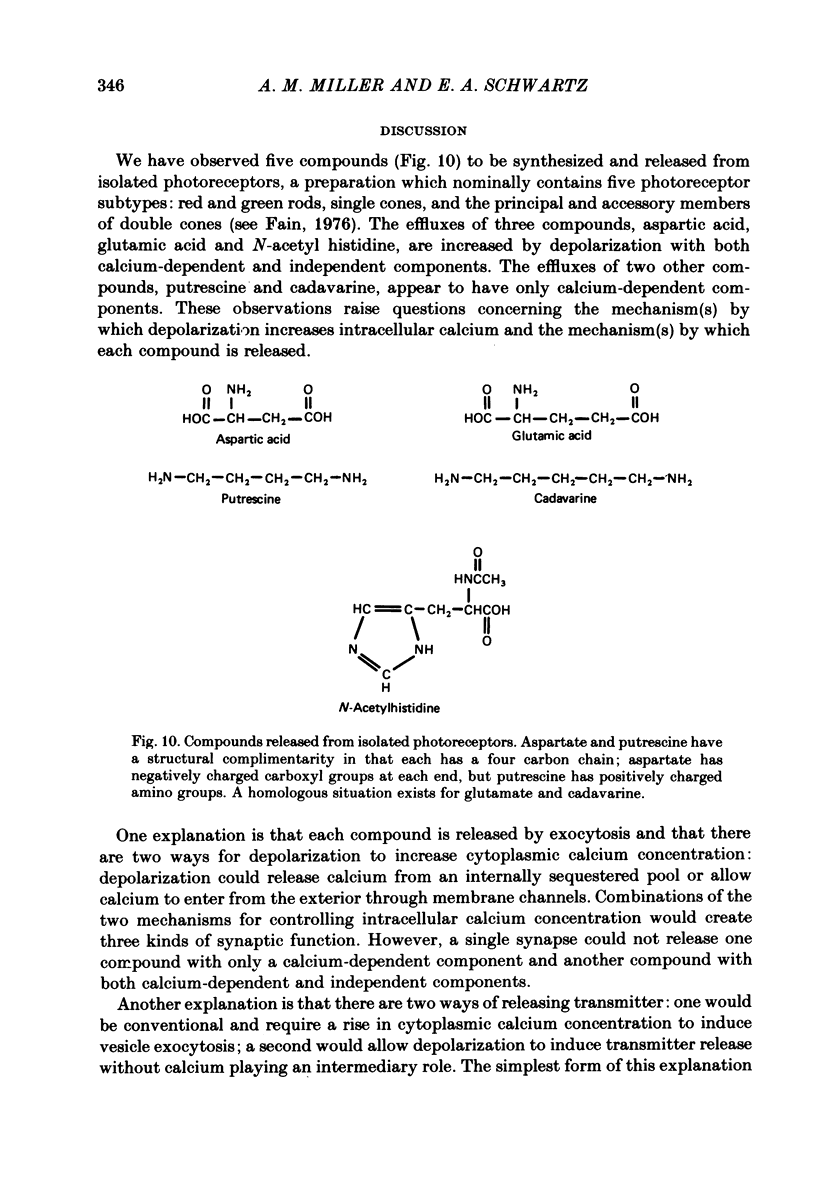
1. When toad retinae were incubated with veratrine, kainic acid, and L-alpha-aminoadipic acid, photoreceptor cells survived and most other neurones died. This preparation of 'isolated' photoreceptor cells accumulated radioactive molecules from the incubation medium and metabolized these into labelled compounds. When a preparation was placed on a filter and superfused, radioactive molecules which were released into the superfusion fluid could be collected and later analysed. Several procedures were used for inducing the release of possible transmitter compounds. Each released compound was chemically identified. 2. Three compounds, aspartic acid, glutamic acid, and N-acetyl histidine, were released when the potassium concentration was increased in media that lacked calcium and contained cobalt. 3. The release of these compounds was further increased when cobalt was removed and calcium returned to the extracellular medium. 4. Two additional compounds, putrescine and cadavarine, were also released during depolarization when calcium was present. 5. The efflux of each of the compounds listed in Section 2 was also increased by homo- and hetero-exchange. For at least aspartate, exchange was sodium-dependent. 6. The post-synaptic effect of released compounds was tested by their ability to increase the efflux of [3H]GABA from 'isolated' horizontal cells. 0 . 1 mM-L-aspartate, or L-glutamate produced an increase in GABA efflux. N-acetyl histidine, putrescine, and cadavarine were ineffective. 7. Isolated photoreceptors and intact retinae were incubated with [3H]aspartate, or [3H]putrescine. Subsequent histology and autoradiography demonstrated that both compounds were selectively accumulated by cones.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byzov A. L., Cervetto L. Effects of applied currents on turtle cones in darkness and during the photoresponse. J Physiol. 1977 Feb;265(1):85–102. doi: 10.1113/jphysiol.1977.sp011706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byzov A. L., Trifonov J. A. The response to electric stimulation of horizontal cells in the carp retina. Vision Res. 1968 Jul;8(7):817–822. doi: 10.1016/0042-6989(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Carmody J. J. Enhancement of acetylcholine secretion by two sulfhydryl reagents. Eur J Pharmacol. 1978 Feb 15;47(4):457–460. doi: 10.1016/0014-2999(78)90127-9. [DOI] [PubMed] [Google Scholar]
- Cervetto L., MacNichol E. F., Jr Inactivation of horizontal cells in turtle retina by glutamate and aspartate. Science. 1972 Nov 17;178(4062):767–768. doi: 10.1126/science.178.4062.767. [DOI] [PubMed] [Google Scholar]
- Cervetto L., Piccolino M. Synaptic transmission between photoreceptors and horizontal cells in the turtle retina. Science. 1974 Feb 1;183(4123):417–419. doi: 10.1126/science.183.4123.417. [DOI] [PubMed] [Google Scholar]
- Davies L. P., Johnston G. A. Uptake and release of D- and L-aspartate by rat brain slices. J Neurochem. 1976 May;26(5):1007–1014. doi: 10.1111/j.1471-4159.1976.tb06485.x. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Ishida A., Poisner A. M. The effect of metabolic inhibitors on the release of vasopressin from the isolated neurohypophysis. J Physiol. 1965 Dec;181(4):753–759. doi: 10.1113/jphysiol.1965.sp007795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E. Synaptic organization of the frog retina: an electron microscopic analysis comparing the retinas of frogs and primates. Proc R Soc Lond B Biol Sci. 1968 Jun 11;170(1019):205–228. doi: 10.1098/rspb.1968.0034. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Falck B. Autoradiography of some suspected neurotransmitter substances: GABA glycine, glutamic acid, histamine, dopamine, and L-dopa. Brain Res. 1971 Oct 8;33(1):157–172. doi: 10.1016/0006-8993(71)90314-3. [DOI] [PubMed] [Google Scholar]
- FURUKAWA T., HANAWA I. Effects of some common cations on electroretinogram of the toad. Jpn J Physiol. 1955 Dec 15;5(4):289–300. doi: 10.2170/jjphysiol.5.289. [DOI] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J., Miledi R. Effects of lanthanum ions on function and structure of frog neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1971 Dec 14;179(1056):247–260. doi: 10.1098/rspb.1971.0096. [DOI] [PubMed] [Google Scholar]
- Iversen L. L. Neuronal uptake processes for amines and amino acids. Adv Biochem Psychopharmacol. 1970;2:109–132. [PubMed] [Google Scholar]
- Kaneko A., Shimazaki H. Synaptic transmission from photoreceptors to bipolar and horizontal cells in the carp retina. Cold Spring Harb Symp Quant Biol. 1976;40:537–546. doi: 10.1101/sqb.1976.040.01.050. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Lateral contacts and interactions of horizontal cell dendrites in the retina of the larval tiger salamander. J Physiol. 1980 Apr;301:59–68. doi: 10.1113/jphysiol.1980.sp013188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J., Voaden M. J. Letter: Further observations on the uptake of (3H)glycine by the isolated retina of the frog. Exp Eye Res. 1976 Feb;22(2):189–191. doi: 10.1016/0014-4835(76)90045-2. [DOI] [PubMed] [Google Scholar]
- Murakami M., Otsu K., Otsuka T. Effects of chemicals on receptors and horizontal cells in the retina. J Physiol. 1972 Dec;227(3):899–913. doi: 10.1113/jphysiol.1972.sp010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Otsuka T., Shimazaki H. Effects of aspartate and glutamate on the bipolar cells in the carp retina. Vision Res. 1975 Mar;15(3):456–458. doi: 10.1016/0042-6989(75)90101-7. [DOI] [PubMed] [Google Scholar]
- Schofield J. G. Effect of sulphydryl reagents on the release of ox growth hormone in vitro. Biochim Biophys Acta. 1971 Dec 21;252(3):516–525. doi: 10.1016/0304-4165(71)90154-1. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Calcium-independent release of GABA from isolated horizontal cells of the toad retina. J Physiol. 1982 Feb;323:211–227. doi: 10.1113/jphysiol.1982.sp014069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Electrical properties of the rod syncytium in the retina of the turtle. J Physiol. 1976 May;257(2):379–406. doi: 10.1113/jphysiol.1976.sp011374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells R. A., Falk G., Naghshineh S. Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature. 1981 Dec 10;294(5841):592–594. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- Wu S. M., Dowling J. E. L-aspartate: evidence for a role in cone photoreceptor synaptic transmission in the carp retina. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5205–5209. doi: 10.1073/pnas.75.10.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]