Abstract
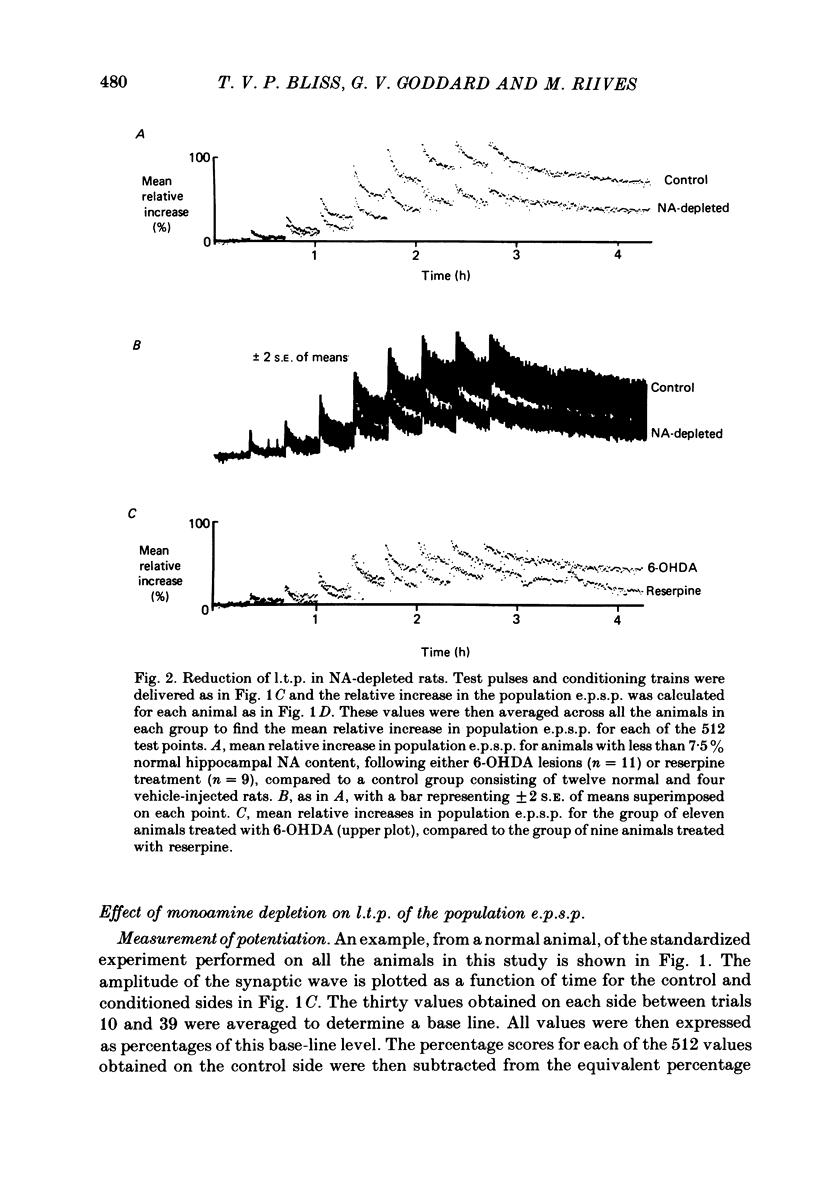
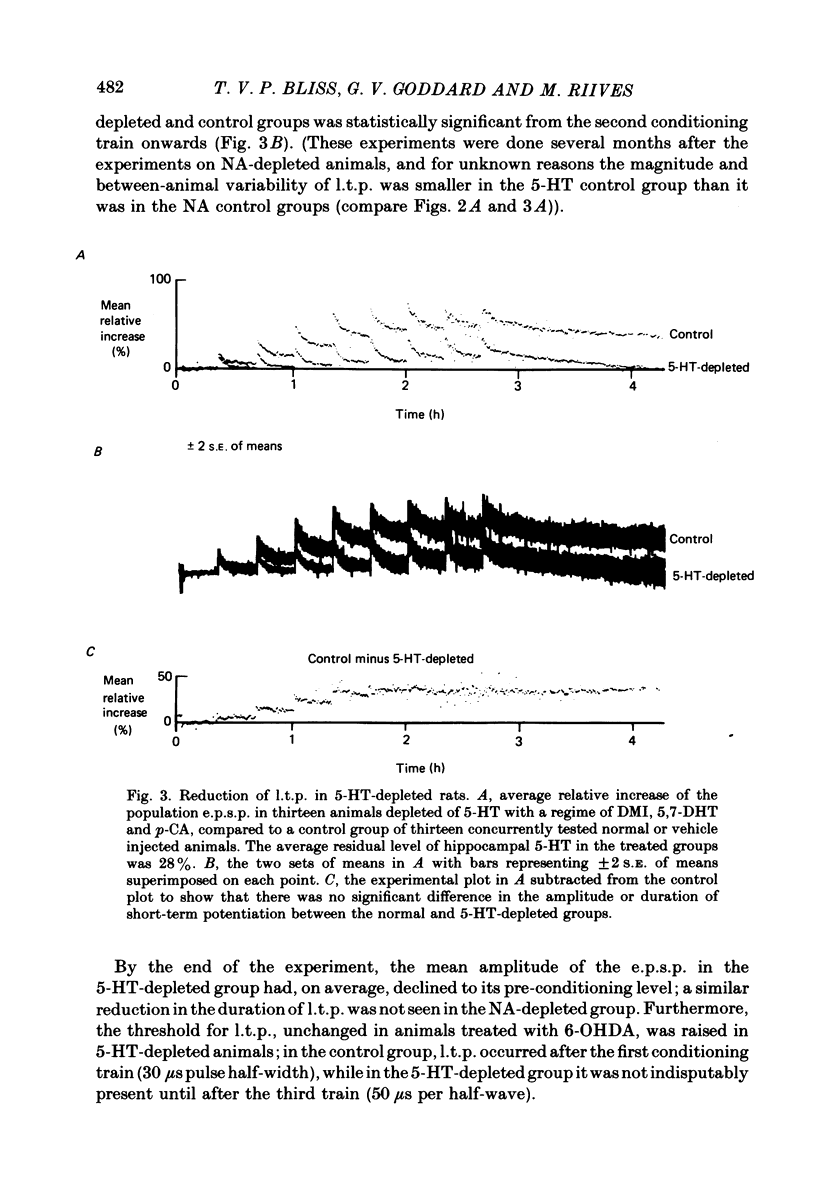
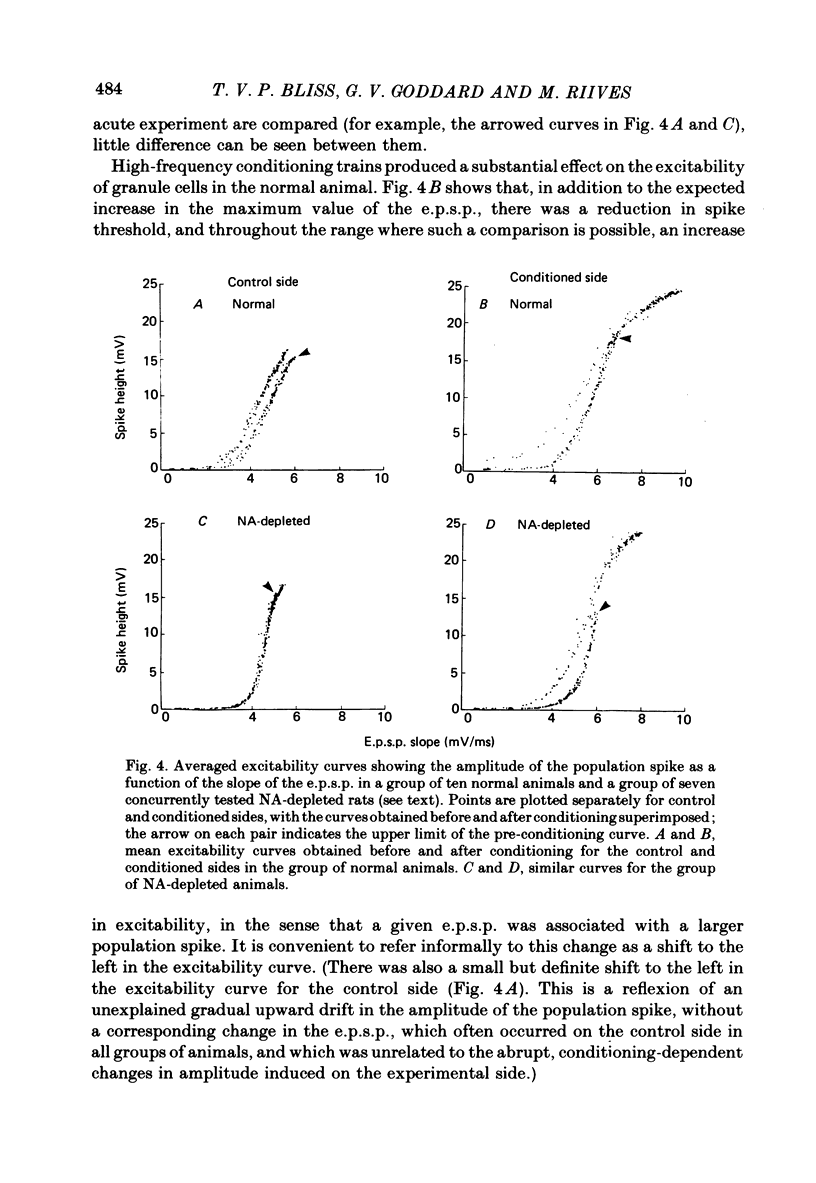
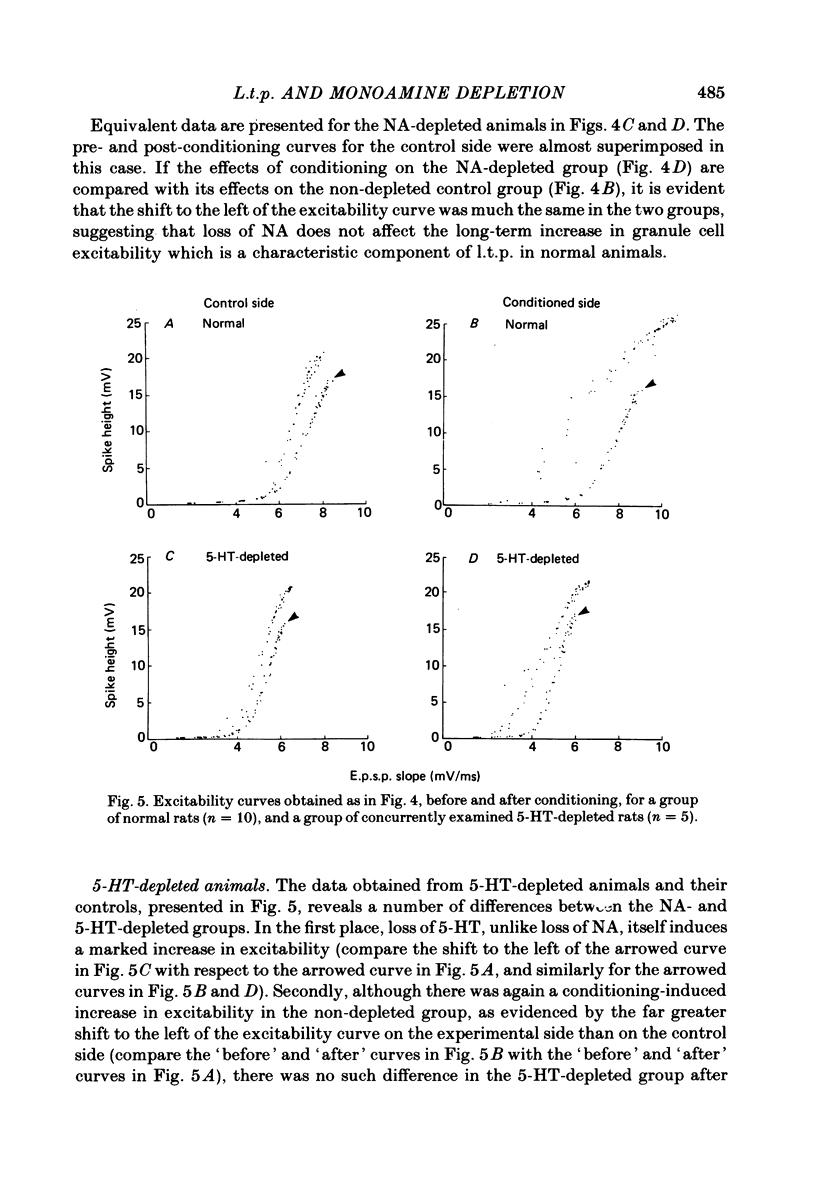
1. Brief, high-frequency stimulation of the perforant path results in a long-term potentiation (l.t.p.) of the field response evoked in the dentate gyrus by single shocks to the perforant path. We have compared the magnitude and duration of l.t.p. in normal, anaesthetized rats with animals depleted of noradrenaline (NA), 5-hydroxytryptamine (5-HT), or both. 2. All animals were exposed to an identical sequence of eight high-frequency trains of increasing intensity given over a period of 140 min to the perforant path of one hemisphere. The potential evoked by test shocks to the perforant path was monitored in both hemispheres throughout this period and for a further 96 min after the last train. 3. Plots of the mean potentiation of the population e.p.s.p. as a function of time were computed for all animals in each group. L.t.p. in the NA-depleted group was about 50% of that in the non-depleted control group throughout the course of the experiment. L.t.p. in the 5-HT-depleted group was more severely affected; mean potentiation did not exceed 30% of that in the control group at any time. 4. The duration of l.t.p. was unaffected by NA depletion and reduced by 5-HT depletion. 5. The threshold for the intensity of high-frequency current pulses necessary to elicit l.t.p. was unaffected by NA depletion and raised by 5-HT depletion. 6. Short-term potentiation of the population e.p.s.p. was unaffected by either NA depletion or 5-HT depletion. 7. The effect of monoamine depletion on granule cell excitability was investigated. 5-HT depletion, but not NA depletion, induced an increase in the excitability of the granule cell population, in the sense that a population e.p.s.p. of a given size was associated with a larger population spike. 8. Long-term potentiation of granule cell excitability was not affected by NA depletion, but was reduced by 5-HT depletion. 9. These results show that monoamines can modulate long-term changes in synaptic function in the dentate gyrus, and suggest that 5-HT is more potent in this respect than NA.
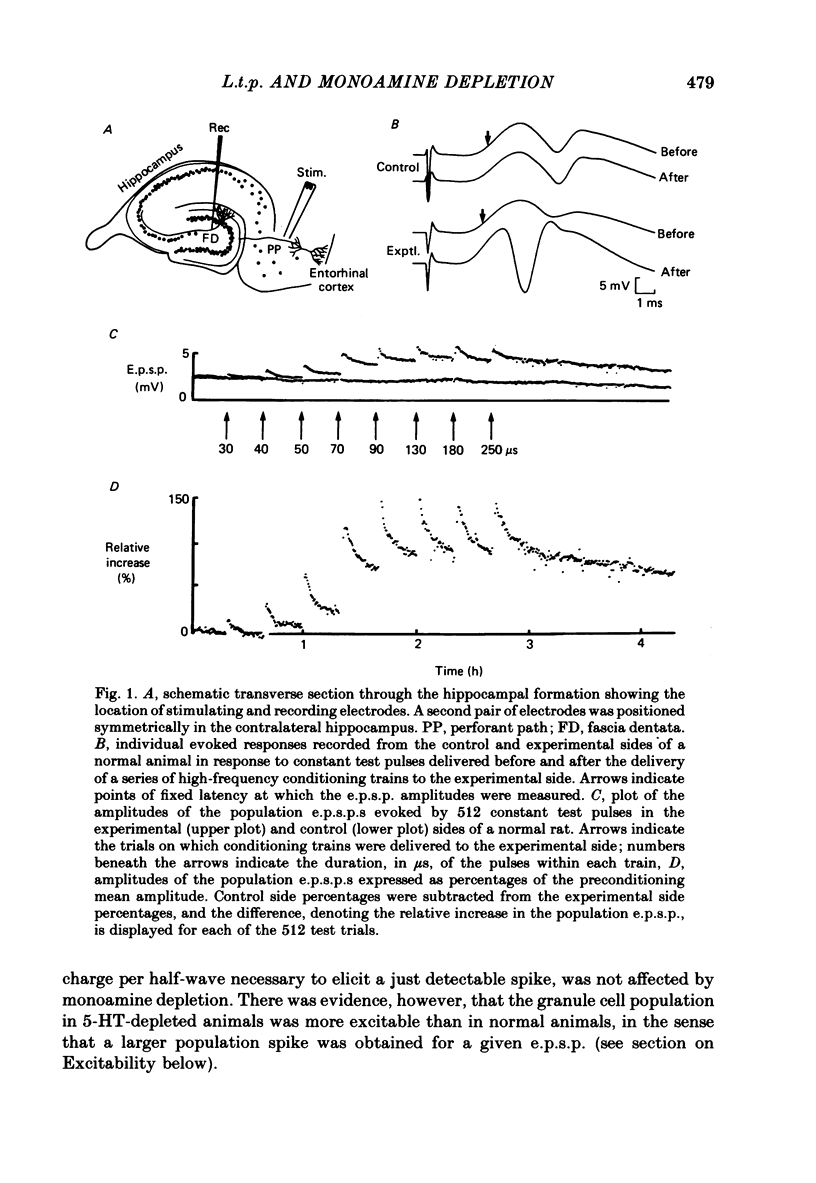
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Sundberg S. H., Sveen O., Swann J. W., Wigström H. Possible mechanisms for long-lasting potentiation of synaptic transmission in hippocampal slices from guinea-pigs. J Physiol. 1980 May;302:463–482. doi: 10.1113/jphysiol.1980.sp013256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anlezark G. M., Crow T. J., Greenway A. P. Impaired learning and decreased cortical norepinephrine after bilateral locus coeruleus lesions. Science. 1973 Aug 17;181(4100):682–684. doi: 10.1126/science.181.4100.682. [DOI] [PubMed] [Google Scholar]
- Assaf S. Y., Crunelli V., Kelly J. S. Action of 5-hydroxytryptamine on granule cells in the rat hippocampal slice. J Physiol (Paris) 1981;77(2-3):377–380. [PubMed] [Google Scholar]
- Assaf S. Y., Mason S. T., Miller J. J. Noradrenergic modulation transmission between the entorhinal cortex and the dentate gyrus of the rat [proceedings]. J Physiol. 1979 Jul;292:52P–52P. [PubMed] [Google Scholar]
- Assaf S. Y., Miller J. J. Neuronal transmission in the dentate gyrus: role of inhibitory mechanisms. Brain Res. 1978 Aug 11;151(3):587–592. doi: 10.1016/0006-8993(78)91091-0. [DOI] [PubMed] [Google Scholar]
- Azmitia E. C., Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978 Jun 1;179(3):641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Barnes C. A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979 Feb;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Gardner-Medwin A. R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese G. R., Cooper B. R. Behavioral and biochemical interactions of 5,7-dihydroxytryptamine with various drugs when administered intracisternally to adult and developing rats. Brain Res. 1975 Nov 21;98(3):517–527. doi: 10.1016/0006-8993(75)90370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli M., Castellucci V., Kandel E. R. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976 Dec 10;194(4270):1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Henry D. Catecholamines in fetal and newborn rat brain. J Neurochem. 1973 Jul;21(1):61–67. doi: 10.1111/j.1471-4159.1973.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Crow T. J. Cortical synapses and reinforcement: a hypothesis. Nature. 1968 Aug 17;219(5155):736–737. doi: 10.1038/219736a0. [DOI] [PubMed] [Google Scholar]
- Curzon G., Green A. R. Rapid method for the determination of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in small regions of rat brain. Br J Pharmacol. 1970 Jul;39(3):653–655. doi: 10.1111/j.1476-5381.1970.tb10373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Errington M. L., Bliss T. V. Long-term potentiation of the perforant path in vivo is associated with increased glutamate release. Nature. 1982 Jun 10;297(5866):496–498. doi: 10.1038/297496a0. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Goddard G. V. Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Res. 1975 Mar 21;86(2):205–215. doi: 10.1016/0006-8993(75)90697-6. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Goddard G. V., Riives M. Inhibitory modulation of long-term potentiation: evidence for a postsynaptic locus of control. Brain Res. 1982 May 27;240(2):259–272. doi: 10.1016/0006-8993(82)90221-9. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- Jahnsen H. The action of 5-hydroxytryptamine on neuronal membranes and synaptic transmission in area CA1 of the hippocampus in vitro. Brain Res. 1980 Sep 15;197(1):83–94. doi: 10.1016/0006-8993(80)90436-9. [DOI] [PubMed] [Google Scholar]
- Jones B. E., Moore R. Y. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res. 1977 May 20;127(1):25–53. [PubMed] [Google Scholar]
- Kety S. S. The possible role of the adrenergic systems of the cortex in learning. Res Publ Assoc Res Nerv Ment Dis. 1972;50:376–389. [PubMed] [Google Scholar]
- Kobayashi H., Hashiguchi T., Ushiyama N. Postsynaptic modulation of excitatory process in sympathetic ganglia by cyclic AMP. Nature. 1978 Jan 19;271(5642):268–270. doi: 10.1038/271268a0. [DOI] [PubMed] [Google Scholar]
- Kuba K., Kato E., Kumamoto E., Koketsu K., Hirai K. Sustained potentiation of transmitter release by adrenaline and dibutyryl cyclic AMP in sympathetic ganglia. Nature. 1981 Jun 25;291(5817):654–656. doi: 10.1038/291654a0. [DOI] [PubMed] [Google Scholar]
- Langmoen I. A., Segal M., Andersen P. Mechanisms of norepinephrine actions on hippocampal pyramidal cells in vitro. Brain Res. 1981 Mar 16;208(2):349–362. doi: 10.1016/0006-8993(81)90563-1. [DOI] [PubMed] [Google Scholar]
- Libet B., Kobayashi H., Tanaka T. Synaptic coupling into the production and storage of a neuronal memory trace. Nature. 1975 Nov 13;258(5531):155–157. doi: 10.1038/258155a0. [DOI] [PubMed] [Google Scholar]
- Lindvall O., Björklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl. 1974;412:1–48. [PubMed] [Google Scholar]
- Loy R., Koziell D. A., Lindsey J. D., Moore R. Y. Noradrenergic innervation of the adult rat hippocampal formation. J Comp Neurol. 1980 Feb 15;189(4):699–710. doi: 10.1002/cne.901890406. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L., Douglas R. M., Goddard G. V. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978 Nov 24;157(2):277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Halaris A. E. Hippocampal innervation by serotonin neurons of the midbrain raphe in the rat. J Comp Neurol. 1975 Nov 15;164(2):171–183. doi: 10.1002/cne.901640203. [DOI] [PubMed] [Google Scholar]
- Ogren S. O., Archer T., Ross S. B. Evidence for a role of the locus coeruleus noradrenaline system in learning. Neurosci Lett. 1980 Dec;20(3):351–356. doi: 10.1016/0304-3940(80)90173-1. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Kasamatsu T. Local perfusion of noradrenaline maintains visual cortical plasticity. Nature. 1978 Feb 23;271(5647):761–763. doi: 10.1038/271761a0. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E., Bushing J. A., Sulser F. Long-term effects of p-chloroamphetamine on tryptophan hydroxylase activity and on the levels of 5-hydroxytryptamine and 5-hydroxyindole acetic acid in brain. Eur J Pharmacol. 1972 Dec;20(3):385–388. doi: 10.1016/0014-2999(72)90204-x. [DOI] [PubMed] [Google Scholar]
- Segal M., Bloom F. E. The action of norepinephrine in the rat hippocampus. I. Iontophoretic studies. Brain Res. 1974 May 31;72(1):79–97. doi: 10.1016/0006-8993(74)90652-0. [DOI] [PubMed] [Google Scholar]
- Segal M. Physiological and pharmacological evidence for a serotonergic projection to the hippocampus. Brain Res. 1975 Aug 22;94(1):115–131. doi: 10.1016/0006-8993(75)90881-1. [DOI] [PubMed] [Google Scholar]
- Segal M. The action of norepinephrine in the rat hippocampus: intracellular studies in the slice preparation. Brain Res. 1981 Feb 9;206(1):107–128. doi: 10.1016/0006-8993(81)90104-9. [DOI] [PubMed] [Google Scholar]
- Segal M. The action of serotonin in the rat hippocampal slice preparation. J Physiol. 1980 Jun;303:423–439. doi: 10.1113/jphysiol.1980.sp013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimahara T., Tauc L. Cyclic AMP induced by serotonin modulates the activity of an identified synapse in Aplysia by facilitating the active permeability to calcium. Brain Res. 1977 May 20;127(1):168–172. doi: 10.1016/0006-8993(77)90389-4. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E., Cowan W. M. Evidence that the commissural, associational and septal projections of the regio inferior of the hippocampus arise from the same neurons. Brain Res. 1980 Sep 15;197(1):207–212. doi: 10.1016/0006-8993(80)90446-1. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Vizi E. S., Hársing L. G., Jr, Zsilla G. Evidence of the modulatory role of serotonin in acetylcholine release from striatal interneurons. Brain Res. 1981 May 11;212(1):89–99. doi: 10.1016/0006-8993(81)90035-4. [DOI] [PubMed] [Google Scholar]
- Vizi S. E., Rónai A., Hársing L., Jr, Knoll J. Inhibitory effect of dopamine on acetylcholine release from caudate nucleus. Pol J Pharmacol Pharm. 1977 May-Jun;29(3):201–211. [PubMed] [Google Scholar]
- Wilson R. C. Changes in translation of synaptic excitation to dentate granule cell discharge accompanying long-term potentiation. I. Differences between normal and reinnervated dentate gyrus. J Neurophysiol. 1981 Aug;46(2):324–338. doi: 10.1152/jn.1981.46.2.324. [DOI] [PubMed] [Google Scholar]
- Wilson R. C., Levy W. B., Steward O. Changes in translation of synaptic excitation to dentate granule cell discharge accompanying long-term potentiation. II. An evaluation of mechanisms utilizing dentate gyrus dually innervated by surviving ipsilateral and sprouted crossed temporodentate inputs. J Neurophysiol. 1981 Aug;46(2):339–355. doi: 10.1152/jn.1981.46.2.339. [DOI] [PubMed] [Google Scholar]
- Winson J. Influence of raphe nuclei on neuronal transmission from perforant pathway through dentate gyrus. J Neurophysiol. 1980 Nov;44(5):937–950. doi: 10.1152/jn.1980.44.5.937. [DOI] [PubMed] [Google Scholar]


