Abstract
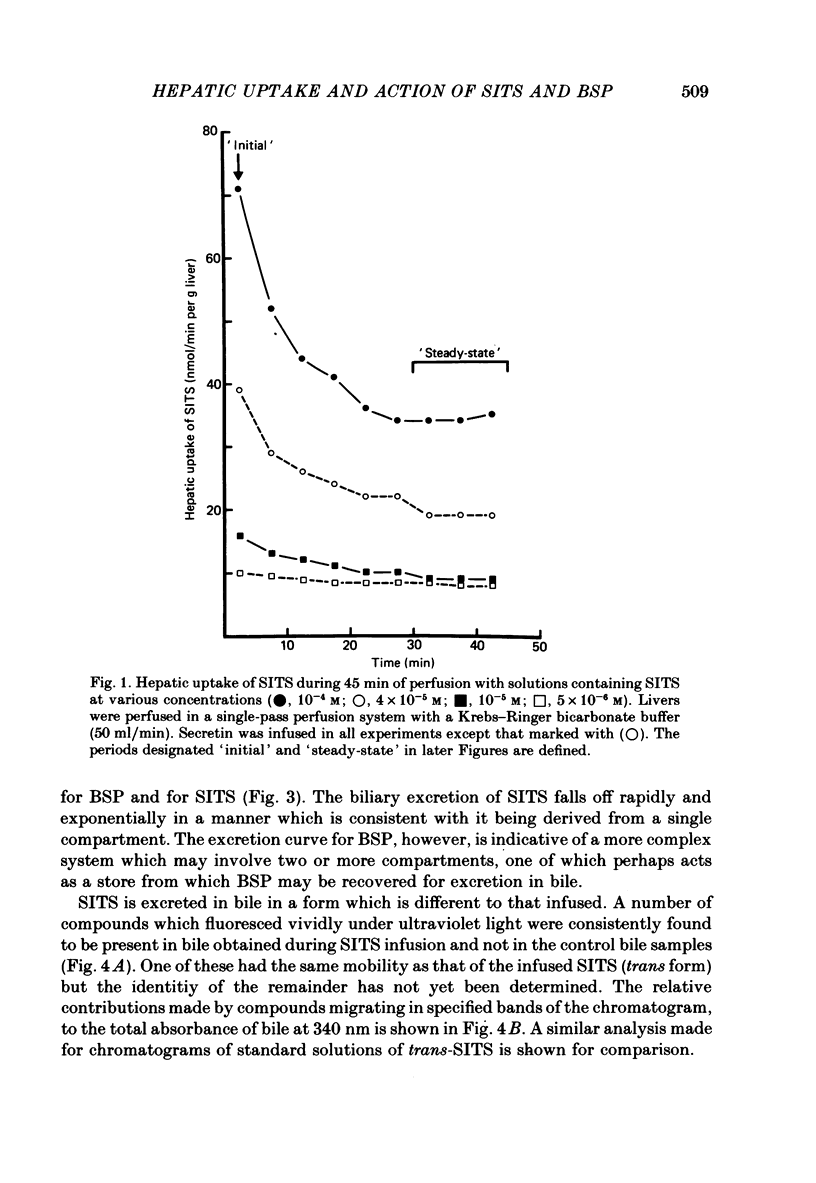
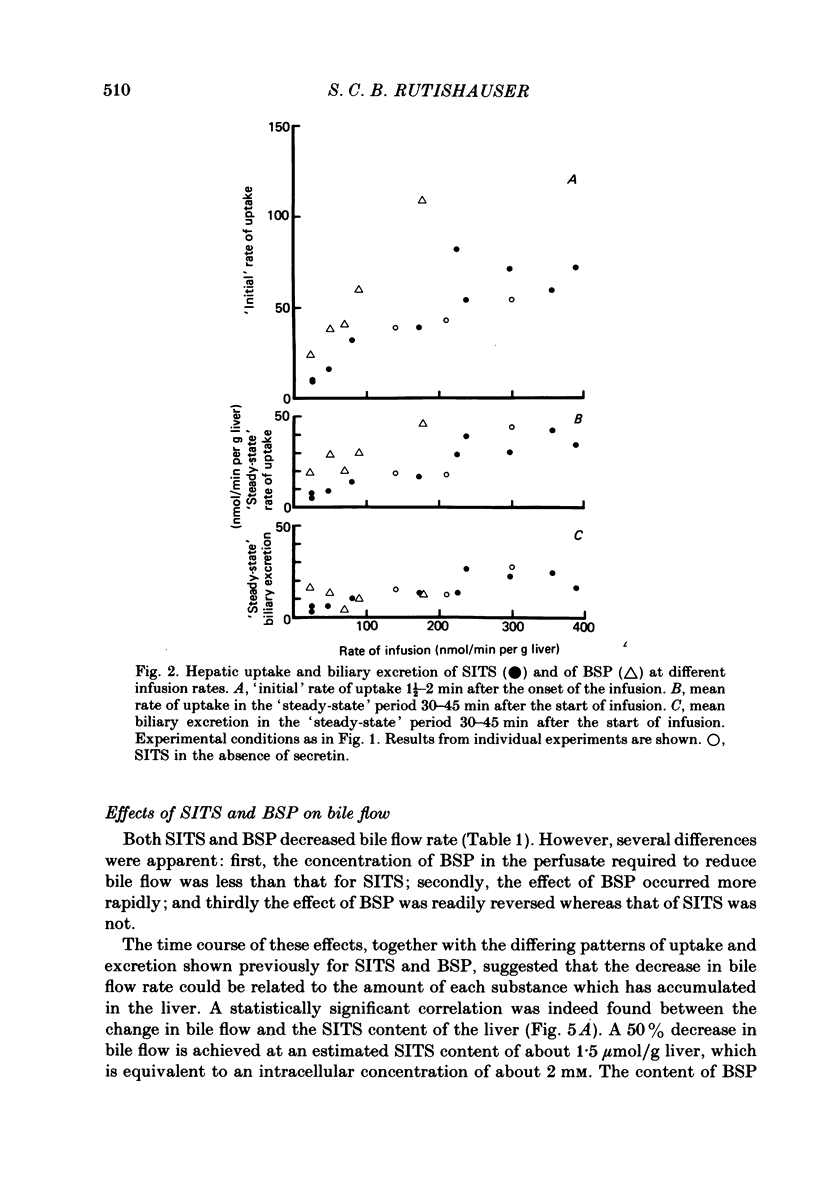
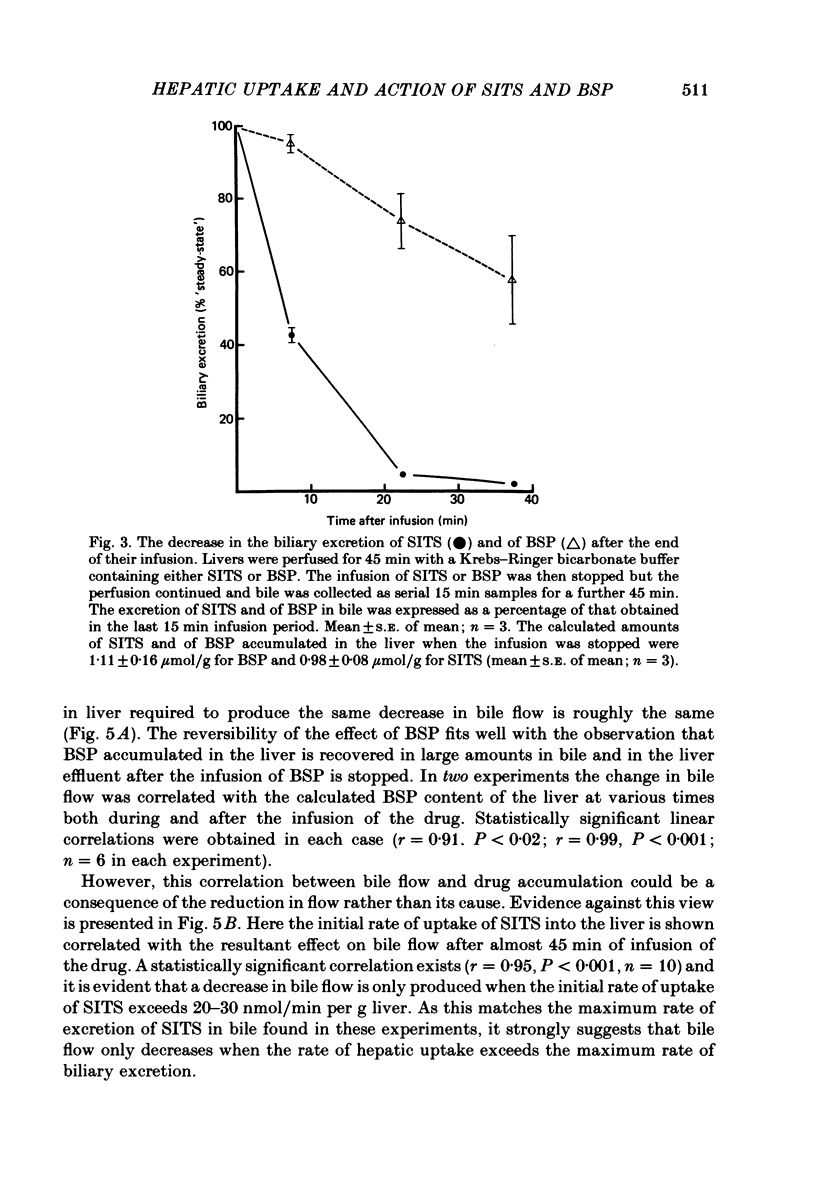
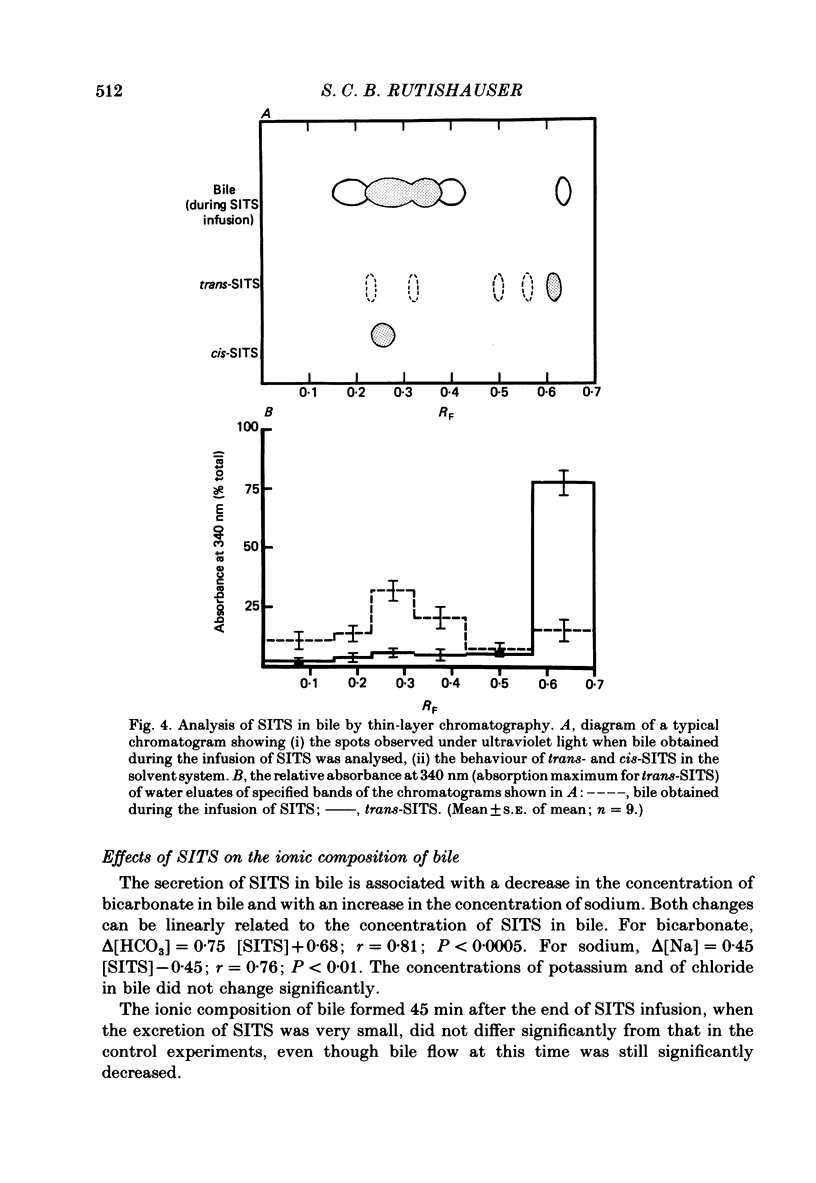
1. Livers were perfused with a Krebs-Ringer bicarbonate buffer in a single-pass perfusion system. Bile secretion was maintained by infusion of secretin. 4-Acetamido-4'-isothiocyano-2,2'-stilbene disulphonic acid (SITS) was added to the perfusate to give concentrations ranging between 5 x 10(-6) and 10(-4) M. 2. SITS was extracted from the perfusate by the liver (V, 0 . 15 mumol/min per g liver; Km 8 . 6 x 10(-5) M) and excreted in bile in a modified form (bile/plasma ratio: 50-170; maximum rate of excretion: 25 nmol/min per g liver wet wt). 3. The rates of uptake and excretion of bromsulphthalein (BSP) were similar to those for SITS, with the exception that the affinity of BSP for hepatic uptake was greater (Km 1 . 8 x 10(-5) M). 4. Both SITS and BSP decreased the rate of bile flow. A 50% reduction in bile flow was attained in each case at an estimated drug content of the liver of 1 . 5 mumol/g wet wt. 5. Unlike other cells the hepatocyte appears to be readily penetrated by SITS, and it is suggested that SITS inhibits bile secretion by inhibiting an intracellular mechanism which could be mitochondrial in location.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodsky W. A., Durham J., Ehrenspeck G. The effects of a disulphonic stilbene on chloride and bicarbonate transport in the turtle bladder. J Physiol. 1979 Feb;287:559–573. doi: 10.1113/jphysiol.1979.sp012677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr R., Schwenk M., Pfaff E. Interaction of bromosulfophthalein with mitochondrial membranes--inhibition of respiration. Biochem Pharmacol. 1977 Mar 15;26(6):461–466. doi: 10.1016/0006-2952(77)90317-3. [DOI] [PubMed] [Google Scholar]
- Burr R., Schwenk M., Pfaff E. Interaction of bromosulfophthalein with mitochondrial membranes--uptake of bromosulfophthalein and effect on ANS-fluorescence. Biochem Pharmacol. 1977 Mar 15;26(6):457–460. doi: 10.1016/0006-2952(77)90316-1. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Cornelius C. E., Ben-Ezzer J., Arias I. M. Binding of sulfobromophthalein sodium (BSP) and other organic anions by isolated hepatic cell plasma membranes in vitro. Proc Soc Exp Biol Med. 1967 Feb;124(2):665–667. doi: 10.3181/00379727-124-31819. [DOI] [PubMed] [Google Scholar]
- Ehrenspeck G., Brodsky W. A. Effects of 4-acetamido-4'-isothiocyano-2,2-disulfonic stilbene on ion transport in turtle bladders. Biochim Biophys Acta. 1976 Feb 6;419(3):555–558. doi: 10.1016/0005-2736(76)90268-6. [DOI] [PubMed] [Google Scholar]
- Elmamlouk T. H., Mukhtar H. trans-Stilbene oxide: a new inducer of rat liver microsomal UDP-glucuronyltransferase. Biochem Pharmacol. 1979;28(4):539–542. doi: 10.1016/0006-2952(79)90250-8. [DOI] [PubMed] [Google Scholar]
- Eveloff J., Kinne R., Kinter W. B. p-Aminohippuric acid transport into brush border vesicles isolated from flounder kidney. Am J Physiol. 1979 Oct;237(4):F291–F298. doi: 10.1152/ajprenal.1979.237.4.F291. [DOI] [PubMed] [Google Scholar]
- Guthenberg C., Morgenstern R., DePierre J. W., Mannervik B. Induction of glutathione S-transferases A, B and C in rat liver cytosol by trans-stilbene oxide. Biochim Biophys Acta. 1980 Aug 1;631(1):1–10. doi: 10.1016/0304-4165(80)90047-1. [DOI] [PubMed] [Google Scholar]
- Heintze K., Olles P., Petersen K. U., Wood J. R. Effects of a disulphonic stilbene on fluid and electrolyte transport in guinea-pig isolated gall-bladder [proceedings]. J Physiol. 1978 Nov;284:152P–153P. [PubMed] [Google Scholar]
- Hong S. K., Goldinger J. M., Song Y. K., Koschier F. J., Lee S. H. Effect of SITS on organic anion transport in the rabbit kidney cortical slice. Am J Physiol. 1978 Apr;234(4):F302–F307. doi: 10.1152/ajprenal.1978.234.4.F302. [DOI] [PubMed] [Google Scholar]
- Killenberg P. G., Hoppel C. L. Inhibition of rat liver mitochondrial oxidative phosphorylation by sulfobromophthalein. Mol Pharmacol. 1974 Jan;10(1):108–118. [PubMed] [Google Scholar]
- Knauf P. A., Rothstein A. Chemical modification of membranes. I. Effects of sulfhydryl and amino reactive reagents on anion and cation permeability of the human red blood cell. J Gen Physiol. 1971 Aug;58(2):190–210. doi: 10.1085/jgp.58.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschier F. J., Stokols M. F., Goldinger J. M., Acara M., Hong S. K. Effect of DIDS on renal tubular transport. Am J Physiol. 1980 Feb;238(2):F99–106. doi: 10.1152/ajprenal.1980.238.2.F99. [DOI] [PubMed] [Google Scholar]
- Laperche Y., Oudea P. Inhibition by sulfobromophthalein of mitochondrial translocation of anions and adenine nucleotides: effects upon liver adenosine triphosphate and possible correlation with inhibition of bile flow in the rat. J Pharmacol Exp Ther. 1976 Apr;197(1):235–244. [PubMed] [Google Scholar]
- Marinetti G. V., Gray G. M. A fluorescent chemical marker for the liver cell plasma membrane. Biochim Biophys Acta. 1967 Sep 9;135(4):580–590. doi: 10.1016/0005-2736(67)90090-9. [DOI] [PubMed] [Google Scholar]
- McCandless M., Nishiyama A., Petersen O. H., Philpott H. G. Mouse pancreatic acinar cells: voltage-clamp study of acetylcholine-evoked membrane current. J Physiol. 1981 Sep;318:57–71. doi: 10.1113/jphysiol.1981.sp013850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell G. M., Jones J. G., Curtis C. G. Kinetic measurements of the biliary excretion of metabolized compounds. Biochem J. 1980 May 15;188(2):561–564. doi: 10.1042/bj1880561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestly B. G., Plaa G. L. Reduced bile flow after sulfobromophthalein administration in the rat. Proc Soc Exp Biol Med. 1970 Nov;135(2):373–376. doi: 10.3181/00379727-135-35054. [DOI] [PubMed] [Google Scholar]
- Reichen J., Blitzer B. L., Berk P. D. Binding of unconjugated and conjugated sulfobromophthalein to rat liver plasma membrane fractions in vitro. Biochim Biophys Acta. 1981 Jan 8;640(1):298–312. doi: 10.1016/0005-2736(81)90554-x. [DOI] [PubMed] [Google Scholar]
- Reichen J., Paumgartner G. Excretory function of the liver. Int Rev Physiol. 1980;21:103–150. [PubMed] [Google Scholar]
- Schulze P. J., Czok G. Studies on the decrease in bile flow produced by sulfobromophthalein. Toxicol Appl Pharmacol. 1974 Jun;28(3):406–417. doi: 10.1016/0041-008x(74)90226-9. [DOI] [PubMed] [Google Scholar]
- Tiribelli C., Lunazzi G., Luciani M., Panfili E., Gazzin B., Liut G., Sandri G., Sottocasa G. Isolation of a sulfobromophthalein-binding protein from hepatocyte plasma membrane. Biochim Biophys Acta. 1978 Jan 25;532(1):105–112. doi: 10.1016/0005-2795(78)90453-1. [DOI] [PubMed] [Google Scholar]
- Uptake of bromosulfophthalein by isolated liver cells. Eur J Biochem. 1976 Apr 15;64(1):189–197. [PubMed] [Google Scholar]
- Waitman A. M., Dyck W. P., Janowitz H. D. Effect of secretin and acetazolamide on the volume and electrolyte composition of hepatic bile in man. Gastroenterology. 1969 Feb;56(2):286–294. [PubMed] [Google Scholar]
- Whelan G., Combes B. Competition by unconjugated and conjugated sulfobromophthalein sodium (BSP) for transport into bile. Evidence for a single excretory system. J Lab Clin Med. 1971 Aug;78(2):230–244. [PubMed] [Google Scholar]
- Whelan G. The influence of diethylmaleate on the biliary excretion of infused sulphobromophthalein sodium and its glutathione conjugate in guinea-pigs. Clin Exp Pharmacol Physiol. 1980 Nov;7(6):595–601. doi: 10.1111/j.1440-1681.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Wolkoff A. W., Chung C. T. Identification, purification, and partial characterization of an organic anion binding protein from rat liver cell plasma membrane. J Clin Invest. 1980 May;65(5):1152–1161. doi: 10.1172/JCI109770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccoli M. A., Karnovsky M. L. Effect of two inhibitors of anion transport on the hydrolysis of glucose 6-phosphate by rat liver microsomes. Covalent modification of the glucose 6-P transport component. J Biol Chem. 1980 Feb 10;255(3):1113–1119. [PubMed] [Google Scholar]



