Abstract
Effects on single lumbar gamma-motoneurones, mediated via fibres running in the ventral roots, were studied by micro-electrode recording in cats anaesthetized with chloralose. Graded electrical stimulation of ventral roots or of peripheral nerves was used. The cells were identified as gamma-motoneurones by antidromic stimulation and by measurement of their axonal conduction velocity. Some of the cells were classified as static or dynamic. The findings confirm the previously demonstrated existence of low-threshold, presumed recurrent, inhibition of both static and dynamic gamma-motoneurones. Strong evidence for the occurrence of high-threshold recurrent inhibition of gamma-motoneurones is also presented. In addition, excitatory effects on gamma-cells, also mediated via fibres in the ventral roots, are described. The low-threshold effects from ventral root fibres are attributed to recurrent alpha-collateral activity and the high-threshold effects to gamma-collateral activity. The significance of recurrent inhibition of gamma-motoneurones is discussed in relation to the 'gain regulator' concept proposed by Hultborn, Lindström & Wigström (1979).
Full text
PDF


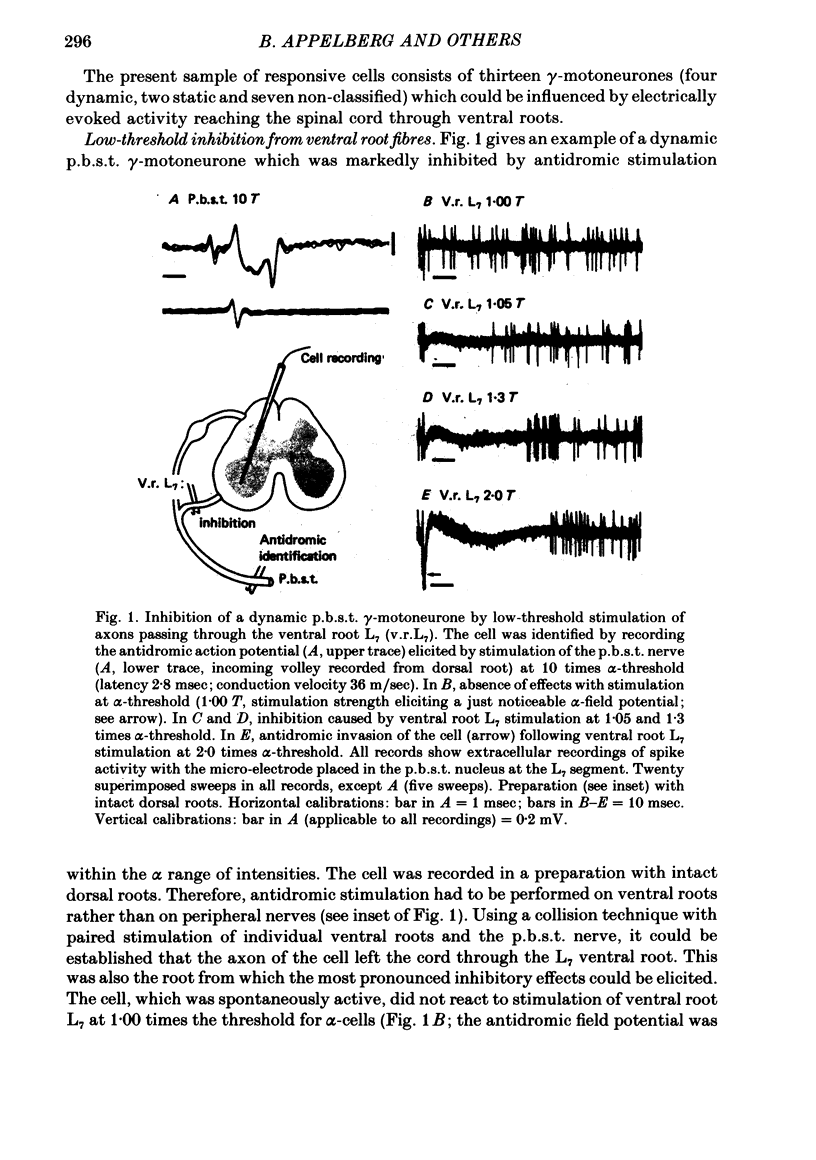

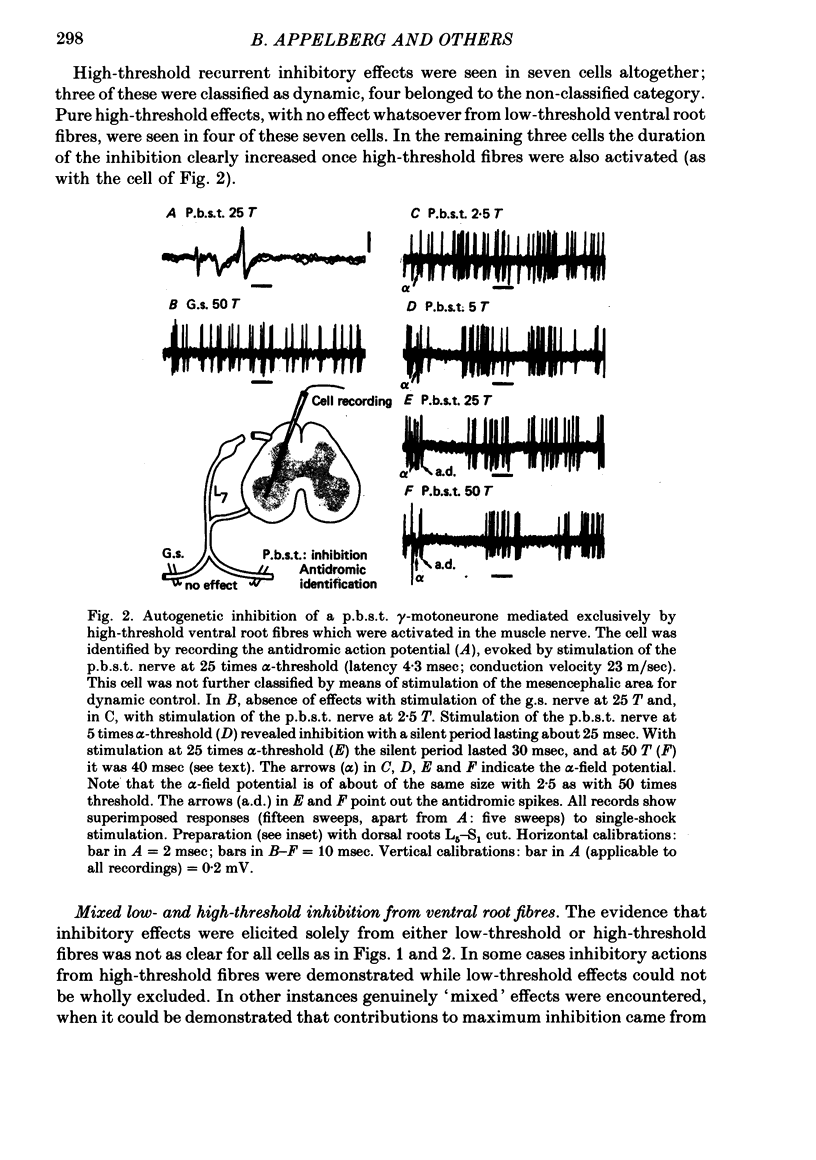







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg B., Hulliger M., Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group I muscle afferent fibres in the hind limb of the cat. J Physiol. 1983 Feb;335:237–253. doi: 10.1113/jphysiol.1983.sp014531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group II muscle afferent fibres in the hind limb of the cat. J Physiol. 1983 Feb;335:255–273. doi: 10.1113/jphysiol.1983.sp014532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group III muscle afferent fibres in the hind limb of the cat. J Physiol. 1983 Feb;335:275–292. doi: 10.1113/jphysiol.1983.sp014533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Fusimotor reflexes in triceps surae elicited by natural stimulation of muscle afferents from the cat ipsilateral hind limb. J Physiol. 1982 Aug;329:211–229. doi: 10.1113/jphysiol.1982.sp014299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Johansson H., Kalistratov G. The influence of group II muscle afferents and low threshold skin afferents on dynamic fusimotor neurones to the triceps surae of the cat. Brain Res. 1977 Aug 19;132(1):153–158. doi: 10.1016/0006-8993(77)90713-2. [DOI] [PubMed] [Google Scholar]
- Bergmans J., Grillner S. Changes in dynamic sensitivity of primary endings of muscle spindle afferents induced by DOPA. Acta Physiol Scand. 1968 Dec;74(4):629–636. doi: 10.1111/j.1748-1716.1968.tb04273.x. [DOI] [PubMed] [Google Scholar]
- Bergmans J., Grillner S. Reciprocal control of spontaneous activity and reflex effects in static and dynamic flexor gamma-motoneurones revealed by an injection of DOPA. Acta Physiol Scand. 1969 Sep-Oct;77(1):106–124. doi: 10.1111/j.1748-1716.1969.tb04557.x. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Lawrence D. G., Matthews P. B. Antidromic inhibition of presumed fusimotor neurones by repetitive stimulation of the ventral root in the decerebrate cat. Experientia. 1968 Dec 15;24(12):1210–1212. doi: 10.1007/BF02146625. [DOI] [PubMed] [Google Scholar]
- Coggeshall R. E. Law of separation of function of the spinal roots. Physiol Rev. 1980 Jul;60(3):716–755. doi: 10.1152/physrev.1980.60.3.716. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Ulfhake B. Observations on the morphology of intracellularly stained gamma-motoneurons in relation to their axon conduction velocity. Neurosci Lett. 1979 Jun;13(1):47–50. doi: 10.1016/0304-3940(79)90073-9. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Murphy P. R. A comparison of the recurrent inhibition of alpha- and gamma-motoneurones in the cat. J Physiol. 1981 Jun;315:43–58. doi: 10.1113/jphysiol.1981.sp013731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. Recurrent inhibition of fusimotor neurones exhibiting background discharges in the decerebrate and the spinal cat. J Physiol. 1971 Jul;216(2):419–439. doi: 10.1113/jphysiol.1971.sp009533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. The influence of DOPA on the static and the dynamic fusimotor activity to the triceps surae of the spinal cat. Acta Physiol Scand. 1969 Dec;77(4):490–509. doi: 10.1111/j.1748-1716.1969.tb04592.x. [DOI] [PubMed] [Google Scholar]
- Haase J., Cleveland S., Ross H. G. Problems of postsynaptic autogenous and recurrent inhibition in the mammalian spinal cord. Rev Physiol Biochem Pharmacol. 1975;73:73–129. doi: 10.1007/BFb0034660. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S., Roberts W. Neuronal pathway of the recurrent facilitation of motoneurones. J Physiol. 1971 Oct;218(2):495–514. doi: 10.1113/jphysiol.1971.sp009630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Lindström S., Wigström H. On the function of recurrent inhibition in the spinal cord. Exp Brain Res. 1979 Oct;37(2):399–403. doi: 10.1007/BF00237722. [DOI] [PubMed] [Google Scholar]
- Kato M., Fukushima K. Effect of differential blocking of motor axons on antidromic activation of Renshaw cells in the cat. Exp Brain Res. 1974;20(2):135–143. doi: 10.1007/BF00234008. [DOI] [PubMed] [Google Scholar]
- Loeb G. E. Ventral root projections of myelinated dorsal root ganglion cells in the cat. Brain Res. 1976 Apr 16;106(1):159–165. doi: 10.1016/0006-8993(76)90081-0. [DOI] [PubMed] [Google Scholar]
- Nothe J. Recurrente Hemmung der Extensor-Fusimotoneurone. Pflugers Arch. 1971;329(1):23–33. doi: 10.1007/BF00586898. [DOI] [PubMed] [Google Scholar]
- Pugh P. M., Stone S. L. The effect of 2,4-dinitrophenol and related compounds on bile secretion. J Physiol. 1968 Sep;198(1):39–49. doi: 10.1113/jphysiol.1968.sp008592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall R. W. Renshaw cell mediated inhibition of Renshaw cells: patterns of excitation and inhibition from impulses in motor axon collaterals. J Neurophysiol. 1970 Mar;33(2):257–270. doi: 10.1152/jn.1970.33.2.257. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., BURGESS P. R. Disinhibition in the cat spinal cord. J Neurophysiol. 1962 May;25:392–404. doi: 10.1152/jn.1962.25.3.392. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., BURGESS P. R. Effects of antidromic conditioning on some motoneurons and interneurons. J Neurophysiol. 1962 Sep;25:636–650. doi: 10.1152/jn.1962.25.5.636. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., DIECKE F. P., TALBOT W. H. Action of tetanus toxin on conditioning of spinal motoneurons. J Neurophysiol. 1960 Nov;23:659–666. doi: 10.1152/jn.1960.23.6.659. [DOI] [PubMed] [Google Scholar]
- WILSON V. J. Recurrent facilitation of spinal reflexes. J Gen Physiol. 1959 Mar 20;42(4):703–713. doi: 10.1085/jgp.42.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand P., Pompeiano O. Contribution of different size motoneurons to Renshaw cell discharge during stretch and vibration reflexes. Prog Brain Res. 1979;50:45–60. doi: 10.1016/S0079-6123(08)60806-7. [DOI] [PubMed] [Google Scholar]
- Westbury D. R. Lack of a contribution from gamma motoneurone axons to Renshaw inhibition in the cap spinal cord. Brain Res. 1980 Mar 17;186(1):217–221. doi: 10.1016/0006-8993(80)90269-3. [DOI] [PubMed] [Google Scholar]