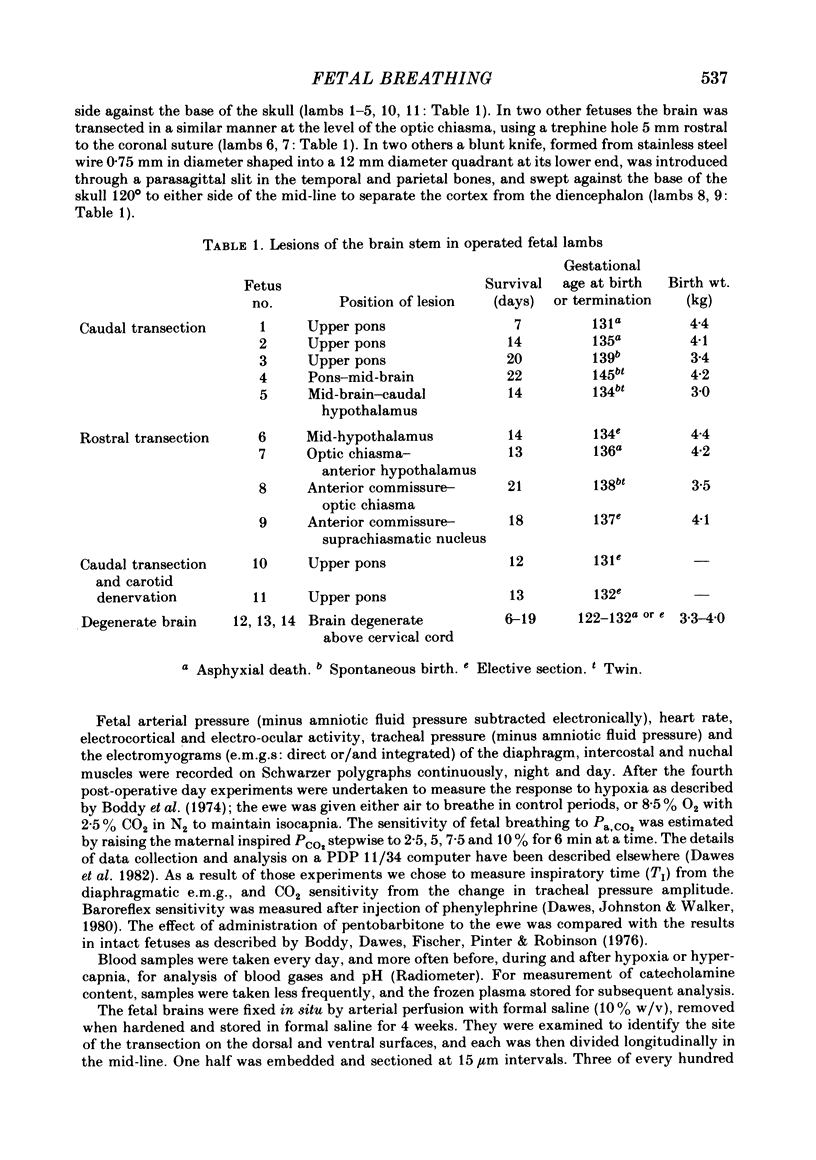
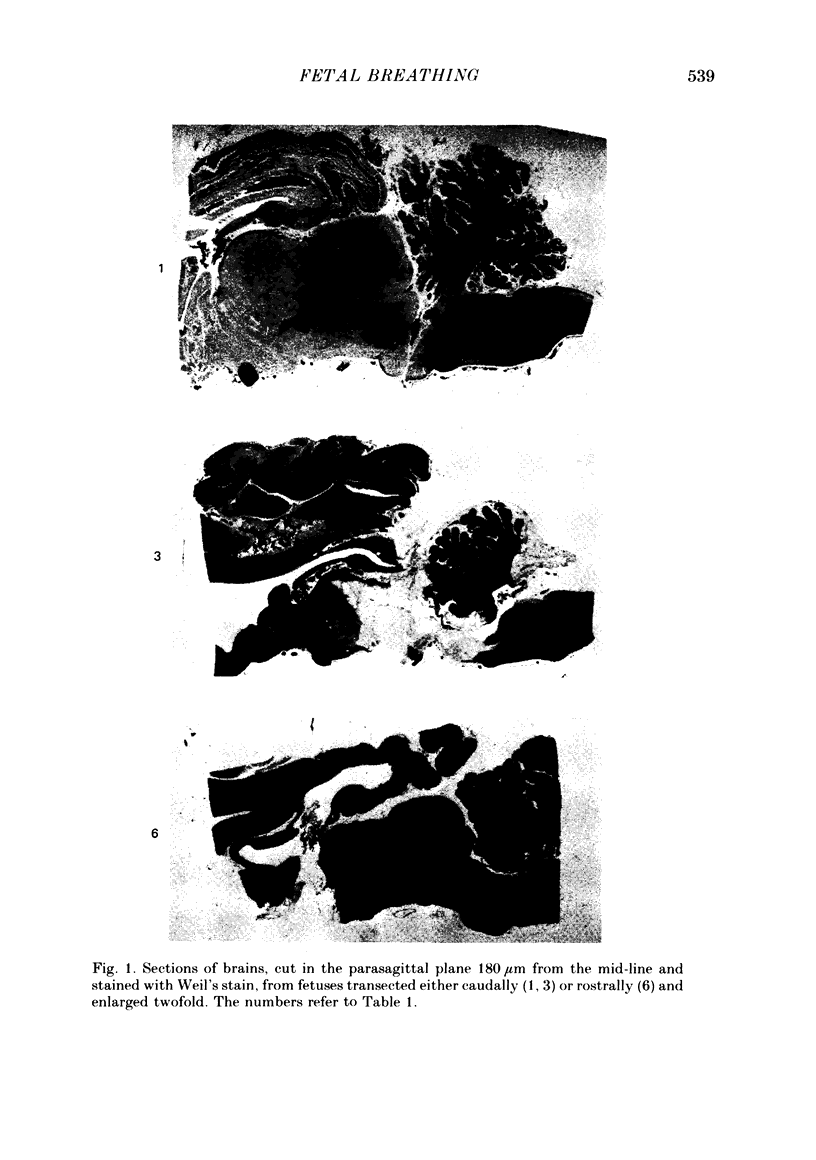
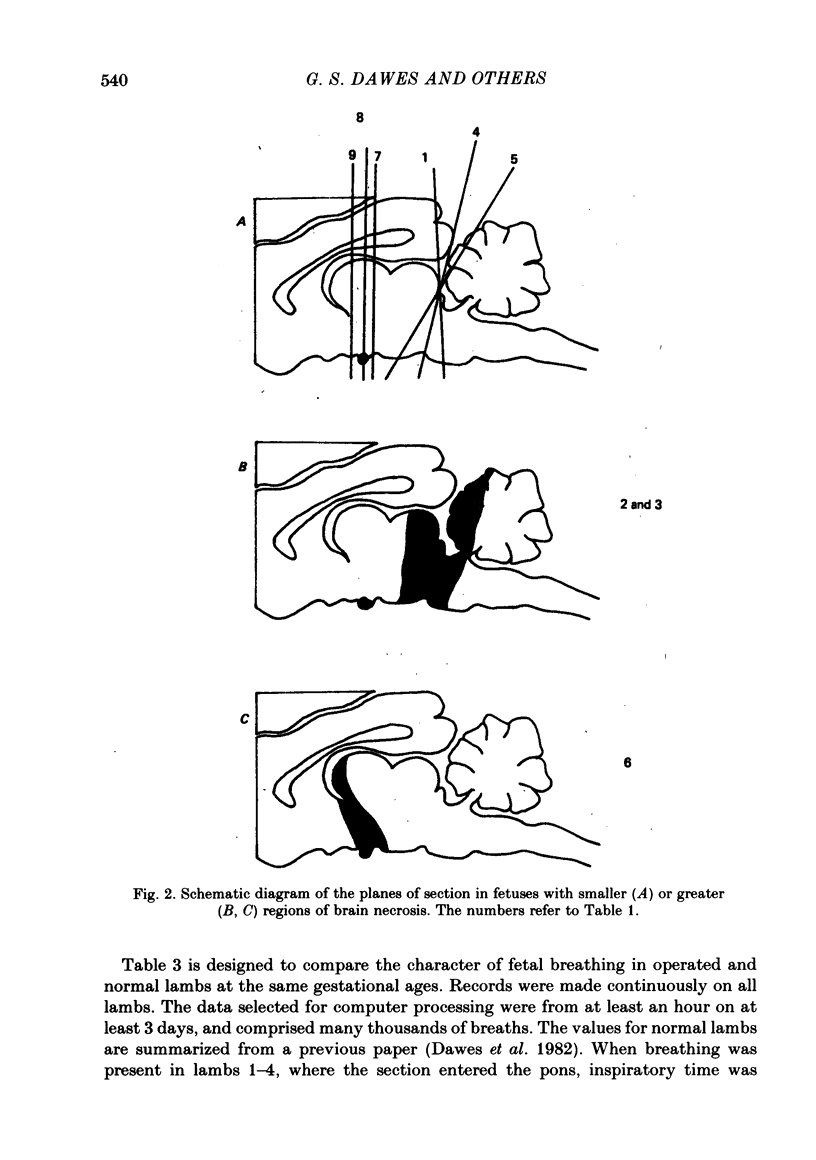
Abstract
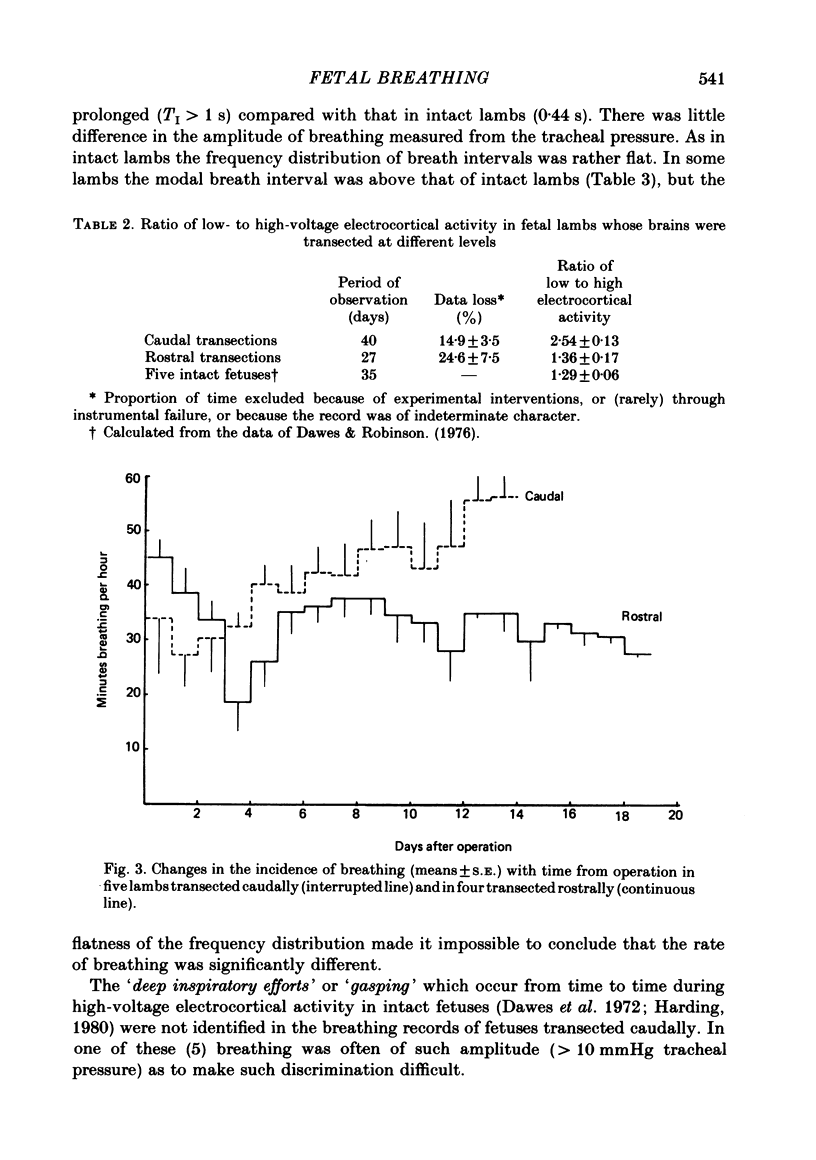
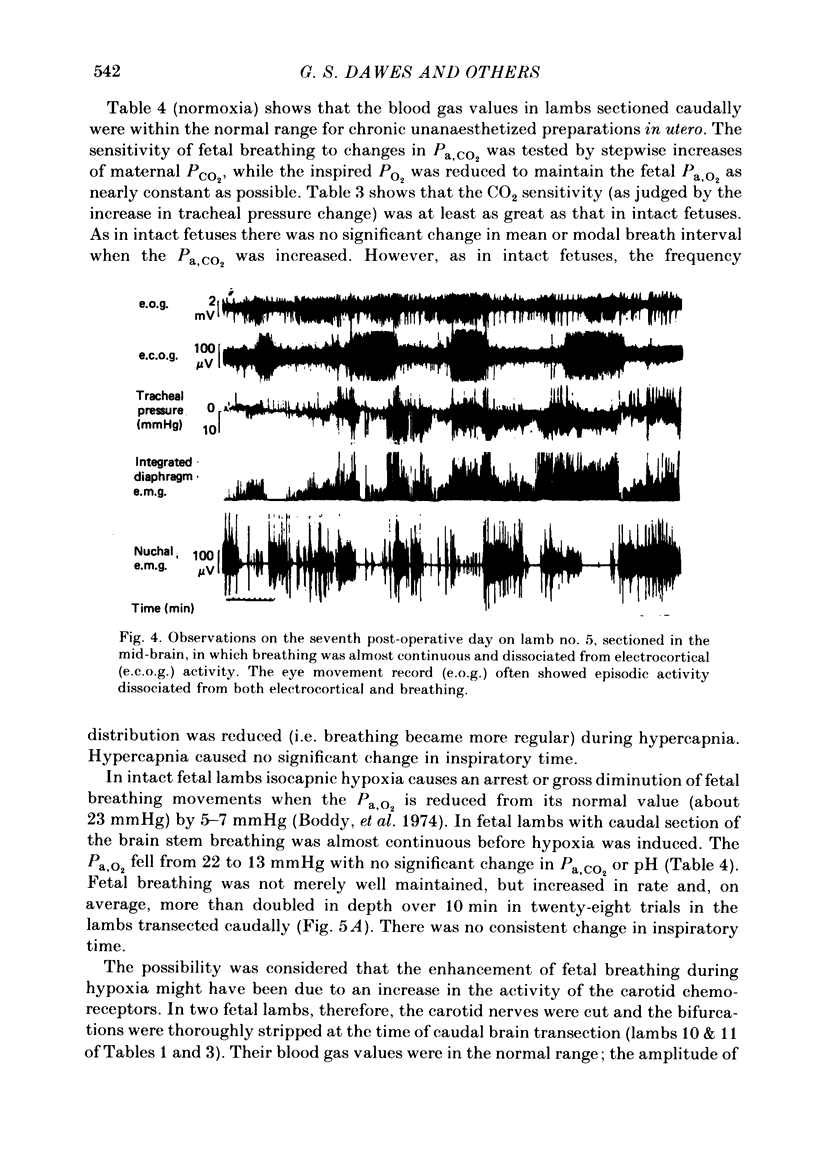
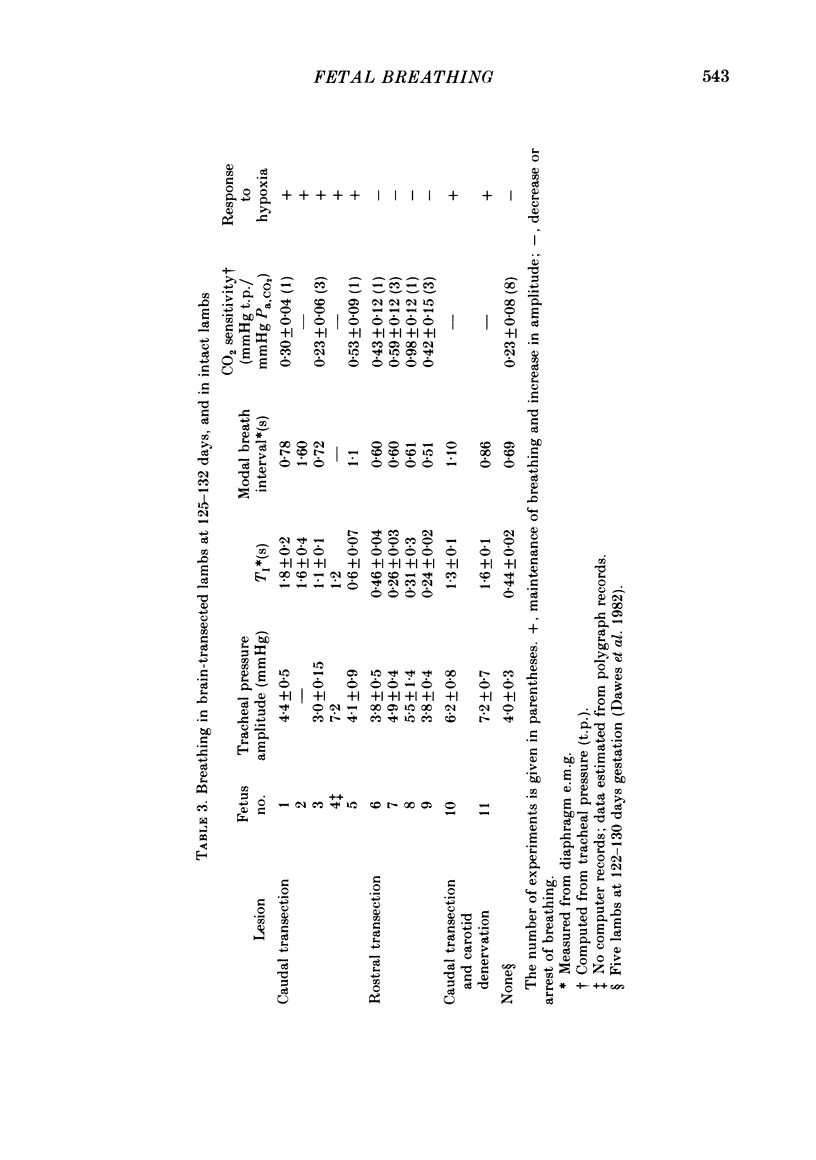
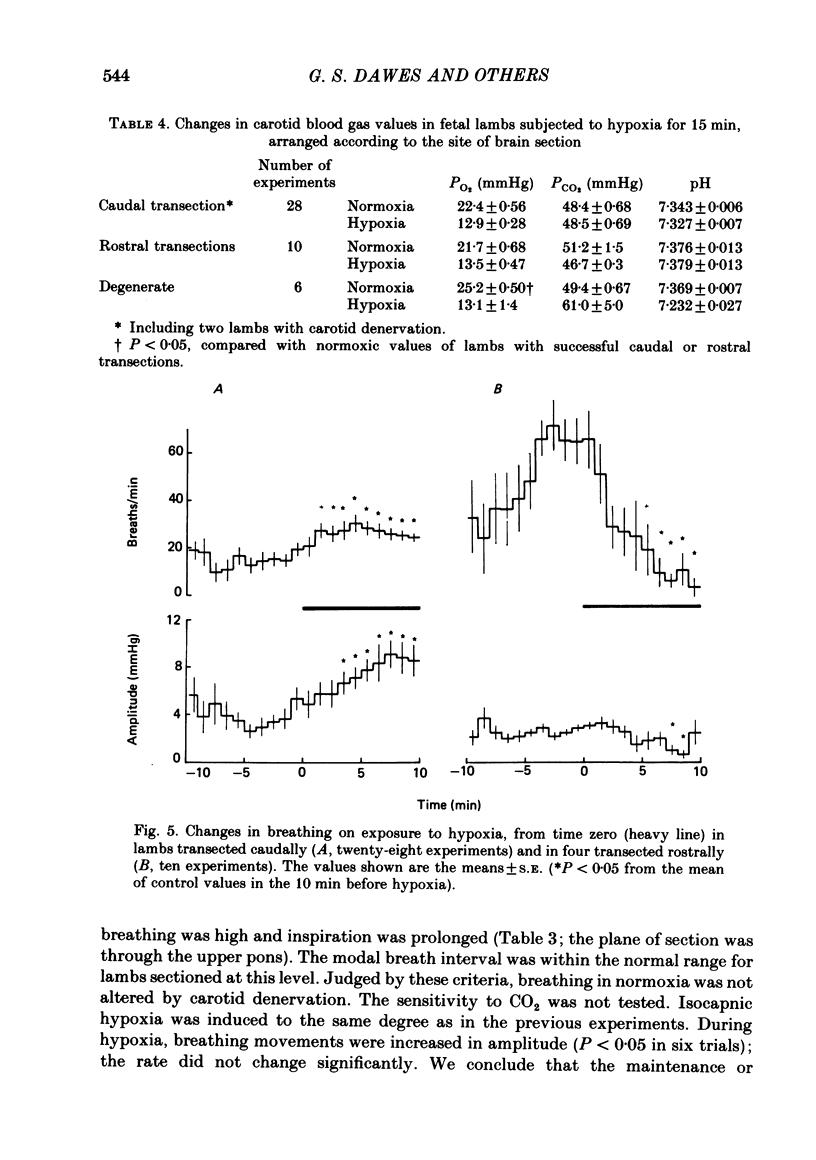
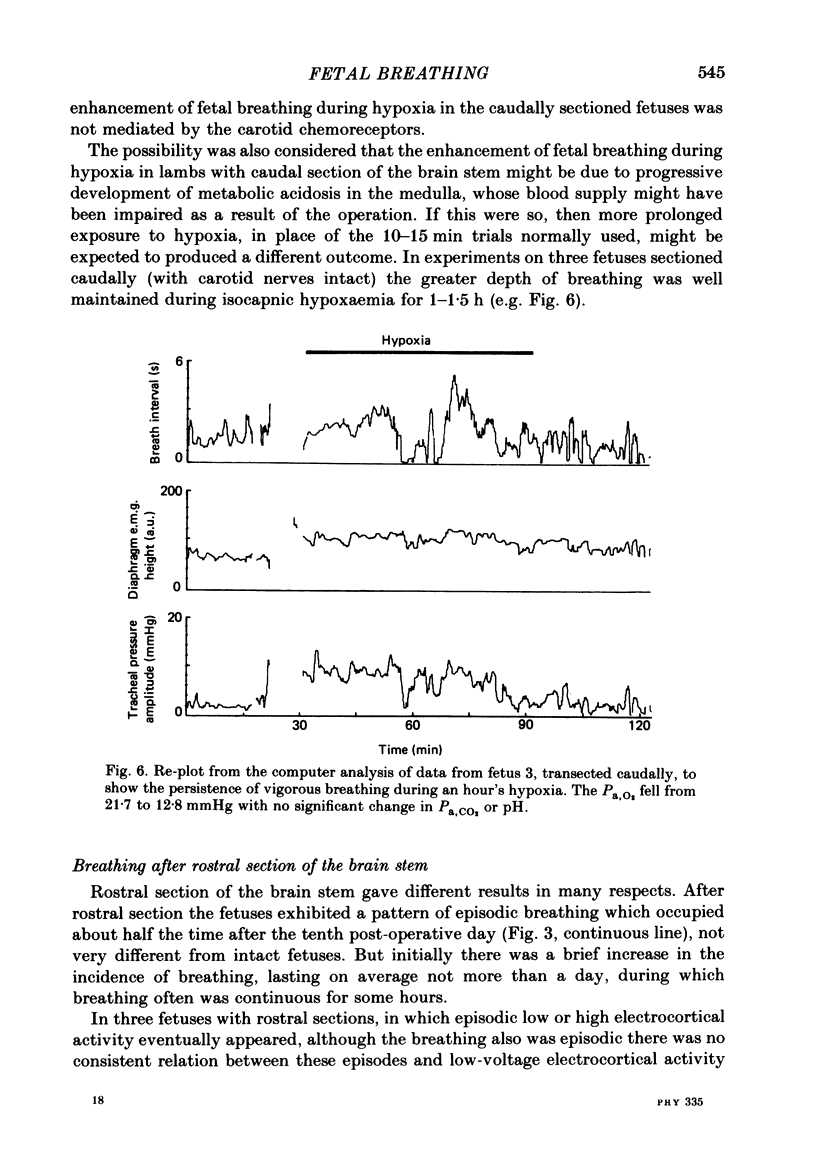
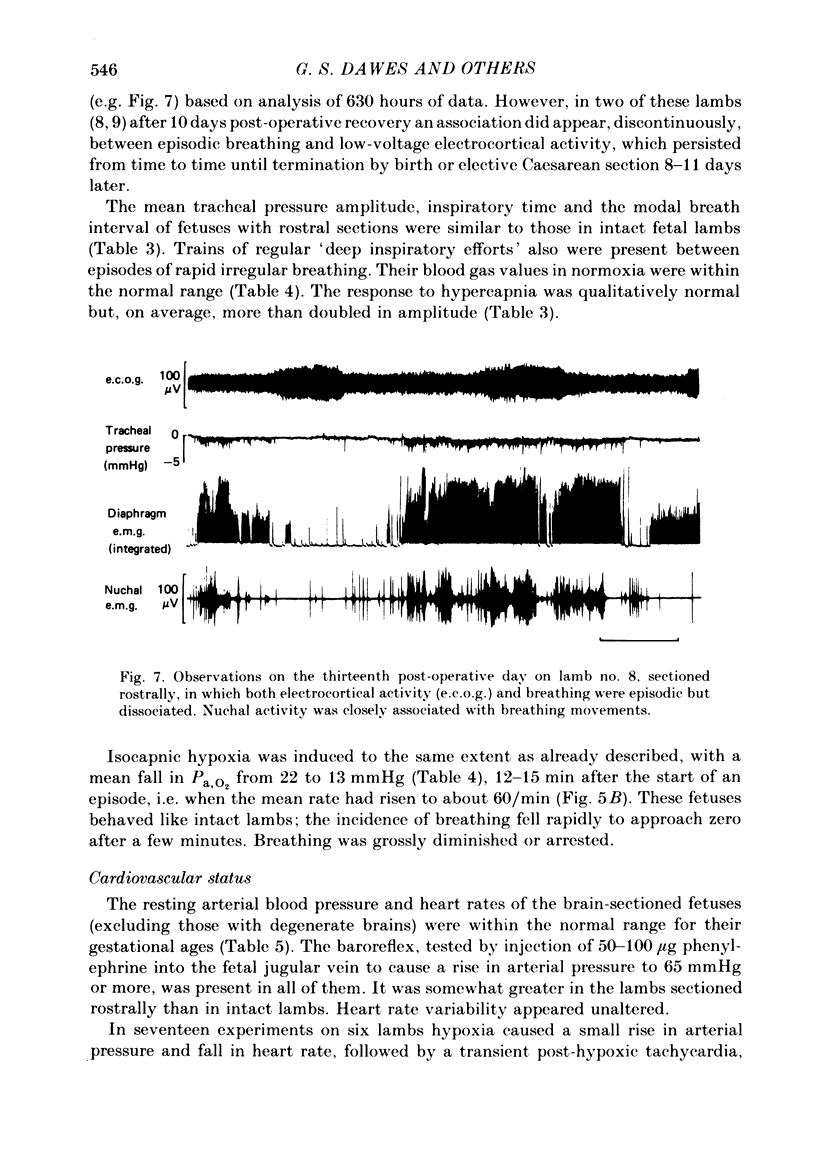
The effects of section of the brain stem caudally (through the upper pons or mid-collicular) or rostrally (through the caudal hypothalamus or anterior commissure-suprachiasmatic nucleus) were studied in fetal lambs from 118 days gestation, after recovery in utero. In lambs sectioned caudally, breathing movements and electrocortical activity were dissociated. After some days recovery breathing tended to become continuous, with an abnormal prolongation of inspiratory time. Isocapnic hypoxia caused an increase in the rate and amplitude of breathing. After carotid denervation hypoxia still caused an increase in the amplitude of breathing. In lambs sectioned rostrally, there was also dissociation between breathing movements and electrocortical activity. Breathing remained episodic, with an incidence similar to that of intact fetal lambs. In two lambs after 10 days of recovery the breathing and electrocortical rhythms returned, from time to time, to their normal phasic relationship. Isocapnic hypoxia caused a diminution or arrest of breathing, as in intact lambs. The cardiovascular effects of transection were examined. Baroreflex sensitivity was normal in those lambs sectioned caudally and enhanced in those sectioned rostrally. It is concluded first that as a result of rostral section, independent episodic rhythms of fetal breathing and electrocortical activity can be dissociated. Secondly, moderate isocapnic hypoxia causes arrest of fetal breathing indirectly, requiring the integrity of a suprapontine mechanism. And thirdly, after caudal section of the brain stem, hypoxia causes enhancement of fetal breathing efforts, independently of the carotid chemoreceptors. Possible mechanisms are discussed.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barcroft J., Barron D. H. Movements in midfoetal life in the sheep embryo. J Physiol. 1937 Dec 14;91(3):329–351. doi: 10.1113/jphysiol.1937.sp003563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Järhult J. The effect of somatostatin on pancreatic endocrine responses mediated via the parasympathetic innervation in the conscious calf. J Physiol. 1980 Nov;308:29–38. doi: 10.1113/jphysiol.1980.sp013459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy K., Dawes G. S., Fisher R. L., Pinter S., Robinson J. S. The effects of pentobarbitone and pethidine on foetal breathing movements in sheep. Br J Pharmacol. 1976 Jun;57(2):311–317. doi: 10.1111/j.1476-5381.1976.tb07481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy K., Dawes G. S., Fisher R., Pinter S., Robinson J. S. Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. J Physiol. 1974 Dec;243(3):599–618. doi: 10.1113/jphysiol.1974.sp010768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G., Adamson T. M., Ritchie B. C., Dowling M., Wilkinson M. H., Maloney J. E. Development of patterns of respiratory activity in unanesthetized fetal sheep in utero. J Appl Physiol Respir Environ Exerc Physiol. 1981 Apr;50(4):693–700. doi: 10.1152/jappl.1981.50.4.693. [DOI] [PubMed] [Google Scholar]
- Dawes G. S., Duncan S. L., Lewis B. V., Merlet C. L., Owen-Thomas J. B., Reeves J. T. Cyanide stimulation of the systemic arterial chemoreceptors in foetal lambs. J Physiol. 1969 Mar;201(1):117–128. doi: 10.1113/jphysiol.1969.sp008746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Fox H. E., Leduc B. M., Liggins G. C., Richards R. T. Respiratory movements and rapid eye movement sleep in the foetal lamb. J Physiol. 1972 Jan;220(1):119–143. doi: 10.1113/jphysiol.1972.sp009698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Gardner W. N., Johnston B. M., Walker D. W. Effects of hypercapnia on tracheal pressure, diaphragm and intercostal electromyograms in unanaesthetized fetal lambs. J Physiol. 1982 May;326:461–474. doi: 10.1113/jphysiol.1982.sp014206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Johnston B. M., Walker D. W. Relationship of arterial pressure and heart rate in fetal, new-born and adult sheep. J Physiol. 1980 Dec;309:405–417. doi: 10.1113/jphysiol.1980.sp013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Robinson J. S. Rhythmic phenomena in prenatal life. Prog Brain Res. 1976;45:383–389. doi: 10.1016/s0079-6123(08)60999-1. [DOI] [PubMed] [Google Scholar]
- Harding R. State-related and developmental changes in laryngeal function. Sleep. 1980;3(3-4):307–322. doi: 10.1093/sleep/3.3-4.307. [DOI] [PubMed] [Google Scholar]
- Moruzzi G. The sleep-waking cycle. Ergeb Physiol. 1972;64:1–165. doi: 10.1007/3-540-05462-6_1. [DOI] [PubMed] [Google Scholar]
- Robinson J. S., Kingston E. J., Thorburn G. D. Increased fetal breathing activity after fetal hypophysectomy. Am J Obstet Gynecol. 1980 Jul 15;137(6):729–734. doi: 10.1016/s0002-9378(15)33250-6. [DOI] [PubMed] [Google Scholar]
- Sprague J. M. The effects of chronic brainstem lesions on wakefulness, sleep and behavior. Res Publ Assoc Res Nerv Ment Dis. 1967;45:148–194. [PubMed] [Google Scholar]
- VILLABLANCA J. Electroencephalogram in the permanently isolated forebrain of the cat. Science. 1962 Oct 5;138(3536):44–46. doi: 10.1126/science.138.3536.44. [DOI] [PubMed] [Google Scholar]
- Yamasaki K., Lico M. C. Electroencephalographic serotonin synchronization: area postrema and solitary tract nucleus. Am J Physiol. 1981 Sep;241(3):R158–R162. doi: 10.1152/ajpregu.1981.241.3.R158. [DOI] [PubMed] [Google Scholar]