Abstract
1. The effects of muscarinic agonists, luteinizing hormone-releasing hormone (LHRH) analogues, uridine triphosphate (UTP) and divalent cations on K+-currents in voltage-clamped bullfrog sympathetic neurones have been studied.
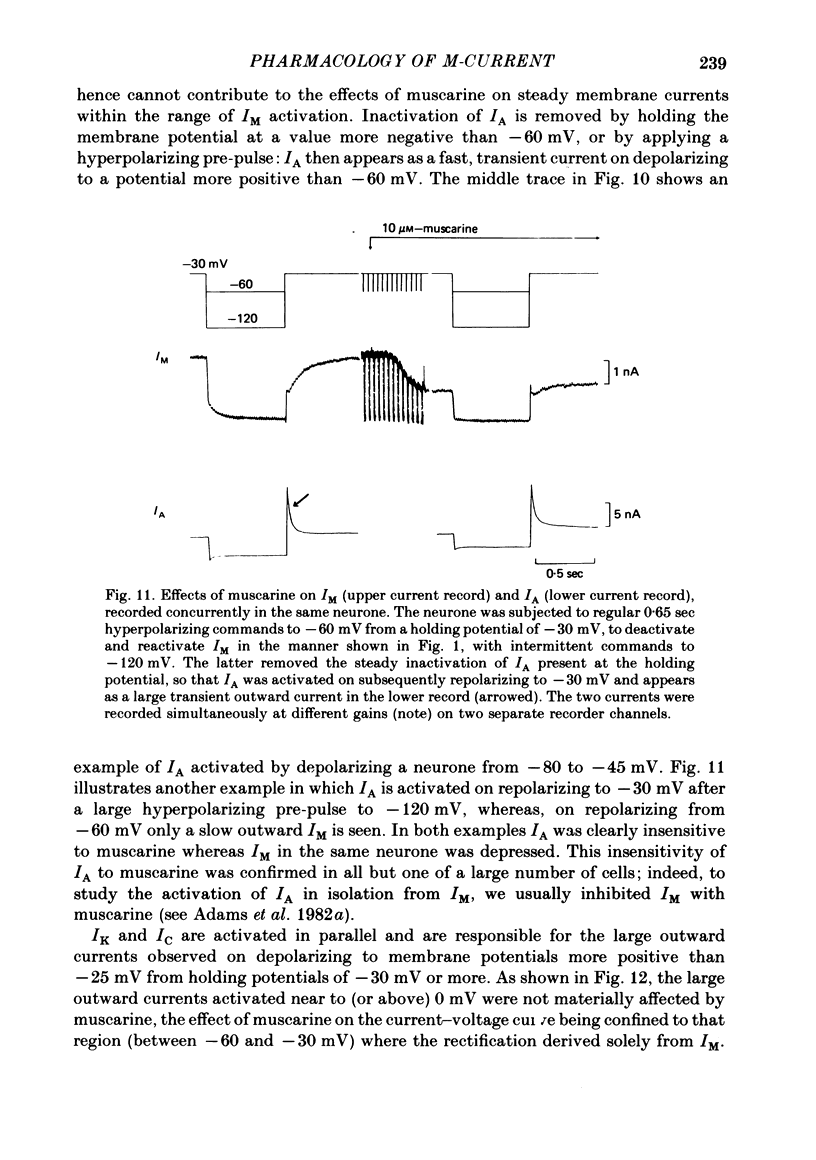
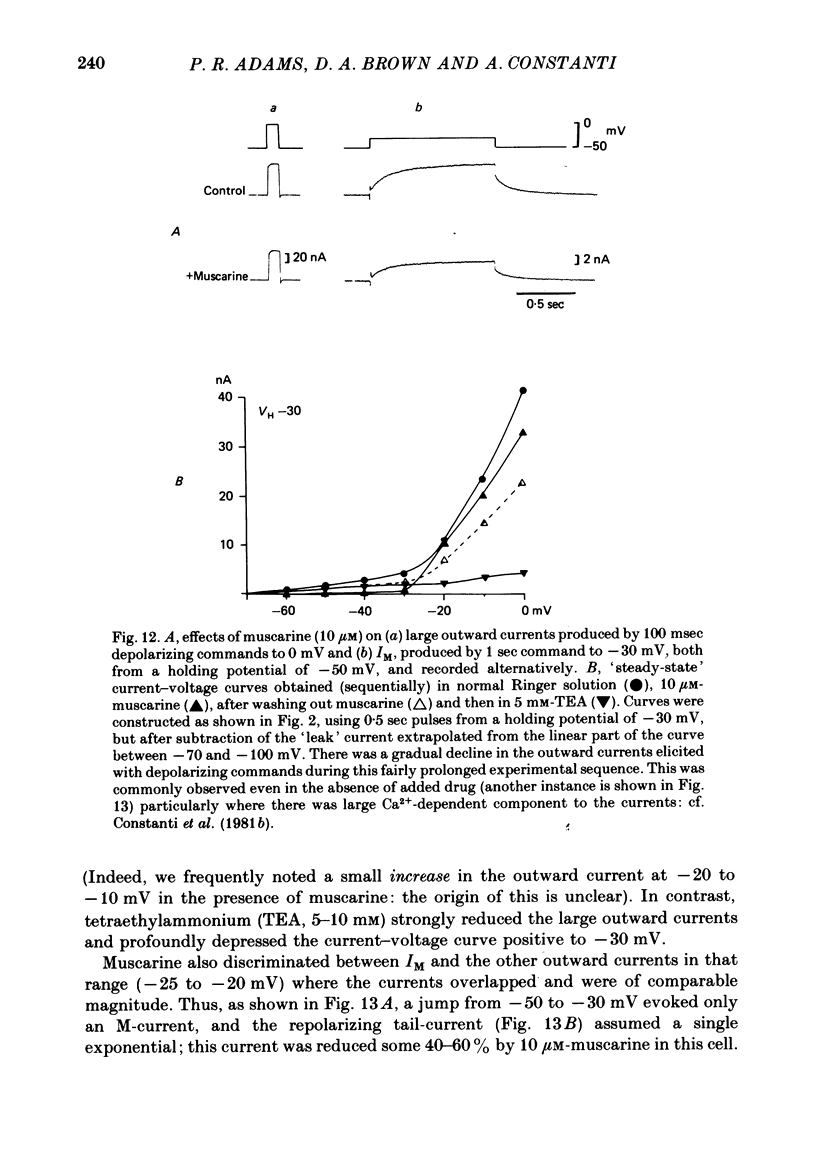
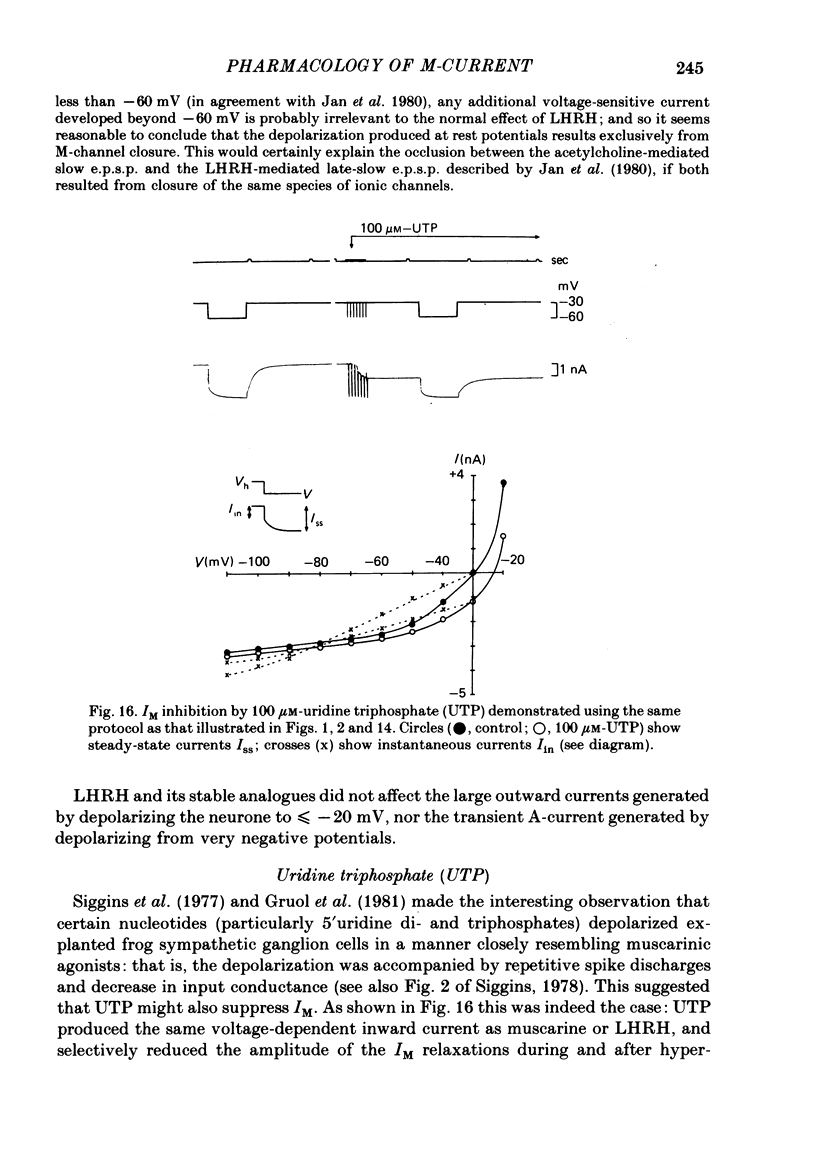
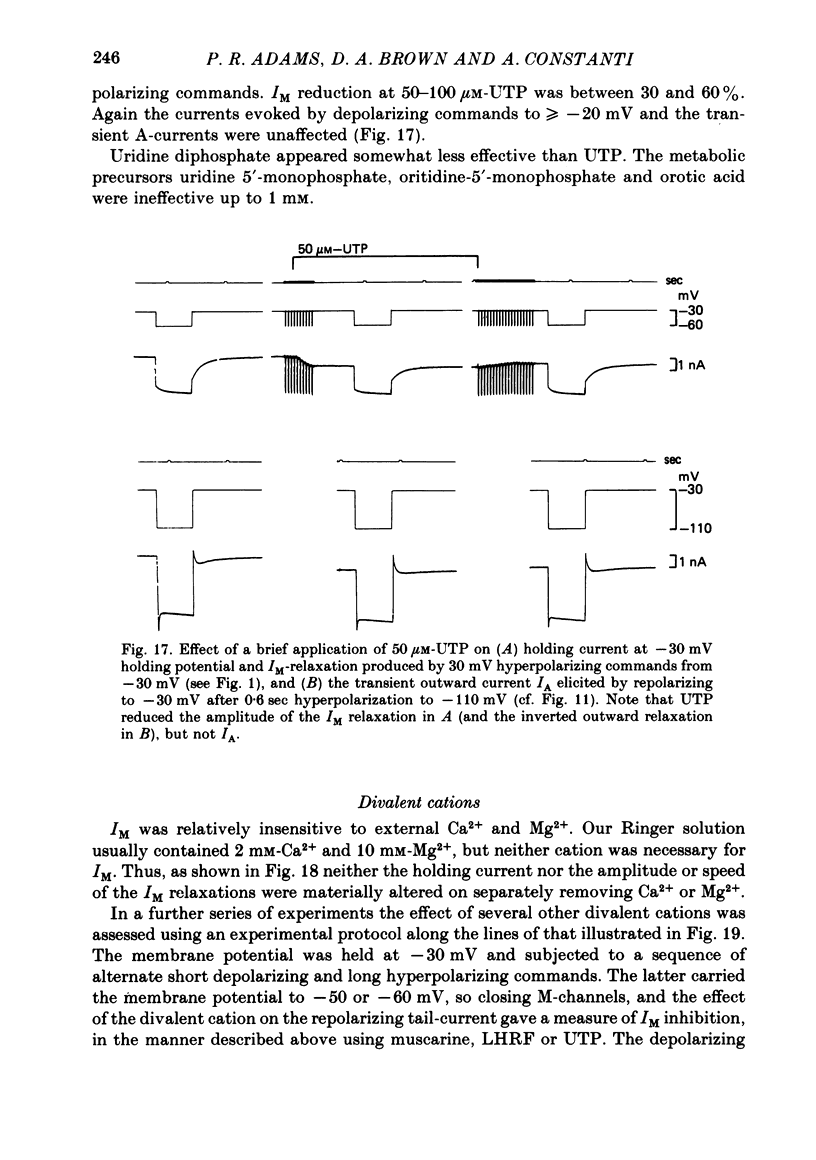
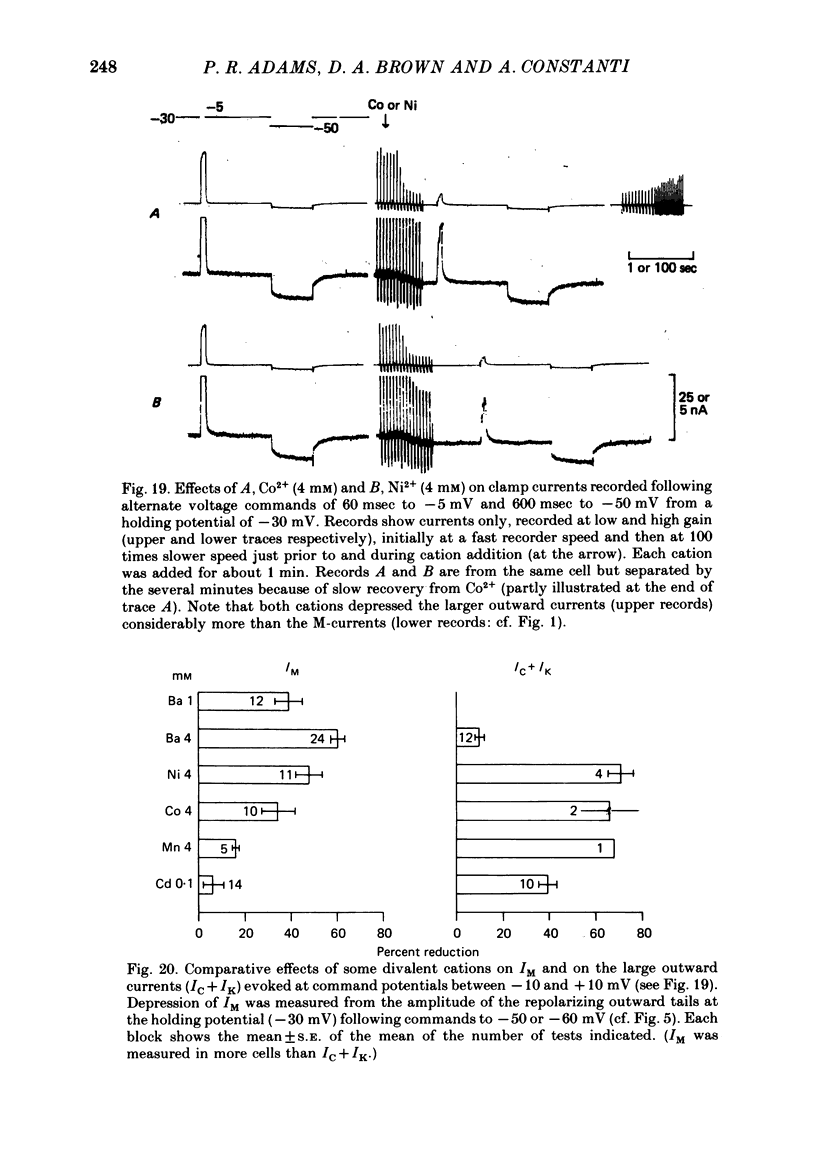
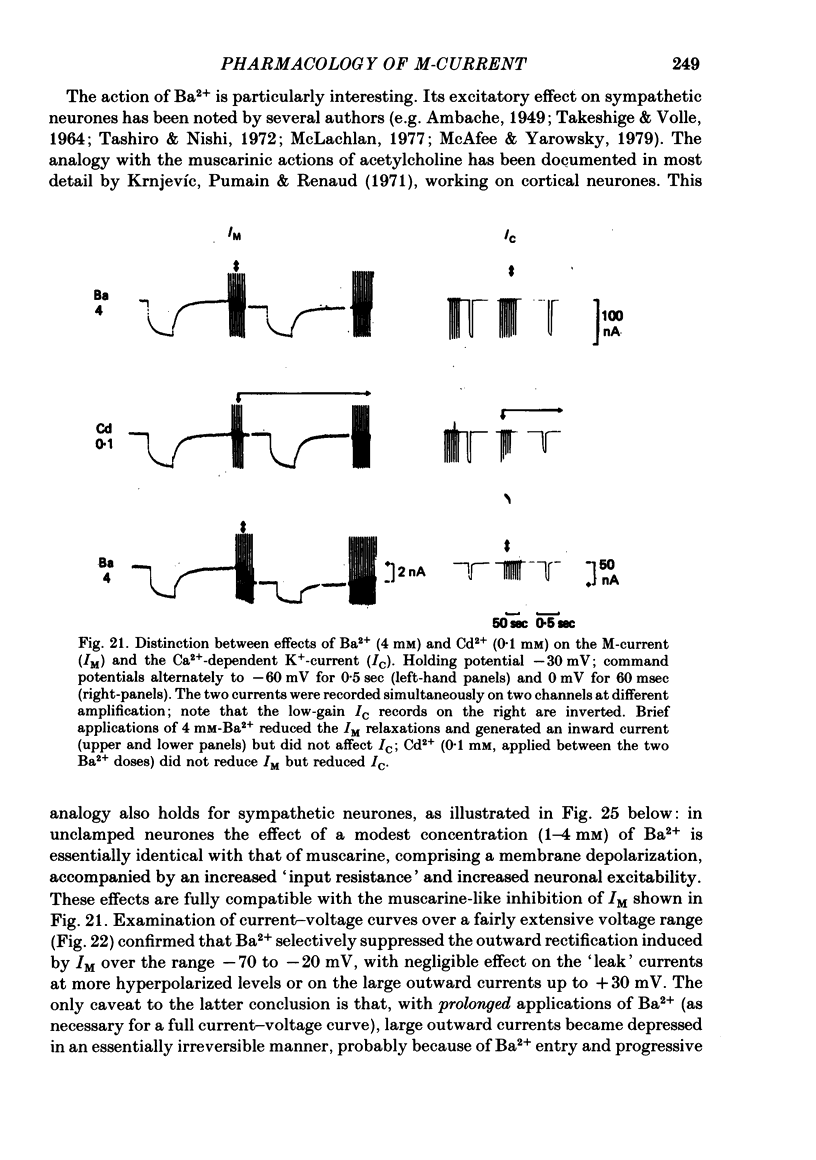
2. Muscarine (1-10 μM), D-ala6 LHRH (1-5 μM), UTP (50-100 μM) and Ba2+ (1-4 mM) selectively depressed the M-current (IM), without appreciable effect on the delayed rectifier, Ca2+-activated or transient outward currents (IK, IC or IA).
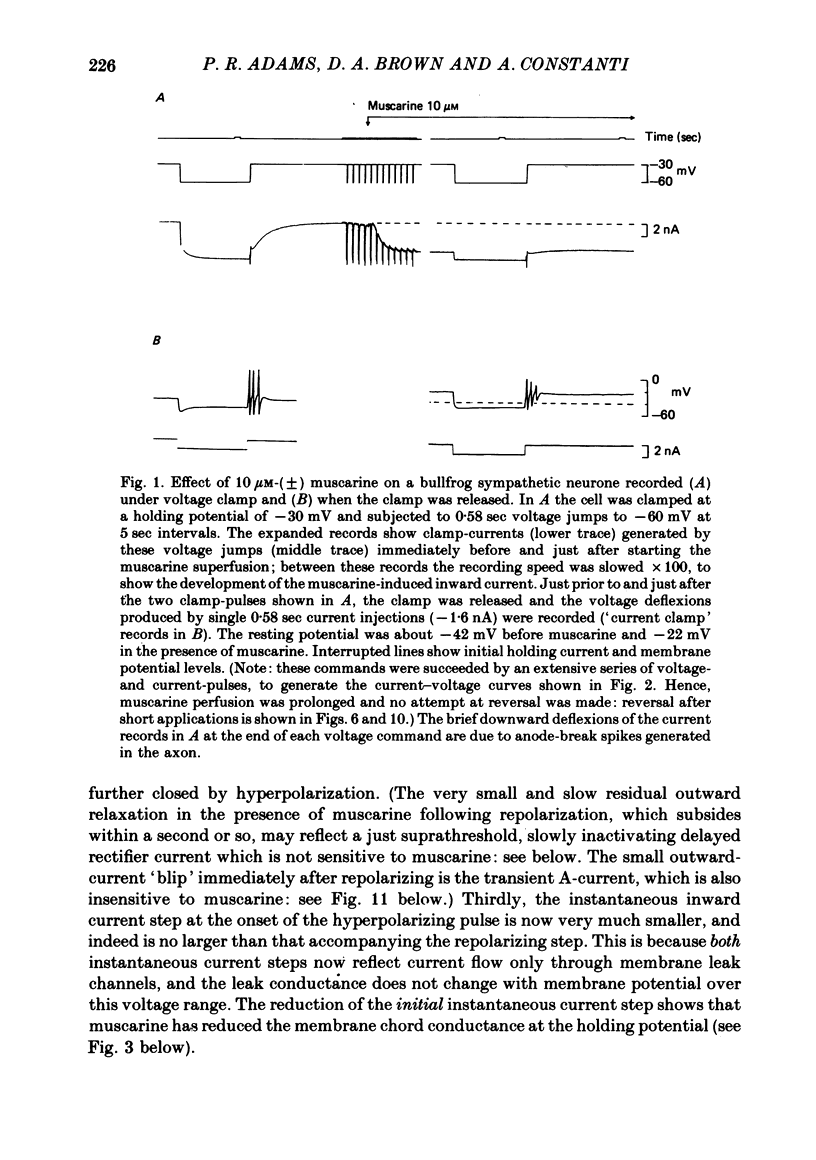
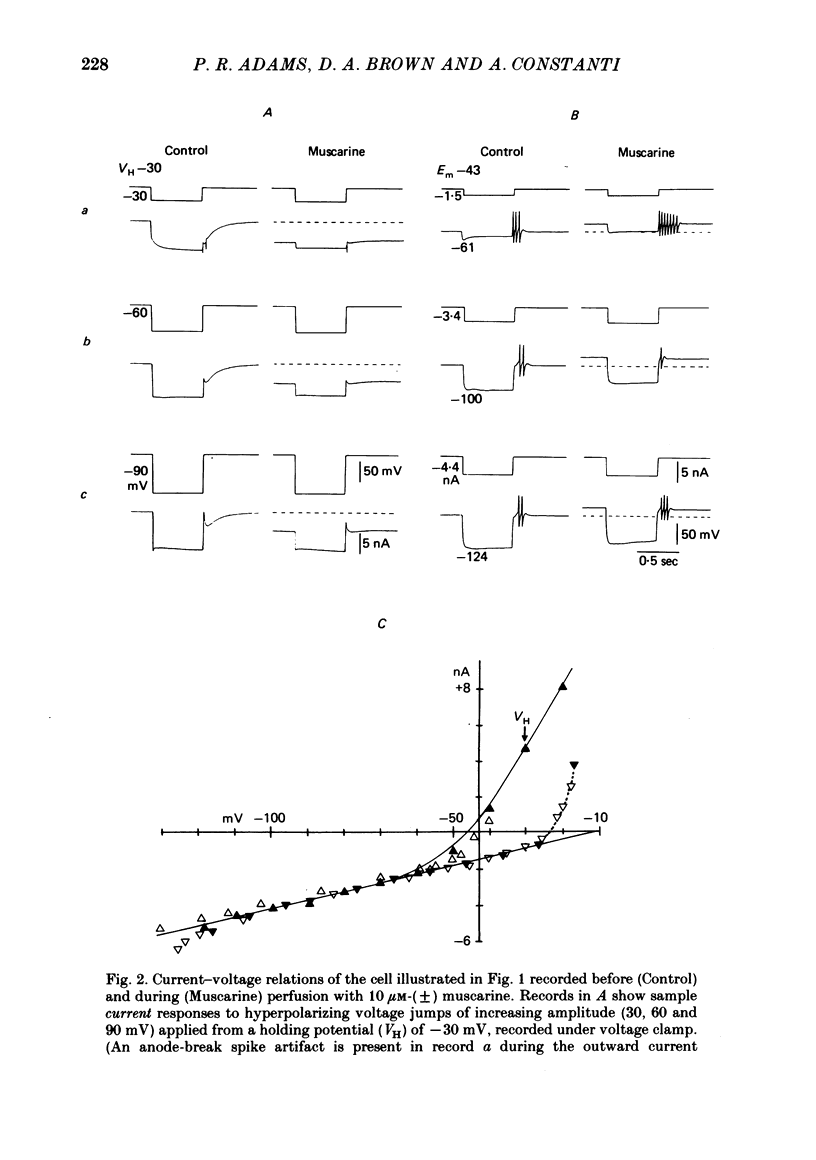
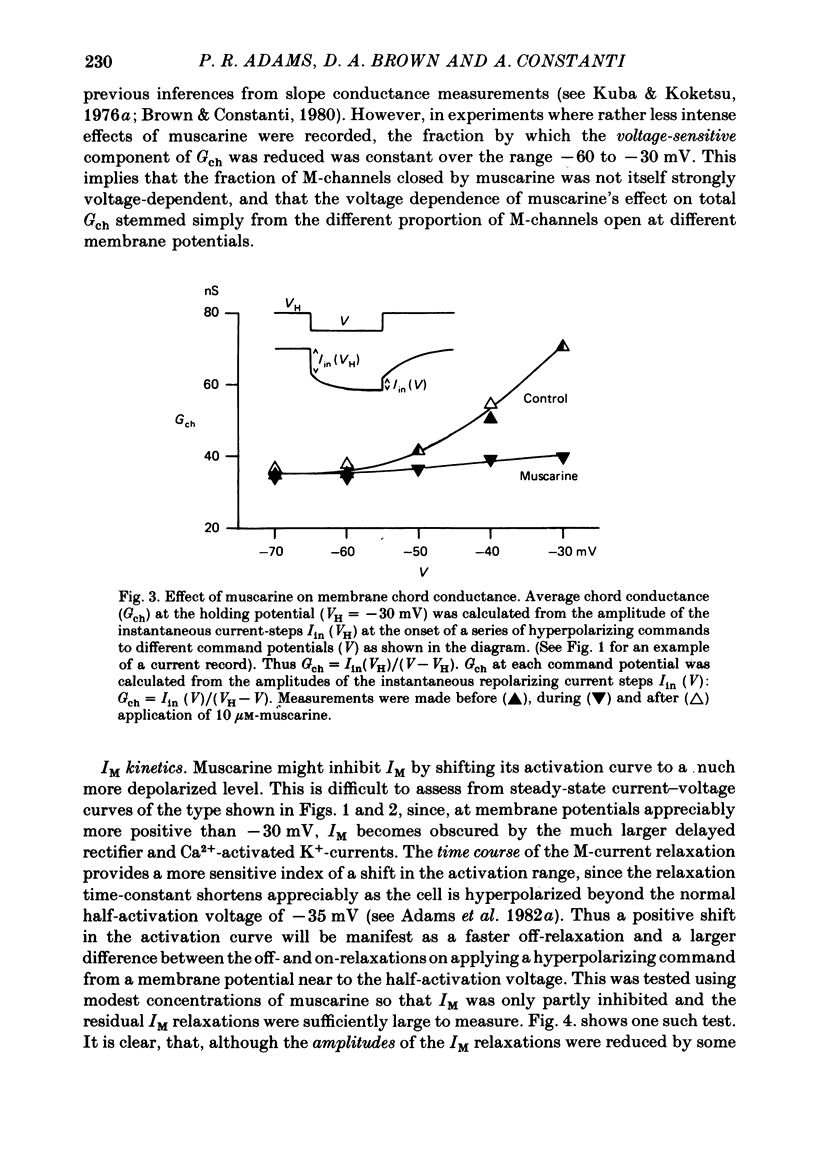
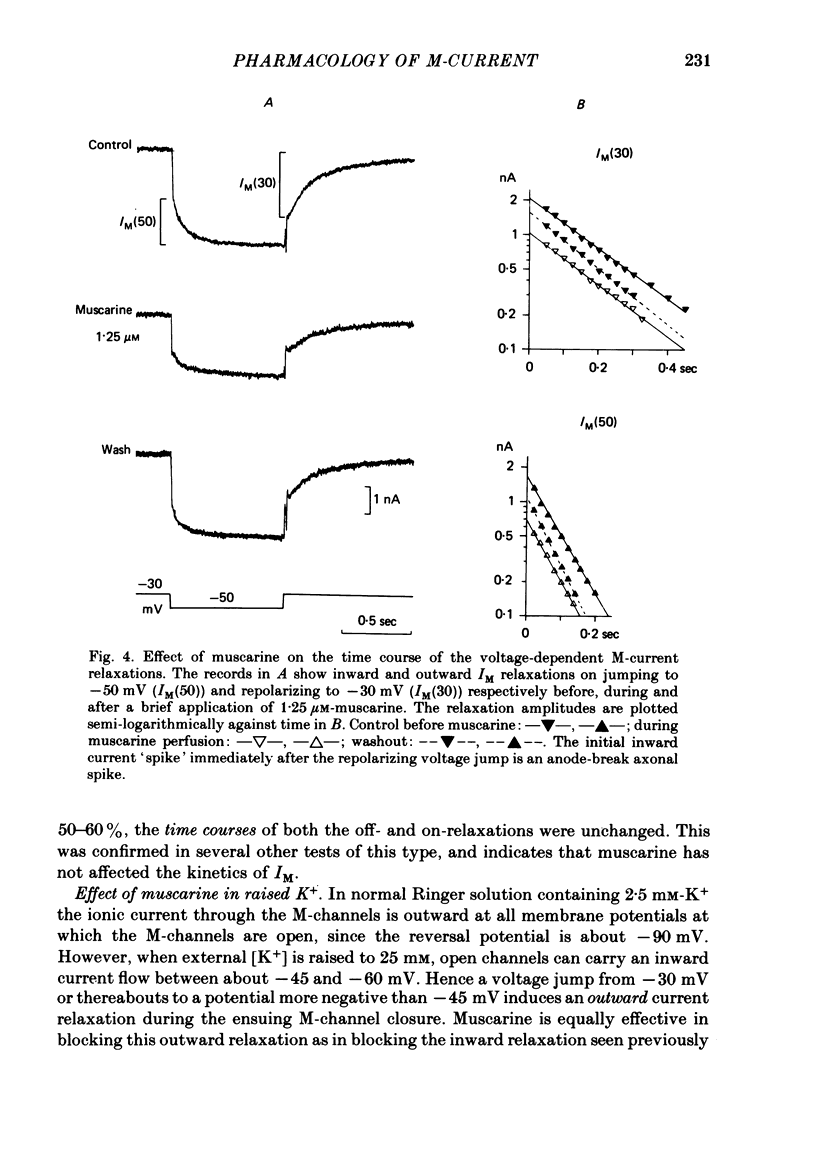
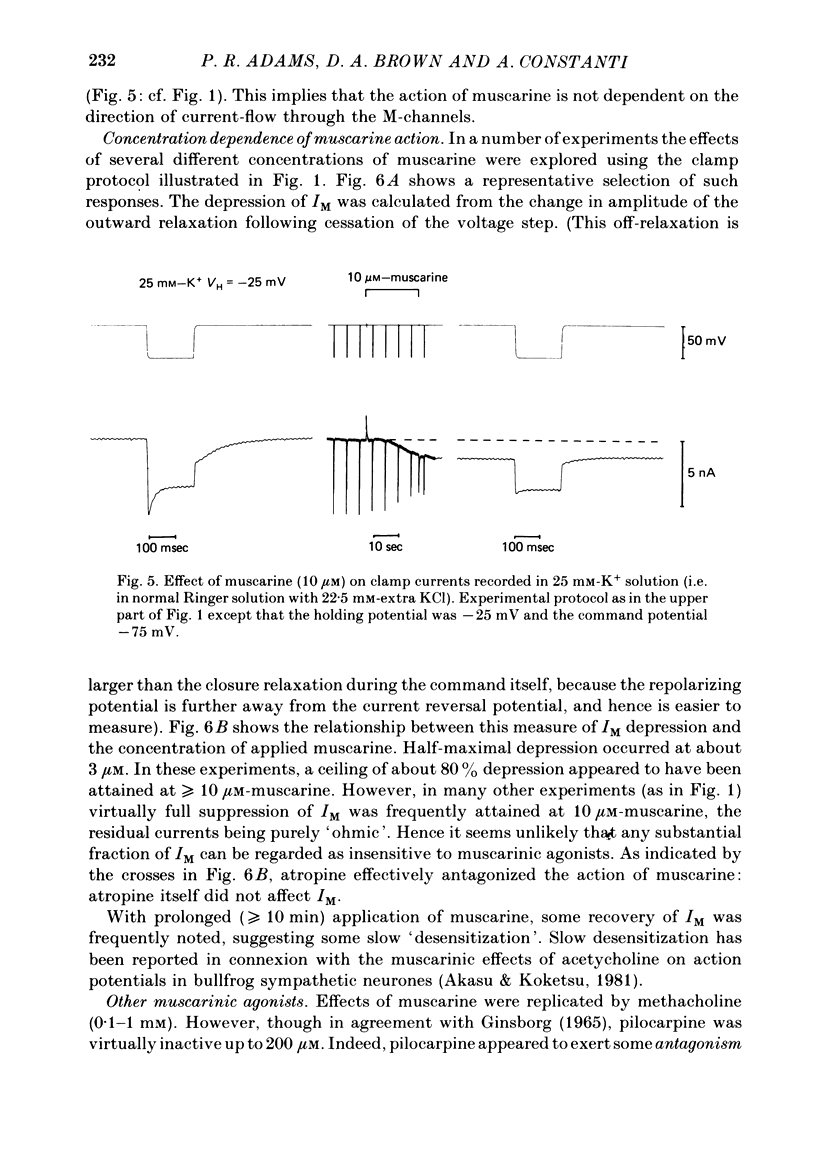
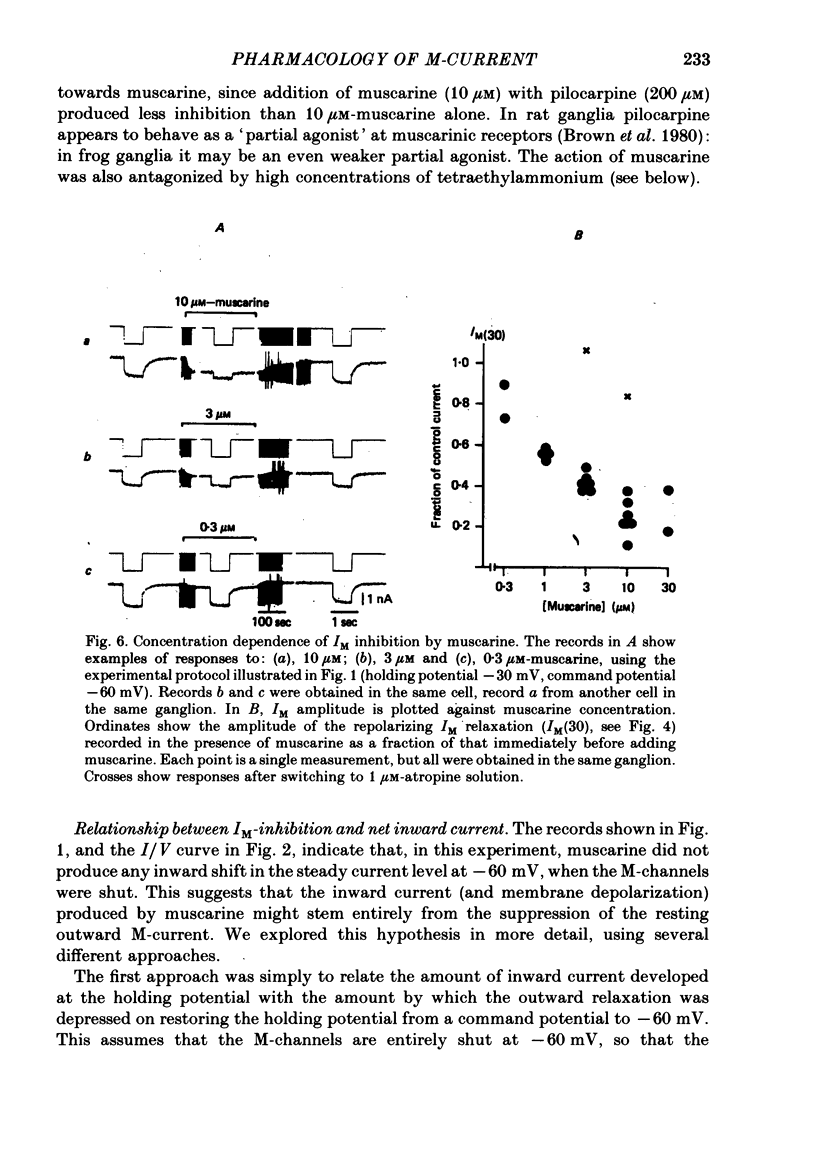
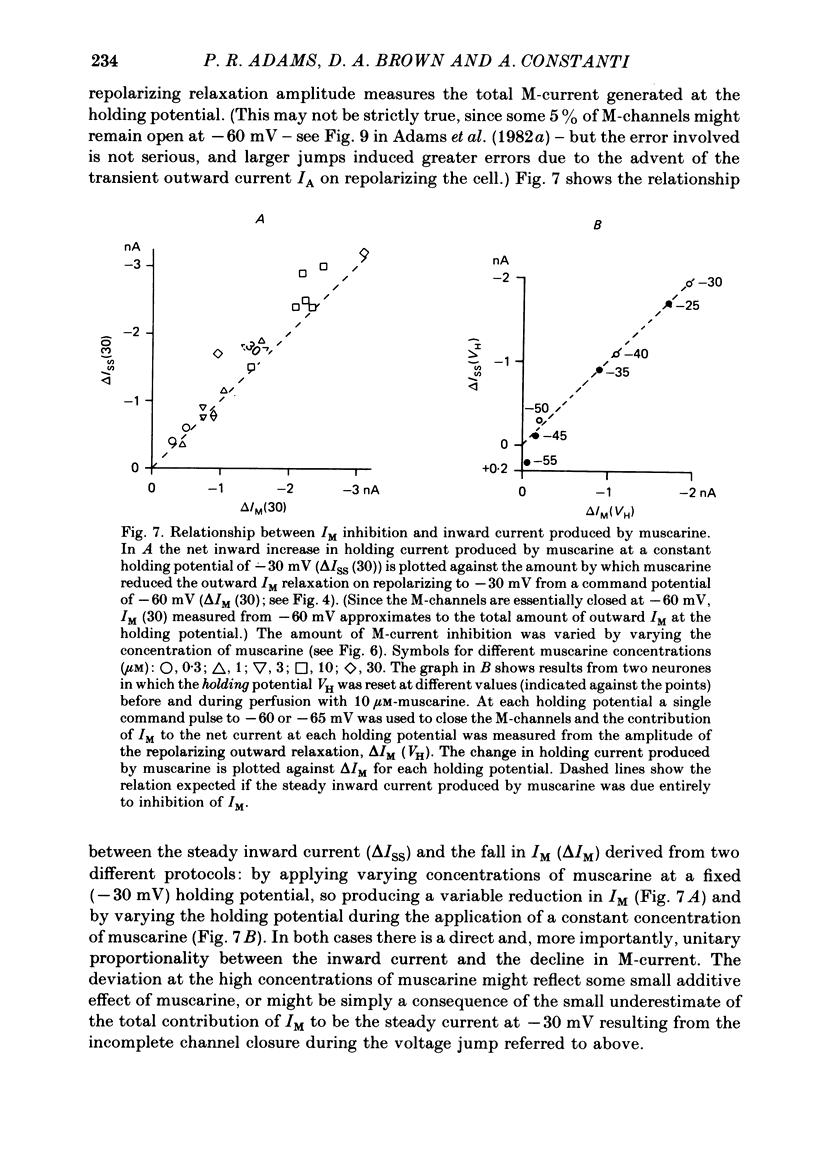
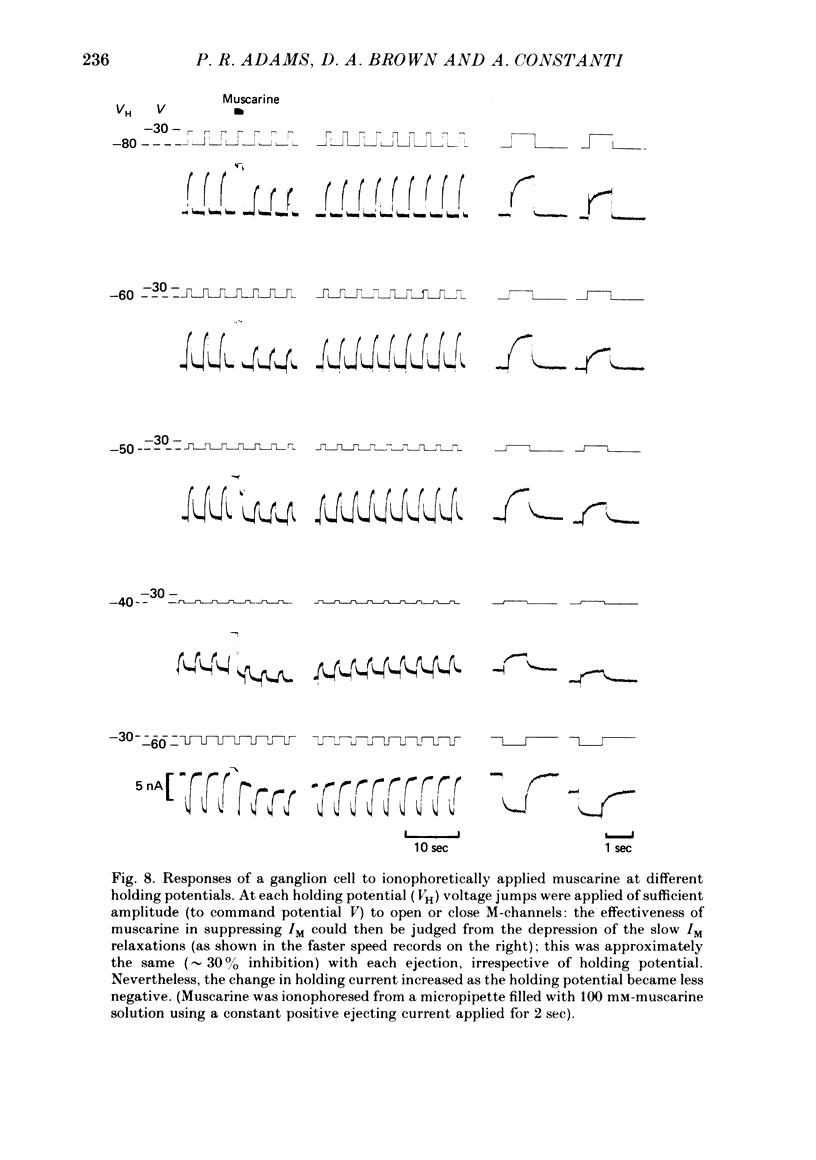
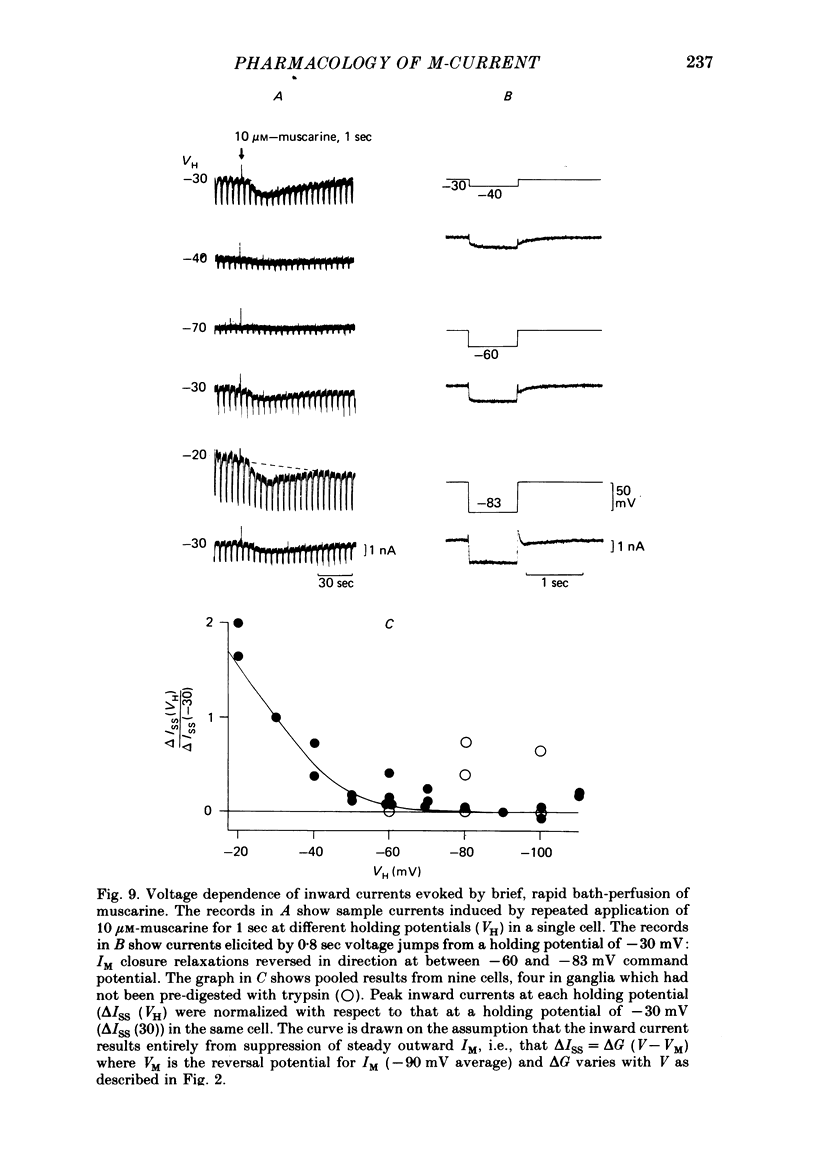
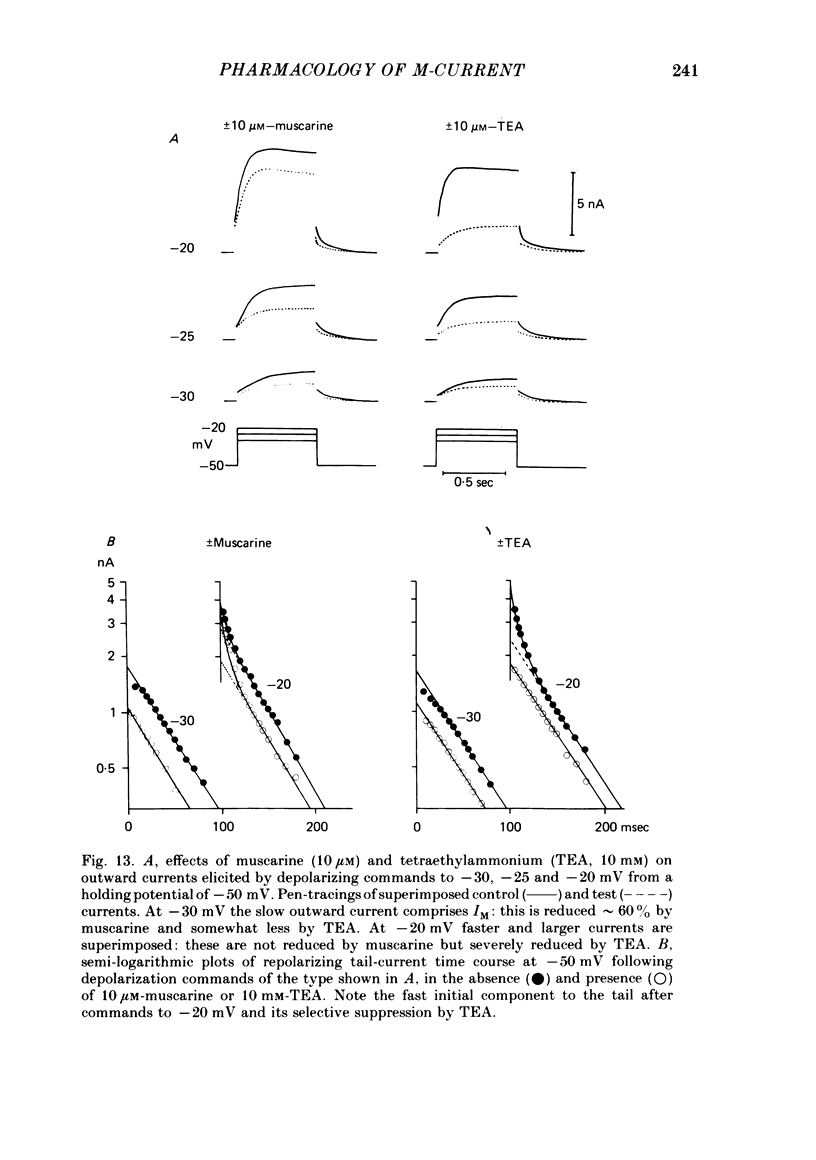
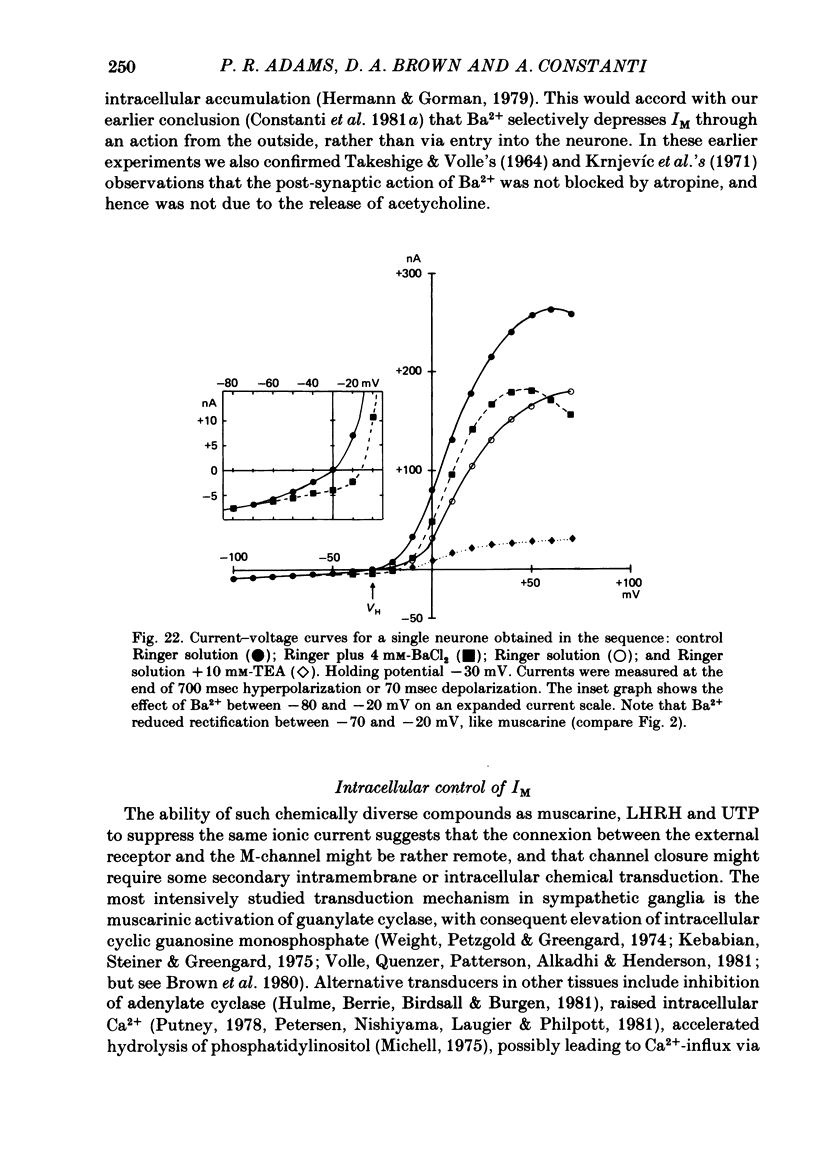
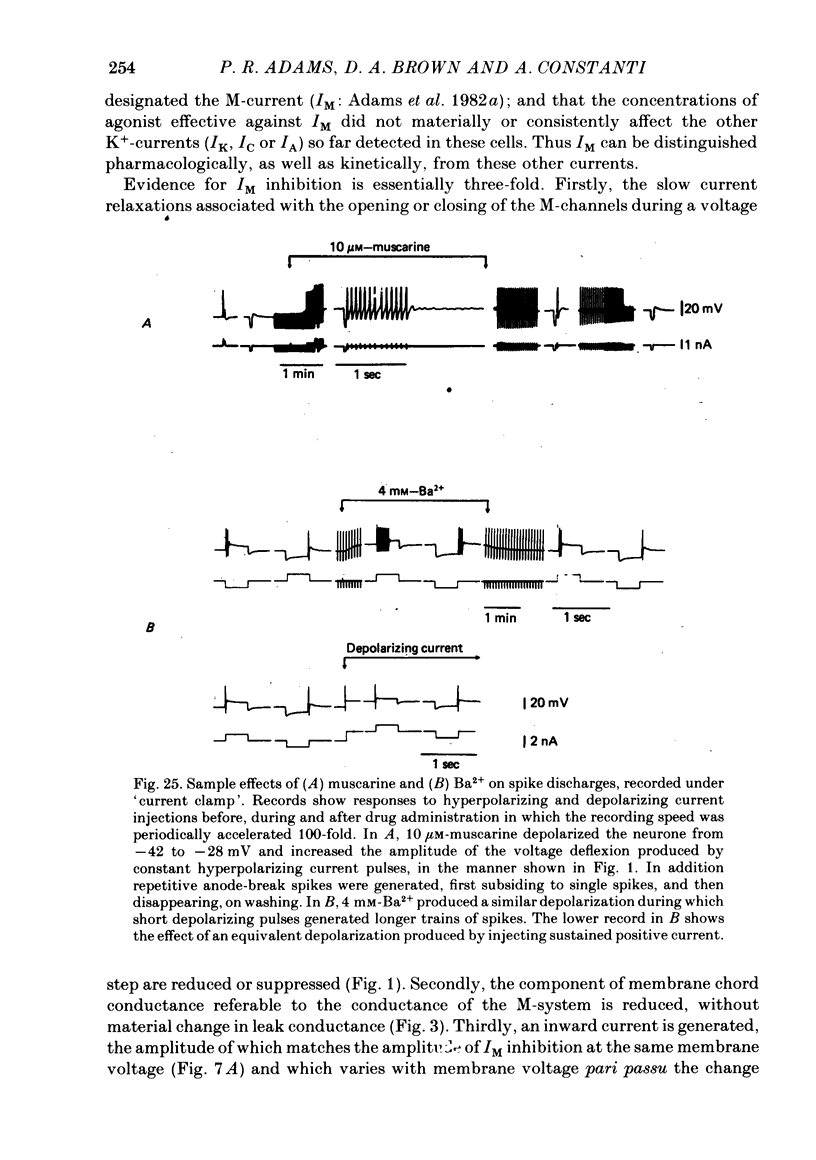
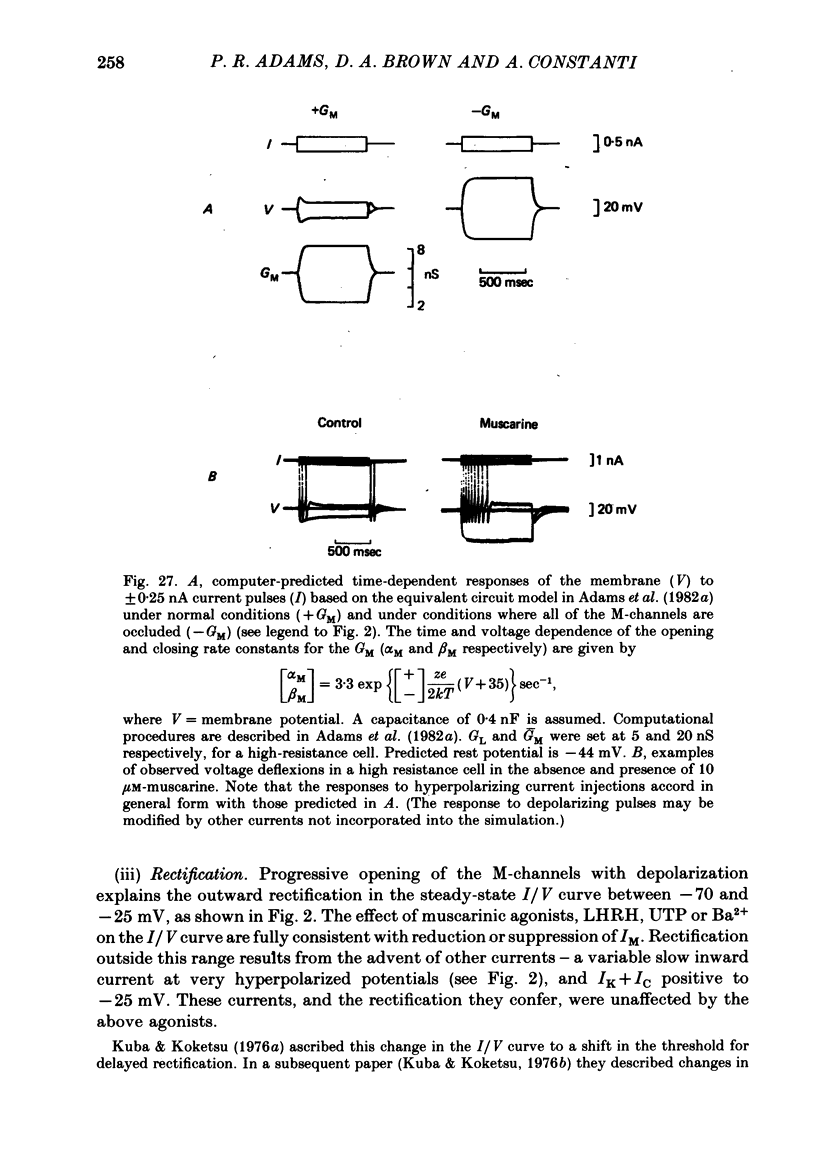
3. IM-inhibition was characterized by: (a) elimination of slow current relaxations accompanying voltage jumps in the membrane potential range -30 to -60 mV; (b) reduced voltage-dependent chord conductance over this range with no change in the voltage-independent chord conductance at more negative membrane potentials; (c) suppression of outward rectification in the steady-state current—voltage curve between -70 and -25 mV; and (d) development of an inward current which increased in amplitude between -70 and -20 mV in proportion to the decrease in steady-state IM. The kinetics and voltage sensitivity of residual IM were unchanged.
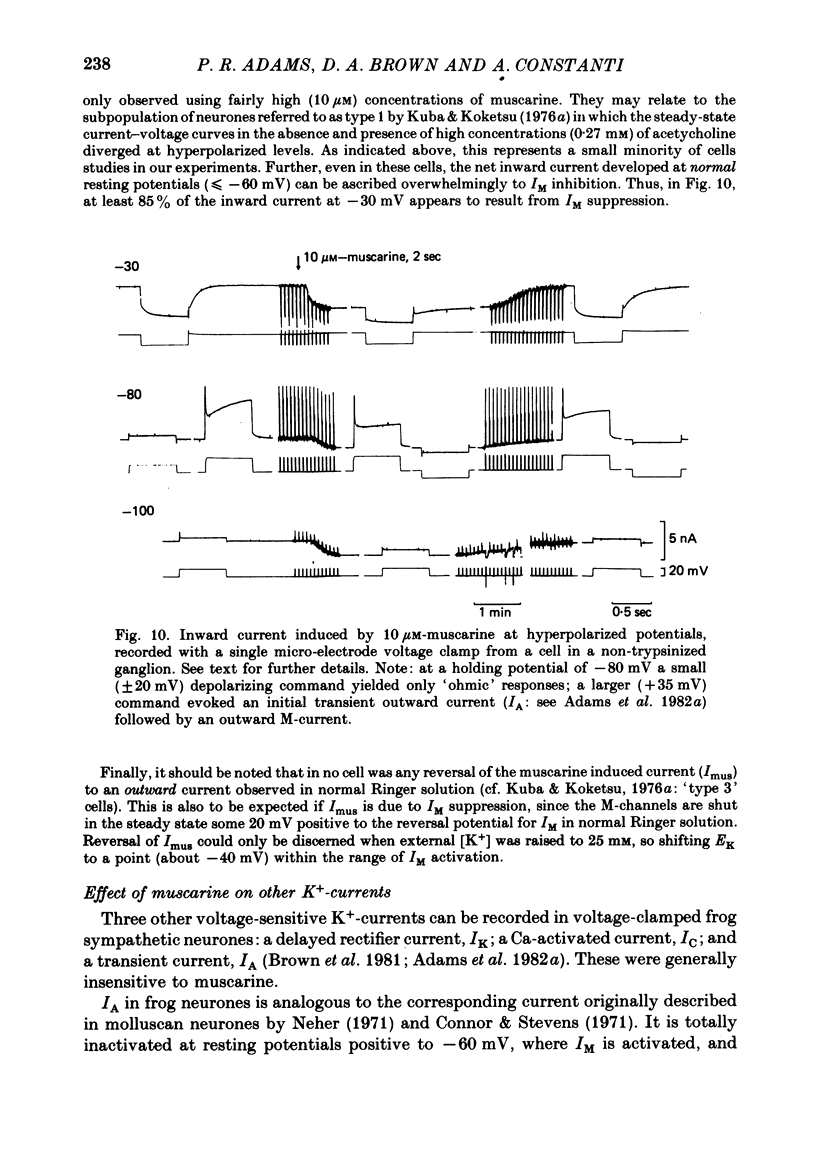
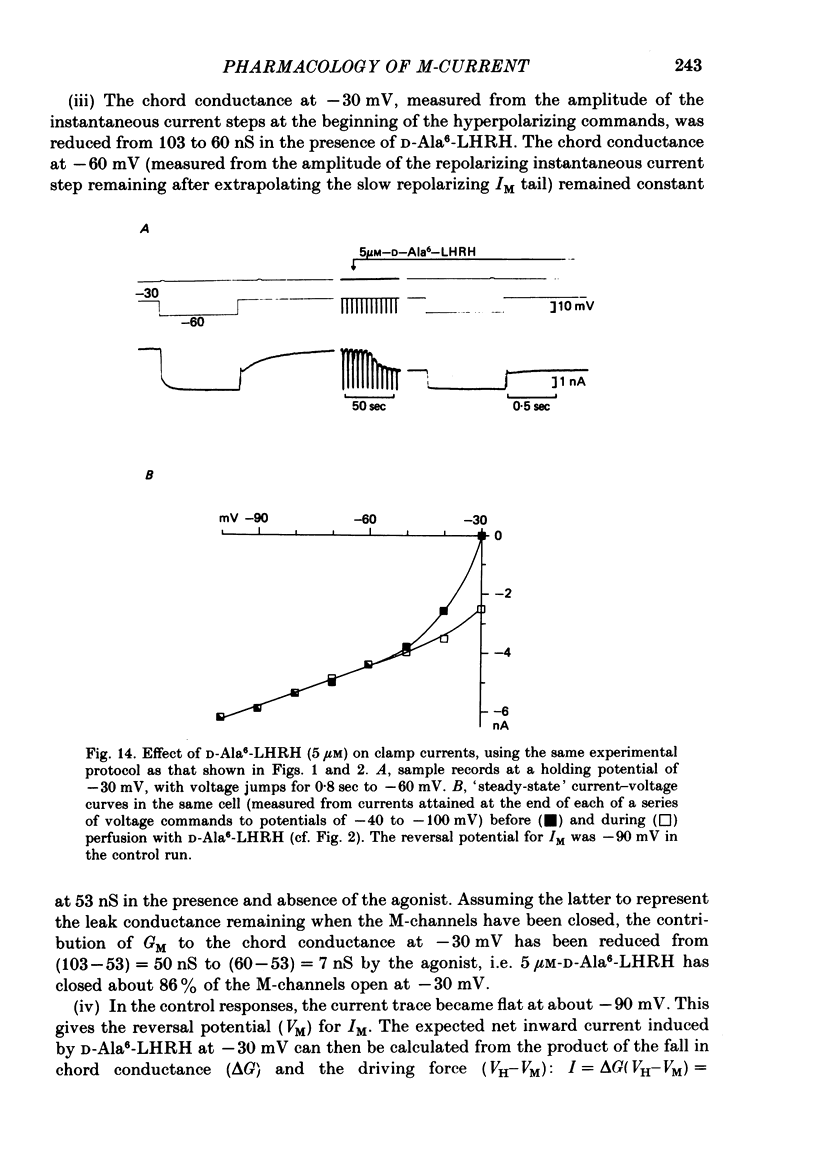
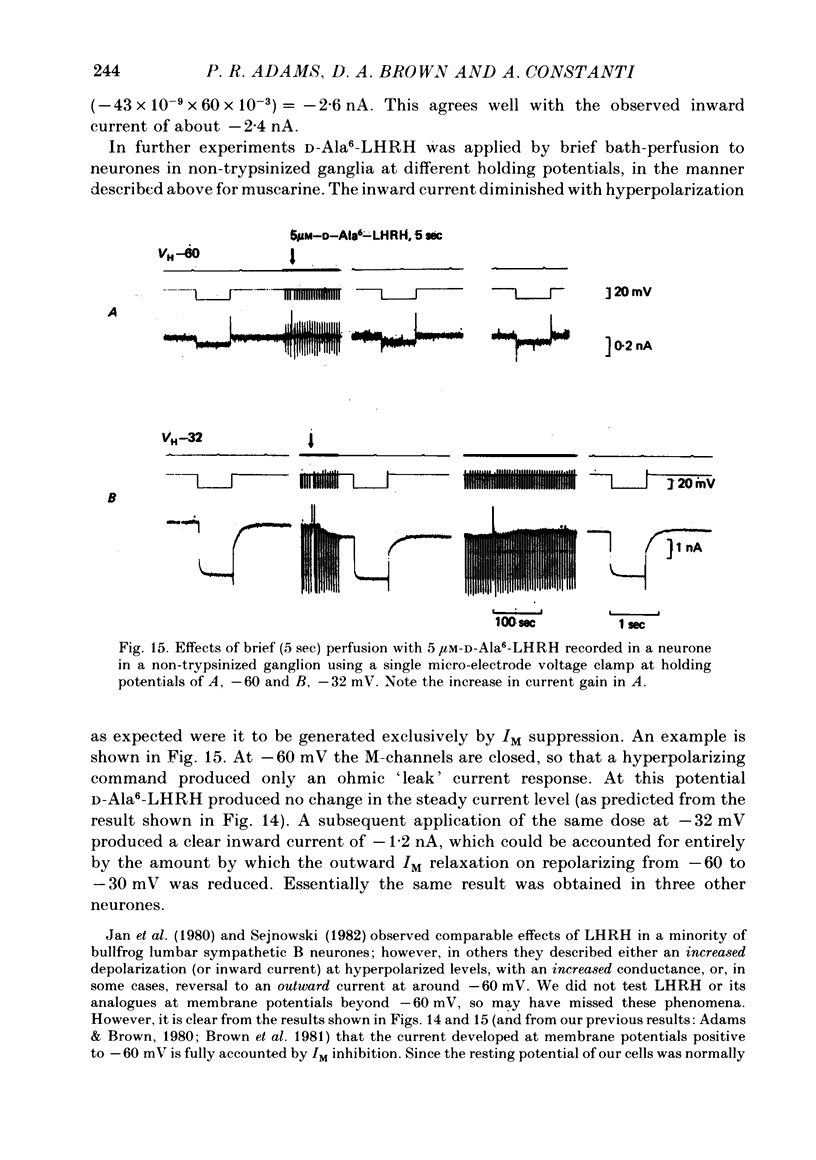
4. The magnitude of the inward current produced by muscarine or LHRH could be accounted for quantitatively by the reduction in steady-state IM. No increase in leak current could be detected in the range -60 to -30 mV. In two cells muscarine (10 μM) increased the leak current and conductance at -70 to -100 mV, but not at more depolarized levels.
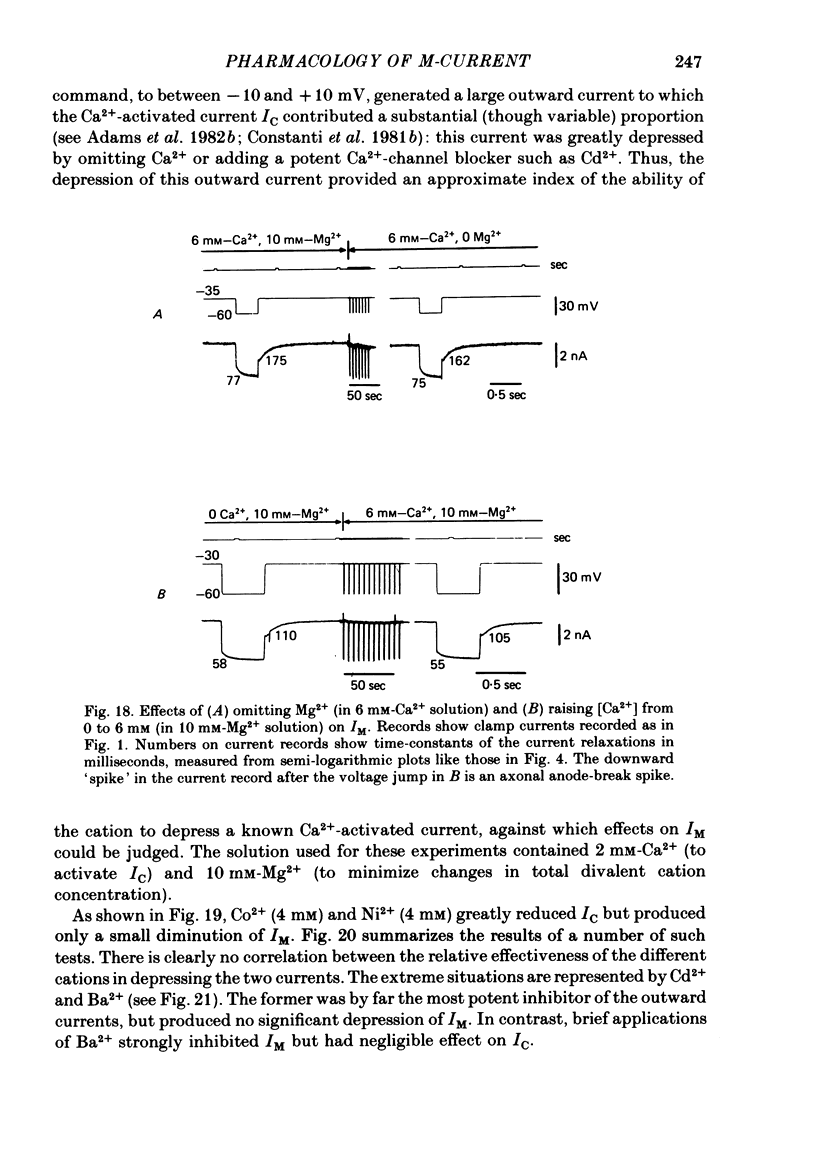
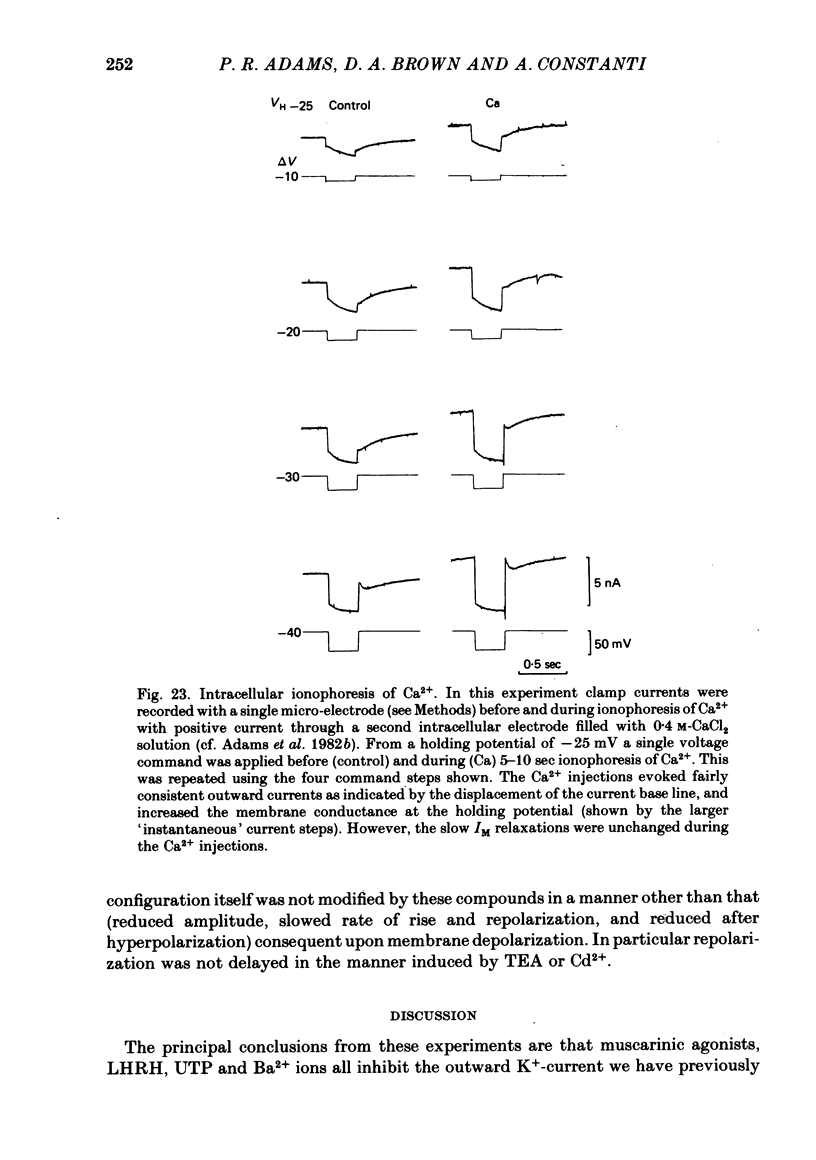
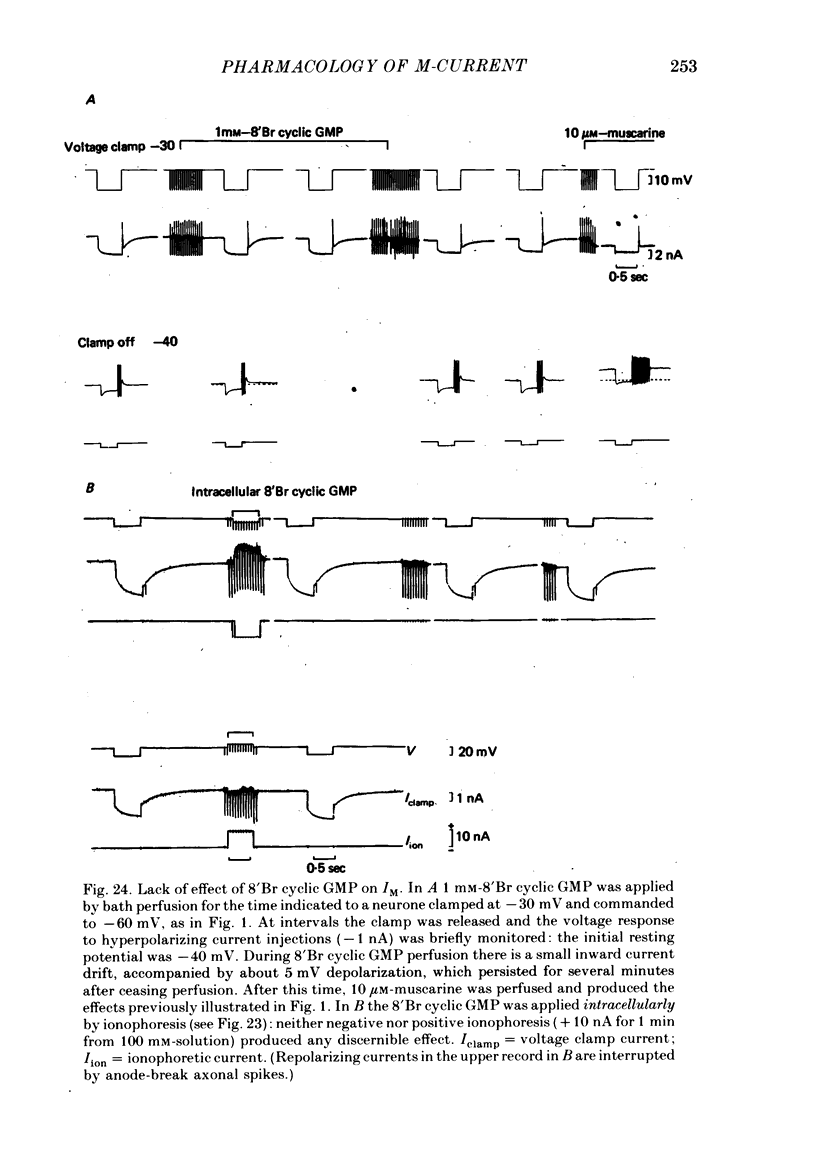
5. IM was not modified by removing extracellular Ca2+, adding a selective Ca2+-channel blocker (Cd2+), adding 1 mM-dibutyryl cyclic AMP or 8′Br cyclic GMP, or by intracellular ionophoresis of Ca2+, 8′Br cyclic GMP, dibutyryl cyclic AMP, GTP-γ-S or S-adenosylmethionine.
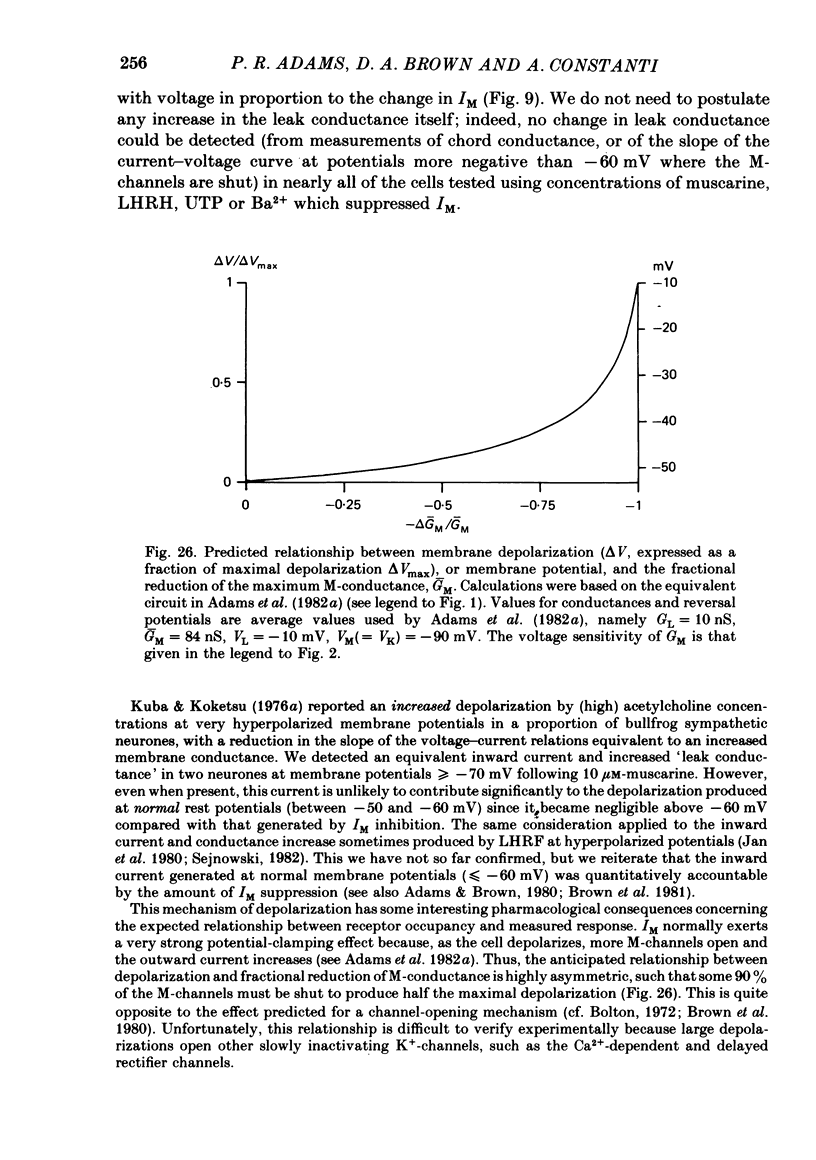
6. It is concluded that the principal effects of these agents in unclamped neurones — depolarization, increased input resistance, reduced outward rectification and increased excitability — are due entirely to a selective inhibition of IM. The intracellular transduction mechanism for IM inhibition is unknown.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBACHE N., PERRY W. L., ROBERTSON P. A. The effect of muscarine on perfused superior cervical ganglia of cats. Br J Pharmacol Chemother. 1956 Dec;11(4):442–448. doi: 10.1111/j.1476-5381.1956.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMBACHE N. The nicotinic action of substances supposed to be purely smooth-muscle stimulating; effect of BaCl2 and pilocarpine on the superior cervical ganglion. J Physiol. 1949 Dec 15;110(1-2):164–172. doi: 10.1113/jphysiol.1949.sp004429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A. Luteinizing hormone-releasing factor and muscarinic agonists act on the same voltage-sensitive K+-current in bullfrog sympathetic neurones. Br J Pharmacol. 1980 Mar;68(3):353–355. doi: 10.1111/j.1476-5381.1980.tb14547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Akasu T., Koketsu K. Desensitization of the muscarinic receptor controlling action potentials of sympathetic ganglion cells in bullfrogs. Life Sci. 1980 Dec 8;27(23):2261–2267. doi: 10.1016/0024-3205(80)90393-8. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Constanti A. Intracellular observations on the effects of muscarinic agonists on rat sympathetic neurones. Br J Pharmacol. 1980 Dec;70(4):593–608. doi: 10.1111/j.1476-5381.1980.tb09778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Fatherazi S., Garthwaite J., White R. D. Muscarinic receptors in rat sympathetic ganglia. Br J Pharmacol. 1980 Dec;70(4):577–592. doi: 10.1111/j.1476-5381.1980.tb09777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Adams P. R., Brown D. A. Who do barium ions imitate acetylcholine? Brain Res. 1981 Feb 9;206(1):244–250. doi: 10.1016/0006-8993(81)90125-6. [DOI] [PubMed] [Google Scholar]
- Constanti A., Brown D. A. M-Currents in voltage-clamped mammalian sympathetic neurones. Neurosci Lett. 1981 Jul 17;24(3):289–294. doi: 10.1016/0304-3940(81)90173-7. [DOI] [PubMed] [Google Scholar]
- Freschi J. E., Shain W. G. Slow muscarinic depolarization in neurons of dissociated rat superior cervical ganglia can be evoked by iontophoresis of acetylcholine. Brain Res. 1980 Mar 10;185(2):429–434. doi: 10.1016/0006-8993(80)91081-1. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. The actions of McN-A-343, pilocarpine and acetyl-beta-methylcholine on sympathetic ganglion cells of the frog. J Pharmacol Exp Ther. 1965 Nov;150(2):216–219. [PubMed] [Google Scholar]
- Gruol D. L., Siggins G. R., Padjen A. L., Forman D. S. Explant cultures of adult amphibian sympathetic ganglia: electrophysiological and pharmacological investigation of neurotransmitter and nucleotide action. Brain Res. 1981 Oct 26;223(1):81–106. doi: 10.1016/0006-8993(81)90808-8. [DOI] [PubMed] [Google Scholar]
- Hauswirth O., Noble D., Tsien R. W. Adrenaline: mechanism of action on the pacemaker potential in cardiac Purkinje fibers. Science. 1968 Nov 22;162(3856):916–917. doi: 10.1126/science.162.3856.916. [DOI] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Blockade of voltage-dependent and Ca2+-dependent K+ current components by internal Ba2+ in molluscan pacemaker neurons. Experientia. 1979 Feb 15;35(2):229–231. doi: 10.1007/BF01920633. [DOI] [PubMed] [Google Scholar]
- Horn R., Brodwick M. S. Acetylcholine-induced current in perfused rat myoballs. J Gen Physiol. 1980 Mar;75(3):297–321. doi: 10.1085/jgp.75.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1501–1505. doi: 10.1073/pnas.76.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. Further evidence for peptidergic transmission in sympathetic ganglia. Proc Natl Acad Sci U S A. 1980 Aug;77(8):5008–5012. doi: 10.1073/pnas.77.8.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONZETT H., WASER P. G. Zur ganglionären Wirkung von Muscarin. Helv Physiol Pharmacol Acta. 1956;14(2):202–206. [PubMed] [Google Scholar]
- Kebabian J. W., Steiner A. L., Greengard P. Muscarinic cholinergic regulation of cyclic guanosine 3,5-monophosphate in autonomic ganglia: possible role in synaptic transmission. J Pharmacol Exp Ther. 1975 May;193(2):474–488. [PubMed] [Google Scholar]
- Kobayashi H., Libet B. Actions of noradrenaline and acetylcholine on sympathetic ganglion cells. J Physiol. 1970 Jun;208(2):353–372. doi: 10.1113/jphysiol.1970.sp009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Pumain R., Renaud L. Effects of Ba2+ and tetraethylammonium on cortical neurones. J Physiol. 1971 May;215(1):223–245. doi: 10.1113/jphysiol.1971.sp009466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Analysis of the slow excitatory postsynaptic potential in bullfrog sympathetic ganglion cells. Jpn J Physiol. 1976;26(6):651–669. doi: 10.2170/jjphysiol.26.651. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Postsynaptic potentiation of the slow muscarinic excitatory response by tetraethylammonium chloride in the bullfrog sympathetic ganglion cells. Brain Res. 1977 Dec 2;137(2):381–386. doi: 10.1016/0006-8993(77)90351-1. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. The muscarinic effects of acetylcholine on the action potential of bullfrog sympathetic ganglion cells. Jpn J Physiol. 1976;26(6):703–716. doi: 10.2170/jjphysiol.26.703. [DOI] [PubMed] [Google Scholar]
- McAfee D. A., Yarowsky P. J. Calcium-dependent potentials in the mammalian sympathetic neurone. J Physiol. 1979 May;290(2):507–523. doi: 10.1113/jphysiol.1979.sp012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. The effects of strontium and barium ions at synapses in sympathetic ganglia. J Physiol. 1977 May;267(2):497–518. doi: 10.1113/jphysiol.1977.sp011823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., Soeda H., Koketsu K. Unusual nature of ganglionic slow EPSP studied by a voltage-clamp method. Life Sci. 1969 Jan 1;8(1):33–42. doi: 10.1016/0024-3205(69)90290-2. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Stimulus-permeability coupling: role of calcium in the receptor regulation of membrane permeability. Pharmacol Rev. 1978 Jun;30(2):209–245. [PubMed] [Google Scholar]
- Putney J. W., Jr, Weiss S. J., Van De Walle C. M., Haddas R. A. Is phosphatidic acid a calcium ionophore under neurohumoral control? Nature. 1980 Mar 27;284(5754):345–347. doi: 10.1038/284345a0. [DOI] [PubMed] [Google Scholar]
- Salmon D. M., Honeyman T. W. Proposed mechanism of cholinergic action in smooth muscle. Nature. 1980 Mar 27;284(5754):344–345. doi: 10.1038/284344a0. [DOI] [PubMed] [Google Scholar]
- Schulman J. A., Weight F. F. Synaptic transmission: long-lasting potentiation by a postsynaptic mechanism. Science. 1976 Dec 24;194(4272):1437–1439. doi: 10.1126/science.188131. [DOI] [PubMed] [Google Scholar]
- Siggins G. R., Gruol D. L., Padjen A. L., Formans D. S. Purine and pyrimidine mononucleotides depolarise neurones of explanted amphibian sympathetic ganglia. Nature. 1977 Nov 17;270(5634):263–265. doi: 10.1038/270263a0. [DOI] [PubMed] [Google Scholar]
- TAKESHIGE C., VOLLE R. L. THE EFFECTS OF BARIUM AND OTHER INORGANIC CATIONS ON SYMPATHETIC GANGLIA. J Pharmacol Exp Ther. 1964 Dec;146:327–334. [PubMed] [Google Scholar]
- Volle R. L., Quenzer L. F., Patterson B. A., Alkadhi K. A., Henderson E. G. Cyclic guanosine 3':5'-monophosphate accumulation and 45Ca-uptake by rat superior cervical ganglia during preganglionic stimulation. J Pharmacol Exp Ther. 1981 Nov;219(2):338–343. [PubMed] [Google Scholar]
- Weight F. F., Petzold G., Greengard P. Guanosine 3',5'-monophosphate in sympathetic ganglia: increase assoicated with synaptic transmission. Science. 1974 Dec 6;186(4167):942–944. doi: 10.1126/science.186.4167.942. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]



