Abstract
1. The effects of the barbiturate anaesthetics, pentobarbitone and thiopentone, on the membrane properties and the γ-aminobutyric acid (GABA)-induced responses of cat primary afferent neurones were studied with intracellular recording and voltageclamp techniques.
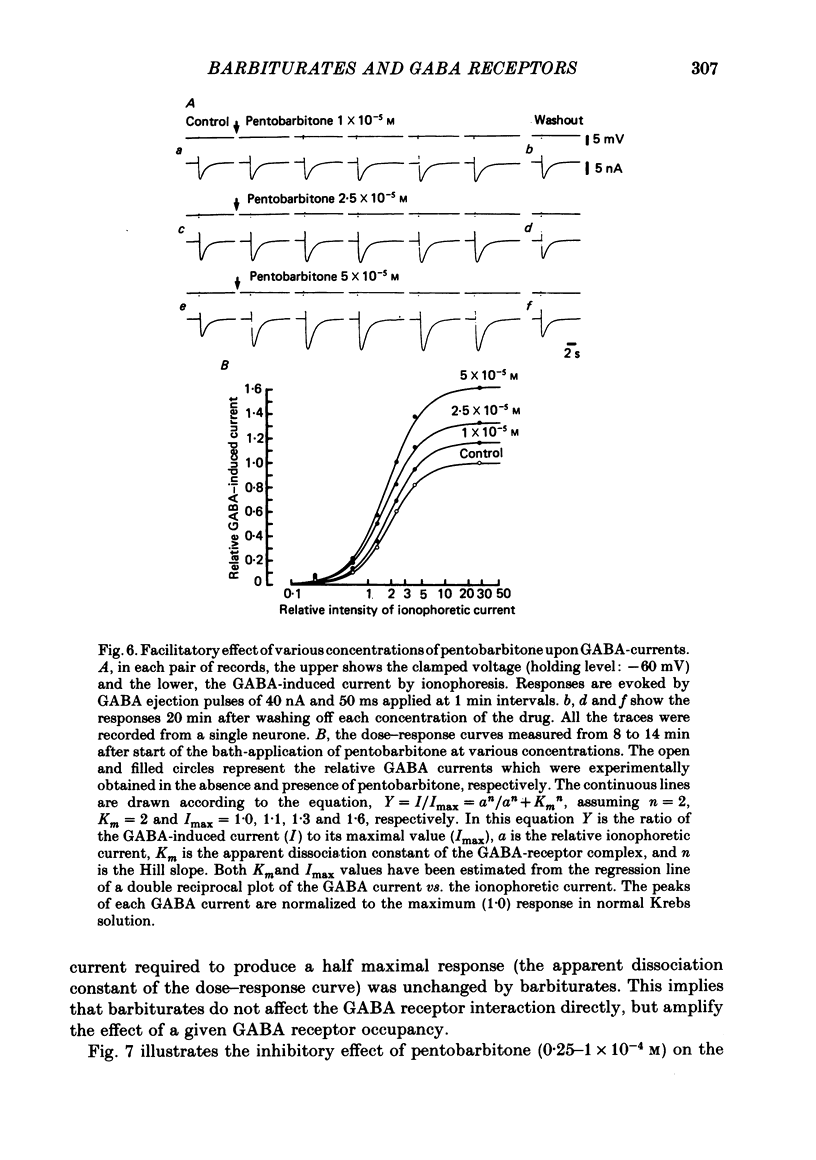
2. At low concentrations (10-7-10-5 M) both barbiturates slightly enhanced and prolonged GABA-induced depolarizations or currents without affecting the membrane properties. At these concentrations, barbiturates have no effect on the apparent dissociation constant of the GABA-GABA receptor interaction or the reversal potential for GABA-induced depolarizations or currents.
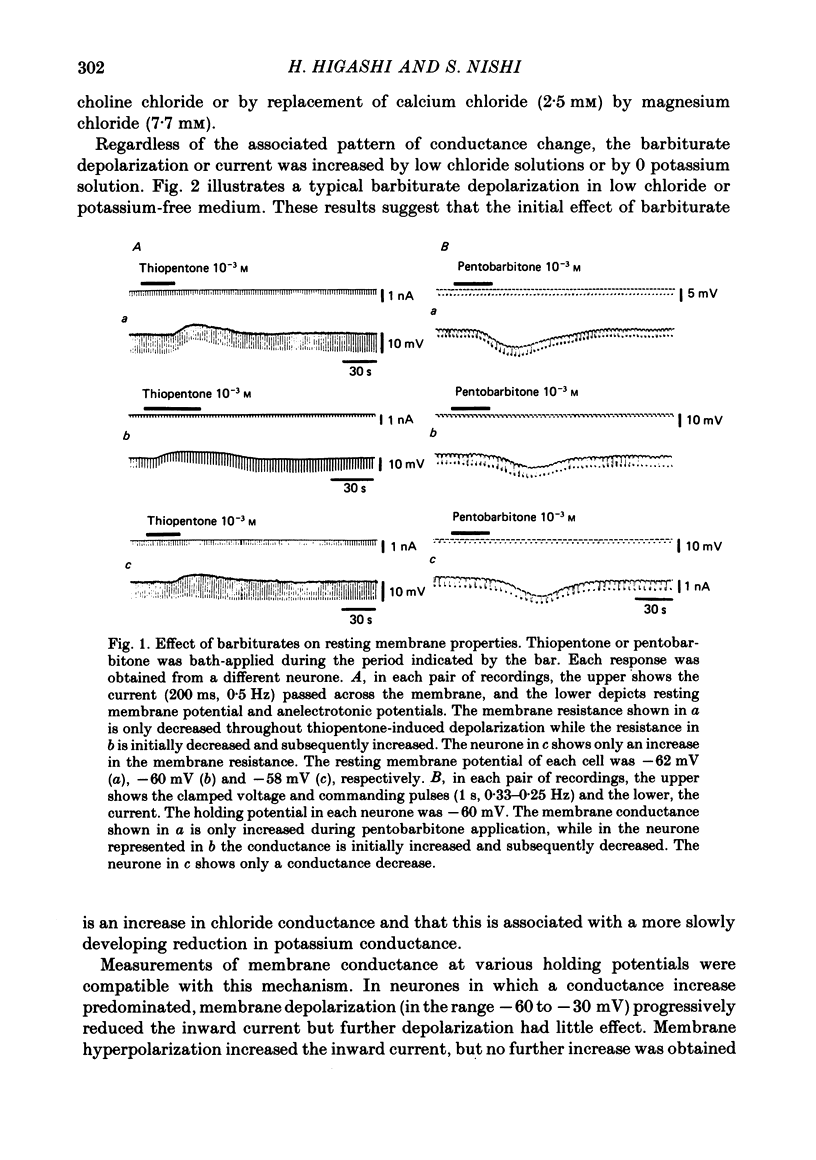
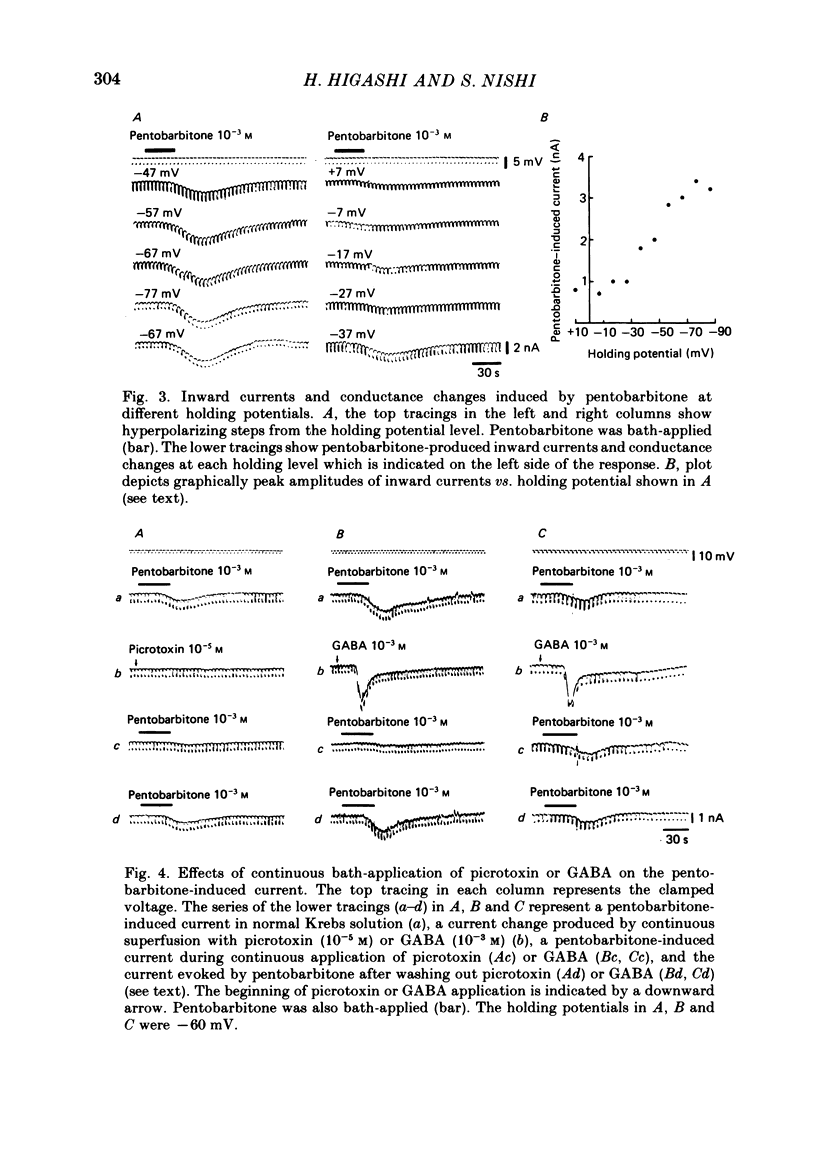
3. At high concentrations (10-4-10-3 M) barbiturates produced a few millivolts reduction in the resting membrane potential. Voltage-clamp analysis revealed that the depolarization was associated with one of the three types of conductance change, i.e., an initial increase followed by a decrease (40% of neurones examined), only an increase (40%) and only a decrease (20%).
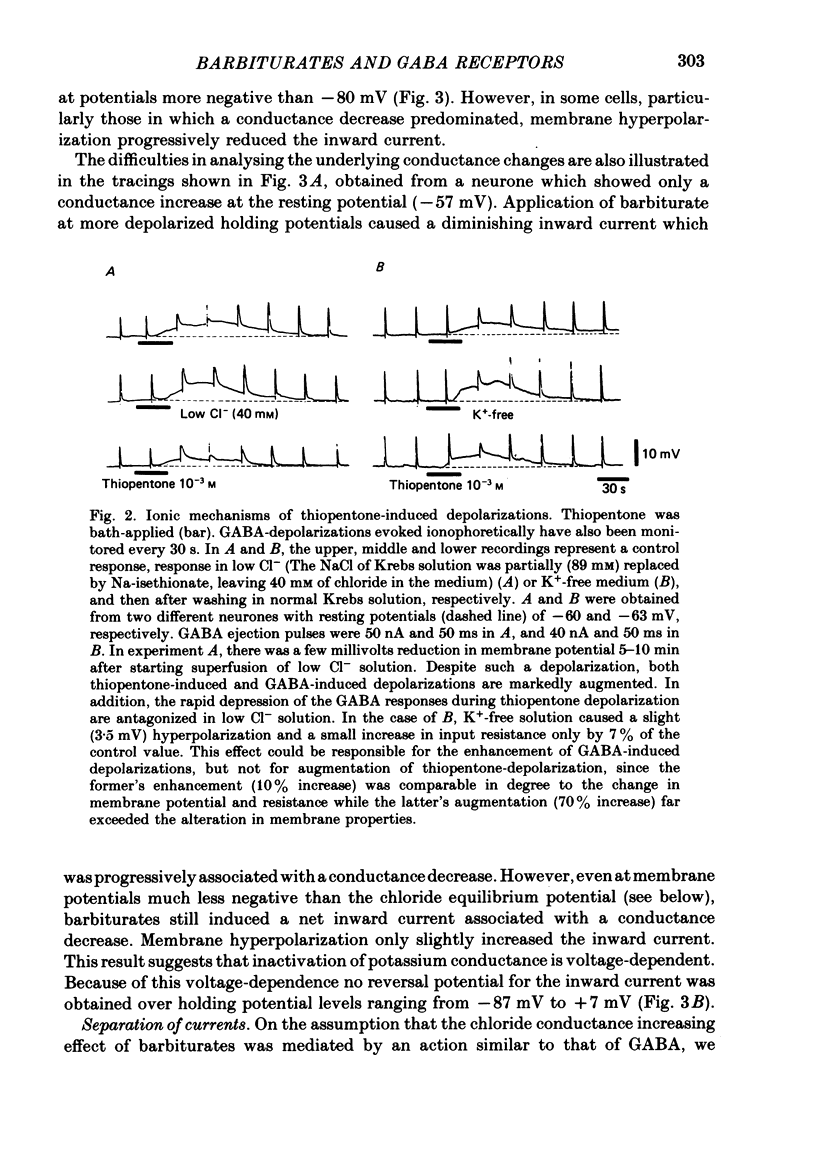
4. Analysis in different ionic media indicated that the depolarization with a reduced membrane resistance is associated with an increased chloride conductance and that the one with an increased membrane resistance is accompanied by a reduction in potassium conductance. Bath-application of GABA (10-3 M) or picrotoxin (10-5 M) inhibited the increase in chloride conductance but not the reduction in potassium conductance.
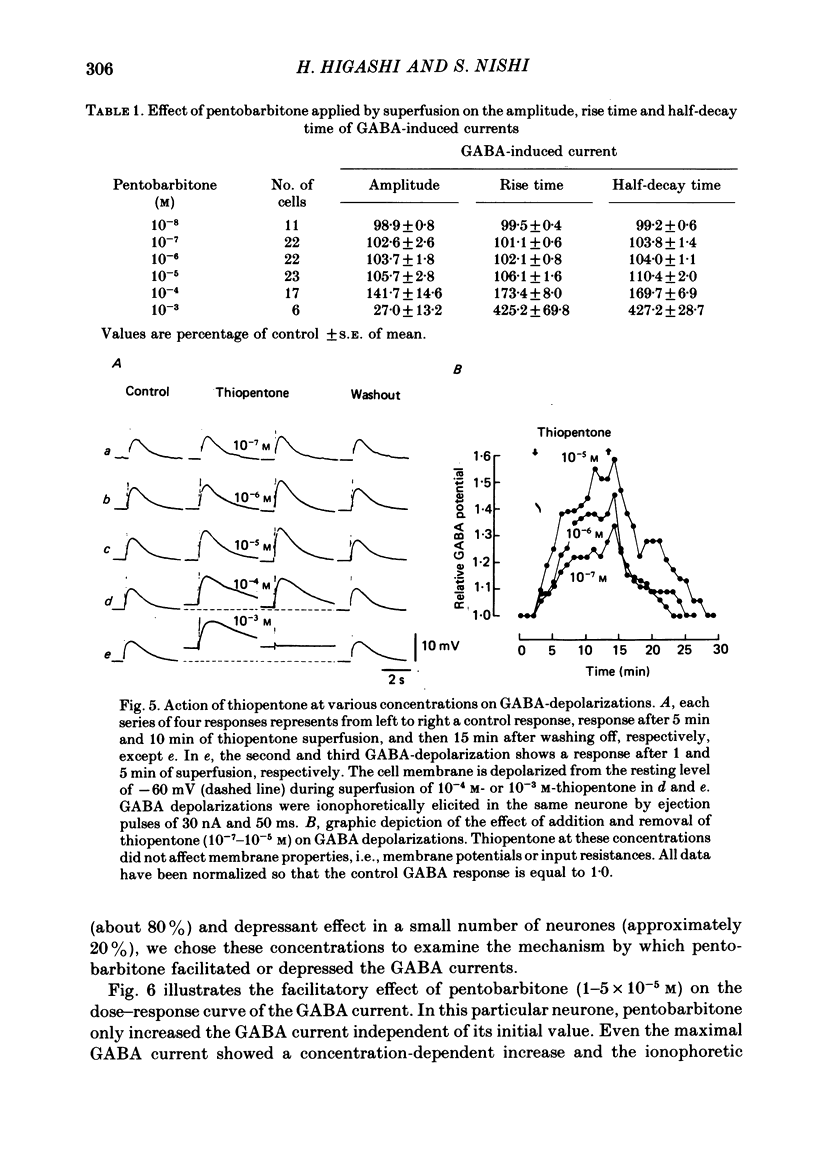
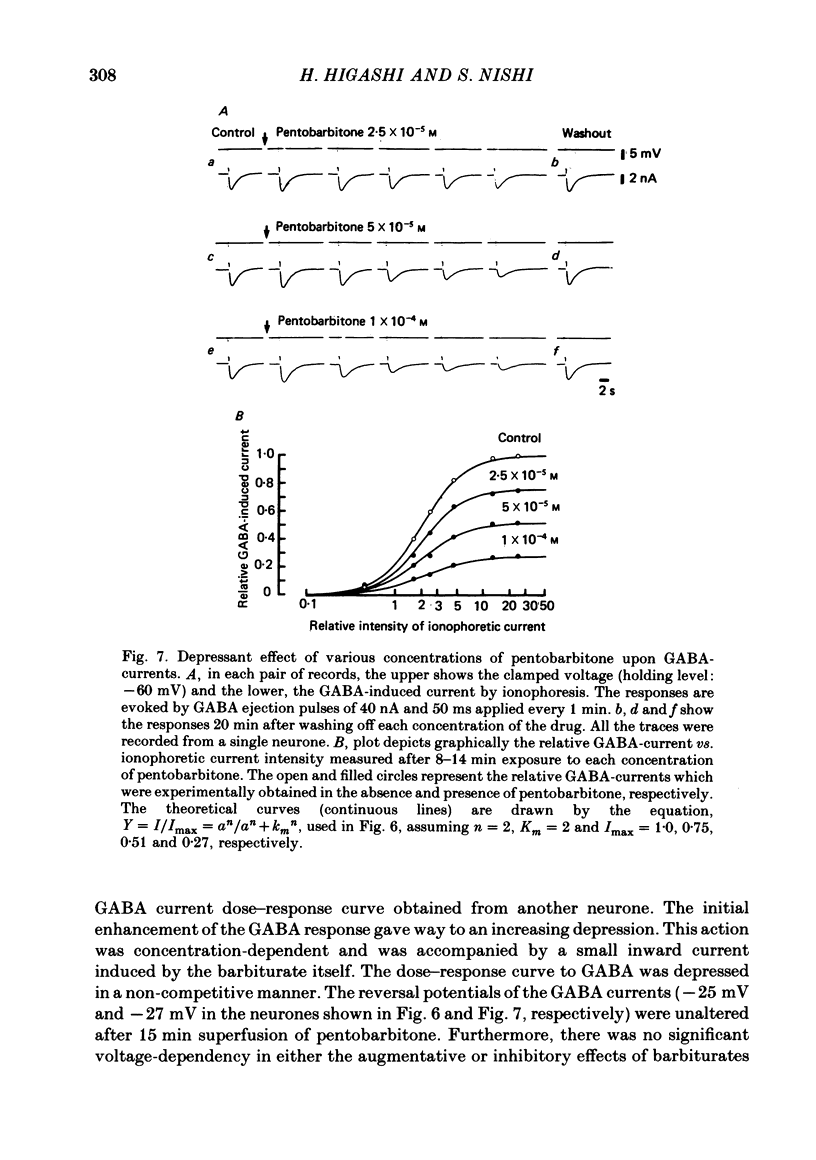
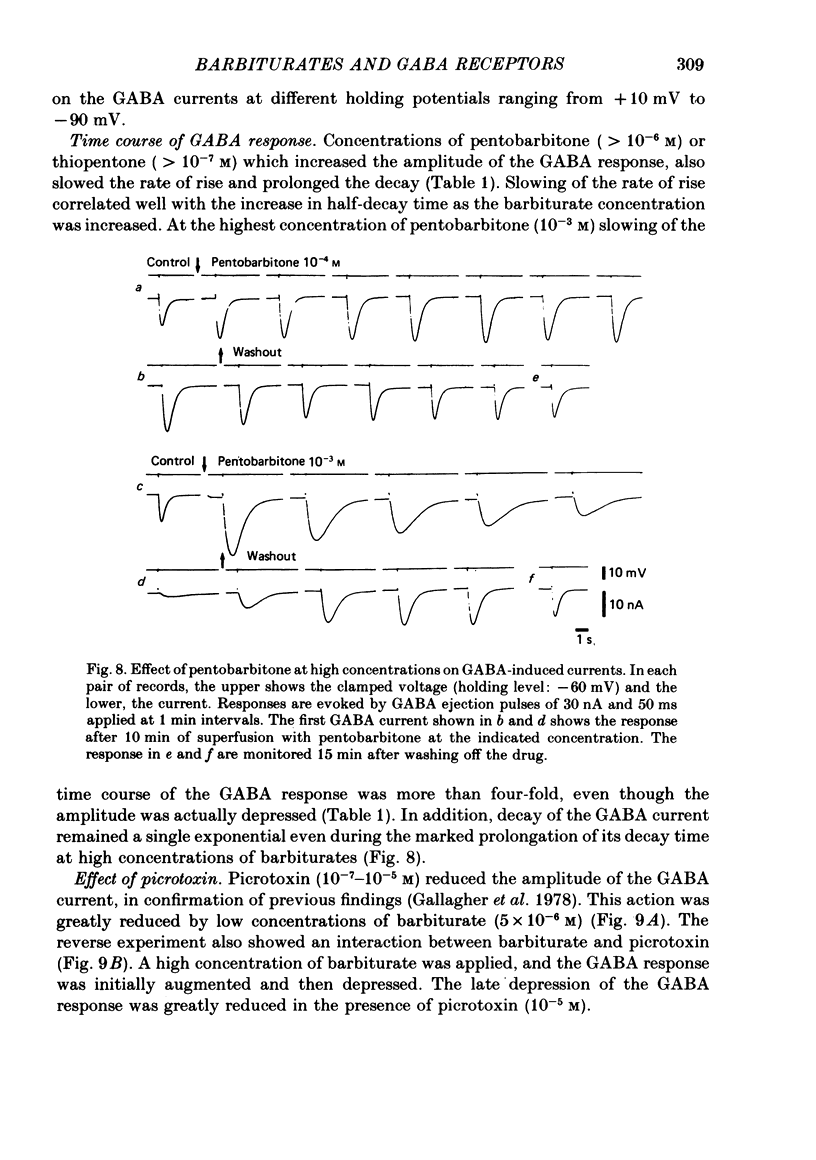
5. Barbiturates at these high concentrations initially caused a marked augmentation and prolongation of GABA responses; this was followed by a depression. The depressant action did not appear to be voltage-dependent. These actions of barbiturates were not accompanied by changes in the apparent dissociation constant of the GABA-current dose—response curve or the reversal potential for GABA currents. In addition, the single exponential decay of GABA current was not changed despite a marked prolongation of its decay time.
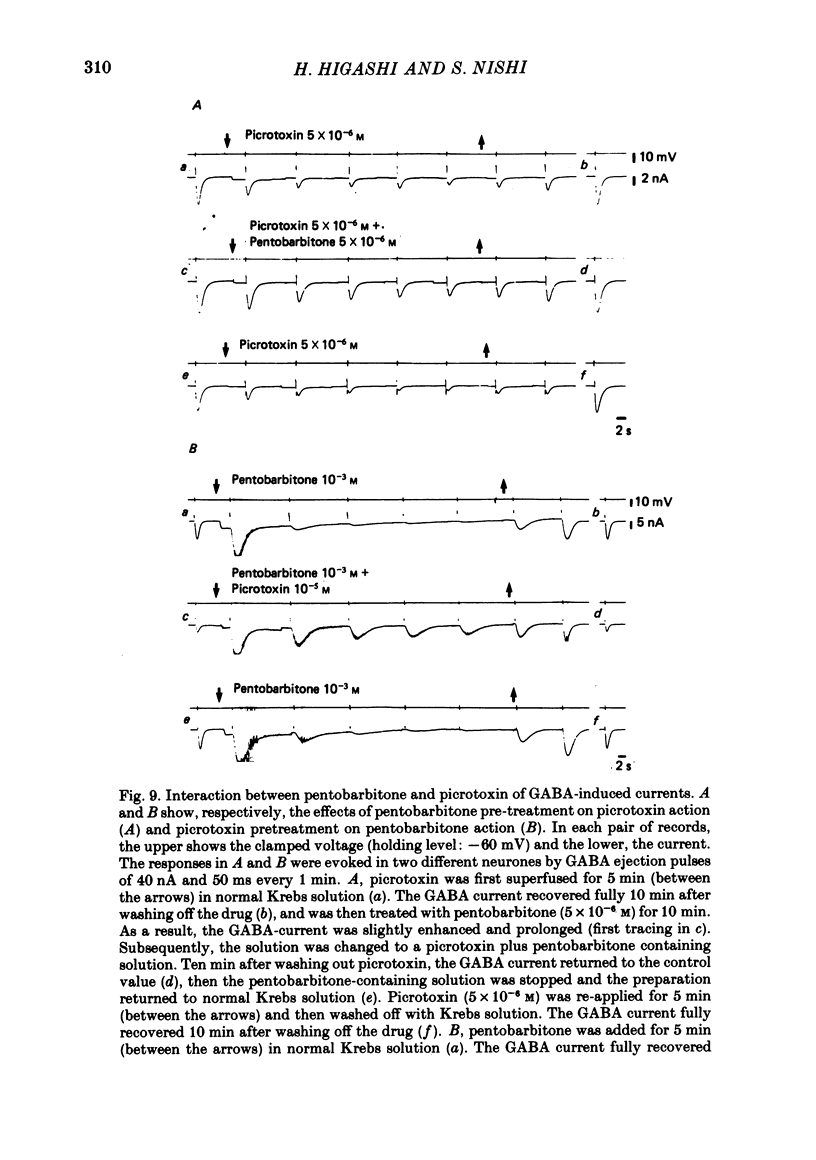
6. Picrotoxin (10-5 M) antagonized the depressant effect of barbiturates at high concentrations on GABA currents, and barbiturates (5 × 10-6 M) reduced the inhibitory action of picrotoxin (5 × 10-6 M) on the GABA-currents.
7. From all these results, it is suggested that the site of barbiturate actions on GABA-responses is mainly the allosteric site (the ionic conductance regulatory subunit) but not the agonist recognition site or the chloride channels linked with GABA receptors.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Aickin C. C., Deisz R. A. Pentobarbitone interference with inhibitory synaptic transmission in crayfish stretch receptor neurones. J Physiol. 1981 Jun;315:175–187. doi: 10.1113/jphysiol.1981.sp013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N. Phenobarbitone modulation of postsynaptic GABA receptor function on cultured mammalian neurons. Proc R Soc Lond B Biol Sci. 1979 Dec 31;206(1164):319–327. doi: 10.1098/rspb.1979.0108. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Pentobarbitone pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:355–372. doi: 10.1113/jphysiol.1978.sp012388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. Barbiturates block calcium uptake by stimulated and potassium-depolarized rat sympathetic ganglia. J Pharmacol Exp Ther. 1976 Jan;196(1):80–86. [PubMed] [Google Scholar]
- Bowery N. G., Dray A. Barbiturate reversal of amino acid antagonism produced by convulsant agents. Nature. 1976 Nov 18;264(5583):276–278. doi: 10.1038/264276a0. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Dray A. Reversal of the action of amino acid antagonists by barbiturates and other hypnotic drugs. Br J Pharmacol. 1978 May;63(1):197–215. doi: 10.1111/j.1476-5381.1978.tb07790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Galvan M. Influence of neuroglial transport on the action of gamma-aminobutyric acid on mammalian ganglion cells. Br J Pharmacol. 1977 Feb;59(2):373–378. doi: 10.1111/j.1476-5381.1977.tb07502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W. A comparison of the effects of pentobarbital and diphenylhydantoin on the GABA sensitivity and excitability of adult sensory ganglion cells. Brain Res. 1981 Mar 2;207(2):357–369. doi: 10.1016/0006-8993(81)90370-x. [DOI] [PubMed] [Google Scholar]
- Deschenes M., Feltz P., Lamour Y. A model for an estimate in vivo of the ionic basis of presynaptic inhibition: an intracellular analysis of the GABA-induced depolarization in rat dorsal root ganglia. Brain Res. 1976 Dec 24;118(3):486–493. doi: 10.1016/0006-8993(76)90318-8. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H. Potentiation of the effects of GABA by pentobarbitone. Brain Res. 1979 Jul 27;171(1):113–120. doi: 10.1016/0006-8993(79)90736-4. [DOI] [PubMed] [Google Scholar]
- Feltz P., Rasminsky M. A model for the mode of action of GABA on primary afferent terminals: depolarizing effects of GABA applied iontophoretically to neurones of mammalian dorsal root ganglia. Neuropharmacology. 1974 Jun;13(6):553–563. doi: 10.1016/0028-3908(74)90145-2. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Higashi H., Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol. 1978 Feb;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L., Kado R. T. Voltage-current relationship of a carbachol-induced potassium-ion pathway in Aplysia neurones. J Physiol. 1975 Mar;245(3):713–725. doi: 10.1113/jphysiol.1975.sp010870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock J. W., Levy W. B., Cotman C. W. Pentobarbital depression of stimulus-secretion coupling in brain--selective inhibition of depolarization-induced calcium-dependent release. Biochem Pharmacol. 1977 Jan 15;26(2):159–161. doi: 10.1016/0006-2952(77)90389-6. [DOI] [PubMed] [Google Scholar]
- LOYNING Y., OSHIMA T., YOKOTA T. SITE OF ACTION OF THIAMYLAL SODIUM ON THE MONOSYNAPTIC SPINAL REFLEX PATHWAY IN CATS. J Neurophysiol. 1964 May;27:408–428. doi: 10.1152/jn.1964.27.3.408. [DOI] [PubMed] [Google Scholar]
- Mathers D. A., Barker J. L. (-)Pentobarbital opens ion channels of long duration in cultured mouse spinal neurons. Science. 1980 Jul 25;209(4455):507–509. doi: 10.1126/science.6248961. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Presynaptic action of barbiturates in the frog spinal cord. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1460–1463. doi: 10.1073/pnas.72.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Wojtowicz J. M. The effects of pentobarbital and related compounds on frog motoneurons. Brain Res. 1980 Jun 2;191(1):225–237. doi: 10.1016/0006-8993(80)90325-x. [DOI] [PubMed] [Google Scholar]
- Nishi S., Minota S., Karczmar A. G. Primary afferent neurones: the ionic mechanism of GABA-mediated depolarization. Neuropharmacology. 1974 Mar;13(3):215–219. doi: 10.1016/0028-3908(74)90110-5. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Leeb-Lundberg F. Convulsant and anticonvulsant drug binding sites related to GABA-regulated chloride ion channels. Adv Biochem Psychopharmacol. 1981;26:93–102. [PubMed] [Google Scholar]
- Richards C. D. On the mechanism of barbiturate anaesthesia. J Physiol. 1972 Dec;227(3):749–767. doi: 10.1113/jphysiol.1972.sp010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT R. F. PHARMACOLOGICAL STUDIES ON THE PRIMARY AFFERENT DEPOLARIZATION OF THE TOAD SPINAL CORD. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963 Jul 2;277:325–346. doi: 10.1007/BF00362515. [DOI] [PubMed] [Google Scholar]
- Somjen G. Effects of anesthetics on spinal cord of mammals. Anesthesiology. 1967 Jan-Feb;28(1):135–143. doi: 10.1097/00000542-196701000-00015. [DOI] [PubMed] [Google Scholar]
- Ticku M. K., Olsen R. W. Interaction of barbiturates with dihydropicrotoxinin binding sites related to the GABA receptor-ionophore system. Life Sci. 1978 May 8;22(18):1643–1651. doi: 10.1016/0024-3205(78)90061-9. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. Effect of barbiturates on 'quantal' synaptic transmission in spinal motoneurones. J Physiol. 1969 Sep;204(1):63–77. doi: 10.1113/jphysiol.1969.sp008898. [DOI] [PMC free article] [PubMed] [Google Scholar]



