Abstract
1. This report describes selected histochemical and physiological properties of the motor units of adult cat soleus muscle approximately one year after self- and cross-reinnervation with the nerve of the heterogenous flexor hallucis longus (f.h.l.). Self-reinnervated f.h.l. motor units are also considered. Whole muscles were tested for fibre reaction to alkaline pre-incubated ATPase, α-glycerophosphate dehydrogenase (α-GPD) and reduced nicotinamide adenine dinucleotide diaphorase (NADH-D). Motor units were isolated and studied by splitting the ventral root in acute preparations.
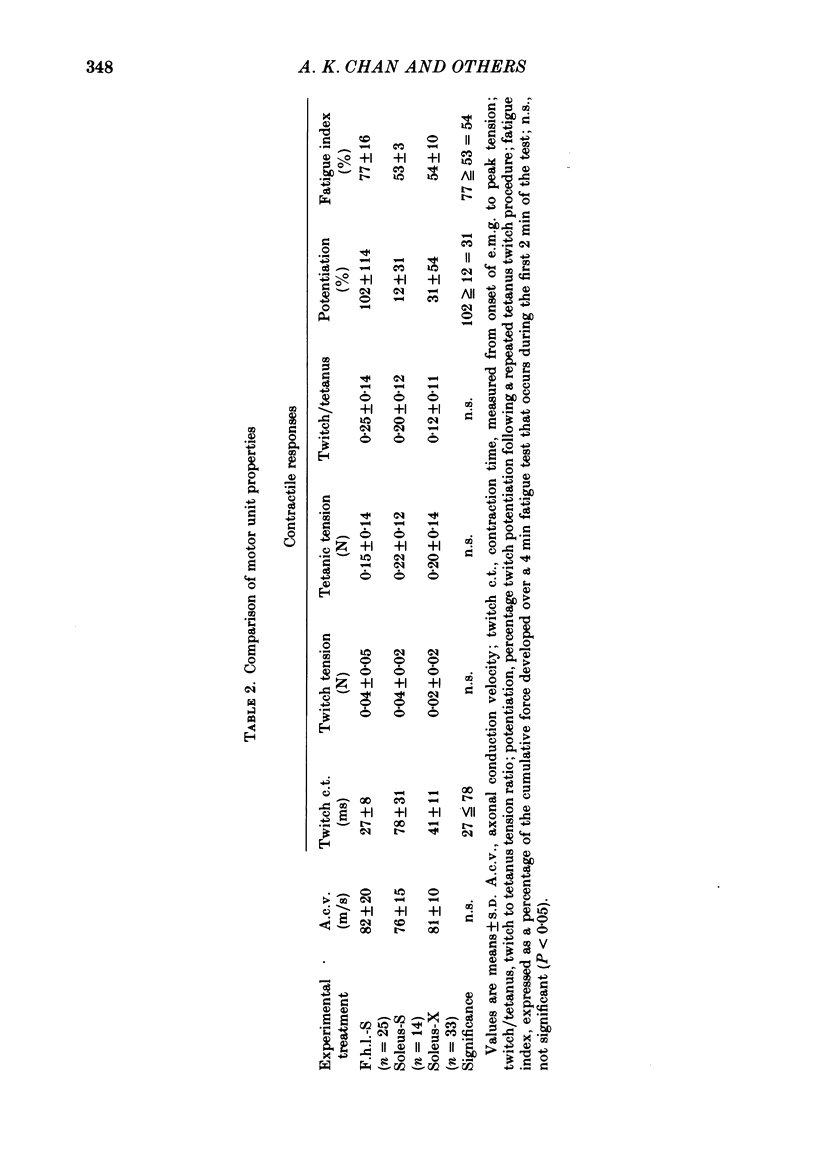
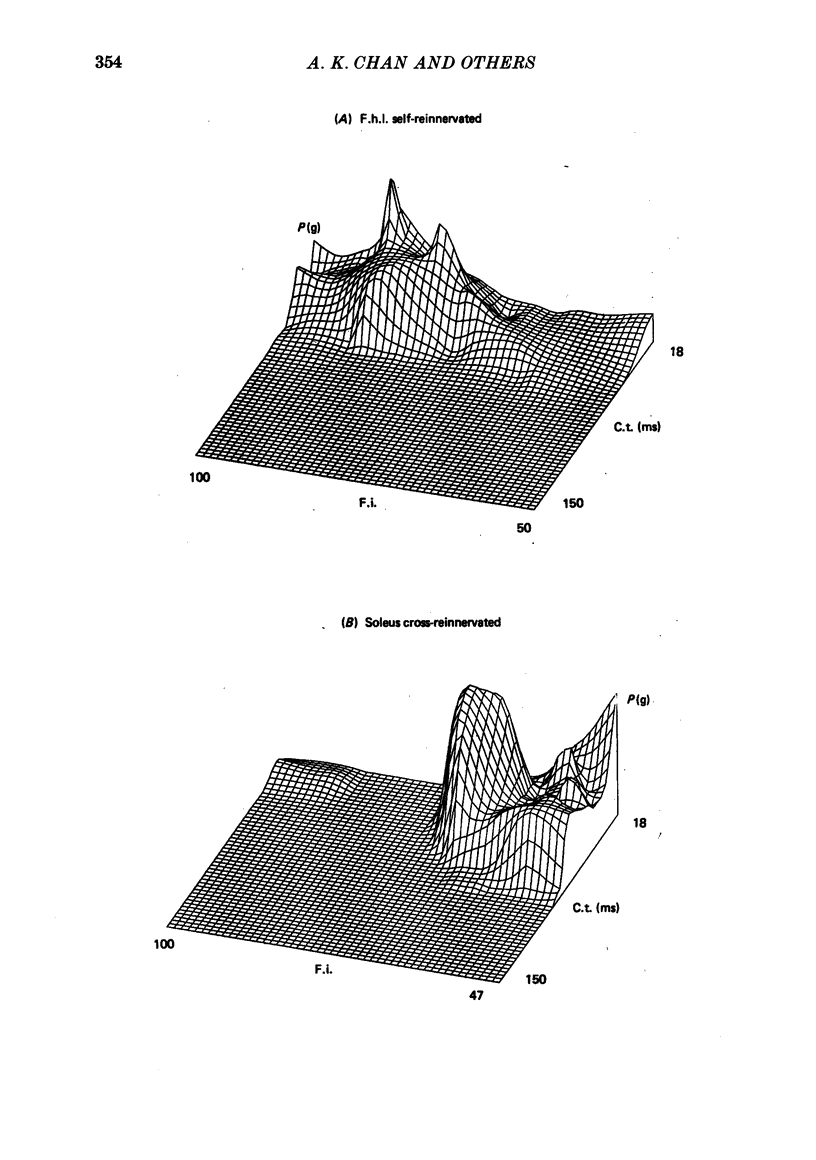
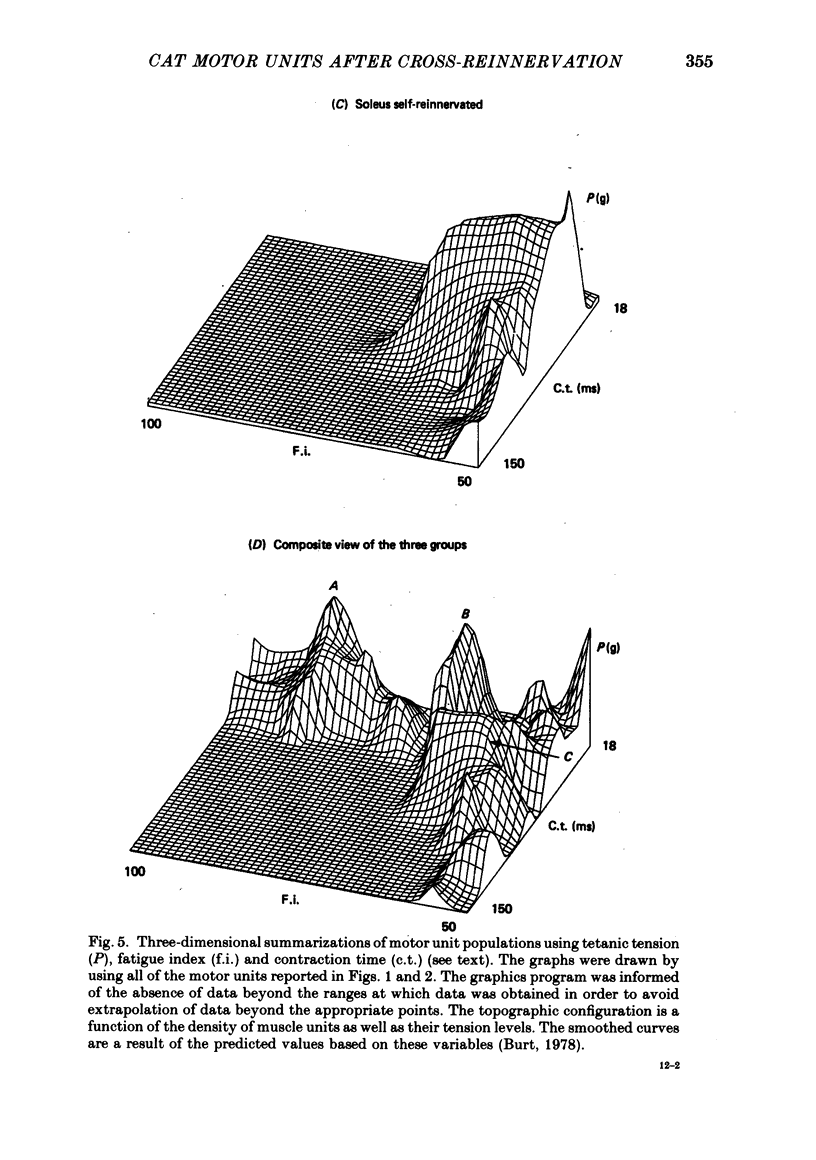
2. The histochemical fibre type profile in the self-reinnervated muscle was comparable to normal muscle as was mean twitch contraction time, twitch—tetanus ratio and fatigue index. The mean tetanic tension of the soleus self- and cross-reinnervated motor units appeared close to a normal soleus whereas the mean tetanic tension of the f.h.l. self-reinnervated units was significantly less than a normal f.h.l.
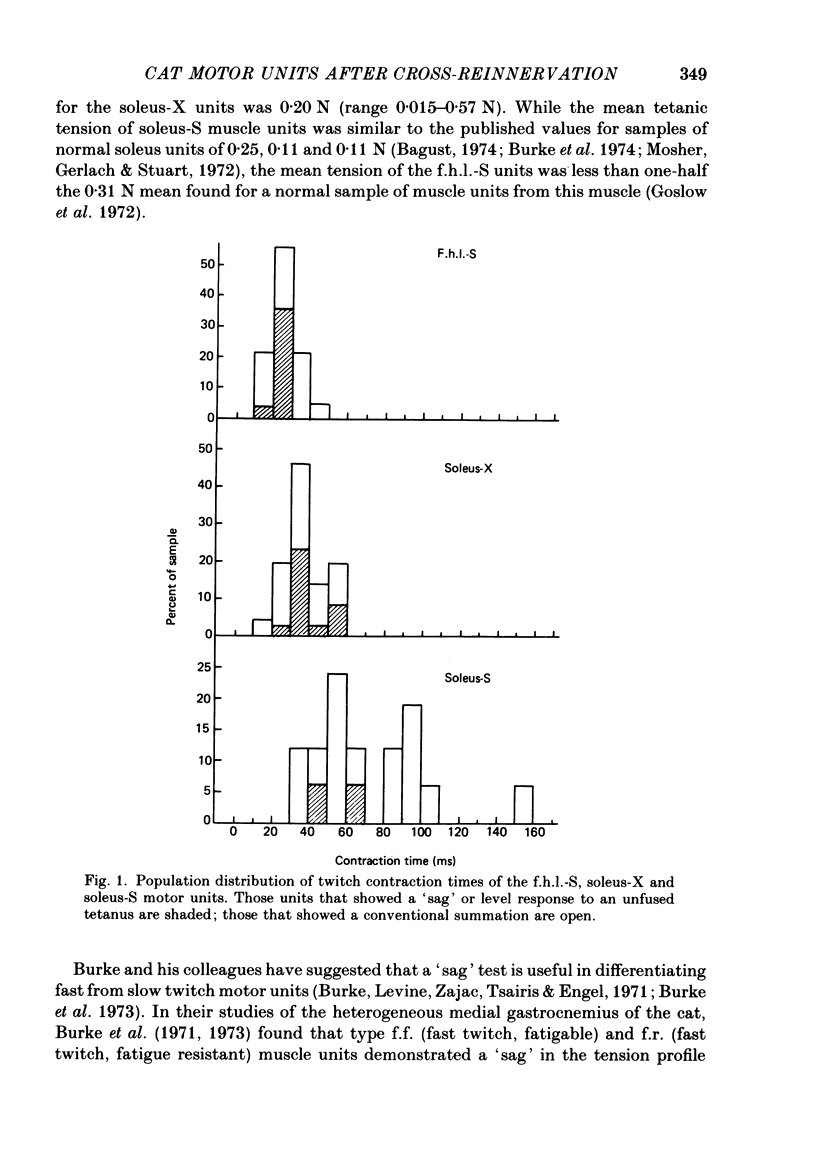
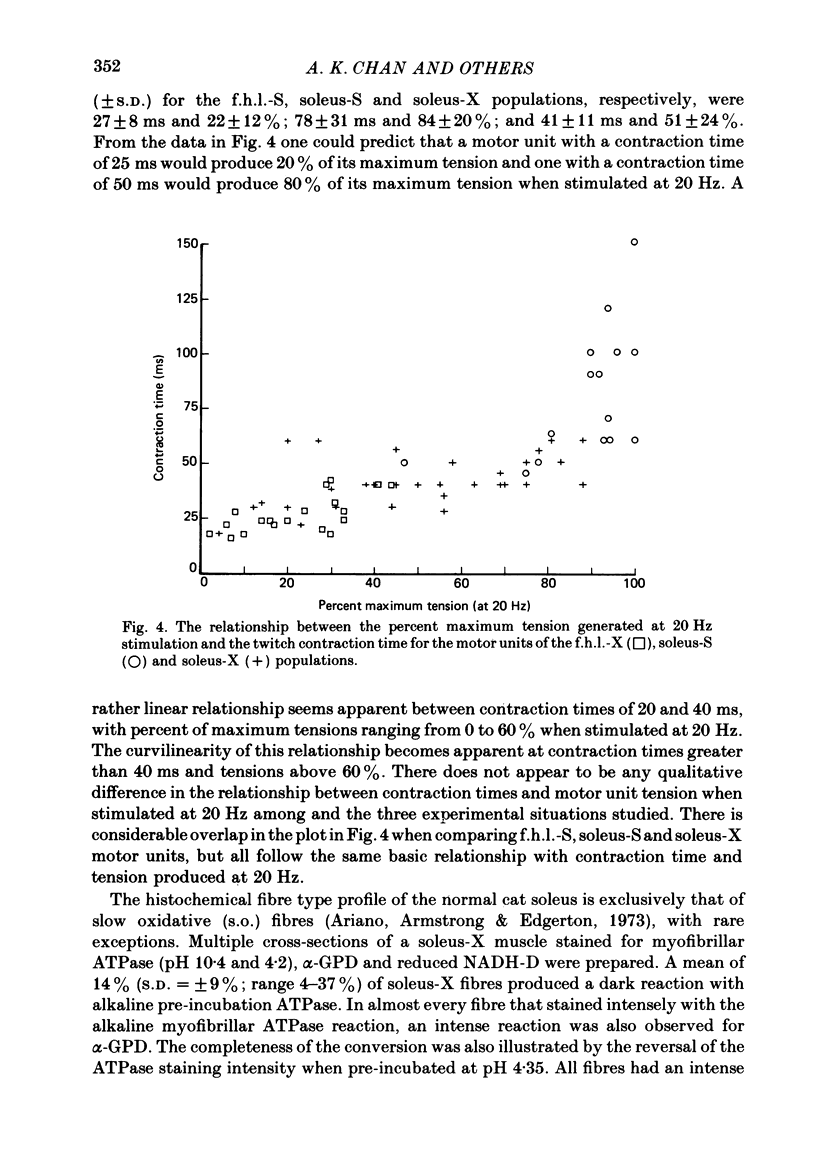
3. An average of 14% of the fibres of the soleus cross-reinnervated muscles had high ATPase and a α-GPD staining intensity in contrast to normal and self-reinnervated soleus in which such fibres are absent. Thus alkaline lability of myofibrillar ATPase increased in some fibres of what was originally a homogeneous population. The small increase in the number of densely staining fibres for ATPase at an alkaline pH (14%) was associated with a 73% decrease in (mean) contraction time (41 ± 11 ms) of the thirty-three cross-reinnervated muscle units studied, with no unit's contraction time greater than 60 ms. Mean contraction times for the self-reinnervated soleus and f.h.l. muscles were 78 ± 31 ms and 27 ± 8 ms respectively.
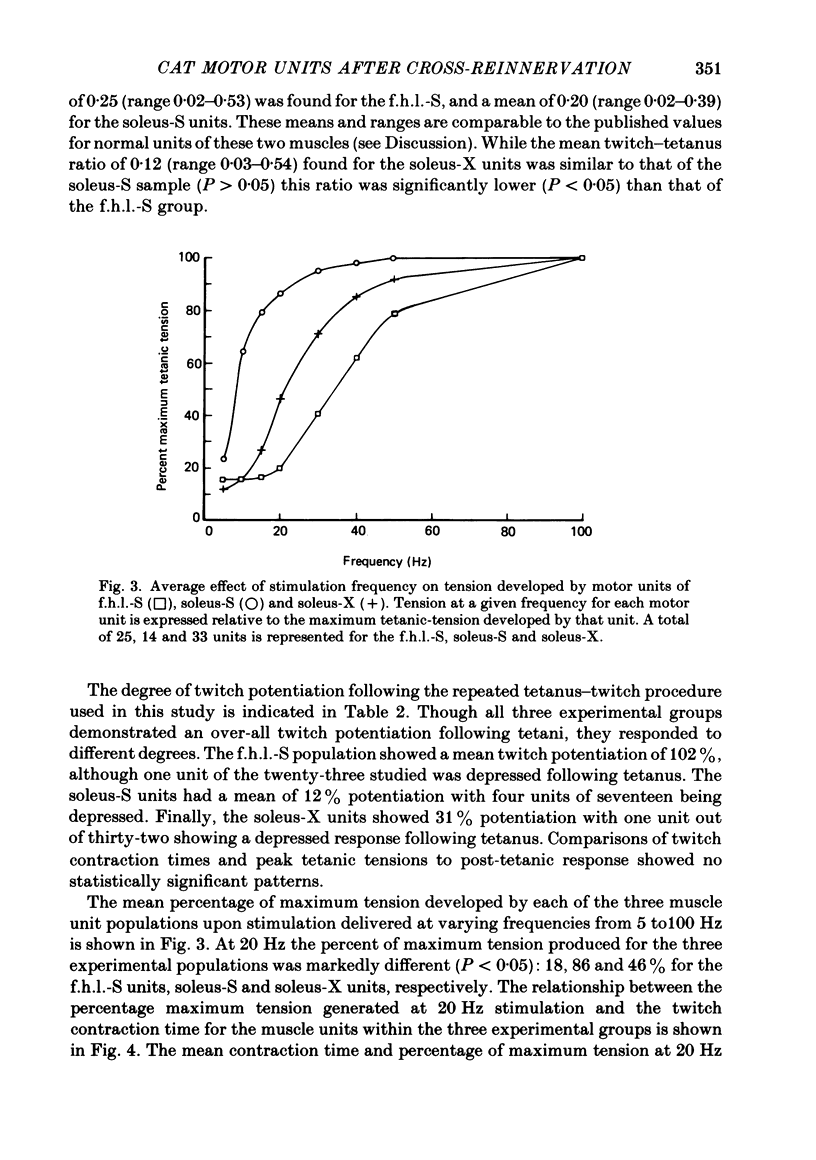
4. All fibres of the soleus cross-reinnervated muscles showed intense reaction to NADH-D, as was true of self-reinnervated soleus. This staining pattern is typical of normal soleus. In concordance, these motor units consistently demonstrated a high resistance to fatigue when stimulated for a four-minute period.
5. These results suggest that in the adult self-and cross-reinnervated soleus muscle, there is some active mechanism which regulates the eventual size of motor units as reflected by tetanic tension.
6. Change in contraction time from that typical for a soleus unit to that similar to an f.h.l. unit remains incomplete one year after cross-reinnervation. Within this time this partial change in single motor units reflects incomplete neural control of this property rather than a mixture of self- and foreign-innervation.
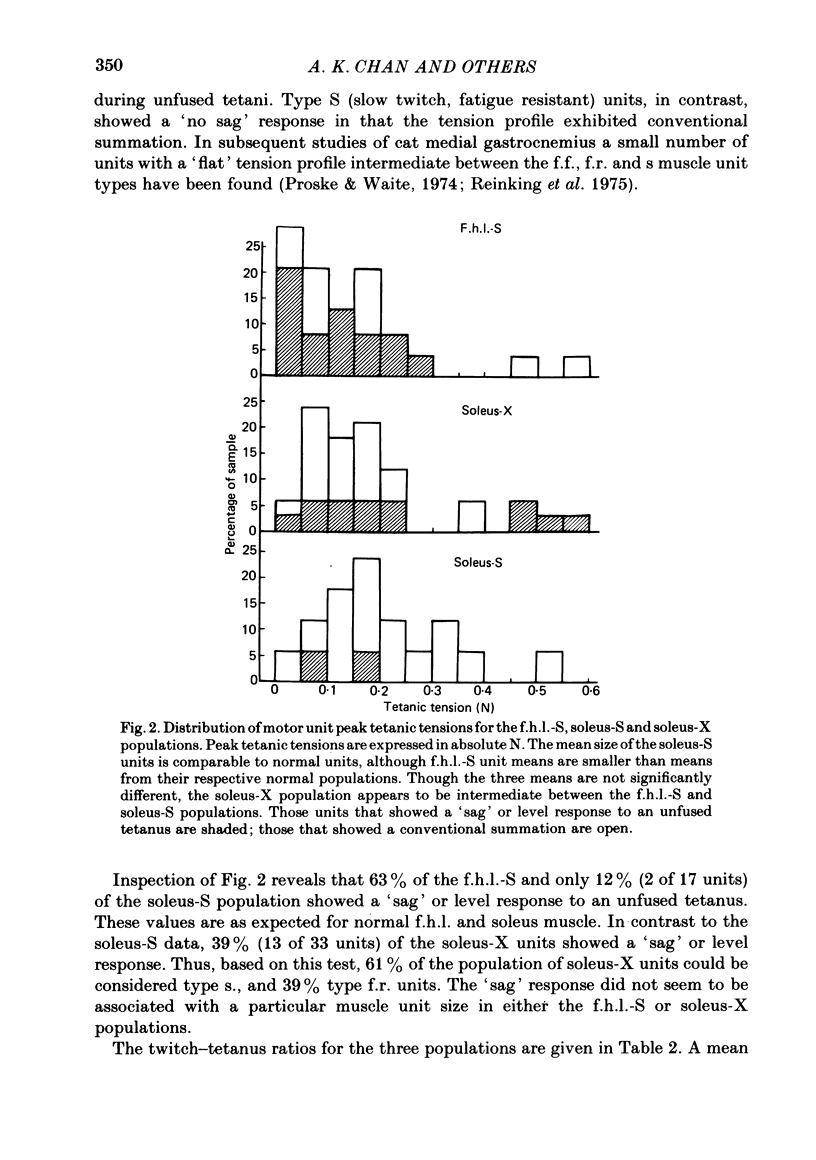
7. A greater degree of independence from neural control to conversion of the histochemically demonstrated myofibrillar ATPase activity exists than is the case for contraction time.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- BULLER A. J., ECCLES J. C., ECCLES R. M. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960 Feb;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust J., Lewis D. M. Isometric contractions of motor units in self-reinnervated fast and slow twitch muscles of the cat. J Physiol. 1974 Feb;237(1):91–102. doi: 10.1113/jphysiol.1974.sp010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust J., Lewis D. M., Westerman R. A. Motor units in cross-reinnervated fast and slow twitch muscle of the cat. J Physiol. 1981;313:223–235. doi: 10.1113/jphysiol.1981.sp013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust J. Relationships between motor nerve conduction velocities and motor unit contraction characteristics in a slow twitch muscle of the cat. J Physiol. 1974 Apr;238(2):269–278. doi: 10.1113/jphysiol.1974.sp010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin K. M., Winder W. W., Holloszy J. O. Adaptation of actomyosin ATPase in different types of muscle to endurance exercise. Am J Physiol. 1975 Aug;229(2):422–426. doi: 10.1152/ajplegacy.1975.229.2.422. [DOI] [PubMed] [Google Scholar]
- Booth F. W., Kelso J. R. Effect of hind-limb immobilization on contractile and histochemical properties of skeletal muscle. Pflugers Arch. 1973 Aug 27;342(3):231–238. doi: 10.1007/BF00591371. [DOI] [PubMed] [Google Scholar]
- Buller A. J., Mommaerts W. F., Seraydarian K. Enzymic properties of myosin in fast and slow twitch muscles of the cat following cross-innervation. J Physiol. 1969 Dec;205(3):581–597. doi: 10.1113/jphysiol.1969.sp008984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Salcman M., Tsairis P. Motor units in cat soleus muscle: physiological, histochemical and morphological characteristics. J Physiol. 1974 May;238(3):503–514. doi: 10.1113/jphysiol.1974.sp010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Zajac F. E., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971 Nov 12;174(4010):709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Close R. I., Luff A. R. Dynamic properties of inferior rectus muscle of the rat. J Physiol. 1974 Jan;236(2):259–270. doi: 10.1113/jphysiol.1974.sp010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett J. L., Edgerton V. R. Exercise and restricted activity effects on reinnervated and cross-innervated skeletal muscles. J Neurol Sci. 1975 May;25(1):1–9. doi: 10.1016/0022-510x(75)90181-1. [DOI] [PubMed] [Google Scholar]
- ENGEL W. K., CUNNINGHAM G. G. RAPID EXAMINATION OF MUSCLE TISSUE. AN IMPROVED TRICHROME METHOD FOR FRESH-FROZEN BIOPSY SECTIONS. Neurology. 1963 Nov;13:919–923. doi: 10.1212/wnl.13.11.919. [DOI] [PubMed] [Google Scholar]
- Edgerton V. R., Goslow G. E., Jr, Rasmussen S. A., Spector S. A. Is resistance of a muscle to fatigue controlled by its motoneurones? Nature. 1980 Jun 19;285(5766):589–590. doi: 10.1038/285589a0. [DOI] [PubMed] [Google Scholar]
- Golisch G., Pette D., Pichlmaier H. Metabolic differentiation of rabbit skeletal muscle as induced by specific innervation. Eur J Biochem. 1970 Sep;16(1):110–116. doi: 10.1111/j.1432-1033.1970.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Goslow G. E., Jr, Stauffer E. K., Nemeth W. C., Stuart D. G. Digit flexor muscles in the cat: their action and motor units. J Morphol. 1972 Jul;137(3):335–342. doi: 10.1002/jmor.1051370305. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- Kugelberg E. Histochemical composition, contraction speed and fatiguability of rat soleus motor units. J Neurol Sci. 1973 Oct;20(2):177–198. doi: 10.1016/0022-510x(73)90029-4. [DOI] [PubMed] [Google Scholar]
- MCPHEDRAN A. M., WUERKER R. B., HENNEMAN E. PROPERTIES OF MOTOR UNITS IN A HOMOGENEOUS RED MUSCLE (SOLEUS) OF THE CAT. J Neurophysiol. 1965 Jan;28:71–84. doi: 10.1152/jn.1965.28.1.71. [DOI] [PubMed] [Google Scholar]
- Maier A., Crockett J. L., Simpson D. R., Saubert CW I. V., Edgerton V. R. Properties of immobilized guinea pig hindlimb muscles. Am J Physiol. 1976 Nov;231(5 Pt 1):1520–1526. doi: 10.1152/ajplegacy.1976.231.5.1520. [DOI] [PubMed] [Google Scholar]
- Moshier C. G., Gerlach R. L., Stuart D. G. Soleus and anterior tibial motor units of the cat. Brain Res. 1972 Sep 15;44(1):1–11. doi: 10.1016/0006-8993(72)90361-7. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B., SHIN W. Y., DRUCKER J. Mitochondrial localization of oxidative enzymes: staining results with two tetrazolium salts. J Biophys Biochem Cytol. 1961 Jan;9:47–61. doi: 10.1083/jcb.9.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Pette D., Ramirez B. U., Müller W., Simon R., Exner G. U., Hildebrand R. Influence of intermittent long-term stimulation on contractile, histochemical and metabolic properties of fibre populations in fast and slow rabbit muscles. Pflugers Arch. 1975 Dec 19;361(1):1–7. doi: 10.1007/BF00587333. [DOI] [PubMed] [Google Scholar]
- Prewitt M. A., Salafsky B. Effect of cross innervation on biochemical characteristics of skeletal muscles. Am J Physiol. 1967 Jul;213(1):295–300. doi: 10.1152/ajplegacy.1967.213.1.295. [DOI] [PubMed] [Google Scholar]
- Proske U., Waite P. M. Properties of types of motor units in the medial gastrochemius muscle of the cat. Brain Res. 1974 Feb 15;67(1):89–101. doi: 10.1016/0006-8993(74)90300-x. [DOI] [PubMed] [Google Scholar]
- Reinking R. M., Stephens J. A., Stuart D. G. The motor units of cat medial gastrocnemius: problem of their categorisation on the basis of mechanical properties. Exp Brain Res. 1975 Sep 29;23(3):301–313. doi: 10.1007/BF00239742. [DOI] [PubMed] [Google Scholar]
- Romanul F. C., Van der Meulen J. P. Slow and fast muscles after cross innervation. Enzymatic and physiological changes. Arch Neurol. 1967 Oct;17(4):387–402. doi: 10.1001/archneur.1967.00470280053006. [DOI] [PubMed] [Google Scholar]
- Sturart D. G., Mosher C. G., Gerlach R. I., Reinking R. M. Mechanical arrangement and transducing properties of Golgi tendon organs. Exp Brain Res. 1972;14(3):274–292. doi: 10.1007/BF00816163. [DOI] [PubMed] [Google Scholar]
- Thompson W., Jansen J. K. The extent of sprouting of remaining motor units in partly denervated immature and adult rat soleus muscle. Neuroscience. 1977;2(4):523–535. doi: 10.1016/0306-4522(77)90049-5. [DOI] [PubMed] [Google Scholar]
- WATTENBERG L. W., LEONG J. L. Effects of coenzyme Q10 and menadione on succinic dehydrogenase activity as measured by tetrazolium salt reduction. J Histochem Cytochem. 1960 Jul;8:296–303. doi: 10.1177/8.4.296. [DOI] [PubMed] [Google Scholar]


