Abstract
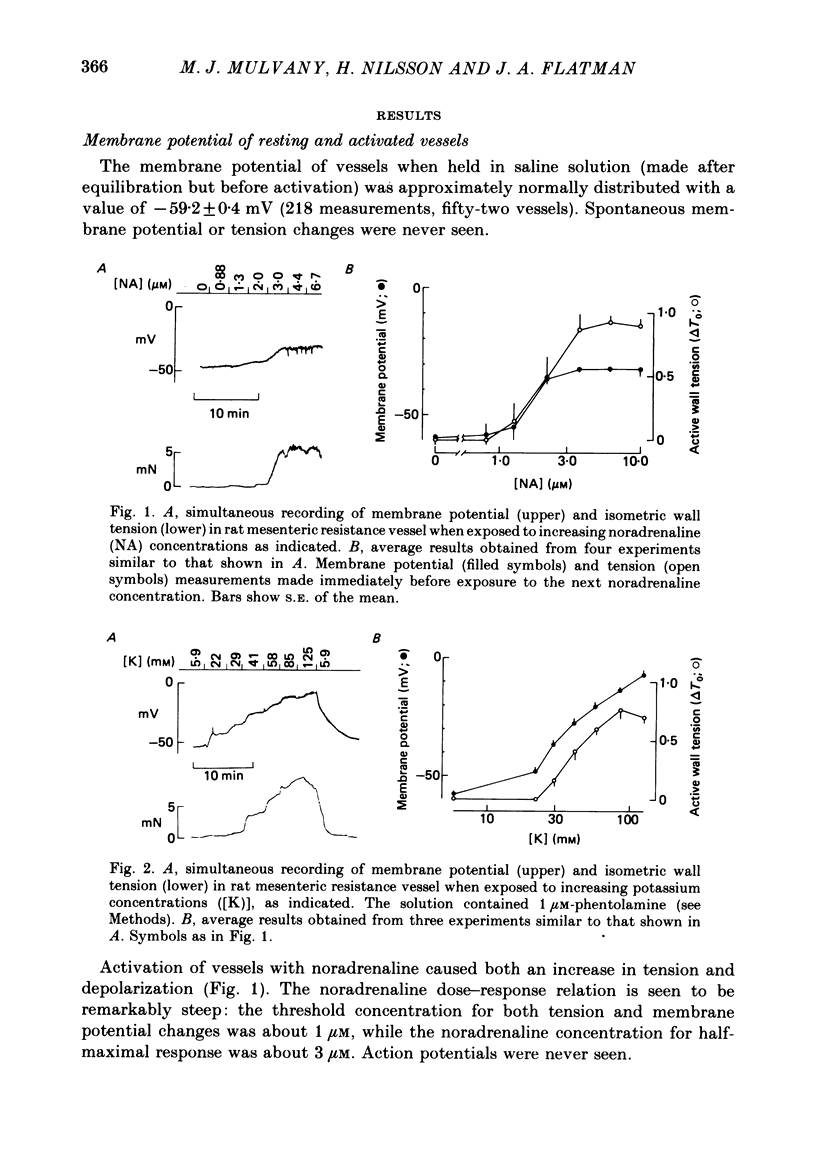
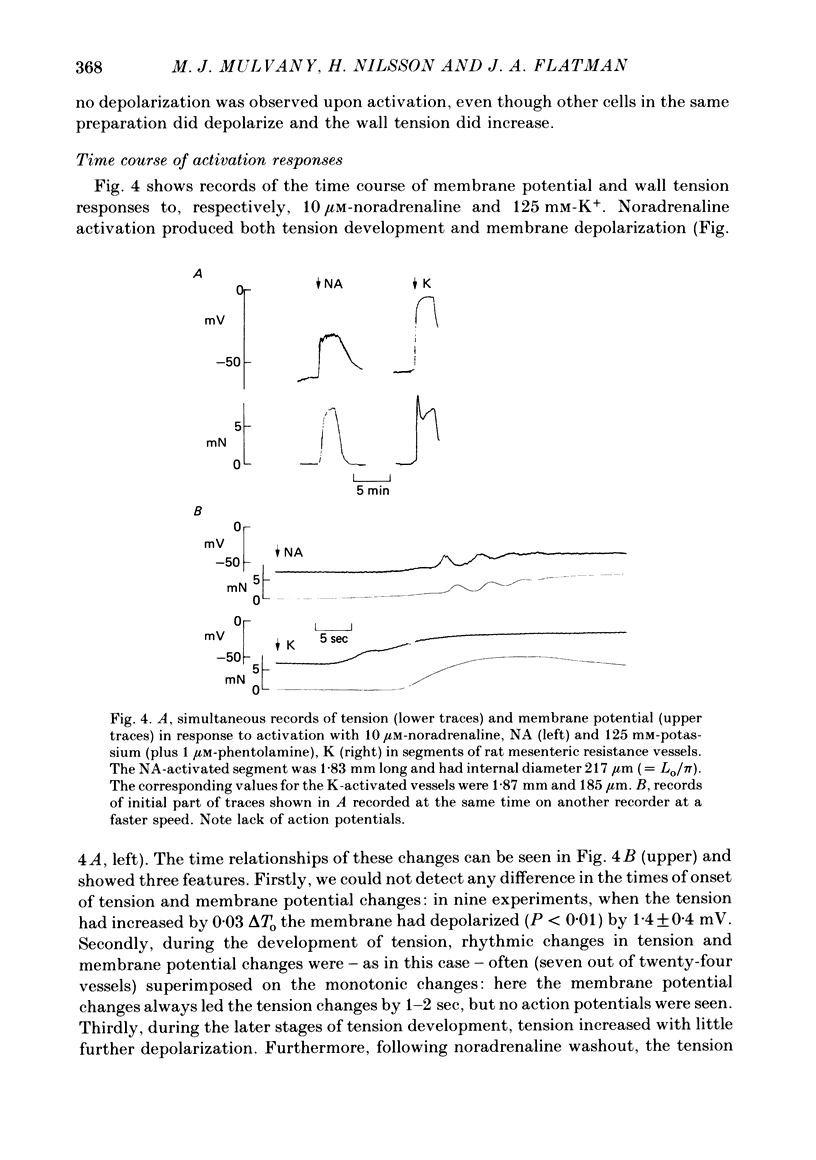
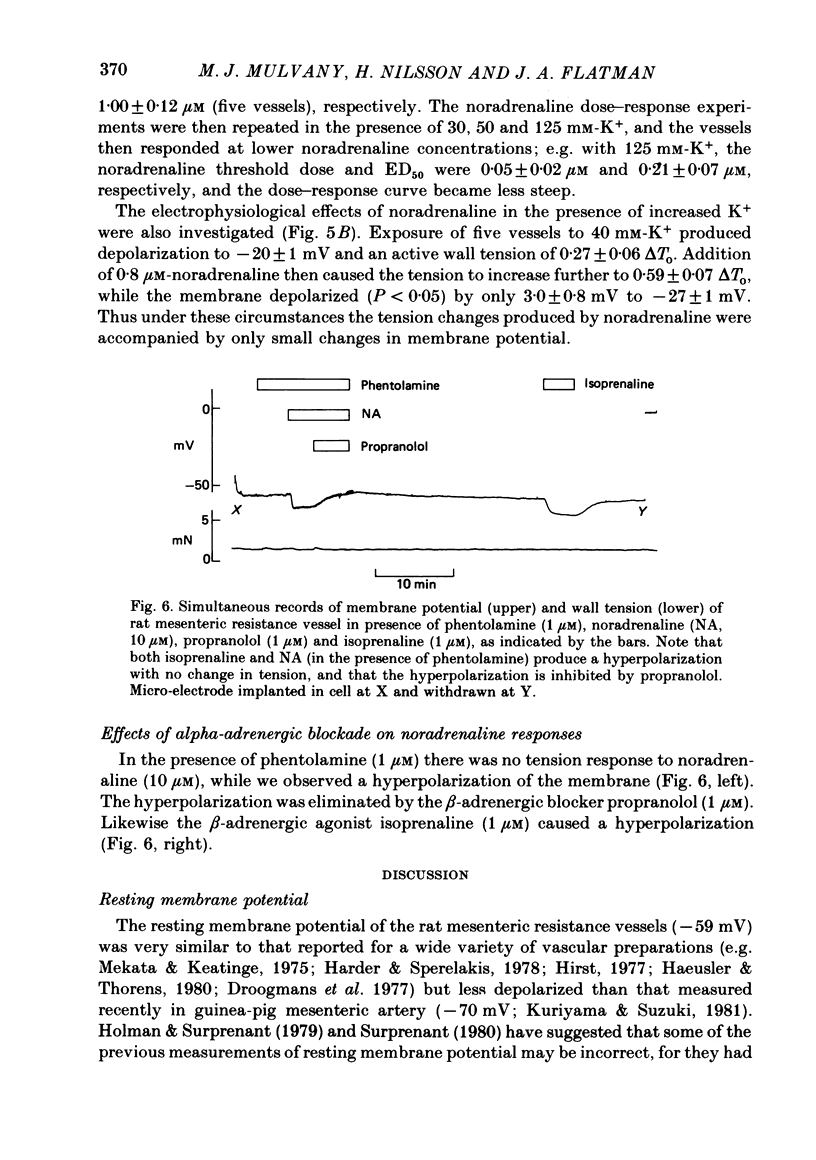
1. We have made simultaneous measurements of membrane potential and wall tension in rat 200 microns mesenteric arteries. 2. The resting membrane potential was -59.2 +/- 0.4 mV and stable (218 measurements, fifty-two vessels). 3. With maximal exogenous noradrenaline stimulation (10 microM) the membrane depolarized to about -34 mV. During the onset of tension development oscillations (period about 6 sec) in both tension and membrane potential were often seen; the membrane potential changes led the tension changes by about 1.2 sec. 4. In the presence of increased K+ (e.g. 40 mM), vessels had an increased noradrenaline sensitivity, and here noradrenaline stimulation produced little change in membrane potential. 5. With maximal K+ stimulation (85 mM), in the presence of phentolamine (1 microM), the membrane depolarized to about -17 mV, the tension being about 70% of the maximal noradrenaline response. 6. In the presence of phentolamine (1 microM), noradrenaline caused hyperpolarization without tension development. The hyperpolarization was inhibited by propranolol and mimicked by isoprenaline. 7. The results suggest that in these small vessels membrane potential variations are not essential to, but have an important modulating influence on, the tension response to exogenous noradrenaline.
Full text
PDF


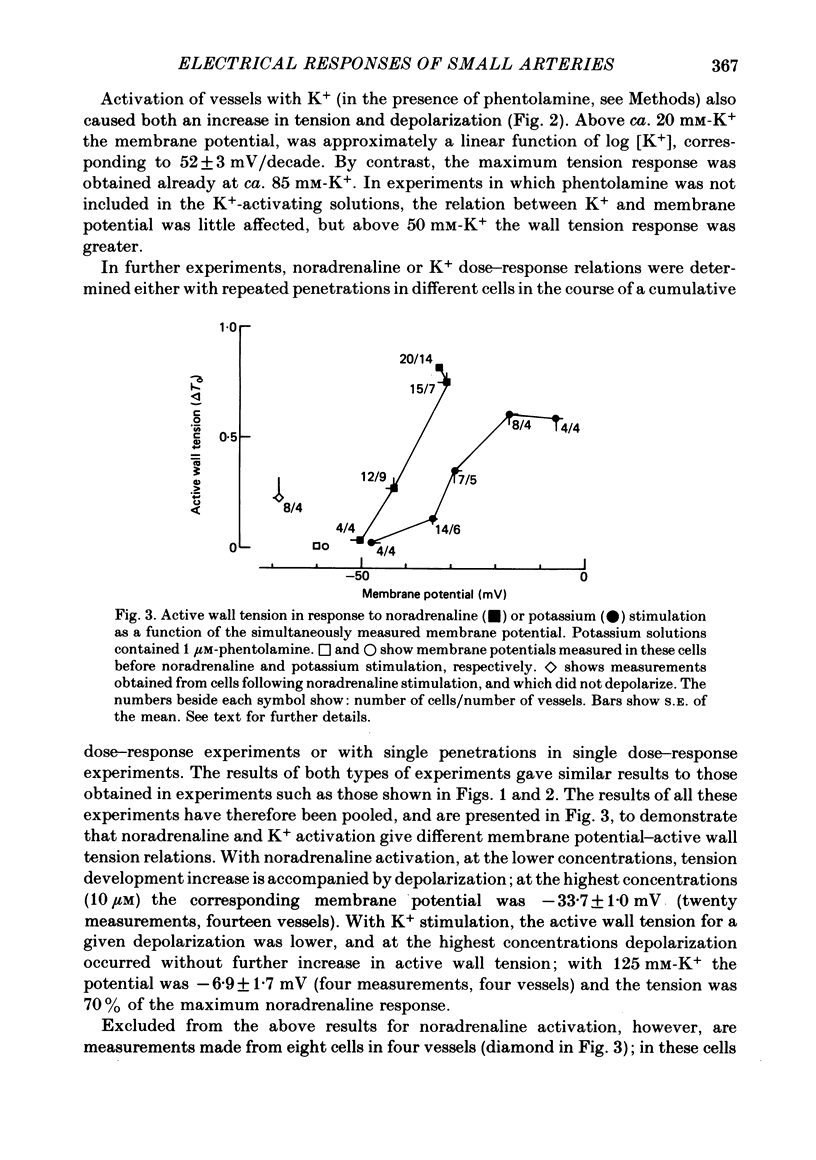
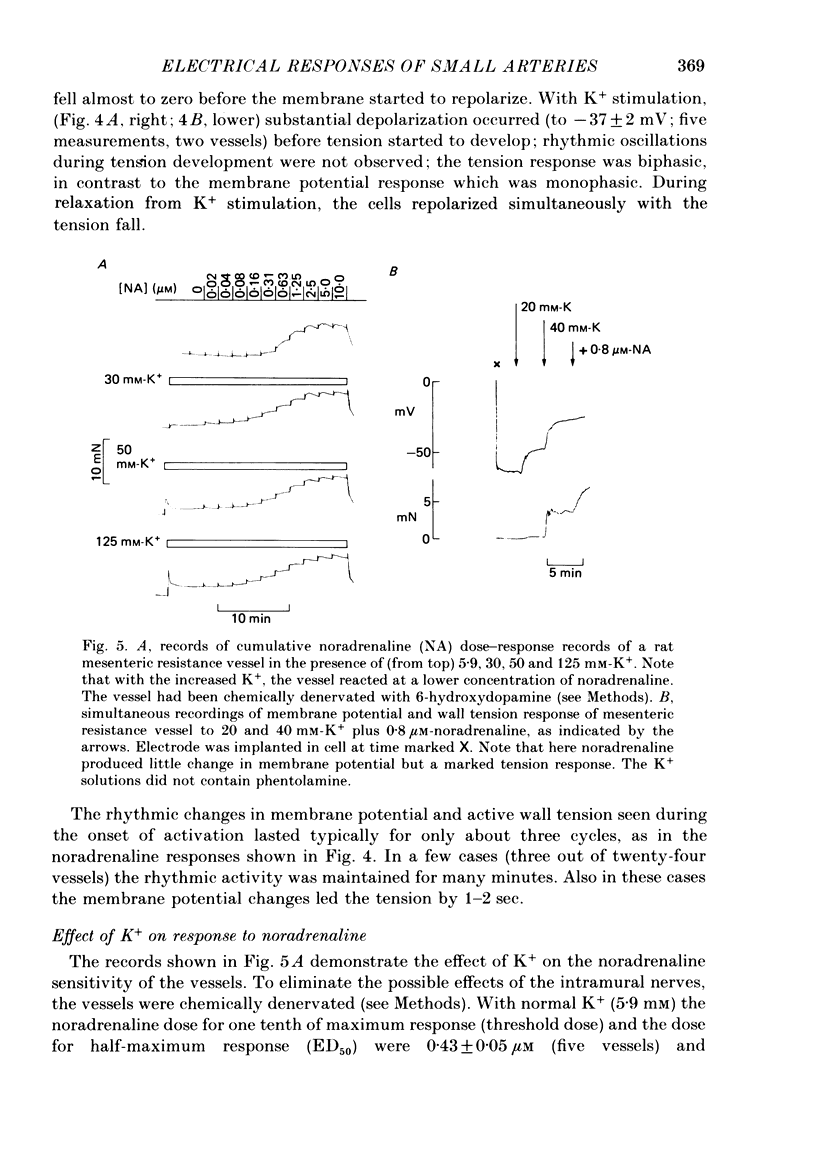



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aprigliano O., Hermsmeyer K. In vitro denervation of the portal vein and caudal artery of the rat. J Pharmacol Exp Ther. 1976 Sep;198(3):568–577. [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):41–61. doi: 10.1113/jphysiol.1977.sp011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming W. W. The electrogenic Na+, K+-pump in smooth muscle: physiologic and pharmacologic significance. Annu Rev Pharmacol Toxicol. 1980;20:129–149. doi: 10.1146/annurev.pa.20.040180.001021. [DOI] [PubMed] [Google Scholar]
- Haeusler G. Relationship between noradrenaline-induced depolarization and contraction in vascular smooth muscle. Blood Vessels. 1978;15(1-3):46–54. doi: 10.1159/000158152. [DOI] [PubMed] [Google Scholar]
- Haeusler G., Thorens S. Effects of tetraethylammonium chloride on contractile, membrane and cable properties of rabbit artery muscle. J Physiol. 1980 Jun;303:203–224. doi: 10.1113/jphysiol.1980.sp013281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder D. R., Contney S. J., Willems W. J., Stekiel W. J. Norepinephrine effect on in situ venous membrane potential in spontaneously hypertensive rats. Am J Physiol. 1981 Jun;240(6):H837–H842. doi: 10.1152/ajpheart.1981.240.6.H837. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Sperelakis N. Membrane electrical properties of vascular smooth muscle from the guinea pig superior mesenteric artery. Pflugers Arch. 1978 Dec 28;378(2):111–119. doi: 10.1007/BF00584443. [DOI] [PubMed] [Google Scholar]
- Hermsmeyer K. Electrogenesis of increased norepinephrine sensitivity of arterial vascular muscle in hypertension. Circ Res. 1976 May;38(5):362–367. doi: 10.1161/01.res.38.5.362. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Localization of specialized noradrenaline receptors at neuromuscular junctions on arterioles of the guinea-pig. J Physiol. 1981;313:343–350. doi: 10.1113/jphysiol.1981.sp013669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D. Neuromuscular transmission in arterioles of guinea-pig submucosa. J Physiol. 1977 Dec;273(1):263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Neild T. O. Membrane properties. Br Med Bull. 1979 Sep;35(3):235–241. doi: 10.1093/oxfordjournals.bmb.a071583. [DOI] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kitamura K., Kuriyama H. Effects of acetylcholine and catecholamines on the smooth muscle cell of the porcine coronary artery. J Physiol. 1979 Sep;294:595–611. doi: 10.1113/jphysiol.1979.sp012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara M., Kitamura K., Kuriyama H. Neuromuscular transmission and smooth muscle membrane properties in the guinea-pig ear artery. J Physiol. 1981 Jun;315:283–302. doi: 10.1113/jphysiol.1981.sp013748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. Adrenergic transmissions in the guinea-pig mesenteric artery and their cholinergic modulations. J Physiol. 1981 Aug;317:383–396. doi: 10.1113/jphysiol.1981.sp013831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O., Björklund A. The glyoxylic acid fluorescence histochemical method: a detailed account of the methodology for the visualization of central catecholamine neurons. Histochemistry. 1974 Apr 22;39(2):97–127. doi: 10.1007/BF00492041. [DOI] [PubMed] [Google Scholar]
- Mekata F., Keatinge W. R. Electrical behaviour of inner and outer smooth muscle of sheep carotid artery. Nature. 1975 Dec 11;258(5535):534–535. doi: 10.1038/258534a0. [DOI] [PubMed] [Google Scholar]
- Mekata F., Niu H. Biophysical effects of adrenaline on the smooth muscle of the rabbit common carotid artery. J Gen Physiol. 1972 Jan;59(1):92–102. doi: 10.1085/jgp.59.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Warshaw D. M. The active tension-length curve of vascular smooth muscle related to its cellular components. J Gen Physiol. 1979 Jul;74(1):85–104. doi: 10.1085/jgp.74.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEDEN R. N. ELECTRICAL ACTIVITY OF SINGLE SMOOTH MUSCLE CELLS OF THE MESENTERIC ARTERY PRODUCED BY SPLANCHNIC NERVE STIMULATION IN THE GUINEA PIG. Nature. 1964 Apr 11;202:193–194. doi: 10.1038/202193a0. [DOI] [PubMed] [Google Scholar]
- Scheid C. R., Honeyman T. W., Fay F. S. Mechanism of beta-adrenergic relaxation of smooth muscle. Nature. 1979 Jan 4;277(5691):32–36. doi: 10.1038/277032a0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Vascular smooth muscle. I. Normal structure, pathology, biochemistry, and biophysics. Pharmacol Rev. 1968 Dec;20(4):197–272. [PubMed] [Google Scholar]
- Surprenant A. A comparative study of neuromuscular transmission in several mammalian muscular arteries. Pflugers Arch. 1980 Jul;386(1):85–91. doi: 10.1007/BF00584192. [DOI] [PubMed] [Google Scholar]
- Vanhoutee P. M., Verbeuren T. J. Inhibition by acetylcholine of the norepinephrine release evoked by potassium in canine saphenous veins. Circ Res. 1976 Aug;39(2):263–269. doi: 10.1161/01.res.39.2.263. [DOI] [PubMed] [Google Scholar]
- von Loh D., Bohr D. F. Membrane potentials of smooth muscle cells of isolated resistance vessels. Proc Soc Exp Biol Med. 1973 Nov;144(2):513–516. doi: 10.3181/00379727-144-37625. [DOI] [PubMed] [Google Scholar]



