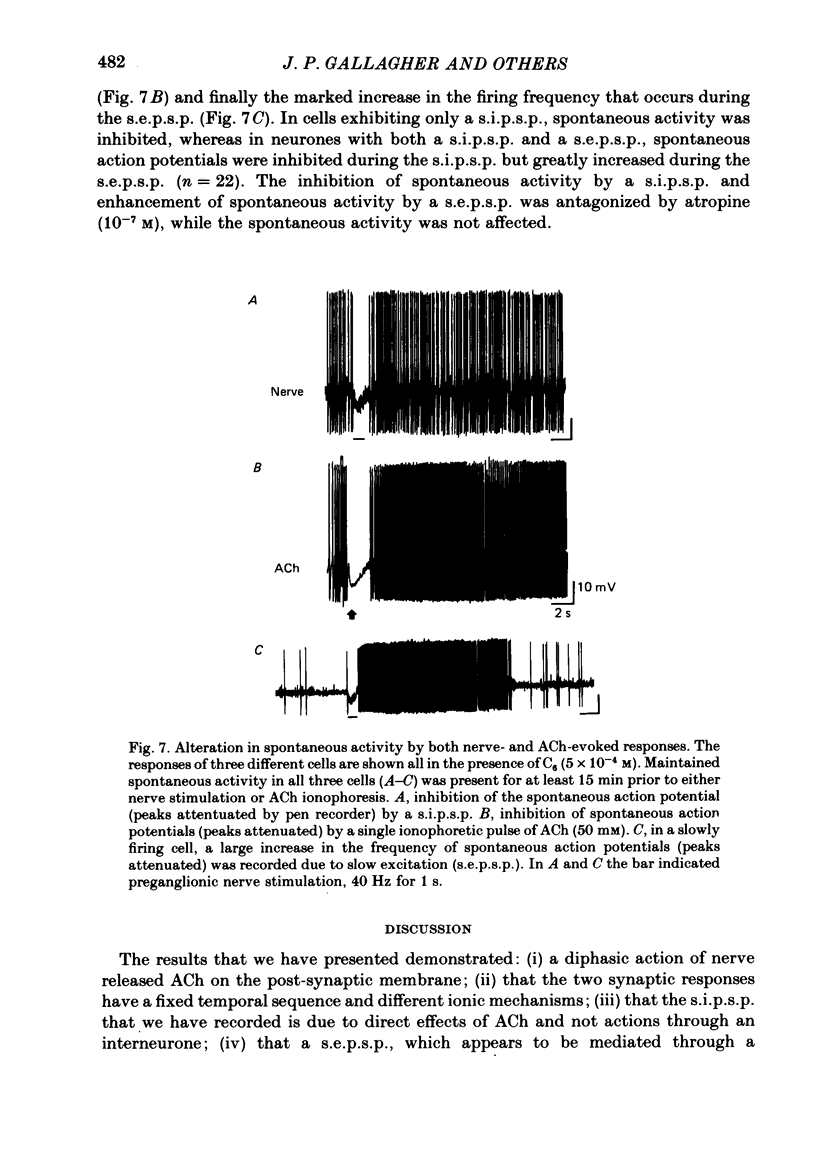
Abstract
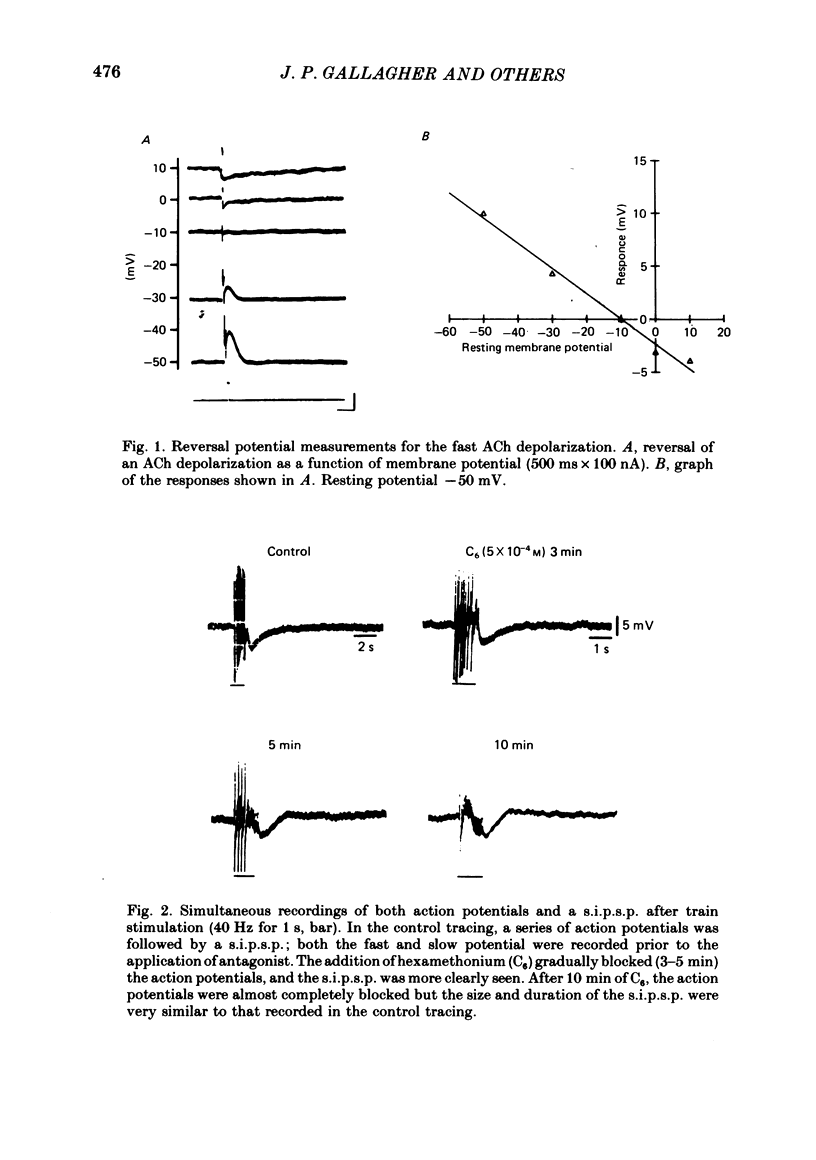
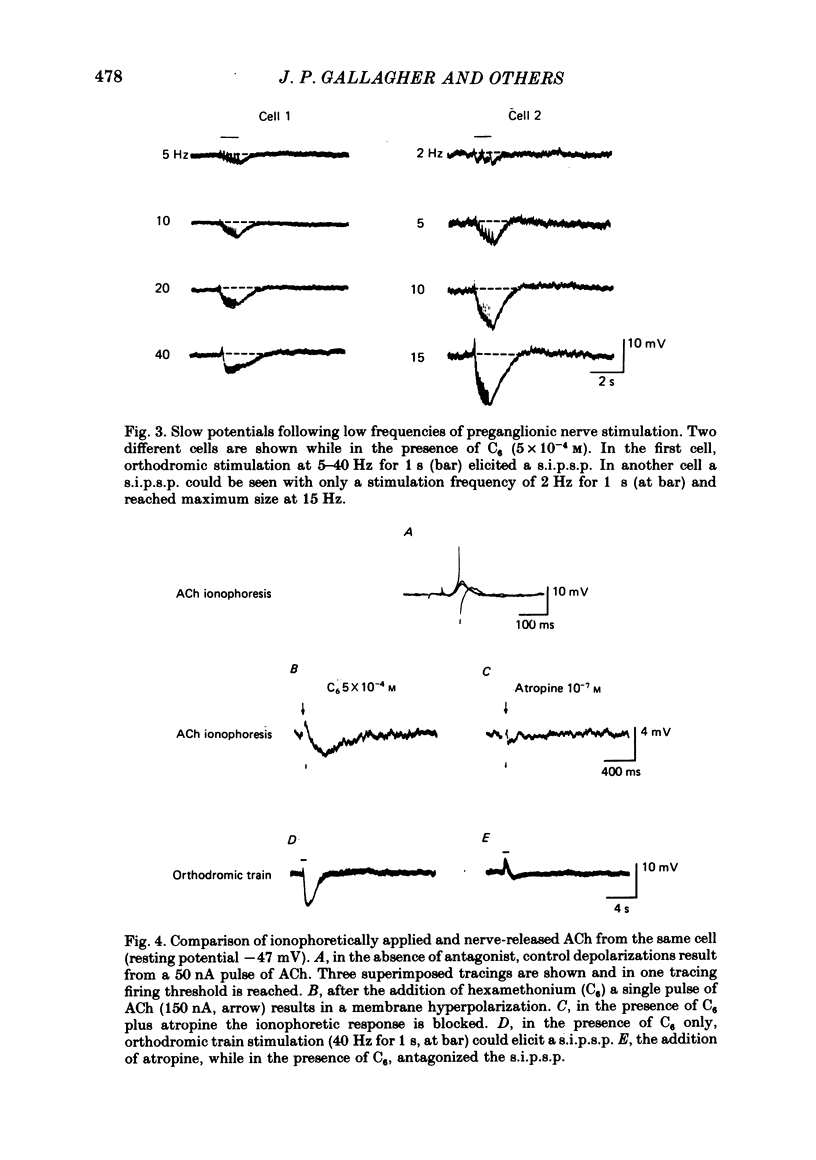
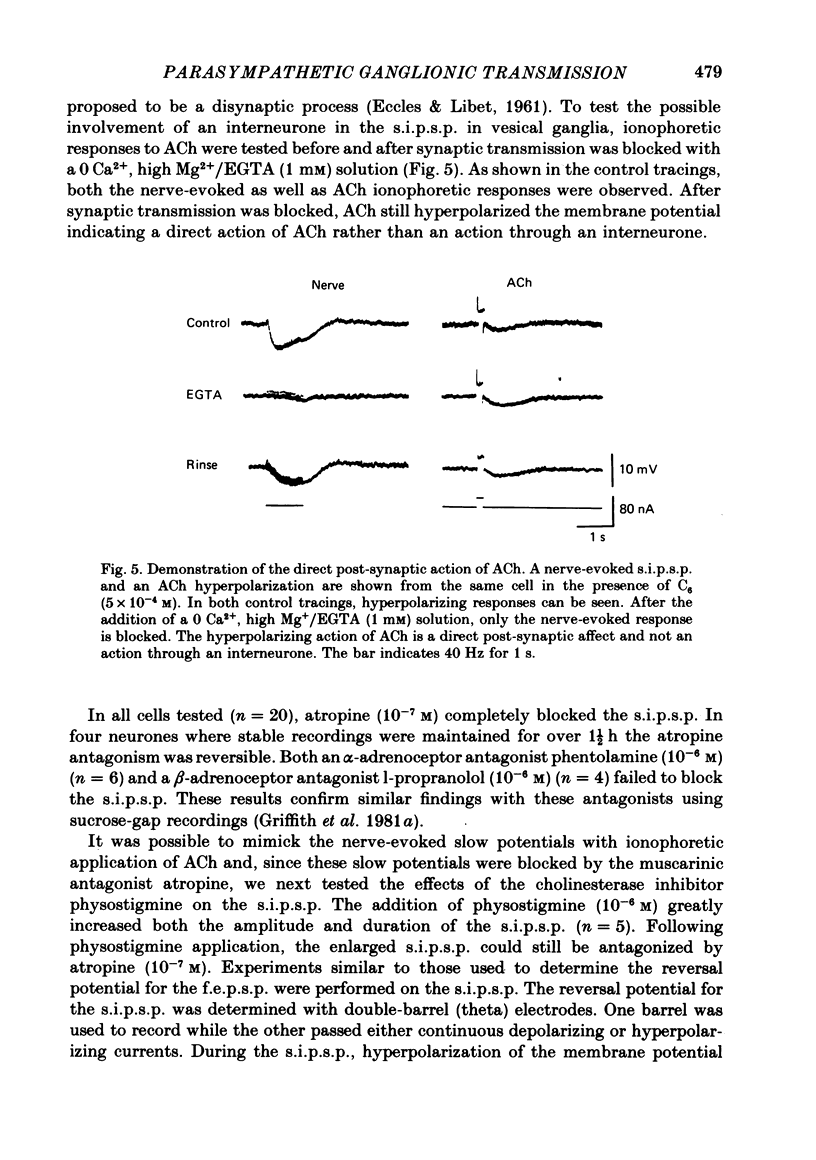
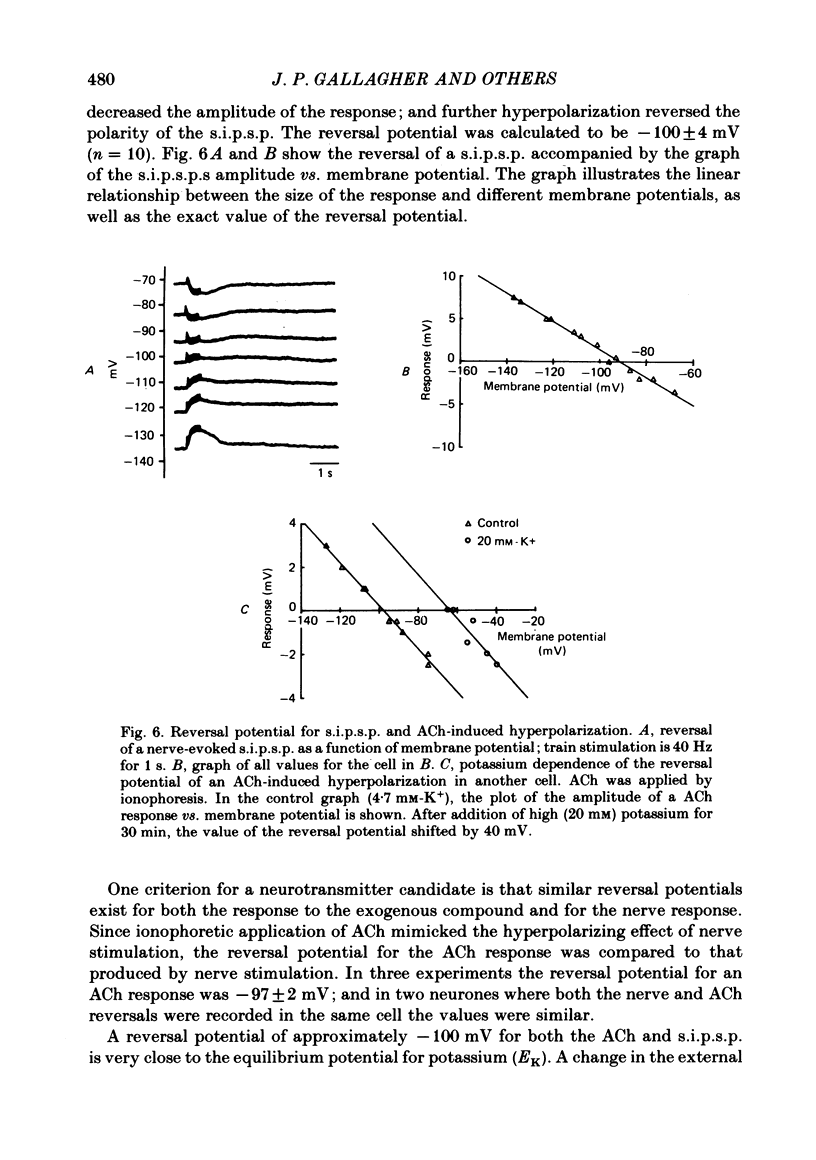
1. Intracellular electrical recording techniques were used to study the ionic mechanisms of cholinergic synaptic transmission in cat vesical pelvic ganglia (v.p.g.). 2. Orthodromic nerve stimulation as well as ionophoretic application of acetylcholine (ACh) resulted in, first, a fast excitatory post-synaptic potential (f.e.p.s.p.) and secondly, a slow inhibitory post-synaptic potential (s.i.p.s.p). These distinct post-synaptic responses were direct actions of ACh and not mediated through an interneurone. In addition, a slow excitatory post-synaptic potential (s.e.p.s.p.) was observed in 44% of the cells. 3. The f.e.p.s.p., mediated via nicotinic receptors, had a reversal potential of -10 mV and resembled the conventional rapid depolarization in other ganglia. The s.i.p.s.p., mediated by muscarinic receptors, had a reversal potential of about -100 mV and resulted from an increase in potassium conductance. 4. The slow muscarinic hyperpolarization could be observed in the absence of antagonists and it was elicited at stimulus frequencies in the physiological range (2-10 Hz). the s.i.p.s.p. induced orthodromically or ionophoretically inhibited firing in spontaneously active neurones. These observations suggest that the muscarinic hyperpolarization may occur under physiological conditions and has sufficient magnitude to be inhibitory to neuronal activity.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Large W. A., Rang H. P. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol. 1979 Oct;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. M., DeGroat W. C. A study of facilitation in vesical parasympathetic ganglia of the cat using intracellular recording techniques. Brain Res. 1979 Jun 22;169(2):388–392. doi: 10.1016/0006-8993(79)91039-4. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Constanti A. Intracellular observations on the effects of muscarinic agonists on rat sympathetic neurones. Br J Pharmacol. 1980 Dec;70(4):593–608. doi: 10.1111/j.1476-5381.1980.tb09778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A. E., Shinnick-Gallagher P. Alpha-adrenoceptor and dopamine receptor antagonists do not block the slow inhibitory postsynaptic potential in sympathetic ganglia. Brain Res. 1980 Apr 7;187(1):226–230. doi: 10.1016/0006-8993(80)90510-7. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Shinnick-Gallagher P. Comparison of the receptors mediating the catecholamine hyperpolarization and slow inhibitory postsynaptic potential in sympathetic ganglia. J Pharmacol Exp Ther. 1981 May;217(2):440–444. [PubMed] [Google Scholar]
- De Groat W. C., Saum W. R. Sympathetic inhibition of the urinary bladder and of pelvic ganglionic transmission in the cat. J Physiol. 1972 Jan;220(2):297–314. doi: 10.1113/jphysiol.1972.sp009708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroat W. C., Saum W. R. Synaptic transmission in parasympathetic ganglia in the urinary bladder of the cat. J Physiol. 1976 Mar;256(1):137–158. doi: 10.1113/jphysiol.1976.sp011316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Karczmar A. G. Involvement of an interneuron in the generation of the slow inhibitory postsynaptic potential in mammalian sympathetic ganglia. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4029–4032. doi: 10.1073/pnas.75.8.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LIBET B. Origin and blockade of the synaptic responses of curarized sympathetic ganglia. J Physiol. 1961 Aug;157:484–503. doi: 10.1113/jphysiol.1961.sp006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith W. H., 3rd, Gallagher J. P., Shinnick-Gallagher P. An intracellular investigation of cat vesical pelvic ganglia. J Neurophysiol. 1980 Feb;43(2):343–354. doi: 10.1152/jn.1980.43.2.343. [DOI] [PubMed] [Google Scholar]
- Griffith W. H., 3rd, Gallagher J. P., Shinnick-Gallagher P. Sucrose-gap recordings of nerve-evoked potentials in mammalian parasympathetic ganglia. Brain Res. 1981 Mar 30;209(2):446–451. doi: 10.1016/0006-8993(81)90168-2. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Mechanisms of slow postsynaptic potentials. Nature. 1981 Jun 18;291(5816):539–544. doi: 10.1038/291539a0. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Dodd J. Monosynaptic muscarinic activation of K+ conductance underlies the slow inhibitory postsynaptic potential in sympathetic ganglia. Nature. 1981 Aug 13;292(5824):625–627. doi: 10.1038/292625a0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11(2):77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Libet B. Generation of slow inhibitory and excitatory postsynaptic potentials. Fed Proc. 1970 Nov-Dec;29(6):1945–1956. [PubMed] [Google Scholar]
- Libet B., Weight F. F., Votava J. Inactivation of potassium conductance in slow postsynaptic excitation. Science. 1971 Apr 30;172(3982):503–504. doi: 10.1126/science.172.3982.503-a. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Connor E. A., Dionne V. E., Parsons R. L. Voltage clamp study of fast excitatory synaptic currents in bullfrog sympathetic ganglion cells. J Gen Physiol. 1980 Jan;75(1):39–60. doi: 10.1085/jgp.75.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P. The characteristics of synaptic currents and responses to acetylcholine of rat submandibular ganglion cells. J Physiol. 1981 Feb;311:23–55. doi: 10.1113/jphysiol.1981.sp013571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saum W. R., De Groat W. C. Parasympathetic ganglia: activation of an adrenergic inhibitory mechanism by cholinomimetic agents. Science. 1972 Feb 11;175(4022):659–661. doi: 10.1126/science.175.4022.659. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- Wachtel H., Kandel E. R. A direct synaptic connection mediating both excitation and inhibition. Science. 1967 Dec 1;158(3805):1206–1208. doi: 10.1126/science.158.3805.1206. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Padjen A. Acetylcholine and slow synaptic inhibition in frog sympathetic ganglion cells. Brain Res. 1973 May 30;55(1):225–228. doi: 10.1016/0006-8993(73)90506-4. [DOI] [PubMed] [Google Scholar]
- de Groat W. C., Booth A. M. Inhibition and facilitation in parasympathetic ganglia of the urinary bladder. Fed Proc. 1980 Oct;39(12):2990–2996. [PubMed] [Google Scholar]



