Abstract
Background
Sacral neuromodulation (SNM) is an established therapy in urology and gastroenterological surgery for treatment of overactive bladder symptoms, urge urinary incontinence or fecal incontinence. SNM has also been used with good results in patients with chronic pelvic pain (CPP). Our aim was to analyze long-term results of SNM in Finnish patients with endometriosis related CPP.
Methods
This is a register-based retrospective study including all the endometriosis patients treated with SNM for CPP in Finland between 2004 and 2017. There were four centers where these procedures were performed, two University Hospitals and two Central Hospitals. Long-term results were assessed by phone interview in spring 2021.
Results
A total of 16 women with endometriosis, with a median age of 39 (25–50) years, underwent SNM treatment for chronic pelvic pain (CPP), with the median follow-up time of 73 (48–85) months. The Implantable Pulse Generator (IPG) was implanted to 14 patients (88%). By the end of the follow-up period, 10 patients (62,5% of all patients and 71% of those who received IPG) had a functional SNM. Pain was assessed by numeral rating scale (NRS) and decreased from a median of 7.4 (3.6–10) to 2.3 (0-6.5).
Conclusions
SNM could be a good option in the treatment of endometriosis related chronic pelvic pain when standard therapy is not enough.
Keywords: Endometriosis, Chronic pelvic pain, Sacral neuromodulation
Introduction
Sacral neuromodulation (SNM) is mostly used in urology to improve bladder control and also in gastroenterological surgery to improve bowel control [1]. Recent systematic reviews and meta-analysis show that SNM could be an effective treatment of chronic pelvic pain (CPP), significantly reducing pain and increasing patients’ quality of life with immediate to long-term effects [2, 3].
CPP is a multifactorial disorder with pain originating in any of the urogynecological, gastrointestinal, pelvic musculoskeletal, or nervous systems [4]. Endometriosis is an estrogen-dependent chronic inflammatory disease defined by the presence of functional endometrial tissue outside the uterine cavity. Among women who underwent laparoscopy for CPP, endometriosis is found in about 1/3 of the cases, while only 25% of women with histological confirmed endometriosis are asymptomatic [5]. Endometriosis has a massive financial impact. Estimated direct medical costs for outpatient visits for chronic pelvic pain for the U.S population of women aged 18–50 years are $881.5 million per year [6].
SNM involves the electrical stimulation of sacral nerve roots S3 or S4 with low electrical current via an electrode placed percutaneously through the sacral foramen. Patients usually undergo a temporary evaluation period, and if successful, an implantable pulse generator (IPG) is inserted. Although the mechanism of action of sacral neuromodulation (SNM) is still not fully elucidated, it seems to involve modulation of spinal cord reflexes and brain networks by peripheral afferents. Nevertheless, motor effects mediated via efferents on direct stimulation cannot be fully excluded [7].
We have already indicated that SNM may be effective in the treatment of endometriosis patients with CPP [8–10]. This study aims to assess the long-term efficacy and safety of sacral neuromodulation (SNM) in the treatment of chronic pelvic pain (CPP) associated with endometriosis, a challenging condition to manage post-surgery. To our best knowledge, this is the first study reporting long-term results of SNM in patients with CPP related to endometriosis.
Materials and methods
All the endometriosis patients treated with SNM for CPP in Finland between 2004 and 2017 were included. There were four centers in which SNM was performed: Oulu and Turku University Hospitals, as well as Jyväskylä and Seinäjoki Central Hospitals. SNM was offered to patients who did not respond to conventional medical and surgical therapies as an off-label treatment and followed the generally accepted contraindications: cognitive disorders, severe or rapidly progressive neurological disease, pregnancy, abnormal sacral anatomy, bleeding disorders and other general contraindications for surgery. SNM was performed in two stages using standard programs. In the evaluation stage I, a tined lead was placed in the S3 or S4 foramina with the patient in prone Jack knife position under local or general anesthesia. After a trial period of 14 days, stage II was performed. The internal pulse generator (IPG) was implanted after a successful test period, meaning at least a 50% clinical improvement of symptoms.
SNM related data was collected from patient records in 2017 and was later completed with endometriosis related data in autumn 2023. Long-term results were assessed by phone questionnaire during spring 2021. Patients were asked the following questions: Does the SNM device still work? If the device was removed, why did this happen and when? What is the intensity of the current worst pain using a numeric rating scale (NRS; range 0–10 with 0 meaning no pain and 10 the worst pain imaginable)? Was the internal pulse generator (IPG) changed?
The primary outcome was successfully sustained SNM therapy and the decrease of NRS pain score. Success was defined as having a permanent stimulator (IPG) implanted and as having a working SNM by the end of the follow-up. Secondary outcomes were failure of the treatment and postoperative morbidity.
Statistical analyses were performed using SPSS Statistics version 22 for Windows (IBM Corp, Armonk, NY, USA). Non-categorical variables were expressed as medians with minimum and maximum values unless otherwise stated. Continuous variables were analyzed using Student’s t-test or Mann–Whitney U-test, the latter for non-normally distributed data. Chi-square or Fisher’s exact test was used for categorical variables. Two-tailed P values were reported and a P value < 0.05 was deemed statistically significant.
This study was conducted in accordance with Finnish Medical Research Act 488/199, 295/2004 and approved by the Ethics Committee of the Hospital District of Southwest Finland (ETMK: 163/1801/2015, ETMK 7/2019).
Results
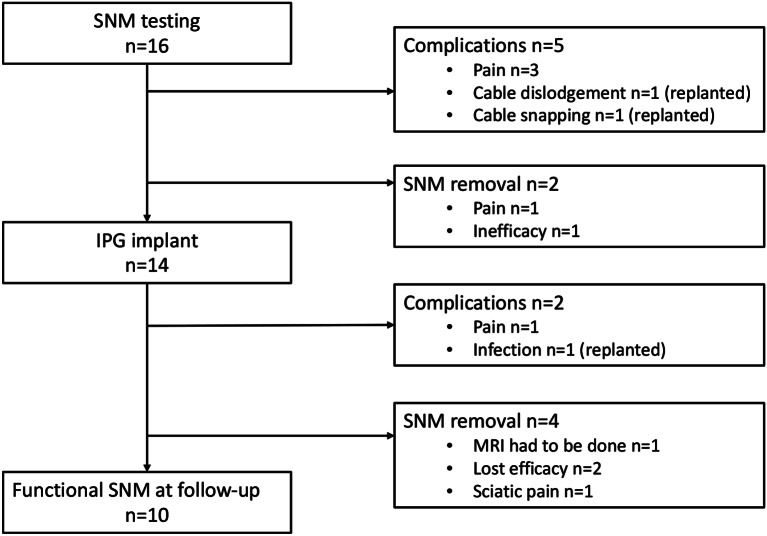
A total of 16 women, with a median age of 39 (25–50) years, underwent SNM treatment for CPP related to endometriosis, with a median follow-up time of 73 (48–85) months. All the patients had previously undergone at least one surgery due to endometriosis, with a median of 3 [1–6] operations. Deep endometriosis (88%) was more frequent than superficial endometriosis (38%) and endometrioma (44%). A significant number of patients have undergone extensive surgery including hysterectomy (44%), adnexectomy (56%) and sigmoid resection or anterior resection of the rectum (44%). Baseline characteristics are presented in Table 1. The patient flow chart is presented in Fig. 1.
Table 1.
Baseline characteristics
| Variable | |
|---|---|
| Age, median (min-max) | 39 (25–50) |
| Follow-up time, median (min-max) | 73 months (48–85) |
| G, median (min-max) | 1 (0–3) |
| P, median (min-max) | 0 (0–2) |
| Superficial endometriosis, n (%) | 6 (38) |
| Deep endometriosis, n (%) | 14 (88) |
| Endometrioma, n (%) | 7 (44) |
| Endometrioma enucleation, n (%) | 7 (44) |
| Hysterectomy, n (%) | 7 (44) |
| Salpingectomy, n (%) | 9 (56) |
| Ovariectomy, n (%) | 9 (56) |
| Superficial peritoneal endometriosis resection, n (%) | 6 (38) |
| Anterior resection of rectum or sigmoid resection, n (%) | 7 (44) |
| Number of endometriosis surgeries, median (min-max) | 3 (1–6) |
| Light opiods, n (%) 1 | 10 (63) |
| Strong opioids, n (%) 2 | 6 (38) |
1 Light opioids, i.e. codein, tramadol
2 Strong opioids, i.e. oxycodone
Fig. 1.
Patient flow chart
The Implantable Pulse Generator (IPG) was implanted to 14 patients (88%). The majority of the patients had the SNM electrode implanted in S3 right (38%) or S4 right (38%) foramens, S3 left (19%) and S4 left (6.3%) were less commonly used. Postoperative complications were registered in five patients (31%) after Stage 1 (pain in three patients, cable dislodgement in one patient and cable snapping in one patient) and in two patients (14%) after Stage 2 (one with pain and one with infection, where IPG and electrode were replanted).
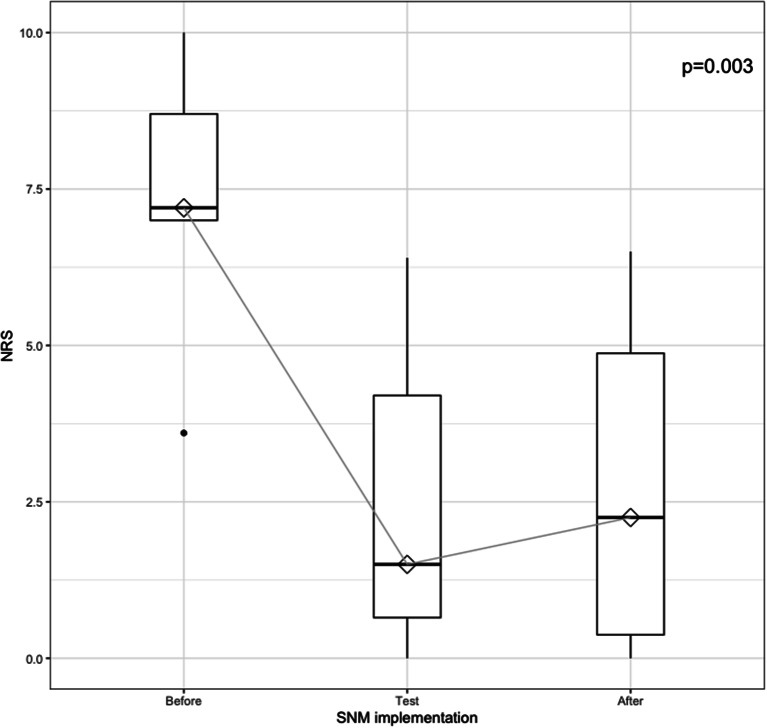
By the end of the follow-up period 10 patients (62,5% of all patients and 71% of those who received IPG) had a functional SNM. Pain NRS value dropped from median 7.4 (3.6–10) before SNM to 2.3 (0-6.5) by the end of the follow-up (Fig. 2). Postoperative outcomes are illustrated in Table 2.
Fig. 2.
Box-plot illustrating current worst pain scores reported by using Numeric Rating Scale (NRS, range 0–10 with 0 no pain and 10 worst imaginable pain) before SNM (n = 16), during test-phase (n = 16) and at the end of the follow-up (n = 10)
Table 2.
Postoperative SNM outcomes
| Variable | |
|---|---|
| Pre-test NRS, median (min-max) | 7.4 (3.6–10) |
| Stage 1 test | 16 (100) |
| Stage 1 complications | 5 (31) |
| Stage 2 implantation rate | 14 (88) |
| Stage 2 complications | 2 (14) |
| Working SNM at the end of follow-up | 10 (71) |
| NRS at the end of the follow-up, median (min-max) | 2.3 (0-6.5) |
Analysis of the two groups of patients with working SNM vs. failed SNM therapy by the end of the follow up showed no statistically significant differences when the type of endometriosis, the type of surgery or the number of surgeries were taken into consideration (Table 3). The number of functional electrodes and the location of the electrodes did not influence the final outcome. Median number [4, 2, 3, 4] of functional electrodes was the same in both groups (p = 0.940).
Table 3.
Comparative analysis of patients with working SNM vs. failed SNM therapy
| Working SNM | Failed therapy | p-value | |
|---|---|---|---|
| Age | 33 (29–40) | 26 (25–27) | 0.562 |
| Preoperative pain | 7 (3.6–8.4) | 9.5 (9–10) | 0.095 |
| Superficial endometriosis | 2 (33) | 4 (67) | 0.118 |
| Deep endometriosis | 8 (57) | 6 (43) | 0.500 |
| Endometrioma | 6 (86) | 1 (14) | 0.145 |
| Endometrioma enucleation | 6 (86) | 1 (14) | 0.145 |
| Hysterectomy | 5 (71) | 2 (29) | 0.633 |
| Salpingectomy | 7 (78) | 2 (22) | 0.302 |
| Ovariectomy | 7 (78) | 2 (22) | 0.302 |
| Superficial peritoneal endometriosis resection | 2 (33) | 4 (67) | 0.118 |
| Anterior resection of rectum or sigmoid resection | 4 (57) | 3 (43) | 0.999 |
| Number of endometriosis surgeries, median (min-max) | 4 (1–6) | 3 (1–6) | 0.635 |
| Light opioids 1 | 7 (70) | 3 (30) | 0.726 |
| Strong opioids 2 | 4 (67) | 2 (33) | 0.915 |
| Number of functional electrodes | 4 (2–4) | 4 (2–4) | 0.940 |
| Foramen S3 | 5 (56%) | 4 (44%) | 0.091 |
| Foramen S4 | 1 (14%) | 6 (86%) |
1 Light opioids, i.e. codein, tramadol
2 Strong opioids, i.e. oxycodone
Discussion
CPP is not currently an approved US Food and Drug Administration (FDA) indication for SNM. There is emerging data showing that SNM is effective in the treatment of CPP with multifactorial etiology [2, 3, 12]. There are only a few studies showing good short-term results of SNM in the treatment of endometriosis related CPP [8, 9, 10], but long-term data is lacking. This Finnish national study is the first to show promising long-term results in the difficult to treat group of endometriosis patients with CPP after surgery.
The long-term efficacy of SNM in our study is reflected by the fact that 71% of the patients that received the IPG had a functioning device by the end of the follow-up and this is better than the reported SNM long-term outcomes with different etiologies [13]. It is also illustrated by the decrease of the pain related NRS value from a median of 7.4 (3.6–10) before SNM to 2.3 (0-6.5) by the time of follow-up. This NRS drop is significant, as usually a reduction of pain by 50% or an absolute pain reduction of 3 units are accurate in evaluating a successful pain reduction after a given treatment [11].
The complication rates are comparable with those reported in earlier studies [2, 14]. The overall implantation rate of 88% in our study is better than the implantation rate of 64.3% reported by a recent meta-analysis and systematic review of SNM in CPP, with a quite diverse etiology of pain [2]. The implantation rate is also better than the one of 54,8% reported by another meta-analysis and systematic review of SNM in CPP [3].
CPP affects women’s quality of life deeply. CPP is often associated with migraine and headache, regardless if CPP is related or not to endometriosis [15]. Endometriosis and CPP was reported to negatively impact all domains of life including physical, psychological, social, sexual, education and employment [16]. A large multi-center study across Europe, UK and the USA found that the total cost per woman with endometriosis per year was €9579 with the bulk of costs (€6298) being due to absence from work [17]. It has been shown that as little as a 10% reduction on a pain scale is needed to improve productivity [18].
All the patients in our study underwent standard medical therapy and at least one surgery for endometriosis before SNM. According to literature, surgery does not reduce pain in 20–28% of patients [19]. Post-surgical hormonal therapy has been advocated to improve the effectiveness of surgery and prevent recurrences, but it has been proven to be of limited or no benefit for endometriosis in general and for deep peritoneal endometriosis in particular [20]. Given the amount of non-responders to surgery and the recurrence of pain, even though there is no evident recurrence of endometriosis in 23%, there is a need for evidence-based approaches that do not require surgery or taking hormones [16]. Diet, exercise, physiotherapy, acupuncture, psychotherapy could be considered [16] and this is the area where SNM might play a role. It was suggested that a major contributing factor for endometriosis-associated pain are not the ectopic growths themselves, but rather nerve activity from surrounding tissues, which affects the activity of neurons in the spinal cord and brain [21]. This could explain why SNM is effective. These findings encourage the consideration of SNM as a long-term treatment option for patients with refractory CPP due to endometriosis, particularly in cases where standard surgical and hormonal therapies fail.
This study has a few limitations. This is a retrospective study. The total number of the patients is relatively small. There are potential biases from reliance on phone questionnaires for long-term follow-up. Furthermore, unfortunately, QoL data was not available. On the other hand this is a comprehensive national study including all the patients that underwent SNM therapy for endometriosis-associated CPP between 2004 and 2017. More studies are definitely needed in order to standardize the role of SNM in the treatment of endometriosis patients with persisting CPP after surgery.
Conclusion
Endometriosis patients with chronic pelvic pain may benefit from SNM therapy after failure of standard medical and surgical therapy, regardless of the type of endometriosis or the extent of surgery performed. More multi-centre, larger, prospective studies are needed, potentially including QoL metrics, cost-effectiveness, and biomarkers predictive of SNM success.
Acknowledgements
Not applicable.
Abbreviations
- SNM
Sacral neuromodulation
- CPP
Chronic pelvic pain
- IPG
Implantable pulse generator
- FDAL
US Food and Drug Administration
Author contributions
AZ: Manuscript writing, Data analysis, Project development, EO: Data collection, JK: Data collection, Project development, PS: Data collection, PV: Project development, JM-K: Data collection, revision, TR: Project development, JS: Data collection, MU: Data analysis, revision ML: Project development and supervision, TP: Project development and supervision.
Funding
This study was conducted without external funding.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with Finnish Medical Research Act 488/199, 295/2004 and approved by the Ethics Committee of the Hospital District of Southwest Finland (ETMK: 163/1801/2015, ETMK 7/2019). Informed consent to participate was obtained from all of the participants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.StatPearls. 2021.
- 2.Greig J, Mak Q, Furrer MA, Sahai A, Raison N. Sacral neuromodulation in the management of chronic pelvic pain: A systematic review and meta-analysis. Neurourol Urodyn. 2023;42(4):822–36. 10.1002/nau.25167. Epub 20230306. [DOI] [PubMed] [Google Scholar]
- 3.Mahran A, Baaklini G, Hassani D, Abolella HA, Safwat AS, Neudecker M et al. Sacral neuromodulation treating chronic pelvic pain: a meta-analysis and systematic review of the literature. Int Urogynecol J. 2019. Epub 2019/03/16. 10.1007/s00192-019-03898-w. PubMed PMID: 30874835. [DOI] [PubMed]
- 4.Grinberg K, Sela Y, Nissanholtz-Gannot R. New Insights about Chronic Pelvic Pain Syndrome (CPPS). Int J Environ Res Public Health. 2020;17(9). Epub 20200426. 10.3390/ijerph17093005. PubMed PMID: 32357440; PubMed Central PMCID: PMC7246747. [DOI] [PMC free article] [PubMed]
- 5.Triolo O, Laganà AS, Sturlese E. Chronic pelvic pain in endometriosis: an overview. J Clin Med Res. 2013;5(3):153–63. Epub 20130423. doi: 10.4021/jocmr1288w. PubMed PMID: 23671540; PubMed Central PMCID: PMC3651065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87(3):321–7. 10.1016/0029-7844(95)00458-0. PubMed PMID: 8598948. [DOI] [PubMed] [Google Scholar]
- 7.De Wachter S, Vaganee D, Kessler TM. Sacral neuromodulation: mechanism of action. Eur Urol Focus. 2020;6(5):823–5. 10.1016/j.euf.2019.11.018. Epub 20200201. [DOI] [PubMed] [Google Scholar]
- 8.Lavonius M, Suvitie P, Varpe P, Huhtinen H. Sacral Neuromodulation: Foray into Chronic Pelvic Pain in End Stage Endometriosis. Case Rep Neurol Med. 2017;2017:2197831. Epub 2017/04/04. 10.1155/2017/2197831. PubMed PMID: 28367344; PubMed Central PMCID: PMC5358435. [DOI] [PMC free article] [PubMed]
- 9.Zegrea A, Kirss J, Pinta T, Rautio T, Varpe P, Kairaluoma M, et al. Outcomes of sacral neuromodulation for chronic pelvic pain: a Finnish National multicenter study. Tech Coloproctol. 2020;24(3):215–20. 10.1007/s10151-020-02148-2. Epub 20200121. [DOI] [PubMed] [Google Scholar]
- 10.Zegrea A, Ojala E, Suvitie P, Varpe P, Huhtinen H, Mäkelä-Kaikkonen J et al. Sacral neuromodulation in endometriosis - A promising treatment option for chronic pelvic pain. Acta Obstet Gynecol Scand. 2023. Epub 20231009. 10.1111/aogs.14690. PubMed PMID: 37814355. [DOI] [PMC free article] [PubMed]
- 11.Forouzanfar T, Weber WE, Kemler M, van Kleef M. What is a meaningful pain reduction in patients with complex regional pain syndrome type 1? Clin J Pain. 2003;19(5):281–5. 10.1097/00002508-200309000-00001. PubMed PMID: 12966253. [DOI] [PubMed] [Google Scholar]
- 12.Bieze M, van Haaps AP, Kapural L, Li S, Ferguson K, de Vries R, et al. Spinal cord stimulation for intractable visceral pain originating from the pelvic and abdominal region: A narrative review on a possible new indication for patients with Therapy-Resistant pain. J Pain Res. 2024;17:691–736. 10.2147/JPR.S445616. Epub 20240219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ismail S, Chartier-Kastler E, Perrouin-Verbe MA, Rose-Dite-Modestine J, Denys P, Phé V. Long-Term functional outcomes of S3 sacral neuromodulation for the treatment of idiopathic overactive bladder. Neuromodulation. 2017;20(8):825–9. Epub 20171002. doi: 10.1111/ner.12696. PubMed PMID: 28967986. [DOI] [PubMed] [Google Scholar]
- 14.Siegel S, Noblett K, Mangel J, Bennett J, Griebling TL, Sutherland SE, et al. Five-Year followup results of a prospective, multicenter study of patients with overactive bladder treated with sacral neuromodulation. J Urol. 2018;199(1):229–36. 10.1016/j.juro.2017.07.010. Epub 20170711. [DOI] [PubMed] [Google Scholar]
- 15.Karp BI, Sinaii N, Nieman LK, Silberstein SD, Stratton P. Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril. 2011;95(3):895–9. 10.1016/j.fertnstert.2010.11.037. Epub 20101210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ball E, Khan KS. Recent advances in understanding and managing chronic pelvic pain in women with special consideration to endometriosis. F1000Res. 2020;9. Epub 20200204. 10.12688/f1000research.20750.1. PubMed PMID: 32089831; PubMed Central PMCID: PMC7001750. [DOI] [PMC free article] [PubMed]
- 17.Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–9. 10.1093/humrep/des073. Epub 20120314. [DOI] [PubMed] [Google Scholar]
- 18.Gerlinger C, Schumacher U, Faustmann T, Colligs A, Schmitz H, Seitz C. Defining a minimal clinically important difference for endometriosis-associated pelvic pain measured on a visual analog scale: analyses of two placebo-controlled, randomized trials. Health Qual Life Outcomes. 2010;8:138. 10.1186/1477-7525-8-138. Epub 2010/11/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbott J, Hawe J, Hunter D, Holmes M, Finn P, Garry R. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil Steril. 2004;82(4):878–84..046. PubMed PMID: 15482763. [DOI] [PubMed] [Google Scholar]
- 20.Somigliana E, Busnelli A, Benaglia L, Viganò P, Leonardi M, Paffoni A, et al. Postoperative hormonal therapy after surgical excision of deep endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:77–80. 10.1016/j.ejogrb.2016.03.030. Epub 20160401. [DOI] [PubMed] [Google Scholar]
- 21.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–46. 10.1093/humupd/dmq050. Epub 2010/11/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.