Abstract
Background
Glucagon-like peptide-1 (GLP-1) receptor agonists and sodium–glucose cotransporter 2 (SGLT2) inhibitors represent a new generation of antihyperglycemic agents that operate through mechanisms distinct from conventional diabetes treatments. Beyond their metabolic effects, these medications have demonstrated neuroprotective properties in preclinical studies. While clinical trials have explored their therapeutic potential in established neurodegenerative conditions, their role in disease prevention remains unclear. We conducted a network meta-analysis (NMA) to comprehensively evaluate the prophylactic benefits of these agents across multiple neurodegenerative diseases and identify the most promising preventive strategies.
Methods
We systematically searched PubMed, Embase, ClinicalKey, Cochrane CENTRAL, ProQuest, ScienceDirect, Web of Science, and ClinicalTrials.gov through October 24th, 2024, for randomized controlled trials (RCTs) of GLP-1 receptor agonists or SGLT2 inhibitors. Our primary outcome was the incidence of seven major neurodegenerative diseases: Parkinson’s disease, Alzheimer’s disease, Lewy body dementia, multiple sclerosis, amyotrophic lateral sclerosis, frontotemporal dementia, and Huntington’s disease. Secondary outcomes included safety profiles assessed through dropout rates. We performed a frequentist-based NMA and evaluated risk of bias with Risk of Bias tool. The main result of the primary outcome in the current study would be re-affirmed via sensitivity test with Bayesian-based NMA.
Results
Our analysis encompassed 22 RCTs involving 138,282 participants (mean age 64.8 years, 36.4% female). Among all investigated medications, only dapagliflozin demonstrated significant prophylactic benefits, specifically in preventing Parkinson’s disease (odds ratio = 0.28, 95% confidence intervals = 0.09 to 0.93) compared to controls. Neither GLP-1 receptor agonists nor other SGLT2 inhibitors showed significant preventive effects for any of the investigated neurodegenerative conditions. Drop-out rates were comparable across all treatments.
Conclusions
This comprehensive NMA reveals a novel and specific prophylactic effect of dapagliflozin against Parkinson’s disease, representing a potential breakthrough in preventive neurology. The specificity of dapagliflozin’s protective effect to Parkinson’s disease might rely on its highly selective inhibition to SGLT2. These findings provide important direction for future research and could inform preventive strategies for populations at risk of Parkinson’s disease.
Trial registration
PROSPERO CRD42021252381.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-025-04018-w.
Keywords: Network meta-analysis, GLP-1 receptor agonist, SGLT2 inhibitor, Neurodegenerative disease, Parkinson’s disease
Background
Glucagon-like peptide-1 (GLP-1) receptor agonists and sodium–glucose cotransporter 2 (SGLT2) inhibitors have emerged as novel glucose-lowering agents, featuring mechanisms of action distinct from those of conventional treatments [1]. GLP-1, an incretin hormone produced by intestinal L cells, enhances insulin release, slows gastric emptying, and suppresses glucagon secretion, which collectively contribute to reduced blood glucose levels [2]. Meanwhile, SGLT2 is produced in the proximal tubules of the kidneys, where SGLT2 inhibitor facilitates the lowering of blood glucose by limiting glucose reabsorption and encouraging its excretion through urine, thereby aiding in improved glycemic control for patients [3].
Beyond their primary role in managing blood sugar, additional therapeutic advantages of GLP-1 receptor agonists and SGLT2 inhibitors have been uncovered in recent years. Notably, SGLT2 inhibitors have shown cardiovascular [4] and renal protective effects in patients with diabetes [5]. Similarly, GLP-1 receptor agonists have been found to provide cardiovascular and renal benefits within the same population [6]. As a result of these expanded benefits, researchers are increasingly viewing GLP-1 receptor agonists and SGLT2 inhibitors as versatile, multi-functional drugs.
Recently, interest has been growing around the potential application of GLP-1 receptor agonists and SGLT2 inhibitors in managing neurodegenerative diseases [7]. Animal studies have demonstrated neuroprotective effects for these medications across various disease models [8, 9]. Clinicians are now focusing on the therapeutic potential of GLP-1 receptor agonists and SGLT2 inhibitors in addressing symptoms of Parkinson’s disease [10, 11] and Alzheimer’s disease [12]. Specifically, Mulvaney and colleagues observed that GLP-1 receptor agonists might improve motor symptoms in individuals with Parkinson’s disease [13]. This clinical observation could be supported by the basic evidence that GLP-1 receptor were not only expressed in gastrointestinal tract but also in several brain regions, such as hypothalamus, subfornical organ, nucleus of the solitary tract, and area postrema [14]. Some of these regions played an important role in some neurodegenerative diseases. For example, Zhou and the colleague demonstrated that the microstructural degeneration in hypothalamus may be associated with development of Parkinson’s disease [15]. In contrast, Vijiaratnam et al., by adding on exenatide to subjects with Parkinson’s disease, suggested that exenatide could not modify the Parkinson’s disease progression [16]. In a recent one traditional pair-wise meta-analysis by Albuquerque et al., the authors showed that overall GLP-1 receptor agonists relieve the motor symptoms in subjects with Parkinson’s disease [17]. Moreover, dapagliflozin, a particular SGLT2 inhibitor, has been shown to exhibit anti-inflammatory properties [18], which may hypothetically be significant in managing diverse neurodegenerative diseases. This proposed neuroprotective effect is supported by data from several large-scale trials on neurodegenerative conditions, including Parkinson’s disease and Alzheimer’s disease [19–21]. However, the precise mechanisms and physiological impacts of these medications remain largely unexplored.
Following these clinical trials, several meta-analyses have shown a beneficial effect of GLP-1 receptor agonists and SGLT2 inhibitors on symptoms of Parkinson’s disease [22] and Alzheimer’s disease [23]. As the adage goes, “Prevention is better than cure” [24]. From a public health perspective, prevention holds particular importance, as most neurodegenerative diseases are irreversible [25]. Although there have been traditional pairwise meta-analyses assessing the protective effects of these newer glucose-lowering drugs on neurodegenerative diseases [26–28], conclusive evidence remains elusive due to methodological limitations. Traditional pairwise meta-analyses, which group various medications together, yield an overall efficacy but lack the specificity needed for individual comparisons, leading to heterogeneity among the medications and diluting statistical significance. Network meta-analysis (NMA), which allows for direct comparisons among different medications, enhances the ability to make multiple treatment efficacy comparisons and assess the potential superiority of specific interventions at various dosages [29]. This approach offers a more detailed and evidence-based framework for guiding future clinical practices.
Given this context, a well-constructed NMA could provide comparative efficacy estimates and offer fresh perspectives on the relative benefits of these medications. To the best of our knowledge, no NMA has yet assessed the preventive potential of various GLP-1 receptor agonists and SGLT2 inhibitors in neurodegenerative diseases. Therefore, this NMA aims to (1) compare the preventive efficacy of GLP-1 receptor agonists and SGLT2 inhibitors across multiple neurodegenerative diseases; (2) identify the most effective agents for prevention; and (3) assess their relative safety profiles in preventive use.
Methods
This network meta-analysis (NMA) followed the guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) with the extension for network meta-analyses (PRISMA NMA) [30] (Additional file: Tab. S1A-S1B). The study was registered in PROSPERO under registration number CRD42021252381 and received ethical approval from the Institutional Review Board at the Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan (TSGHIRB No. B-109–29).
Database searches and study identification
We performed comprehensive database searches in PubMed, Embase, ClinicalKey, Cochrane CENTRAL, ProQuest, ScienceDirect, Web of Science, and ClinicalTrials.gov (Additional file: Tab. S2) for studies published up to October 24, 2024. Two independent authors (PT Tseng and BY Zeng) conducted these searches, screened the titles and abstracts, and resolved any disagreements about study inclusion through consensus. Additionally, we manually reviewed reference lists from relevant review articles and meta-analyses to identify additional studies [7, 13, 22, 23, 26–28, 31–41]. No language restrictions were applied in the search.
Inclusion and exclusion criteria
Since the main goal of the current NMA was to evaluate the prophylactic effect, the participants to be included should not have pre-existed neurodegenerative diseases at baseline. Therefore, the inclusion criteria for this NMA were based on the following PICOS model (Population, Intervention, Comparison, Outcome, and Study design): Population: Adults (≥ 18 years) without pre-existing neurodegenerative diseases; Intervention: prescription of GLP-1 receptor agonist or SGLT2 inhibitor at any dose; Comparison: Placebo, standard care, or active comparator; Outcomes: Incident cases of neurodegenerative diseases; Study design: Randomized controlled trials.
This NMA focused on assessing prophylactic effects; therefore, only participants without neurodegenerative diseases at baseline were included. To limit heterogeneity, we included only studies comparing GLP-1 receptor agonists or SGLT2 inhibitors. Eligible studies were limited to peer-reviewed randomized controlled trials (RCTs) and included (1) RCTs with participants free of neurodegenerative diseases at baseline; (2) RCTs involving GLP-1 receptor agonists or SGLT2 inhibitors; (3) studies on human participants; and (4) RCTs that systematically screened for adverse events or specifically targeted these outcomes.
Exclusion criteria included (1) studies that were not RCTs or peer-reviewed; (2) RCTs involving participants with pre-existing neurodegenerative conditions; (3) RCTs not directly comparing GLP-1 receptor agonists or SGLT2 inhibitors; (4) after checking full text, not report target outcome, either in primary/secondary outcome or in adverse event profile; and (5) animal studies. Because the currently available RCTs regarding such medications were designed to evaluate their treatment efficacy but not to detect incidence of neurodegenerative diseases, it would easily miss the occurrence of neurodegenerative diseases and result in potential reporting bias if they were not designed as systematically screening for adverse events. Only RCTs with systematic screening for adverse events or those directly assessing our target outcomes were included to enhance reliability and reduce selective reporting bias [42].
Methodological quality appraisal
Two independent authors evaluated the risk of bias for each study using the Cochrane Risk of Bias Tool 1.0 [43], achieving an inter-rater reliability of 0.85. Any differences were resolved by consulting a third author.
Outcome definition
Due to the variability in the methods used to record targeted events, we defined our primary outcome as the “event numbers in registry systems.” Specifically, we counted total event occurrences rather than the number of affected patients. The primary outcome was the total number of overall neurodegenerative disease events recorded in registry systems. Based on the book by Suescun [44] and review article by Koenig [45], the neurodegenerative diseases were defined to include (1) Parkinson’s disease, (2) Alzheimer’s disease, (3) Lewy body dementia, (4) multiple sclerosis, (5) amyotrophic lateral sclerosis, (6) Frontotemporal dementia, and (7) Huntington’s disease. The safety profile was assessed through drop-out rates (i.e., participants who withdrew from the study before completion for any reason).
Data extraction, management, and conversion
Data extraction was independently performed by two authors (PT Tseng and BY Zeng), recording demographic data, study design, treatment details, primary outcomes, and safety information. If essential data were missing, we reached out to corresponding authors. The data extraction adhered to protocols from the Cochrane Handbook for Systematic Reviews of Interventions and other pertinent medical literature [46].
Dose definitions followed original RCT classifications [19–21, 47–65]: canagliflozin (low: 100 mg, and high: 300 mg); ertugliflozin (low: 5 mg, and high: 15 mg); injectable semaglutide (low: 0.5 mg, and high: 1.0 mg); empagliflozin (low: 1–10 mg, and high: 25–50 mg).
Statistical analyses
For analysis with multiple treatment arms, a random-effects model was used in the NMA [66], employing MetaInsight (version 4.0.2, Complex Reviews Support Unit, National Institute for Health Research, London, UK) within a frequentist framework. MetaInsight is a web-based platform for conducting NMAs via the netmeta package in R software, designed for frequentist statistical analysis [67].
For categorical data, a continuity correction of single-zero-event studies was applied in the meta-analytical procedure. However, for studies with zero event in both the intervention and the control arms, such a correction was not applied to avoid increasing the bias. Rather, we would exclude that comparison instead [68, 69]. Forest plots were created to present odds ratios (ORs) with 95% confidence intervals (95%CIs) for effect size calculation [70]. We then generated treatment rankings and effect sizes for direct and indirect comparisons, tabulated accordingly. A two-tailed p-value less than 0.05 indicated statistical significance.
Inconsistency evaluation
The “node-splitting” method was applied to evaluate the potential inconsistency between direct and indirect evidence, a method particularly beneficial in NMA when trial-level data are available. To be specific, the inconsistency test of node-splitting method in MetaInsight was conducted based on R package netmeta (Gerta Rücker, Guido Schwarzer, Ulrike Krahn and Jochem König 2017) in the platform of R software-based webpage [67, 71].
Sensitivity analyses
To assess the robustness of our findings, we conducted a subgroup analysis by grouping RCTs by seven primary outcome categories (e.g., Parkinson’s disease, Alzheimer’s disease, Lewy body dementia, multiple sclerosis, amyotrophic lateral sclerosis, frontotemporal dementia, and Huntington’s disease).
Further, to re-affirm the reliability and the convergence of the investigated treatment estimates, we arrange sensitivity analysis with Bayesian-based NMA to re-run the analytic process of the main primary outcome. Further, we arranged Bayesian-based surface under the cumulative ranking (SUCRA) evaluations by Litmus Rank-O-Gram and radial SUCRA plots [72] to evaluate the rank of superiority of individual regimen. Finally, we used a deviance model to evaluate the fit and influence of treatment effect estimates by comparing deviance from the NMA and the unrelated mean effects inconsistency model, examining residual deviance across study arms, and analyzing leverage versus residual deviance [73].
General declaration
This study complies with the principles outlined in the Declaration of Helsinki.
Results
Eligibility of the studies
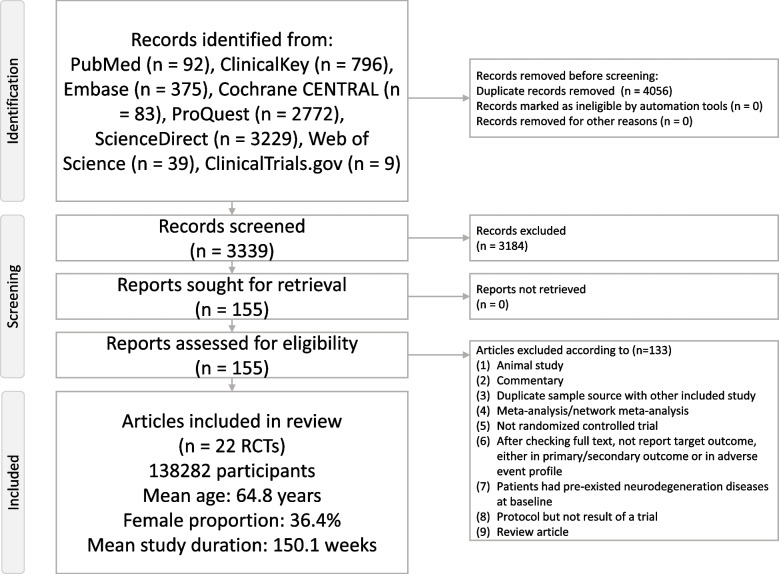
Figure 1 illustrates the flowchart summarizing the literature search and screening process for this NMA. After excluding 133 articles for various reasons (Additional file: Tab. S3) [7–13, 16, 22, 23, 26–28, 31–41, 50, 51, 53, 54, 61, 63, 74–176], a total of 22 RCTs were included in the analysis [19–21, 47–65]. The selected studies involved 138,282 participants (mean age = 64.8 years, range 57.1 to 71.9 years; mean female proportion = 36.4%, range 23.4 to 46.5%) (Additional file: Tab. S4). The average study duration was 150.1 weeks (range 24 to 281 weeks). In total, 17 experimental arms were examined, comprising 1 placebo/control arm and 16 various dosage ranges of different GLP-1 receptor agonists/SGLT2 inhibitors arms. The investigated GLP-1 receptor agonists included liraglutide, albiglutide, dulaglutide, exenatide, semaglutide, and lixisenatide. The investigated SGLT2 inhibitors included canagliflozin, empagliflozin, ertugliflozin, dapagliflozin, and sotagliflozin.
Fig. 1.
PRISMA2020 Flowchart of current network meta-analysis
Primary outcome: overall events of neurodegenerative diseases
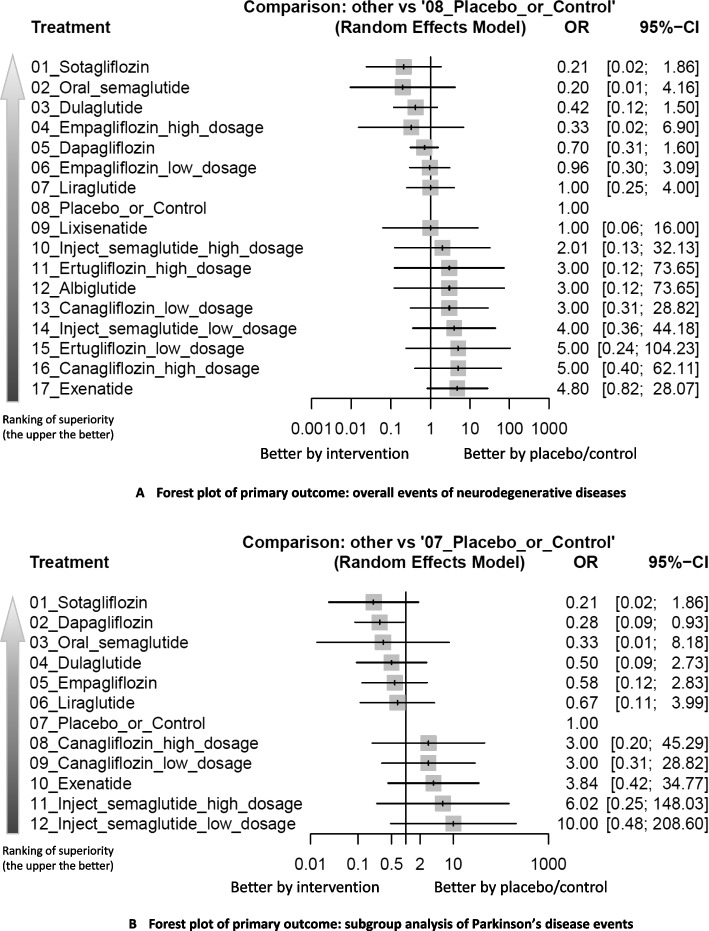
Analysis of overall neurodegenerative disease events revealed no statistically significant differences between any investigated treatments and the control group. However, several interventions showed promising trends: Sotagliflozin demonstrated a trend of favorable profile, OR = 0.21 (95%CIs = 0.02 to 1.86), which had a consistent trend across subgroups although not achieved statistical significance. Oral semaglutide ranked second (OR = 0.20, 95%CIs = 0.01 to 4.16), demonstrating similar magnitude of effect to sotagliflozin. Dulaglutide ranked third (OR = 0.42, 95%CIs = 0.12 to 1.50), showing consistent effects across sensitivity analyses (Figs. 2A, 3A, and Table 1).
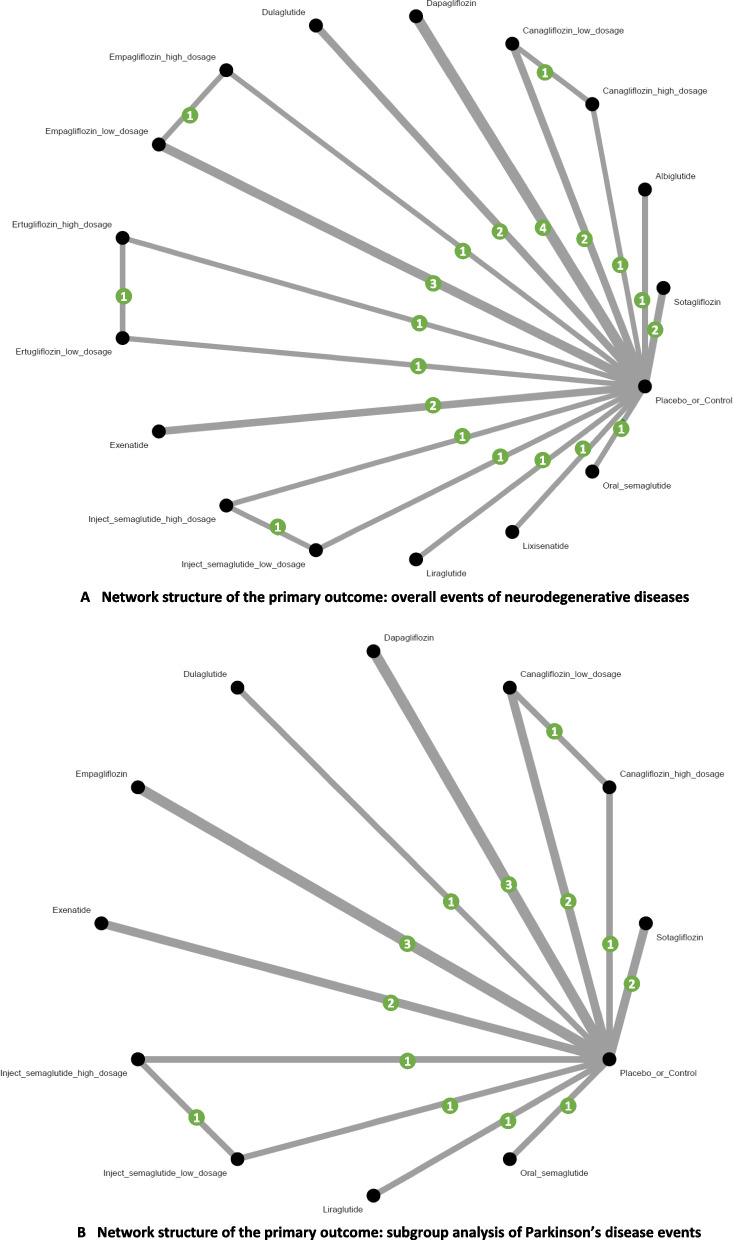
Fig. 2.
A Network structure of the primary outcome: overall events of neurodegenerative diseases. A depicts the structure of the overall network meta-analysis of primary outcome. The lines between nodes represent direct comparisons from various trials, with the numbers over the lines indicating the number of trials providing these comparisons for each specific treatment. The thickness of the lines corresponds to the number of trials linked to the network. B Network structure of the primary outcome: subgroup analysis of Parkinson’s disease events. B depicts the structure of the subgroup analysis focusing on Parkinson’s disease. The lines between nodes represent direct comparisons from various trials, with the numbers over the lines indicating the number of trials providing these comparisons for each specific treatment. The thickness of the lines corresponds to the number of trials linked to the network
Fig. 3.
A Forest plot of primary outcome: overall events of neurodegenerative diseases. When the effect size (expressed as odds ratio) is less than 1, the specified treatment is associated with fewer neurodegenerative disease events compared to placebo/controls. B Forest plot of primary outcome: subgroup analysis of Parkinson’s disease events. When the effect size (expressed as odds ratio) is less than 1, the specified treatment is associated with fewer Parkinson’s disease events compared to placebo/controls. Dosage definition: canagliflozin (low: 100 mg, and high: 300 mg); ertugliflozin (low: 5 mg, and high: 15 mg); injectable semaglutide (low: 0.5 mg, and high: 1.0 mg); empagliflozin (low: 1–10 mg, and high: 25–50 mg). Abbreviations: 95%CIs: 95% confidence intervals; GLP-1 agonist: glucagon-like peptide-1 agonist; NMA: network meta-analysis; OR: odds ratio; RCT: randomized controlled trial; SGLT2 inhibitor: sodium–glucose cotransporter 2 inhibitor
Table 1.
League table of the primary outcome: overall events of neurodegenerative diseases
| Sotagliflozin | 0.21 [0.02; 1.86] | |||||||||||||||
| Sotagliflozin | 0.21 [0.02; 1.86] | |||||||||||||||
| 1.06 [0.03; 44.45] |
Oral_ semaglutide |
0.20 [0.01; 4.16] | ||||||||||||||
| 0.50 [0.04; 6.25] | 0.47 [0.02; 12.78] | Dulaglutide | 0.42 [0.12; 1.50] | |||||||||||||
| 0.65 [0.02; 27.48] | 0.61 [0.01; 45.29] | 1.29 [0.05; 35.13] |
Empagliflozin_ high_dosage |
0.33 [0.01; 8.19] | 0.33 [0.01; 8.15] | |||||||||||
| 0.30 [0.03; 3.10] | 0.29 [0.01; 6.65] | 0.60 [0.13; 2.74] | 0.47 [0.02; 11.01] | Dapagliflozin | 0.70 [0.31; 1.60] | |||||||||||
| 0.22 [0.02; 2.59] | 0.21 [0.01; 5.37] | 0.44 [0.08; 2.45] | 0.34 [0.02; 7.16] | 0.73 [0.17; 3.03] |
Empagliflozin_ low_dosage |
0.96 [0.30; 3.09] | ||||||||||
| 0.21 [0.02; 2.79] | 0.20 [0.01; 5.62] | 0.42 [0.06; 2.76] | 0.33 [0.01; 9.31] | 0.70 [0.14; 3.51] | 0.96 [0.16; 5.88] | Liraglutide | 1.00 [0.25; 4.00] | |||||||||
| 0.21 [0.02; 1.86] | 0.20 [0.01; 4.16] | 0.42 [0.12; 1.50] | 0.33 [0.02; 6.90] | 0.70 [0.31; 1.60] | 0.96 [0.30; 3.09] | 1.00 [0.25; 4.00] |
Placebo_ or_Control |
1.00 [0.06; 15.99] | 0.50 [0.03; 7.97] | 0.33 [0.01; 8.19] | 0.33 [0.01; 8.19] | 0.33 [0.03; 3.21] | 0.25 [0.02; 2.76] | 0.20 [0.01; 4.17] | 0.20 [0.01; 4.17] | 0.21 [0.04; 1.22] |
| 0.21 [0.01; 7.18] | 0.20 [0.00; 12.20] | 0.42 [0.02; 8.89] | 0.33 [0.01; 20.13] | 0.70 [0.04; 12.62] | 0.96 [0.05; 19.47] | 1.00 [0.05; 22.20] | 1.00 [0.06; 15.99] | Lixisenatide | ||||||||
| 0.11 [0.00; 3.58] | 0.10 [0.00; 6.08] | 0.21 [0.01; 4.43] | 0.16 [0.00; 10.04] | 0.35 [0.02; 6.29] | 0.48 [0.02; 9.71] | 0.50 [0.02; 11.07] | 0.50 [0.03; 7.97] | 0.50 [0.01; 25.15] |
Inject_ semaglutide_ high_dosage |
0.50 [0.05; 5.55] | ||||||
| 0.07 [0.00; 3.39] | 0.07 [0.00; 5.49] | 0.14 [0.00; 4.40] | 0.11 [0.00; 9.06] | 0.23 [0.01; 6.36] | 0.32 [0.01; 9.68] | 0.33 [0.01; 10.92] | 0.33 [0.01; 8.19] | 0.33 [0.00; 23.03] | 0.67 [0.01; 46.23] |
Ertugliflozin_ high_dosage |
0.60 [0.08; 4.54] | |||||
| 0.07 [0.00; 3.39] | 0.07 [0.00; 5.49] | 0.14 [0.00; 4.40] | 0.11 [0.00; 9.06] | 0.23 [0.01; 6.36] | 0.32 [0.01; 9.68] | 0.33 [0.01; 10.92] | 0.33 [0.01; 8.19] | 0.33 [0.00; 23.02] | 0.67 [0.01; 46.22] | 1.00 [0.01; 92.45] | Albiglutide | |||||
| 0.07 [0.00; 1.63] | 0.07 [0.00; 2.94] | 0.14 [0.01; 1.89] | 0.11 [0.00; 4.86] | 0.23 [0.02; 2.60] | 0.32 [0.03; 4.10] | 0.33 [0.02; 4.75] | 0.33 [0.03; 3.21] | 0.33 [0.01; 11.96] | 0.67 [0.02; 24.02] | 1.00 [0.02; 50.47] | 1.00 [0.02; 50.47] |
Canagliflozin_ low_dosage |
0.60 [0.08; 4.54] | |||
| 0.05 [0.00; 1.35] | 0.05 [0.00; 2.40] | 0.11 [0.01; 1.59] | 0.08 [0.00; 3.96] | 0.17 [0.01; 2.22] | 0.24 [0.02; 3.47] | 0.25 [0.02; 4.01] | 0.25 [0.02; 2.76] | 0.25 [0.01; 9.80] | 0.50 [0.05; 5.55] | 0.75 [0.01; 41.01] | 0.75 [0.01; 41.02] | 0.75 [0.03; 20.32] |
Inject_ semaglutide_ low_dosage |
|||
| 0.04 [0.00; 1.77] | 0.04 [0.00; 2.93] | 0.08 [0.00; 2.26] | 0.07 [0.00; 4.83] | 0.14 [0.01; 3.25] | 0.19 [0.01; 4.98] | 0.20 [0.01; 5.64] | 0.20 [0.01; 4.17] | 0.20 [0.00; 12.21] | 0.40 [0.01; 24.52] | 0.60 [0.08; 4.54] | 0.60 [0.01; 49.44] | 0.60 [0.01; 26.45] | 0.80 [0.02; 38.41] |
Ertugliflozin_ low_dosage |
||
| 0.04 [0.00; 1.18] | 0.04 [0.00; 2.06] | 0.08 [0.01; 1.41] | 0.07 [0.00; 3.41] | 0.14 [0.01; 1.98] | 0.19 [0.01; 3.09] | 0.20 [0.01; 3.54] | 0.20 [0.02; 2.48] | 0.20 [0.00; 8.46] | 0.40 [0.01; 16.99] | 0.60 [0.01; 35.20] | 0.60 [0.01; 35.20] | 0.60 [0.09; 4.19] | 0.80 [0.02; 25.95] | 1.00 [0.02; 51.66] |
Canagliflozin_ high_dosage |
*0.04 [0.00; 0.73]
0.04 [0.00; 1.40]
*0.09 [0.01; 0.77]
0.07 [0.00; 2.31]
0.15 [0.02; 1.03]
0.20 [0.02; 1.67]
0.21 [0.02; 1.97]
0.21 [0.04; 1.22]
0.21 [0.01; 5.58]
0.42 [0.02; 11.21]
0.63 [0.02; 24.21]
0.63 [0.02; 24.20]
0.62 [0.04; 11.03]
0.83 [0.04; 16.45]
1.04 [0.03; 35.00]
1.04 [0.05; 22.62]
Exenatide
Data present as OR [95%CIs]. Pairwise (upper-right portion) and network (lower-left portion) meta-analysis results are presented as estimate effect sizes for the outcome of overall events of neurodegenerative diseases. Interventions are reported in order of mean ranking of beneficially prophylactic effect on overall events of neurodegenerative diseases, and outcomes are expressed as odds ratio (OR) (95% confidence intervals) (95%CIs). For the pairwise meta-analyses, OR of less than 1 indicate that the treatment specified in the row got more beneficial effect than that specified in the column. For the network meta-analysis (NMA), OR of less than 1 indicate that the treatment specified in the column got more beneficial effect than that specified in the row. Bold results marked with * indicate statistical significance
Network meta-analysis results for all treatment comparisons are presented in Fig. 3A and detailed in Table 1. While these results suggest potential protective effects, the wide confidence intervals and lack of statistical significance highlight the need for larger, targeted studies.
Subgroup analyses of seven categories of neurodegenerative diseases
In our analysis of specific neurodegenerative conditions, dapagliflozin emerged as the only intervention showing significant prophylactic benefits against Parkinson’s disease (OR = 0.28, 95%CIs = 0.09 to 0.93) compared to control. While sotagliflozin demonstrated the most favorable point estimate (OR = 0.21, 95%CIs = 0.02 to 1.86) and ranked first in the network, the wide confidence intervals precluded statistical significance. Dapagliflozin ranked second in the network hierarchy for Parkinson’s disease prevention (Figs. 2B, 3B, and Table 2).
Table 2.
League table of the primary outcome: subgroup analysis of Parkinson’s disease events
| Sotagliflozin | 0.21 [0.02; 1.86] | ||||||||||
| 0.75 [0.06; 8.93] | Dapagliflozin | *0.28 [0.09; 0.93] | |||||||||
| 0.64 [0.01; 30.49] | 0.85 [0.03; 25.84] | Oral_semaglutide | 0.33 [0.01; 8.18] | ||||||||
| 0.42 [0.03; 6.68] | 0.57 [0.07; 4.49] | 0.67 [0.02; 24.95] | Dulaglutide | 0.50 [0.09; 2.73] | |||||||
| 0.36 [0.02; 5.33] | 0.48 [0.07; 3.50] | 0.57 [0.02; 20.24] | 0.86 [0.08; 8.70] | Empagliflozin | 0.58 [0.12; 2.83] | ||||||
| 0.32 [0.02; 5.31 | 0.42 [0.05; 3.64] | 0.50 [0.01; 19.55] | 0.75 [0.06; 8.84] | 0.88 [0.08; 9.52] | Liraglutide | 0.67 [0.11; 3.99] | |||||
| 0.21 [0.02; 1.86] | *0.28 [0.09; 0.93] | 0.33 [0.01; 8.18] | 0.50 [0.09; 2.73] | 0.58 [0.12; 2.83] | 0.67 [0.11; 3.99] | Placebo_or_Control | 0.33 [0.01; 8.19] | 0.33 [0.03; 3.21] | 0.26 [0.03; 2.36] | 0.17 [0.01; 4.08] | 0.10 [0.00; 2.08] |
| 0.07 [0.00; 2.29] | 0.09 [0.00; 1.83] | 0.11 [0.00; 7.38] | 0.17 [0.01; 4.10] | 0.19 [0.01; 4.50] | 0.22 [0.01; 5.74] | 0.33 [0.02; 5.03] |
Canagliflozin_ high_dosage |
1.00 [0.10; 9.64] | |||
| 0.07 [0.00; 1.63] | 0.09 [0.01; 1.22] | 0.11 [0.00; 5.61] | 0.17 [0.01; 2.83] | 0.19 [0.01; 3.08] | 0.22 [0.01; 3.99] | 0.33 [0.03; 3.21] | 1.00 [0.11; 8.97] |
Canagliflozin_ low_dosage |
|||
| 0.06 [0.00; 1.22] | *0.07 [0.01; 0.90] | 0.09 [0.00; 4.23] | 0.13 [0.01; 2.11] | 0.15 [0.01; 2.29] | 0.17 [0.01; 2.98] | 0.26 [0.03; 2.36] | 0.78 [0.02; 25.82] | 0.78 [0.03; 18.41] | Exenatide | ||
| 0.04 [0.00; 1.69] | 0.05 [0.00; 1.43] | 0.06 [0.00; 5.12] | 0.08 [0.00; 3.11] | 0.10 [0.00; 3.44] | 0.11 [0.00; 4.34] | 0.17 [0.01; 4.08] | 0.50 [0.01; 33.13] | 0.50 [0.01; 25.10] | 0.64 [0.01; 31.06] |
Inject_semaglutide_ high_dosage |
0.60 [0.08; 4.57] |
| *0.02 [0.00; 0.89] | *0.03 [0.00; 0.74] | 0.03 [0.00; 2.75] | 0.05 [0.00; 1.62] | 0.06 [0.00; 1.79] | 0.07 [0.00; 2.27] | 0.10 [0.00; 2.08] | 0.30 [0.01; 17.63] | 0.30 [0.01; 13.23] | 0.38 [0.01; 16.36] | 0.60 [0.08; 4.57] |
Inject_semaglutide_ low_dosage |
Data present as OR [95%CIs]. Pairwise (upper-right portion) and network (lower-left portion) meta-analysis results are presented as estimate effect sizes for the outcome of events of Parkinson’s disease. Interventions are reported in order of mean ranking of beneficially prophylactic effect on events of Parkinson’s disease, and outcomes are expressed as odds ratio (OR) (95% confidence intervals) (95%CIs). For the pairwise meta-analyses, OR of less than 1 indicate that the treatment specified in the row got more beneficial effect than that specified in the column. For the network meta-analysis (NMA), OR of less than 1 indicate that the treatment specified in the column got more beneficial effect than that specified in the row. Bold results marked with * indicate statistical significance
Dosage definition: Canagliflozin (Low: 100mg, and High: 300mg); Ertugliflozin (Low: 5mg, and High: 15mg); Injectable semaglutide (Low: 0.5mg, and High: 1.0mg); Empagliflozin (Low: 1-10mg, and High: 25-50mg)
Abbreviation: 95%CIs 95% confidence intervals, GLP-1 agonist: glucagon-like peptide-1 agonist, NMA network meta-analysis, OR odds ratio, RCT randomized controlled trial, SGLT2 inhibitor sodium–glucose cotransporter 2 inhibitor
For Alzheimer’s disease, our network meta-analysis revealed no significant preventive effects across all investigated interventions (Additional file: Fig. S1A, Additional file: Fig. S2A, and Additional file: Tab. S5A). This pattern of non-significant findings extended to several other neurodegenerative conditions. Specifically, we found no significant prophylactic benefits for Lewy body dementia (Additional file: Fig. S1B, Additional file: Fig. S2B, and Additional file: Tab. S5B), multiple sclerosis (Additional file: Fig. S1C, Additional file: Fig. S2C, and Additional file: Tab. S5C), or amyotrophic lateral sclerosis (Additional file: Fig. S1D, Additional file: Fig. S2D, and Additional file: Tab. S5D).
The evidence base for frontotemporal dementia was limited to a single RCT, and no eligible trials reported outcomes for Huntington’s disease, preventing meaningful network meta-analysis for these conditions. This paucity of data highlights an important gap in current research regarding the preventive potential of these medications for less common neurodegenerative disorders.
Safety profile: drop-out rate
Only the canagliflozin was associated with significantly less drop-out rates than the control group did (high dosage canagliflozin: OR = 0.57, 95%CIs = 0.40 to 0.83; low dosage canagliflozin OR = 0.65, 95%CIs = 0.47 to 0.91). Among these interventions, high dosage canagliflozin ranked the best (Additional file: Fig. S1E, Additional file: Fig. S2E, and Additional file: Tab. S5E).
Sensitivity analysis with Bayesian-based NMA
The relative ranking of interventions remained stable across different analytical approaches (Additional file: Fig. S3, and Additional file: Fig. S4). Generally, the main results of primary outcome did not differ between frequentist-based NMA and Bayesian-based NMA (Additional file: Fig. S5). The Bayesian-based SUCRA ranking list had been depicted in Additional file: Tab. S6 and Additional file: Fig. S6A-S6B. The deviation-model assessment did not demonstrate significant deviation among the current NMA (Additional file: Fig. S7A-S7C).
Risk of bias and inconsistency
We identified that 82.5% (127/154 items), 14.3% (22/154 items), and 3.2% (5/154 items) of the included studies had low, unclear, and high risks of bias, respectively (Additional file: Fig. S8). The inconsistency test, evaluating the assumption of consistency, showed no significant inconsistencies in the present NMA (Additional file: Tab. S7A-S7G).
Discussion
This comprehensive network meta-analysis revealed a novel and specific prophylactic benefit of dapagliflozin against Parkinson’s disease, marking a potentially important advancement in preventive neurology. While multiple GLP-1 receptor agonists and SGLT2 inhibitors were evaluated across various neurodegenerative conditions, only dapagliflozin demonstrated significant preventive effects (OR = 0.28, 95%CIs = 0.09 to 0.93). This specificity is particularly noteworthy, as no significant prophylactic benefits were observed for other major neurodegenerative conditions, including Alzheimer’s disease, Lewy body dementia, multiple sclerosis, and amyotrophic lateral sclerosis. The evidence base for less common conditions, specifically frontotemporal dementia and Huntington’s disease, proved insufficient for definitive conclusions, highlighting critical gaps in current research. These findings suggest that the neuroprotective mechanisms of these medications may be more selective than previously hypothesized, with particular relevance to Parkinson’s disease pathophysiology.
This study represents the first network meta-analysis to systematically evaluate the prophylactic potential of GLP-1 receptor agonists and SGLT2 inhibitors across neurodegenerative conditions. While previous research has primarily investigated the therapeutic effects of these medications in established neurodegenerative diseases [13, 22, 23], our analysis specifically addresses their preventive capabilities. This distinction is crucial, as the irreversible nature of neurodegenerative processes makes prevention potentially more impactful than treatment from a public health perspective [24]. Our network meta-analytic approach offers several advantages over traditional pair-wise meta-analyses, enabling direct comparisons between individual medications and doses, thus providing more nuanced evidence of their relative prophylactic efficacy [29]. This methodological strength allows us to identify specific agents, such as dapagliflozin, that may offer particular promise for preventive interventions, while also highlighting areas where current evidence remains insufficient.
One key finding of this NMA was that only the dapagliflozin, a highly selective and reversible SGLT2 inhibitor, was associated with significantly less events of Parkinson’s disease than the control group did. As addressed in the method section, the current NMA did not include participants with pre-existed neurodegenerative diseases, including Parkinson’s disease. Further, among the included RCTs, none of them specifically recruit subjects with pre-existed Parkinson’s disease. Therefore, the findings of our NMA might suggest a potential of protective benefit of dapagliflozin to patients who had indications for such medications but without current Parkinson’s disease. Dapagliflozin has been found to exert potential neuroprotective effects against neurodegenerative dysfunctions via ROS-dependent AKT/GSK-3β/NF-κB and DJ-1/Nrf2 pathways in the rotenone-induced Parkinson’s disease rat model [177]. Further, the prescription of dapagliflozin could help in the attenuation of motor dysfunction in Parkinson’s disease animal model [177]. In addition, dapagliflozin could also reduce the histopathologic alterations and α-synuclein expression and increase the tyrosine hydroxylase and dopamine levels [177], which physiopathology had been found to be one of the etiology of Parkinson’s disease [178, 179]. Another potential mechanism which could involve dapagliflozin’s neuroprotective properties relied on its anti-inflammatory property. Previous studies have suggested that Parkinson’s disease might be associated with interleukin-1 related over-oxidative environment [180]. Elevated cytokines, such as interleukin-1 beta (IL-1B) [180], in the brain can alter neural function and lead to neural death. Dapagliflozin has been shown to reduce systemic inflammation, including plasma IL-1B levels, in patients treated with dapagliflozin for 12 months [181]. Finally, different from the other SGLT2 inhibitors, the dapagliflozin exhibited its properties of highly selection to SGLT2 and reversibility [182]. In contrary, the other SGLT2 inhibitors, which also inhibit the SGLT1, might interfere with the neuroprotective effects of SGLT1 in the central nervous system [183]. This pharmacodynamical theory could be supported by the insignificant findings of those SGLT2 inhibitors with SGLT1 affinity. Finally, although no formal reports have directly linked this reduction in inflammation to the prevention of Parkinson’s disease, it may serve as a basis for hypothesizing that dapagliflozin could help prevent this condition. In addition to the above mechanism, the better comparative efficacy on glycated hemoglobin (HbA1c) by dapagliflozin use than other SGLT2 inhibitors might also be another explanation of the preferably protective effects by dapagliflozin on Parkinson’s disease [184]. Since the existence of diabetes mellitus would increase risk of Parkinson’s disease to an extent of 23–85% [185], dapagliflozin would serve as one of the choices of anti-diabetic medications who treating diabetic subjects with risk of Parkinson’s disease.
Regarding other neurodegenerative diseases, such as Alzheimer’s disease, dementia of Lewy body, multiple sclerosis, amyotrophic lateral sclerosis, frontotemporal dementia, and Huntington’s disease, this NMA did not find any significant benefits from the investigated medications. This may be due to the fact that the most neurodegenerative diseases often involve more chronic changes [186], and the duration of the RCTs included in the analysis may not have been long enough to detect meaningful differences.
Strengths and limitations
This network meta-analysis offers several methodological strengths that enhance the reliability and clinical utility of our findings. The NMA design enables direct comparisons between different GLP-1 receptor agonists and SGLT2 inhibitors, providing more comprehensive evidence than traditional pairwise meta-analyses. Our rigorous methodology included exclusive focus on peer-reviewed randomized controlled trials, ensuring high-quality evidence while minimizing potential bias. By specifically excluding participants with pre-existing neurodegenerative conditions, we were able to isolate true prophylactic effects. Furthermore, our detailed subgroup analyses across individual neurodegenerative conditions offer clinicians granular evidence to inform preventive strategies for specific patient populations. Finally, to enhance the reliability, we also arranged sensitivity analysis with Bayesian-based NMA to re-affirm the main result of the current study, which sensitivity analysis revealed similar results.
Despite these strengths, several important limitations warrant consideration. The primary limitation relates to study duration; although the included trials averaged 150.1 weeks of follow-up, this timeframe may be insufficient to fully capture the development of neurodegenerative conditions, which typically evolve over decades [181]. Our stringent focus on RCTs, while ensuring methodological rigor, potentially excluded valuable observational data from long-term cohort studies. Additionally, the variation in diagnostic approaches across multi-country trials presents a notable limitation. The lack of standardized neuropsychiatric assessment and structured diagnostic interviews may have introduced heterogeneity in case identification, potentially affecting the precision of our effect estimates. Besides, since this is a statistical study, we could not know the actual molecular and physiological mechanism between the neuroprotection and dapagliflozin prescription. Finally, since the original data did not provide further information regarding classified outcomes according to achieving glycemic control (i.e. HbA1c < 7 or HbA1c > 7) or gender-related difference, we could not do further sensitivity analysis based on these issues. These limitations suggest the need for longer-term, standardized studies specifically designed to assess preventive effects in neurodegenerative conditions based on levels of glycemic control or gender-specific design.
Conclusions
This comprehensive network meta-analysis reveals a potentially important breakthrough in neurodegenerative disease prevention, demonstrating that dapagliflozin, an SGLT2 inhibitor, significantly reduces the risk of Parkinson’s disease development (OR = 0.28, 95% CIs = 0.09 to 0.93). This finding is particularly noteworthy given the absence of significant prophylactic effects for other investigated agents across multiple neurodegenerative conditions, including Alzheimer’s disease, Lewy body dementia, multiple sclerosis, and amyotrophic lateral sclerosis.
The specificity of dapagliflozin’s preventive effect suggests distinct neuroprotective mechanisms that warrant further investigation. These findings have important implications for clinical practice and future research directions, particularly in Parkinson’s disease prevention. Future studies should focus on elucidating the underlying mechanisms of dapagliflozin’s neuroprotective effects, determining optimal preventive strategies, and identifying patient populations most likely to benefit from prophylactic intervention.
While longer-term studies are needed to fully understand the preventive potential of these medications, our findings provide valuable evidence to guide both clinical decision-making and the design of future preventive trials in neurodegenerative diseases. The results particularly highlight the need for targeted investigation of dapagliflozin’s role in Parkinson’s disease prevention, potentially opening a new avenue in preventive neurology.
Supplementary Information
Supplementary Material 1: Fig. S1. (A) Network structure of primary outcome: subgroup analysis of Alzheimer’s disease events; (B) Network structure of primary outcome: subgroup analysis of dementia of Lewy body events; (C) Network structure of primary outcome: subgroup analysis of multiple sclerosis events; (D) Network structure of primary outcome: subgroup analysis of amyotrophic lateral sclerosis events; (E) Network structure of safety profile: drop-out rate. Fig. S2. (A) Forest plot of primary outcome: subgroup analysis of Alzheimer’s disease events; (B) Forest plot of primary outcome: subgroup analysis of dementia of Lewy body events; (C) Forest plot of primary outcome: subgroup analysis of multiple sclerosis events; (D) Forest plot of primary outcome: subgroup analysis of amyotrophic lateral sclerosis events; (E) Forest plot of safety profile: drop-out rate. Fig. S3. Summary plot of ranking of primary outcome (overall events of neurodegenerative diseases). Fig. S4. Individual study result of primary outcome: overall events of neurodegenerative diseases. Fig. S5. Bayesian-based forest plot of primary outcome: overall events of neurodegenerative diseases. Fig. S6. (A) Bayesian-based Litmus Rank-O-Gram rank plot of primary outcome: overall events of neurodegenerative diseases; (B) Bayesian-based radial surface under the cumulative ranking of primary outcome: overall events of neurodegenerative diseases. Fig. S7. (A) Bayesian-based residual deviance NMA/UME model of primary outcome: overall events of neurodegenerative diseases; (B) Bayesian-based per-arm residual deviance of primary outcome: overall events of neurodegenerative diseases; (C) Bayesian-based leverage plot of primary outcome: overall events of neurodegenerative diseases. Fig. S8. Detailed risk of bias in each study. Tab. S1. (A) PRISMA 2020 checklist of the current network meta-analysis; (B) PRISMA 2020 abstract checklist of the current network meta-analysis. Tab. S2. Keyword used in each database and search results. Tab. S3. Excluded studies and reason. Tab. S4. Characteristics of the included studies. Tab. S5. (A) League table of primary outcome: subgroup of Alzheimer’s disease events; (B) League table of primary outcome: subgroup of dementia of Lewy body events; (C) League table of primary outcome: subgroup of multiple sclerosis events; (D) League table of primary outcome: subgroup of amyotrophic lateral sclerosis events; (E) League table of safety profile: drop-out rate. Tab. S6. Surface under the cumulative ranking of primary outcome: overall events of neurodegenerative diseases. Tab. S7. (A) Inconsistency within the primary outcome: overall events of neurodegenerative diseases; (B) Inconsistency within the primary outcome: subgroup of Parkinson’s disease events; (C) Inconsistency within the primary outcome: subgroup of Alzheimer’s disease events; (D) Inconsistency within the primary outcome: subgroup of dementia of Lewy body events; (E) Inconsistency within the primary outcome: subgroup of multiple sclerosis events; (F) Inconsistency within the primary outcome: subgroup of amyotrophic lateral sclerosis events; (G) Inconsistency within the safety profile: drop-out rate. Legends to Combined Additional files [7–13, 16, 19–23, 26–28, 31–41, 47–65, 74–176, 187].
Acknowledgements
This paper presents independent research. The views expressed in this publication are those of the authors and not necessarily those of the acknowledged institutions.
Abbreviations
- 95%CI
95% confidence interval
- GLP-1
Glucagon-like peptide-1
- HbA1c
Glycated hemoglobin
- NMA
Network meta-analysis
- OR
Odds ratio
- PICOS
Population, intervention, comparison, outcome, and study design
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
Randomized controlled trial
- SGLT2
Sodium–glucose cotransporter 2
- SUCRA
Surface under the cumulative ranking
Authors’ contributions
All authors read and approved the final manuscript. PT T, BY Z, and CW H, who contributed equally as first authors, took the whole responsibility of literature search, data extraction, data analysis, and manuscript drafting. CM H, AF C, B S, YW C, TY C, WT L, JJ C, and KP S contributed to study design, concept formation, and manuscript revision. PT T, YL S, and CS L, who contributed equally as corresponding authors, took the whole responsibility of collection of information from the other authors, manuscript major revision, and manuscript submission.
Authors’ Twitter handles
@tao_tseng (Ping-Tao Tseng).
Funding
None.
Data availability
All the data of the current study were available upon reasonable request to the corresponding authors.
Declarations
Ethics approval and consent to participate
The authors report no financial interests or potential conflicts of interest. The Institutional Review Board of the Tri-Service General Hospital has confirmed that no ethical approval is required (TSGHIRB: B-109–29). The current study did not directly involve individual participant so that we did not have the chance to approach individual participant or explore individual participant’s information. Therefore, it would be impossible to obtain consent to participate in the current study.
The authors of this work were supported by the following grants: Brendon Stubbs is supported by the NIHR. Brendon Stubbs is part funded by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust. Brendon Stubbs is also supported by the Maudsley Charity, King’s College London.
Consent for publication
The current study did not directly involve individual participant so that we did not have the chance to approach individual participant or explore individual participant’s information. Therefore, it would be impossible to obtain consent to publish in the current study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ping-Tao Tseng, Bing-Yan Zeng and Chih-Wei Hsu contributed equally as first authors
Ping-Tao Tseng, Yow-Ling Shiue and Chih-Sung Liang contributed equally as corresponding authors.
Contributor Information
Ping-Tao Tseng, Email: ducktseng@gmail.com.
Yow-Ling Shiue, Email: shirley@imst.nsysu.edu.tw.
Chih-Sung Liang, Email: lcsyfw@gmail.com.
References
- 1.Avogaro A, de Kreutzenberg SV, Morieri ML, Fadini GP, Del Prato S. Glucose-lowering drugs with cardiovascular benefits as modifiers of critical elements of the human life history. Lancet Diabetes Endocrinol. 2022;10(12):882–9. [DOI] [PubMed] [Google Scholar]
- 2.Erbil D, Eren CY, Demirel C, Kucuker MU, Solaroglu I, Eser HY. GLP-1’s role in neuroprotection: a systematic review. Brain Inj. 2019;33(6):734–819. [DOI] [PubMed] [Google Scholar]
- 3.Plosker GL. Canagliflozin: a review of its use in patients with type 2 diabetes mellitus. Drugs. 2014;74(7):807–24. [DOI] [PubMed] [Google Scholar]
- 4.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136(17):1643–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes Diabetes Work G. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5S):S1–127. [DOI] [PubMed] [Google Scholar]
- 6.Pan HC, Chen JY, Chen HY, Yeh FY, Sun CY, Huang TT, et al. GLP-1 receptor agonists’ impact on cardio-renal outcomes and mortality in T2D with acute kidney disease. Nat Commun. 2024;15(1):5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fessel J. All GLP-1 agonists should, theoretically, cure Alzheimer’s dementia but dulaglutide might be more effective than the others. J Clin Med. 2024;13(13):3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holubova M, Hruba L, Popelova A, Bencze M, Prazienkova V, Gengler S, et al. Liraglutide and a lipidized analog of prolactin-releasing peptide show neuroprotective effects in a mouse model of beta-amyloid pathology. Neuropharmacology. 2019;144:377–87. [DOI] [PubMed] [Google Scholar]
- 9.McClean PL, Holscher C. Lixisenatide, a drug developed to treat type 2 diabetes, shows neuroprotective effects in a mouse model of Alzheimer’s disease. Neuropharmacology. 2014;86:241–58. [DOI] [PubMed] [Google Scholar]
- 10.McGarry A, Rosanbalm S, Leinonen M, Olanow CW, To D, Bell A, et al. Safety, tolerability, and efficacy of NLY01 in early untreated Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2024;23(1):37–45. [DOI] [PubMed] [Google Scholar]
- 11.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Kahan J, Ell P, et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis. 2014;4(3):337–44. [DOI] [PubMed] [Google Scholar]
- 12.Gejl M, Gjedde A, Egefjord L, Moller A, Hansen SB, Vang K, et al. Alzheimer’s disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Front Aging Neurosci. 2016;8:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulvaney CA, Duarte GS, Handley J, Evans DJ, Menon S, Wyse R, et al. GLP-1 receptor agonists for Parkinson’s disease. Cochrane Database Syst Rev. 2020;7(7):CD012990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baggio LL, Drucker DJ. Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight. J Clin Invest. 2014;124(10):4223–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou C, You J, Guan X, Guo T, Wu J, Wu H, et al. Microstructural alterations of the hypothalamus in Parkinson’s disease and probable REM sleep behavior disorder. Neurobiol Dis. 2024;194:106472. [DOI] [PubMed] [Google Scholar]
- 16.Vijiaratnam N, Girges C, Auld G, Chau M, Maclagan K, King A, et al. Exenatide once weekly over 2 years as a potential disease-modifying treatment for Parkinson’s disease: protocol for a multicentre, randomised, double blind, parallel group, placebo controlled, phase 3 trial: the “Exenatide-PD3” study. BMJ Open. 2021;11(5):e047993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albuquerque MB, Nunes L, Oliveira Maldonado JV, Melo Ferreira DG, Margato MM, Rabelo LV, et al. GLP-1 receptor agonists for Parkinson’s disease: an updated meta-analysis. Parkinsonism Relat Disord. 2025;130:107220. [DOI] [PubMed] [Google Scholar]
- 18.Abdollahi E, Keyhanfar F, Delbandi AA, Falak R, Hajimiresmaiel SJ, Shafiei M. Dapagliflozin exerts anti-inflammatory effects via inhibition of LPS-induced TLR-4 overexpression and NF-kappaB activation in human endothelial cells and differentiated macrophages. Eur J Pharmacol. 2022;918:174715. [DOI] [PubMed] [Google Scholar]
- 19.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57. [DOI] [PubMed] [Google Scholar]
- 20.Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–98. [DOI] [PubMed] [Google Scholar]
- 21.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30. [DOI] [PubMed] [Google Scholar]
- 22.Wang SY, Wu SL, Chen TC, Chuang CS. Antidiabetic agents for treatment of Parkinson’s disease: a meta-analysis. Int J Environ Res Public Health. 2020;17(13):4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao B, Rosenblat JD, Brietzke E, Park C, Lee Y, Musial N, et al. Comparative efficacy and acceptability of antidiabetic agents for Alzheimer’s disease and mild cognitive impairment: a systematic review and network meta-analysis. Diabetes Obes Metab. 2018;20(10):2467–71. [DOI] [PubMed] [Google Scholar]
- 24.Borysiewicz L. Prevention is better than cure. Clin Med (Lond). 2009;9(6):572–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jellinger KA. Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med. 2010;14(3):457–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang H, Shao H, Shaaban CE, Yang K, Brown J, Anton S, et al. Newer glucose-lowering drugs and risk of dementia: a systematic review and meta-analysis of observational studies. J Am Geriatr Soc. 2023;71(7):2096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuate Defo A, Bakula V, Pisaturo A, Labos C, Wing SS, Daskalopoulou SS. Diabetes, antidiabetic medications and risk of dementia: a systematic umbrella review and meta-analysis. Diabetes Obes Metab. 2024;26(2):441–62. [DOI] [PubMed] [Google Scholar]
- 28.Tang H, Lu Y, Okun MS, Donahoo WT, Ramirez-Zamora A, Wang F, et al. Meta-analysis of association between newer glucose-lowering drugs and risk of Parkinson’s disease. Mov Disord Clin Pract. 2023;10(11):1659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JP, Welton NJ. Network meta-analysis: a norm for comparative effectiveness? Lancet. 2015;386(9994):628–30. [DOI] [PubMed] [Google Scholar]
- 30.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. [DOI] [PubMed] [Google Scholar]
- 31.Jaiswal V, Mashkoor Y, Raj N, Rajak K, Jaiswal A, Fonarow GC. Association between SGLT2 inhibitors and risk of dementia and Parkinson's disease: a meta-analysis of 12 randomized controlled trials. Am J Med. 2024;137(11):1136–41. [DOI] [PubMed]
- 32.Banerjee M, Pal R, Maisnam I, Mukhopadhyay S. GLP-1 receptor agonists, SGLT2 inhibitors and noncardiovascular mortality in type 2 diabetes: Insights from a meta-analysis. Diabetes Metab Syndr. 2024;18(1):102943. [DOI] [PubMed] [Google Scholar]
- 33.Maski K, Trotti LM, Kotagal S, Robert Auger R, Swick TJ, Rowley JA, et al. Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2021;17(9):1895–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aviles-Olmos I, Limousin P, Lees A, Foltynie T. Parkinson’s disease, insulin resistance and novel agents of neuroprotection. Brain. 2013;136(Pt 2):374–84. [DOI] [PubMed] [Google Scholar]
- 35.Ciocca M, Pizzamiglio C. Clinical benefits of therapeutic interventions targeting mitochondria in Parkinson’s disease patients. CNS Neurol Disord Drug Targets. 2024;23(5):554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colin IM, Szczepanski LW, Gerard AC, Elosegi JA. Emerging evidence for the use of antidiabetic drugs, glucagon-like peptide 1 receptor agonists, for the treatment of Alzheimer’s disease. touchREV Endocrinol. 2023;19(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eberhardt O, Topka H. Neurological outcomes of antidiabetic therapy: what the neurologist should know. Clin Neurol Neurosurg. 2017;158:60–6. [DOI] [PubMed] [Google Scholar]
- 38.Glotfelty EJ, Olson L, Karlsson TE, Li Y, Greig NH. Glucagon-like peptide-1 (GLP-1)-based receptor agonists as a treatment for Parkinson’s disease. Expert Opin Investig Drugs. 2020;29(6):595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holscher C. Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection. CNS Drugs. 2012;26(10):871–82. [DOI] [PubMed] [Google Scholar]
- 40.Liang Y, Dore V, Rowe CC, Krishnadas N. Clinical evidence for GLP-1 receptor agonists in Alzheimer’s disease: a systematic review. J Alzheimers Dis Rep. 2024;8(1):777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tharmaraja T, Ho JSY, Sia CH, Lim NA, Chong YF, Lim AYL, et al. Sodium-glucose cotransporter 2 inhibitors and neurological disorders: a scoping review. Ther Adv Chronic Dis. 2022;13:20406223221086996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips R, Hazell L, Sauzet O, Cornelius V. Analysis and reporting of adverse events in randomised controlled trials: a review. BMJ Open. 2019;9(2): e024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.0.2. The Cochrane Collaboration; 2009.
- 44.Suescun J, Chandra S, Schiess MC. Chapter 13 - the role of neuroinflammation in neurodegenerative disorders. In: Actor JK, Smith KC, editors. Translational inflammation: a volume in perspectives in translational cell biology. edn. Houston: Academic Press; 2019. p. 241–67.
- 45.Koenig AM, Nobuhara CK, Williams VJ, Arnold SE. Biomarkers in Alzheimer’s, frontotemporal, lewy body, and vascular dementias. Focus (Am Psychiatr Publ). 2018;16(2):164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Chapter 11: undertaking network meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions. London: Cochrane; 2018. p. 7.
- 47.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61. [DOI] [PubMed] [Google Scholar]
- 48.Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–39. [DOI] [PubMed] [Google Scholar]
- 49.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–28. [DOI] [PubMed] [Google Scholar]
- 50.Blonde L, Jendle J, Gross J, Woo V, Jiang H, Fahrbach JL, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385(9982):2057–66. [DOI] [PubMed] [Google Scholar]
- 51.Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–35. [DOI] [PubMed] [Google Scholar]
- 52.Gallwitz B, Bohmer M, Segiet T, Molle A, Milek K, Becker B, et al. Exenatide twice daily versus premixed insulin aspart 70/30 in metformin-treated patients with type 2 diabetes: a randomized 26-week study on glycemic control and hypoglycemia. Diabetes Care. 2011;34(3):604–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46. [DOI] [PubMed] [Google Scholar]
- 54.Hernandez AF, Green JB, Janmohamed S, D’Agostino RB Sr, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29. [DOI] [PubMed] [Google Scholar]
- 55.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–51. [DOI] [PubMed] [Google Scholar]
- 57.Ji L, Lu Y, Li Q, Fu L, Luo Y, Lei T, et al. Efficacy and safety of empagliflozin in combination with insulin in Chinese patients with type 2 diabetes and insufficient glycaemic control: a phase III, randomized, double-blind, placebo-controlled, parallel study. Diabetes Obes Metab. 2023;25(7):1839–48. [DOI] [PubMed] [Google Scholar]
- 58.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44. [DOI] [PubMed] [Google Scholar]
- 59.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. [DOI] [PubMed] [Google Scholar]
- 61.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. [DOI] [PubMed] [Google Scholar]
- 62.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306. [DOI] [PubMed] [Google Scholar]
- 63.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57. [DOI] [PubMed] [Google Scholar]
- 64.The E-KCG, Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. [DOI] [PubMed] [Google Scholar]
- 66.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. [DOI] [PubMed] [Google Scholar]
- 67.Owen RK, Bradbury N, Xin Y, Cooper N, Sutton A. MetaInsight: an interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res Synth Methods. 2019;10(4):569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng J, Pullenayegum E, Marshall JK, Iorio A, Thabane L. Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: a simulation study. BMJ Open. 2016;6(8): e010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brockhaus AC, Bender R, Skipka G. The Peto odds ratio viewed as a new effect measure. Stat Med. 2014;33(28):4861–74. [DOI] [PubMed] [Google Scholar]
- 70.Converting among effect sizes. https://www.meta-analysis.com/downloads/Meta-analysis%20Converting%20among%20effect%20sizes.pdf.
- 71.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7–8):932–44. [DOI] [PubMed] [Google Scholar]
- 72.Nevill CR, Cooper NJ, Sutton AJ. A multifaceted graphical display, including treatment ranking, was developed to aid interpretation of network meta-analysis. J Clin Epidemiol. 2023;157:83–91. [DOI] [PubMed] [Google Scholar]
- 73.Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Chapter 3: Model fit, model comparison and outlier detection. In: Scott M, Barnett V, editors. Network meta-anlaysis for decision-making. edn. Chichester: John Wiley & Sons Ltd; 2002. p. 59–92.
- 74.Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, et al. fficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258–66. [DOI] [PubMed] [Google Scholar]
- 75.Ahmed S, El-Sayed MM, Kandeil MA, Khalaf MM. Empagliflozin attenuates neurodegeneration through antioxidant, anti-inflammatory, and modulation of alpha-synuclein and Parkin levels in rotenone-induced Parkinson’s disease in rats. Saudi Pharm J. 2022;30(6):863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahren B, Masmiquel L, Kumar H, Sargin M, Karsbol JD, Jacobsen SH, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–54. [DOI] [PubMed] [Google Scholar]
- 77.An FM, Chen S, Xu Z, Yin L, Wang Y, Liu AR, et al. Glucagon-like peptide-1 regulates mitochondrial biogenesis and tau phosphorylation against advanced glycation end product-induced neuronal insult: studies in vivo and in vitro. Neuroscience. 2015;300:75–84. [DOI] [PubMed] [Google Scholar]
- 78.Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724–32. [DOI] [PubMed] [Google Scholar]
- 79.Athauda D, Gulyani S, Karnati HK, Li Y, Tweedie D, Mustapic M, et al. Utility of neuronal-derived exosomes to examine molecular mechanisms that affect motor function in patients with Parkinson disease: a secondary analysis of the exenatide-PD trial. JAMA Neurol. 2019;76(4):420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Athauda D, Maclagan K, Budnik N, Zampedri L, Hibbert S, Skene SS, et al. What effects might exenatide have on non-motor symptoms in Parkinson’s disease: a post hoc analysis. J Parkinsons Dis. 2018;8(2):247–58. [DOI] [PubMed] [Google Scholar]
- 81.Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10103):1664–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Athauda D, Wyse R, Brundin P, Foltynie T. Is exenatide a treatment for Parkinson’s disease? J Parkinsons Dis. 2017;7(3):451–8. [DOI] [PubMed] [Google Scholar]
- 83.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123(6):2730–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Badawi GA, Abd El Fattah MA, Zaki HF, El Sayed MI. Sitagliptin and liraglutide modulate L-dopa effect and attenuate dyskinetic movements in rotenone-lesioned rats. Neurotox Res. 2019;35(3):635–53. [DOI] [PubMed] [Google Scholar]
- 85.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9733):2223–33. [DOI] [PubMed] [Google Scholar]
- 86.Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369–84. [DOI] [PubMed] [Google Scholar]
- 87.Buse JB, Garg SK, Rosenstock J, Bailey TS, Banks P, Bode BW, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 study. Diabetes Care. 2018;41(9):1970–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39–47. [DOI] [PubMed] [Google Scholar]
- 89.Cai HY, Holscher C, Yue XH, Zhang SX, Wang XH, Qiao F, et al. Lixisenatide rescues spatial memory and synaptic plasticity from amyloid beta protein-induced impairments in rats. Neuroscience. 2014;277:6–13. [DOI] [PubMed] [Google Scholar]
- 90.Cefalu WT, Leiter LA, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin’s effects on glycemia and cardiovascular risk factors in high-risk patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. Diabetes Care. 2015;38(7):1218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Charbonnel B, Steinberg H, Eymard E, Xu L, Thakkar P, Prabhu V, et al. Efficacy and safety over 26 weeks of an oral treatment strategy including sitagliptin compared with an injectable treatment strategy with liraglutide in patients with type 2 diabetes mellitus inadequately controlled on metformin: a randomised clinical trial. Diabetologia. 2013;56(7):1503–11. [DOI] [PubMed] [Google Scholar]
- 92.Chen S, Sun J, Zhao G, Guo A, Chen Y, Fu R, et al. Liraglutide improves water maze learning and memory performance while reduces hyperphosphorylation of tau and neurofilaments in APP/PS1/Tau triple transgenic mice. Neurochem Res. 2017;42(8):2326–35. [DOI] [PubMed] [Google Scholar]
- 93.Cherney DZI, Ferrannini E, Umpierrez GE, Peters AL, Rosenstock J, Powell DR, et al. Efficacy and safety of sotagliflozin in patients with type 2 diabetes and stage 3 chronic kidney disease. Diabetes Obes Metab. 2023;25(6):1646–57. [DOI] [PubMed] [Google Scholar]
- 94.Davies M, Faerch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971–84. [DOI] [PubMed] [Google Scholar]
- 95.Davies MJ, Bain SC, Atkin SL, Rossing P, Scott D, Shamkhalova MS, et al. Efficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): a randomized clinical trial. Diabetes Care. 2016;39(2):222–30. [DOI] [PubMed] [Google Scholar]
- 96.Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjoth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–99. [DOI] [PubMed] [Google Scholar]
- 97.Dei Cas A, Micheli MM, Aldigeri R, Gardini S, Ferrari-Pellegrini F, Perini M, et al. Long-acting exenatide does not prevent cognitive decline in mild cognitive impairment: a proof-of-concept clinical trial. J Endocrinol Invest. 2024;47(9):2339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dungan KM, Povedano ST, Forst T, Gonzalez JG, Atisso C, Sealls W, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384(9951):1349–57. [DOI] [PubMed] [Google Scholar]
- 99.Dungan KM, Weitgasser R, Perez Manghi F, Pintilei E, Fahrbach JL, Jiang HH, et al. A 24-week study to evaluate the efficacy and safety of once-weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD-8). Diabetes Obes Metab. 2016;18(5):475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fox CK, Clark JM, Rudser KD, Ryder JR, Gross AC, Nathan BM, et al. Exenatide for weight-loss maintenance in adolescents with severe obesity: a randomized, placebo-controlled trial. Obesity (Silver Spring). 2022;30(5):1105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Frias JP, Choi J, Rosenstock J, Popescu L, Niemoeller E, Muehlen-Bartmer I, et al. Efficacy and safety of once-weekly efpeglenatide monotherapy versus placebo in type 2 diabetes: the AMPLITUDE-M randomized controlled trial. Diabetes Care. 2022;45(7):1592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frias JP, Hsia S, Eyde S, Liu R, Ma X, Konig M, et al. Efficacy and safety of oral orforglipron in patients with type 2 diabetes: a multicentre, randomised, dose-response, phase 2 study. Lancet. 2023;402(10400):472–83. [DOI] [PubMed] [Google Scholar]
- 103.Gallo S, Charbonnel B, Goldman A, Shi H, Huyck S, Darekar A, et al. Long-term efficacy and safety of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: 104-week VERTIS MET trial. Diabetes Obes Metab. 2019;21(4):1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gallwitz B, Guzman J, Dotta F, Guerci B, Simo R, Basson BR, et al. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet. 2012;379(9833):2270–8. [DOI] [PubMed] [Google Scholar]
- 105.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–81. [DOI] [PubMed] [Google Scholar]
- 106.Garvey WT, Batterham RL, Bhatta M, Buscemi S, Christensen LN, Frias JP, et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022;28(10):2083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gejl M, Brock B, Egefjord L, Vang K, Rungby J, Gjedde A. Blood-brain glucose transfer in Alzheimer’s disease: effect of GLP-1 analog treatment. Sci Rep. 2017;7(1):17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385(10):896–907. [DOI] [PubMed] [Google Scholar]
- 109.Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2). Diabetes Care. 2015;38(12):2241–9. [DOI] [PubMed] [Google Scholar]
- 110.Group RC. Empagliflozin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Diabetes Endocrinol. 2023;11(12):905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grunberger G, Camp S, Johnson J, Huyck S, Terra SG, Mancuso JP, et al. Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: the VERTIS RENAL randomized study. Diabetes Ther. 2018;9(1):49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Han L, Holscher C, Xue GF, Li G, Li D. A novel dual-glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide receptor agonist is neuroprotective in transient focal cerebral ischemia in the rat. NeuroReport. 2016;27(1):23–32. [DOI] [PubMed] [Google Scholar]
- 113.Home PD, Ahren B, Reusch JEB, Rendell M, Weissman PN, Cirkel DT, et al. Three-year data from 5 HARMONY phase 3 clinical trials of albiglutide in type 2 diabetes mellitus: long-term efficacy with or without rescue therapy. Diabetes Res Clin Pract. 2017;131:49–60. [DOI] [PubMed] [Google Scholar]
- 114.Hong B, Lee H, Choi A, Kim WJ, Cho YM, Yon DK, et al. Sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase IV inhibitors and risk of dementia among patients with type 2 diabetes and comorbid mental disorders: a population-based cohort study. Diabetes Metab. 2024;50(6): 101581. [DOI] [PubMed] [Google Scholar]
- 115.Jankovic J. Parkinson disease: exenatide - a drug for diabetes and Parkinson disease? Nat Rev Neurol. 2017;13(11):643–4. [DOI] [PubMed] [Google Scholar]
- 116.Januzzi JL Jr, Butler J, Jarolim P, Sattar N, Vijapurkar U, Desai M, et al. Effects of canagliflozin on cardiovascular biomarkers in older adults with type 2 diabetes. J Am Coll Cardiol. 2017;70(6):704–12. [DOI] [PubMed] [Google Scholar]
- 117.Kadowaki T, Isendahl J, Khalid U, Lee SY, Nishida T, Ogawa W, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10(3):193–206. [DOI] [PubMed] [Google Scholar]
- 118.Kaku K, Chin R, Naito Y, Iliev H, Ikeda R, Ochiai K, et al. Safety and effectiveness of empagliflozin in Japanese patients with type 2 diabetes: interim analysis from a post-marketing surveillance study. Expert Opin Drug Saf. 2020;19(2):211–21. [DOI] [PubMed] [Google Scholar]
- 119.Kosiborod MN, Esterline R, Furtado RHM, Oscarsson J, Gasparyan SB, Koch GG, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9(9):586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kovacs CS, Seshiah V, Merker L, Christiansen AV, Roux F, Salsali A, et al. Empagliflozin as add-on therapy to pioglitazone with or without metformin in patients with type 2 diabetes mellitus. Clin Ther. 2015;37(8):1773-88 e1. [DOI] [PubMed] [Google Scholar]
- 121.Koychev I, Reid G, Nguyen M, Mentz RJ, Joyce D, Shah SH, et al. Inflammatory proteins associated with Alzheimer’s disease reduced by a GLP1 receptor agonist: a post hoc analysis of the EXSCEL randomized placebo controlled trial. Alzheimers Res Ther. 2024;16(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56(12):2582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee BW, Cho YM, Kim SG, Ko SH, Lim S, Dahaoui A, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as metformin add-on in a Korean population with type 2 diabetes. Diabetes Ther. 2024;15(2):547–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leiter LA, Cefalu WT, de Bruin TW, Xu J, Parikh S, Johnsson E, et al. Long-term maintenance of efficacy of dapagliflozin in patients with type 2 diabetes mellitus and cardiovascular disease. Diabetes Obes Metab. 2016;18(8):766–74. [DOI] [PubMed] [Google Scholar]
- 125.Li Q, Jia M, Yan Z, Li Q, Sun F, He C, et al. Activation of glucagon-like peptide-1 receptor ameliorates cognitive decline in type 2 diabetes mellitus through a metabolism-independent pathway. J Am Heart Assoc. 2021;10(14): e020734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu J, Shi X, Shao Y. Sodium-glucose cotransporter 1/2 inhibition and risk of neurodegenerative disorders: a Mendelian randomization study. Brain Behav. 2024;14(7): e3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ludvik B, Frias JP, Tinahones FJ, Wainstein J, Jiang H, Robertson KE, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370–81. [DOI] [PubMed] [Google Scholar]
- 128.Mathieu C, Ranetti AE, Li D, Ekholm E, Cook W, Hirshberg B, et al. Randomized, double-blind, phase 3 trial of triple therapy with dapagliflozin add-on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38(11):2009–17. [DOI] [PubMed] [Google Scholar]
- 129.Meissner WG, Remy P, Giordana C, Maltete D, Derkinderen P, Houeto JL, et al. Trial of lixisenatide in early Parkinson’s disease. N Engl J Med. 2024;390(13):1176–85. [DOI] [PubMed] [Google Scholar]
- 130.Meneilly GS, Roy-Duval C, Alawi H, Dailey G, Bellido D, Trescoli C, et al. Lixisenatide therapy in older patients with type 2 diabetes inadequately controlled on their current antidiabetic treatment: the GetGoal-o randomized trial. Diabetes Care. 2017;40(4):485–93. [DOI] [PubMed] [Google Scholar]
- 131.Morel T, Cleanthous S, Andrejack J, Barker RA, Biagioni M, Blavat G, et al. Development and early qualitative evidence of two novel patient-reported outcome instruments to assess daily functioning in people with early-stage Parkinson’s. J Patient Rep Outcomes. 2023;7(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mosenzon O, Blicher TM, Rosenlund S, Eriksson JW, Heller S, Hels OH, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):515–27. [DOI] [PubMed] [Google Scholar]
- 133.Mu Y, Bao X, Eliaschewitz FG, Hansen MR, Kim BT, Koroleva A, et al. Efficacy and safety of once weekly semaglutide 2.4 mg for weight management in a predominantly east Asian population with overweight or obesity (STEP 7): a double-blind, multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2024;12(3):184–95. [DOI] [PubMed] [Google Scholar]
- 134.Mullins RJ, Mustapic M, Chia CW, Carlson O, Gulyani S, Tran J, et al. A pilot study of exenatide actions in Alzheimer’s disease. Curr Alzheimer Res. 2019;16(8):741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32(1):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nauck MA, Stewart MW, Perkins C, Jones-Leone A, Yang F, Perry C, et al. Efficacy and safety of once-weekly GLP-1 receptor agonist albiglutide (HARMONY 2): 52 week primary endpoint results from a randomised, placebo-controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetologia. 2016;59(2):266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Norgaard CH, Friedrich S, Hansen CT, Gerds T, Ballard C, Moller DV, et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimers Dement (N Y). 2022;8(1): e12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Osmanovic Barilar J, Babic Perhoc A, Knezovic A, Homolak J, Virag D, Salkovic-Petrisic M. The effect of the sodium-glucose cotransporter inhibitor on cognition and metabolic parameters in a rat model of sporadic Alzheimer's disease. Biomedicines . 2023;11(4):1025. [DOI] [PMC free article] [PubMed]
- 139.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383(15):1413–24. [DOI] [PubMed] [Google Scholar]
- 140.Parkinson J, Tang W, Johansson CC, Boulton DW, Hamren B. Comparison of the exposure-response relationship of dapagliflozin in adult and paediatric patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2016;18(7):685–92. [DOI] [PubMed] [Google Scholar]
- 141.Pieber TR, Bode B, Mertens A, Cho YM, Christiansen E, Hertz CL, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):528–39. [DOI] [PubMed] [Google Scholar]
- 142.Polidori D, Mari A, Ferrannini E. Canagliflozin, a sodium glucose co-transporter 2 inhibitor, improves model-based indices of beta cell function in patients with type 2 diabetes. Diabetologia. 2014;57(5):891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39–50. [DOI] [PubMed] [Google Scholar]
- 144.Pratley RE, Nauck MA, Barnett AH, Feinglos MN, Ovalle F, Harman-Boehm I, et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2(4):289–97. [DOI] [PubMed] [Google Scholar]
- 145.Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SO, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42(12):2272–81. [DOI] [PubMed] [Google Scholar]
- 146.Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208–19. [DOI] [PubMed] [Google Scholar]
- 147.Rodgers M, Migdal AL, Rodriguez TG, Chen ZZ, Nath AK, Gerszten RE, et al. Weight loss outcomes among early high responders to exenatide treatment: a randomized, placebo controlled study in overweight and obese women. Front Endocrinol (Lausanne). 2021;12: 742873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rosenstock J, Allison D, Birkenfeld AL, Blicher TM, Deenadayalan S, Jacobsen JB, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321(15):1466–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rosenstock J, Fonseca VA, Gross JL, Ratner RE, Ahren B, Chow FC, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37(8):2317–25. [DOI] [PubMed] [Google Scholar]
- 150.Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rubino DM, Greenway FL, Khalid U, O’Neil PM, Rosenstock J, Sorrig R, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327(2):138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36(9):2508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Selvarajah V, Robertson D, Hansen L, Jermutus L, Smith K, Coggi A, et al. A randomized phase 2b trial examined the effects of the glucagon-like peptide-1 and glucagon receptor agonist cotadutide on kidney outcomes in patients with diabetic kidney disease. Kidney Int. 2024;106(6):1170–80. [DOI] [PubMed]
- 154.Shin A, Koo BK, Lee JY, Kang EH. Risk of dementia after initiation of sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase-4 inhibitors in adults aged 40–69 years with type 2 diabetes: population based cohort study. BMJ. 2024;386: e079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Siao WZ, Lin TK, Huang JY, Tsai CF, Jong GP. The association between sodium-glucose cotransporter 2 inhibitors and incident dementia: a nationwide population-based longitudinal cohort study. Diab Vasc Dis Res. 2022;19(3):14791641221098168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Spertus JA, Birmingham MC, Nassif M, Damaraju CV, Abbate A, Butler J, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med. 2022;28(4):809–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stack AG, Han D, Goldwater R, Johansson S, Dronamraju N, Oscarsson J, et al. Dapagliflozin added to verinurad plus febuxostat further reduces serum uric acid in hyperuricemia: the QUARTZ study. J Clin Endocrinol Metab. 2021;106(5):e2347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stenlof K, Cefalu WT, Kim KA, Jodar E, Alba M, Edwards R, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2014;30(2):163–75. [DOI] [PubMed] [Google Scholar]
- 159.Tuttle KR, Lakshmanan MC, Rayner B, Busch RS, Zimmermann AG, Woodward DB, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–17. [DOI] [PubMed] [Google Scholar]
- 160.Tuttle KR, Levin A, Nangaku M, Kadowaki T, Agarwal R, Hauske SJ, et al. Safety of empagliflozin in patients with type 2 diabetes and chronic kidney disease: pooled analysis of placebo-controlled clinical trials. Diabetes Care. 2022;45(6):1445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37(8):2168–76. [DOI] [PubMed] [Google Scholar]
- 162.Vaccari C, Grotto D, Pereira TDV, de Camargo JLV, Lopes LC. GLP-1 and GIP receptor agonists in the treatment of Parkinson’s disease: translational systematic review and meta-analysis protocol of clinical and preclinical studies. PLoS One. 2021;16(8): e0255726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vadini F, Simeone PG, Boccatonda A, Guagnano MT, Liani R, Tripaldi R, et al. Liraglutide improves memory in obese patients with prediabetes or early type 2 diabetes: a randomized, controlled study. Int J Obes (Lond). 2020;44(6):1254–63. [DOI] [PubMed] [Google Scholar]
- 164.Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28(3):568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang J, Li HQ, Xu XH, Kong XC, Sun R, Jing T, et al. The effects of once-weekly dulaglutide and insulin glargine on glucose fluctuation in poorly oral-antidiabetic controlled patients with type 2 diabetes mellitus. Biomed Res Int. 2019;2019:2682657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang X, Plantinga AM, Xiong X, Cromer SJ, Bonzel CL, Panickan V, et al. Comparing insulin against glucagon-like peptide-1 receptor agonists, dipeptidyl peptidase-4 inhibitors, and sodium-glucose cotransporter 2 inhibitors on 5-year incident heart failure risk for patients with type 2 diabetes mellitus: real-world evidence study using insurance claims. JMIR Diabetes. 2024;9: e58137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Watson KT, Wroolie TE, Tong G, Foland-Ross LC, Frangou S, Singh M, et al. Neural correlates of liraglutide effects in persons at risk for Alzheimer’s disease. Behav Brain Res. 2019;356:271–8. [DOI] [PubMed] [Google Scholar]
- 169.Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab. 2015;17(9):849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Weissman PN, Carr MC, Ye J, Cirkel DT, Stewart M, Perry C, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475–84. [DOI] [PubMed] [Google Scholar]
- 171.Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156(6):405–15. [DOI] [PubMed] [Google Scholar]
- 172.Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. [DOI] [PubMed] [Google Scholar]
- 173.Wu CY, Iskander C, Wang C, Xiong LY, Shah BR, Edwards JD, et al. Association of sodium-glucose cotransporter 2 inhibitors with time to dementia: a population-based cohort study. Diabetes Care. 2023;46(2):297–304. [DOI] [PubMed] [Google Scholar]
- 174.Wysham C, Blevins T, Arakaki R, Colon G, Garcia P, Atisso C, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care. 2014;37(8):2159–67. [DOI] [PubMed] [Google Scholar]
- 175.Yu M, Brunt KV, Milicevic Z, Varnado O, Boye KS. Patient-reported outcomes in patients with type 2 diabetes treated with dulaglutide added to titrated insulin glargine (AWARD-9). Clin Ther. 2017;39(11):2284–95. [DOI] [PubMed] [Google Scholar]
- 176.Zhou B, Zissimopoulos J, Nadeem H, Crane MA, Goldman D, Romley JA. Association between exenatide use and incidence of Alzheimer’s disease. Alzheimers Dement (N Y). 2021;7(1):e12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Arab HH, Safar MM, Shahin NN. Targeting ROS-dependent AKT/GSK-3beta/NF-kappaB and DJ-1/Nrf2 pathways by dapagliflozin attenuates neuronal injury and motor dysfunction in rotenone-induced Parkinson’s disease rat model. ACS Chem Neurosci. 2021;12(4):689–703. [DOI] [PubMed] [Google Scholar]
- 178.Stefanis L. alpha-Synuclein in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(2):a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jin M, Matsumoto S, Ayaki T, Yamakado H, Taguchi T, Togawa N, et al. DOPAnization of tyrosine in alpha-synuclein by tyrosine hydroxylase leads to the formation of oligomers. Nat Commun. 2022;13(1):6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stojakovic A, Paz-Filho G, Arcos-Burgos M, Licinio J, Wong ML, Mastronardi CA. Role of the IL-1 pathway in dopaminergic neurodegeneration and decreased voluntary movement. Mol Neurobiol. 2017;54(6):4486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang DD, Naumova AV, Isquith D, Sapp J, Huynh KA, Tucker I, et al. Dapagliflozin reduces systemic inflammation in patients with type 2 diabetes without known heart failure. Cardiovasc Diabetol. 2024;23(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fioretto P, Giaccari A, Sesti G. Efficacy and safety of dapagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in diabetes mellitus. Cardiovasc Diabetol. 2015;14:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tanvir A, Jo J, Park SM. Targeting glucose metabolism: a novel therapeutic approach for Parkinson’s disease. Cells. 2024;13(22):1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shyangdan DS, Uthman OA, Waugh N. SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. BMJ Open. 2016;6(2):e009417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bantounou MA, Shoaib K, Mazzoleni A, Modalavalasa H, Kumar N, Philip S. The association between type 2 diabetes mellitus and Parkinson’s disease; a systematic review and meta-analysis. Brain Disorders. 2024;15:100158. [Google Scholar]
- 186.Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: what is it and where are we? J Clin Invest. 2003;111(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Fig. S1. (A) Network structure of primary outcome: subgroup analysis of Alzheimer’s disease events; (B) Network structure of primary outcome: subgroup analysis of dementia of Lewy body events; (C) Network structure of primary outcome: subgroup analysis of multiple sclerosis events; (D) Network structure of primary outcome: subgroup analysis of amyotrophic lateral sclerosis events; (E) Network structure of safety profile: drop-out rate. Fig. S2. (A) Forest plot of primary outcome: subgroup analysis of Alzheimer’s disease events; (B) Forest plot of primary outcome: subgroup analysis of dementia of Lewy body events; (C) Forest plot of primary outcome: subgroup analysis of multiple sclerosis events; (D) Forest plot of primary outcome: subgroup analysis of amyotrophic lateral sclerosis events; (E) Forest plot of safety profile: drop-out rate. Fig. S3. Summary plot of ranking of primary outcome (overall events of neurodegenerative diseases). Fig. S4. Individual study result of primary outcome: overall events of neurodegenerative diseases. Fig. S5. Bayesian-based forest plot of primary outcome: overall events of neurodegenerative diseases. Fig. S6. (A) Bayesian-based Litmus Rank-O-Gram rank plot of primary outcome: overall events of neurodegenerative diseases; (B) Bayesian-based radial surface under the cumulative ranking of primary outcome: overall events of neurodegenerative diseases. Fig. S7. (A) Bayesian-based residual deviance NMA/UME model of primary outcome: overall events of neurodegenerative diseases; (B) Bayesian-based per-arm residual deviance of primary outcome: overall events of neurodegenerative diseases; (C) Bayesian-based leverage plot of primary outcome: overall events of neurodegenerative diseases. Fig. S8. Detailed risk of bias in each study. Tab. S1. (A) PRISMA 2020 checklist of the current network meta-analysis; (B) PRISMA 2020 abstract checklist of the current network meta-analysis. Tab. S2. Keyword used in each database and search results. Tab. S3. Excluded studies and reason. Tab. S4. Characteristics of the included studies. Tab. S5. (A) League table of primary outcome: subgroup of Alzheimer’s disease events; (B) League table of primary outcome: subgroup of dementia of Lewy body events; (C) League table of primary outcome: subgroup of multiple sclerosis events; (D) League table of primary outcome: subgroup of amyotrophic lateral sclerosis events; (E) League table of safety profile: drop-out rate. Tab. S6. Surface under the cumulative ranking of primary outcome: overall events of neurodegenerative diseases. Tab. S7. (A) Inconsistency within the primary outcome: overall events of neurodegenerative diseases; (B) Inconsistency within the primary outcome: subgroup of Parkinson’s disease events; (C) Inconsistency within the primary outcome: subgroup of Alzheimer’s disease events; (D) Inconsistency within the primary outcome: subgroup of dementia of Lewy body events; (E) Inconsistency within the primary outcome: subgroup of multiple sclerosis events; (F) Inconsistency within the primary outcome: subgroup of amyotrophic lateral sclerosis events; (G) Inconsistency within the safety profile: drop-out rate. Legends to Combined Additional files [7–13, 16, 19–23, 26–28, 31–41, 47–65, 74–176, 187].
Data Availability Statement
All the data of the current study were available upon reasonable request to the corresponding authors.