Abstract
A major challenge for microbiologists is to elucidate the strategies deployed by microorganisms to adapt to and thrive in highly complex and dynamic environments. In vitro studies, including those monitoring genomewide changes, have proven their value, but they can, at best, mimic only a subset of the ensemble of abiotic and biotic stimuli that microorganisms experience in their natural habitats. The widely used gene-to-phenotype approach involves the identification of altered niche-related phenotypes on the basis of gene inactivation. However, many traits contributing to ecological performance that, upon inactivation, result in only subtle or difficult to score phenotypic changes are likely to be overlooked by this otherwise powerful approach. Based on the premise that many, if not most, of the corresponding genes will be induced or upregulated in the environment under study, ecologically significant genes can alternatively be traced using the promoter trap techniques differential fluorescence induction and in vivo expression technology (IVET). The potential and limitations are discussed for the different IVET selection strategies and system-specific variants thereof. Based on a compendium of genes that have emerged from these promoter-trapping studies, several functional groups have been distinguished, and their physiological relevance is illustrated with follow-up studies of selected genes. In addition to confirming results from largely complementary approaches such as signature-tagged mutagenesis, some unexpected parallels as well as distinguishing features of microbial phenotypic acclimation in diverse environmental niches have surfaced. On the other hand, by the identification of a large proportion of genes with unknown function, these promoter-trapping studies underscore how little we know about the secret lives of bacteria and other microorganisms.
INTRODUCTION
The overwhelming focus for microbiology during the last century has been the study of microbes under well-defined laboratory conditions. The value of this approach is evident in the wealth of information now available on physiological and genetic mechanisms, without which the rapid advances in molecular microbiology would not have been possible.
The utility of studying bacteria in vitro remains clear, particularly in light of technologies for genome-scale analysis in conjunction with the ability to carefully control biotic and abiotic environmental factors in the laboratory (184, 199, 238). For example, virulence factors of animal pathogens have been identified by analyzing bacterial responses to changes in temperature (166, 178), iron concentration (146, 182), pH (189), exposure to oxidative stress (282), and phosphate starvation (211). Similarly, the biology of rhizosphere-colonizing bacteria has been studied using simplified in vitro approaches. For example, root exudates have been collected from plant roots and used to study the bacterial response to root-derived factors (68, 176); the response of bacteria to other inhabitants of the rhizosphere has also been studied (294). Likewise, in vitro studies have proved useful for identifying host signal molecules triggering the onset of Agrobacterium tumefaciens pathogenesis (59, 61) and Rhizobium symbiosis (25, 207) upon interaction with plants.
Despite the value of in vitro studies, there is no escape from the fact that the vast majority of microbes exist in complex, dynamic environments that cannot be reproduced in the laboratory. For microbes, irrespective of their life style, there is growing recognition of the need to understand their function in the very environments that they inhabit and thus, ultimately, the causes of their ecological success.
Analysis of ecological success is far from straightforward: it is a complex and multidimensional phenotype determined by interconnected regulatory pathways involving both individual genes and gene networks. Natural selection, which is largely responsible for shaping the determinants of ecological success, does so by operating on interacting systems (more so than on single genes) to generate specific morphologies, physiologies, and behaviors. With this in mind, the value of different experimental approaches can be assessed.
Both bottom-up (genes to population) and top-down (population to genes) approaches have been used. The bottom-up approach is commonly used for studies of bacteria, although it is rarely pursued to the population level. The typical genes-to-phenotype strategy involves identification of traits on the basis of gene inactivation (143). This is a powerful approach that has been fundamental to the majority of advances in molecular microbiology, but, despite its power, insertional mutagenesis is not always appropriate for the analysis of phenotypes as complex as ecological performance. For most organisms, in most environments, there is no primary determinant of ecological performance; this is because it is determined by complex epistatic interactions among many different gene products that each have a long evolutionary history. Traits having the greatest effect on ecological performance are likely to be those that show subtle quantitative variation, and such traits are unlikely to produce “defective” phenotypes when inactivated (143).
Recent advances in gene fusion technologies provide an alternative way to study complex phenotypes. Rather than identifying genes on the basis of function loss, ecologically significant genes can be identified on the basis of their positive contribution to a specific phenotype. A study that aims to understand the mechanistic basis of ecological performance in bacteria colonizing a specific host might, therefore, begin by identifying those genes that are induced in the host environment. One advantage of this approach is that it considers bacteria as integrated organisms rather than as a toolbox of independent genes and phenotypes.
Bacterial gene expression can be determined by direct or indirect measurements of mRNA levels. Reporter gene fusions provide simple indirect methods for assaying transcription by placing a gene that encodes a product that can be readily assayed under the control of the promoter of interest. Two such reporters are lacZ (which encodes β-galactosidase) (116) and gusA (which encodes β-glucuronidase) (247). While reporters such as lacZ and gusA have been used most extensively to study gene expression in vitro, both of these reporters have also been used to study expression in complex environments, such as within the environment of living cells. However, improved reporters that encode luminescent (e.g., lux) or fluorescent (e.g., gfp) proteins have greatly increased the utility of transcriptional reporters to the extent that expression of single cells in complex environments can be studied (34, 41, 53, 85, 242).
In the past decade, many different techniques have been developed to study bacterial genes that are expressed during growth in specific and complex ecological niches (47, 138, 220). In this article we discuss the promoter-trapping techniques differential fluorescence induction (DFI) (279) and in vivo expression technology (IVET) (156), which have been used to identify and study genes showing elevated levels of expression in complex environments. In addition to the information these genes provide about the way that an organism perceives its environment, genes activated in a specific niche are likely to encode (or contribute toward) traits that are important determinants of ecological performance in that environment (201, 212). Complementary strategies such as signature-tagged mutagenesis (STM) (99), differential display using arbitrarily primed PCR (69, 172), subtractive and differential hybridization (111, 112), and selective capture of transcribed sequences (SCOTS) (86), are reviewed elsewhere (37, 94, 96, 154).
IN VIVO EXPRESSION TECHNOLOGY
Development of In Vivo Expression Technology
More than 15 years ago, Osbourn et al. designed the experimental approach now widely known as in vivo expression technology (190). To isolate Xanthomonas campestris genes induced during infection of turnips, the authors used a promoter trap containing a promoterless chloramphenicol resistance gene. In 1993 Mahan et al. (156) described a modified promoter trap and coined the term in vivo expression technology (IVET). This allowed the identification and subsequent analysis of Salmonella genes expressed during infection of mice.
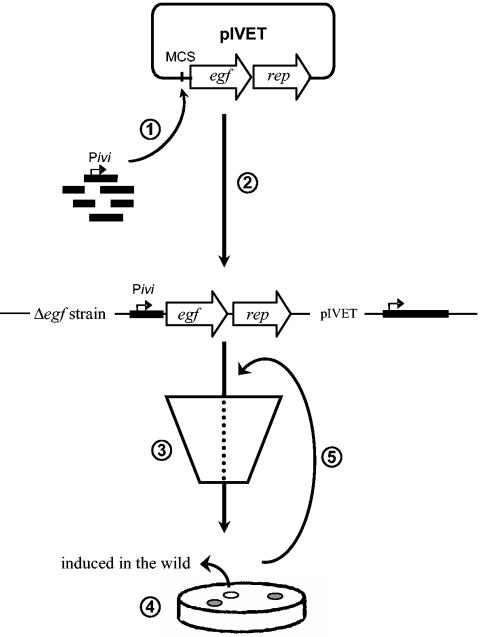
IVET (Fig. 1) is a promoter-trapping technique that selects microbial promoters active in a specified niche, for instance, during the interaction of a microorganism with its host. The first component of IVET is a conditionally compromised strain of the microorganism of interest that is mutated in a gene encoding an essential growth factor (egf) (220). The mutant strain is not able to sustain growth in the environment under study unless the egf gene is expressed. The second component of IVET is a plasmid carrying the promoter trap composed of the promoterless egf gene and a linked reporter gene (rep). Bacterial DNA is cloned randomly into the promoter trap and integrated in the chromosome of the egf mutant strain. Promoters that are specifically induced in the wild are identified by the ability to drive expression of the promoterless egf gene in this environment. This results in complementation of the mutation and, hence, in growth under the conditions encountered in the specified niche. To eliminate fusions with a “constitutive” promoter, recovered bacteria are screened for expression of the linked reporter gene (rep) on a general growth medium.
FIG. 1.
Schematic representation of the basic IVET strategy. This strategy involves the construction of a conditionally compromised strain that is mutated in a gene encoding an essential growth factor (egf). This mutant strain is not able to grow in the environment under study. The second component of IVET is the promoter trap, consisting of a promoterless egf gene and a transcriptionally linked reporter gene (rep). Bacterial DNA is cloned randomly into the promoter trap (step 1) and integrated in the chromosome of the egf mutant strain (step 2). Only in strains that carry a promoter active in the specified niche can the egf mutation be complemented (step 3). After selection in this environment, bacteria are reisolated and spread on a general growth medium that is suitable for monitoring reporter gene activity in vitro (step 4). Accordingly, constitutive promoters are distinguished from promoters that are specifically induced in the wild. Colonies bearing the latter type of transcriptional fusion are subjected to a second IVET screening to eliminate false positives (step 5).
Mahan and colleagues (156) devised the IVET concept to meet three important criteria. First of all, integration of a single copy of the transcriptional fusions into the chromosome avoids gene dosage effects inherent in multicopy plasmid vehicles. However, several authors applied IVET with a promoter trap provided on a stably maintained plasmid. Second, the integration of fusions by a single recombination event in the host chromosome generates a duplication of the cloned DNA, thereby retaining a functional copy of the wild-type gene and avoiding the loss of virulence factors or disruption of genes that may be essential for survival in the wild. Third, the reporter gene for screening promoter activity in vitro, in most cases lacZ, gusA, or gfp, enables the monitoring of promoter activity in vitro and in the wild using a chromogenic substrate or fluorescence detection.
Bacteria harboring promoters that are specifically active in the wild are isolated from the specified niche, and the transcriptional fusions are rescued from the genome by standard molecular cloning procedures. However, this is laborious and can also be problematic. Therefore, alternative methods to recover fusions from the genome have been devised. One method is to recover the fusion by transduction using a suitable phage, e.g., bacteriophage P22 in the case of Salmonella spp. (155), but transducing phages are not widely available. A more generally applicable procedure to rescue chromosomally integrated plasmids is conjugative cloning (219). A helper plasmid supplies the genetic loci necessary for mobilization of the integrated plasmid into a suitable Escherichia coli host (220).
The IVET screening by Mahan et al. (156) relied on a purA or thyA null mutation, resulting in purine and pyrimidine auxotrophy, respectively, that greatly attenuated the growth of Salmonella enterica serovar Typhimurium during mouse infection (98, 156). Since then, a wide variety of genes encoding essential growth factors (Table 1), as well as different reporter genes have been used.
TABLE 1.
Overview of applications of IVET to isolate microbial genes upregulated in complex niches
| Category and gene | Function | Microorganism | Host or environment | Reference(s) |
|---|---|---|---|---|
| Auxotrophy-based selection | ||||
| purA | De novo purine nucleotide biosynthesis | Salmonella enterica | Mouse | 98, 156, 264 |
| Pseudomonas aeruginosa | Mouse | 93 | ||
| purEK | De novo purine nucleotide biosynthesis | Pseudomonas aeruginosa | Burned tissue | 92 |
| Biofilm | 67 | |||
| Mouse | 290 | |||
| thyA | De novo thymidine nucleotide biosynthesis | Salmonella enterica | Mouse | 156 |
| pyrB | De novo pyrimidine nucleotide biosynthesis | Pseudomonas putida | Phytophthora parasitica | 140 |
| ura5 | Uracil biosynthesis | Histoplasma capsulatum | Mouse | 226 |
| asd | Diaminopimelate biosynthesis | Pseudomonas aeruginosa | Mouse | 95 |
| Pseudomonas putida | Maize | 63 | ||
| Shigella flexneri | Macrophage | 244 | ||
| dapB | Diaminopimelate biosynthesis | Pseudomonas fluorescens | Sugar beet | 78, 302, 303 |
| Pseudomonas fluorescens | Bulk soil | 245 | ||
| Pseudomonas stutzeri | Rice | 224 | ||
| panB | Pantothenate biosynthesis | Pseudomonas fluorescens | Sugar beet | 218 |
| trpEG | Tryptophan biosynthesis | Ralstonia solanacearum | Tomato | 26 |
| galU | Galactose metabolism | Klebsiella pneumoniae | Mouse | 133 |
| inhA | Mycolic acid biosynthesis | Mycobacterium tuberculosis | Macrophage | 56 |
| ribBAH | Riboflavin biosynthesis | Actinobacillus pleuropneumoniae | Pig | 76 |
| Antibiotic resistance selection | ||||
| cat | Chloramphenicol resistance | Xanthomonas campestris | Turnip | 190 |
| Burkholderia pseudomallei | Macrophage | 239 | ||
| Shigella flexneri | Macrophage | 14 | ||
| Escherichia coli | Mouse | 126, 191 | ||
| Helicobacter pylori | Mouse | 8 | ||
| Salmonella enterica | Mouse | 98, 157 | ||
| Yersinia enterocolitica | Mouse | 83, 300 | ||
| Streptococcus gordonii | Rabbit | 127, 191 | ||
| Yersinia ruckeri | Fish | 65 | ||
| erm | Erythromycin resistance | Lactobacillus reuteri | Mouse | 285 |
| Streptococcus suis | Pig | 254 | ||
| tet | Tetracycline resistance | Porphyromonas gingivalis | Mouse | 141, 298 |
| kan | Kanamycin resistance | Pasteurella multocida | Mouse | 108 |
| RIVET | ||||
| tnpR | Site-specific recombinase | Vibrio cholerae | Rabbit/mouse | 32, 137, 174, 191 |
| Staphylococcus aureus | Mouse | 152 | ||
| cre | Site-specific recombinase | Salmonella enterica | Epithelial cells | 5 |
| Lactobacillus plantarum | Mouse | 24 | ||
| FLP | Site-specific recombinase | Candida albicans | Mouse | 262 |
| System-specific selection | ||||
| bacA | Membrane protein necessary for bacteroid differentiation | Sinorhizobium meliloti | Alfalfa | 188 |
| hly | Listeriolysin | Listeria monocytogenes | Mouse | 55, 77 |
| metXW | Methionine biosynthesis | Pseudomonas syringae pv. syringae | Bean | 159 |
| hrcC | Component of TTSS | Pseudomonas syringae pv. tomato | Arabidopsis thaliana | 16 |
| gfp | Green fluorescent protein | Erwinia chrysanthemi | Spinach | 299 |
Selection Strategies in IVET
Several variations on the original IVET theme have emerged. These IVET variants involve selection strategies based upon auxotrophy, antibiotic resistance, or recombination events resulting in the excision of a genetic marker. In addition, some highly specific IVET selection strategies have also been devised.
Auxotrophy-based selection.
The auxotrophy-based selection strategy has been widely used in IVET screenings. All IVET studies based on this type of selection require a mutant strain defective in growth in the wild. This growth defect can be complemented by expression of the promoterless essential gene provided on the promoter trap.
As mentioned above, the first studies under the IVET moniker used auxotrophic Salmonella enterica serovar Typhimurium mutants defective in the de novo biosynthesis of purine or pyrimidine nucleotides combined with promoter traps supplying a promoterless purA or thyA gene, respectively, to complement growth of the Salmonella enterica serovar Typhimurium mutants in the wild (98, 156). The purA-based selection strategy was also used to identify Pseudomonas aeruginosa genes specifically induced during infection of mice (93). In another IVET study, purine auxotrophy of P. aeruginosa was obtained using a purEK mutant strain (289, 290).
Since not all bacteria show defective growth upon purA mutation and it is difficult to obtain a purA mutant strain for some microorganisms, several research groups used other essential genes. In principle, any biosynthetic gene that is necessary for growth in the wild can be used. The only prerequisite is that the auxotrophy cannot be complemented by metabolites retrieved from the occupied niche. Nevertheless, the gene to be mutated has to be chosen after careful consideration. For instance, when bacteria are able to reside intracellularly in host tissue, it has to be taken into account that nonsecreted host metabolites might also complement the auxotrophy.
Several authors adapted the auxotrophy-based selection strategy and used other essential metabolic genes (Table 1): panB, involved in pantothenate biosynthesis (218); dapB or asd, involved in diaminopimelate biosynthesis (63, 78, 95, 224, 245); metXW (159) or trpEG (26), necessary for methionine and tryptophan biosynthesis, respectively; inhA, required for mycolic acid biosynthesis (56); pyrB or thyA, necessary for de novo biosynthesis of pyrimidine nucleotides (140, 156); galU, involved in galactose metabolism (133); or ribBAH, involved in riboflavin biosynthesis (76). IVET was also applied to study infection of mice by the pathogenic fungus Histoplasma capsulatum. In this case, uracil auxotrophy was created by mutating the ura5 gene (226).
Antibiotic resistance-based selection.
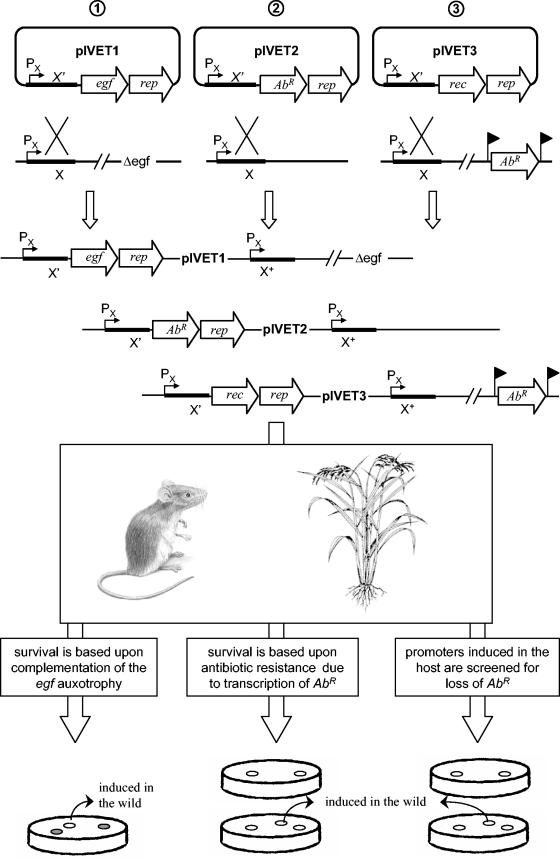
As it is not always easy or possible to construct an auxotrophic mutant, IVET selection based on antibiotic resistance, using antibiotic resistance genes as reporter genes (Fig. 2), is an important variant that expands the applications to a wider variety of microorganisms. While extending the utility of IVET, the use of antibiotic selection typically requires dosing the environment of interest with antibiotic, which inevitably changes aspects of the biological niche studied with implications for the spectrum of genes recovered.
FIG.2.
Schematic overview of the three main IVET selection strategies. Depending on the chosen IVET selection, the promoter trap contains a promoterless reporter gene (rep) transcriptionally linked to (1) a promoterless egf gene, encoding an essential growth factor (auxotrophy-based selection); (2) a promoterless AbR gene, conferring antibiotic resistance (antibiotic resistance-based selection); or (3) a promoterless site-specific recombinase gene (rec), which, when expressed, will splice out the antibiotic resistance (Abr) gene that is integrated elsewhere in the bacterial genome. Fusion libraries are constructed by ligating random genomic fragments (designated gene X) into the IVET vector of choice. Subsequently, the fusion library is transferred to an auxotrophic (egf) mutant strain (1) or a strain harboring the Abr gene flanked by recognition sites for the recombinase (indicated by flags) (3) in the case of auxotrophy-based and RIVET selection, respectively. After transfer of the transcriptional fusions into the microorganism of interest, the suicide plasmid is, in most cases, integrated into the chromosome at the sites of homology to gene X, thereby creating a merodiploid and retaining a functional copy of gene X (indicated with X+). In the case of RIVET, prescreening is required to remove strains harboring in vitro active gene fusions by selecting for AbR during construction of the fusion library. Subsequently, strains carrying the fusions are passed through the specific environment of interest and collected after a period of time. For antibiotic resistance-based selection, the antibiotic must be administered to the environment at a sufficient dose. Strains containing genes induced in the wild are selected by the ability to sustain growth in the environment (auxotrophy- or antibiotic resistance-based selection) or by screening for the loss of antibiotic resistance after recovery (RIVET selection). In the case of auxotrophy-based and antibiotic resistance-based selection, constitutive promoters can be discarded by monitoring the activity of the reporter gene in vitro and for antibiotic sensitivity, respectively, on a general growth medium.
Osbourn et al. (190) used an “IVET avant la lettre,” based on antibiotic selection to isolate genes of the plant pathogen Xanthomonas campestris induced during infection of turnip. The promoter trap was provided on a stably maintained plasmid and consisted of a promoterless cat gene, encoding chloramphenicol acetyltransferase. The chloramphenicol resistance gene was used as a reporter for both in vitro and host-induced promoter activity by screening the bacteria for chloramphenicol sensitivity and resistance, respectively.
Later, the cat gene was also used in IVET studies of Shigella flexneri (14), Salmonella enterica serovar Typhimurium (98, 157), Helicobacter pylori (8), Yersinia enterocolitica (300), Yersinia ruckeri (65), Streptococcus gordonii (127), Escherichia coli (126), and Burkholderia pseudomallei (239). Bacteria harboring promoters that are specifically induced in the wild were selected by administrating chloramphenicol to the host.
The use of antibiotic resistance genes as reporter genes in IVET studies is not limited to cat. To study Porphyromonas gingivalis virulence in mice, the promoterless tet gene was used, conferring tetracycline resistance (141, 298). Induced gene expression during pig infection by Streptococcus suis (254, 255) and mouse infection by Lactobacillus reuteri (285) was analyzed by an erythromycin resistance-based screening. And gene expression by Pasteurella multocida infecting mice was explored using a kanamycin resistance reporter gene (108).
Recombinase-based selection.
Both auxotrophy-based and antibiotic resistance-based selection have to cope with the inability to isolate transiently or weakly expressed genes. These disadvantages are circumvented by a second major modification of IVET screening, recombinase-based in vivo expression technology (RIVET). RIVET is based on the activation of a site-specific DNA resolvase and was initially used to identify Vibrio cholerae genes induced during infection of mice (32, 137). The resolvase used, TnpR from Tnγδ, is able to mediate recombination between two specific target sequences, the so-called res1 sites, and consequently slice out the interjacent DNA fragment from the genome.
In the first RIVET application, a tetracycline resistance gene (tet) was chosen as the reporter gene and was integrated into the chromosome, flanked by two res1 sites. The promoterless tnpR gene was provided on the promoter trap. Active promoters direct transcription of tnpR, and the activity of the resolvase results in excision of the reporter gene from the genome. By selection for tetracycline resistance during construction of the library, promoters that are active in vitro are discarded. After reisolation from the host, bacteria are screened for tetracycline sensitivity, and promoters active during interaction with the host are retained. The RIVET strategy has also been validated to study Staphylococcus aureus infection of mice (152). In this case, a kanamycin resistance gene was used as reporter gene and was integrated into the chromosome, flanked by two res1 sites.
A similar system was used in a RIVET strategy to study Salmonella enterica serovar Typhimurium infection of mice (5). This system consists of a promoterless derivative of cre, encoding the phage P1 recombinase, carried on the promoter trap. The targets of the Cre recombinase are two chromosomally integrated loxP sites flanking the npt gene, conferring kanamycin resistance.
RIVET is applicable to many microorganisms, even those that are difficult to manipulate since only the reporter gene flanked by recognition sites has to be integrated into the chromosome. A Cre-based RIVET system was devised by Bron et al. (24) for lactic acid bacteria. To study infection of mice by the fungal pathogen Candida albicans, Staib et al. devised a RIVET system consisting of an Flp recombinase and a genetic marker, conferring resistance to mycophenolic acid, flanked by the specific recognition sites for the recombinase (262).
System-specific selection.
It can be of interest to identify genes (promoters) differentially expressed during a particular stage of the interaction between a bacterium and its eukaryotic host. To this end, dedicated IVET strategies can be devised in which the promoter trapping gene encodes an “essential interaction factor” (eif) required at a specific stage of the interaction. Bacteria are screened for the ability to establish a firm interaction with the host. It is therefore necessary that the establishment of the microbe-host interaction result in a scorable host phenotype, such as cell lysis, plant disease, or symbiosis.
For example, a specific IVET selection strategy was devised to isolate Sinorhizobium meliloti genes that are specifically induced in the early stages of symbiosis (188). In this study, the bacA and gusA genes were used as reporter genes to assess host-induced and in vitro promoter activity, respectively. BacA is an integral membrane protein that affects the degree of modification of the lipopolysaccharides. BacA is required for intracellular infections during Sinorhizobium meliloti plant symbiosis and Brucella abortus animal pathogenesis (64). BacA is also necessary for differentiation of Sinorhizobium meliloti into nitrogen-fixing differentiated cells (bacteroids) (109). Only when active promoters are inserted in the promoter trap will the bacA gene be expressed, resulting in the differentiation process. Nitrogen-fixing nodules containing bacteroids can readily be distinguished from non-nitrogen-fixing nodules by macroscopic observation. In this way, the screen targets genes that are expressed after the initiation of nodulation but before bacteroid differentiation and nitrogen fixation take place. Isolation of genes known to be involved in nodulation (e.g., nifS) suggests that the strategy functions as expected. Moreover, it enabled identification of genes that were not previously associated with nodulation (188).
A similar strategy was developed to isolate genes involved in the early stage of Pseudomonas syringae pv. tomato infection of Arabidopsis thaliana leaves (16). This IVET consists of a Pseudomonas syringae hrcC mutant strain with a deficient type III secretion system (TTSS). TTSS is necessary for infection and growth in susceptible plants. Subsequently, a promoterless hrcC gene was used as the reporter in the promoter trap. Only genes expressed during establishment of infection can be isolated with this modified IVET. The approach used here proved useful, since 40% of the transcriptional fusions revealed genes already known to be involved in pathogenesis. Validation was obtained by isolation of hrp/hrc and avr genes, encoding proteins of the TTSS, as they are known to be induced upon inoculation and hence during the early stage of infection. A similar system is being developed with a Xanthomonas campestris pv. vesicatoria hrpB1 mutant also defective in expression of a functional TTSS (U. Bonas, personal communication).
To isolate Listeria monocytogenes virulence genes, a modified IVET was devised based on hly, encoding a hemolysin (listeriolysin) (55, 77). Listeriolysin is a virulence factor absolutely required for intracellular survival and growth in mice. Disruption of hly results in the loss of the hemolytic phenotype on blood agar plates and a severe decrease in virulence. Consequently, infection of mice by hly mutants can only occur when the hly gene provided on the promoter trap (lacking its cognate promoter) is expressed. After isolation of the infecting bacteria, the same reporter gene (hly) was used to screen promoter activity in vitro, since hemolysis is apparent as a zone surrounding Hly+ bacteria on blood agar plates. Again, this modified IVET focuses on genes that are induced and necessary in the early stages of infection rather than genes that enable bacteria to adapt to and survive in the new environment.
In an IVET application to study the plant pathogen Pseudomonas syringae pv. syringae, a methionine auxotrophy-based selection strategy was devised. In moist plant leaves, the metXW mutant used displays normal growth, but shows severely attenuated growth on plants in dry conditions (159). In this way, the auxotrophy-based selection only occurs when plants are transferred to dry growth conditions, and the timing and degree of selection pressure can be altered accordingly. For instance, by growing the plants in wet conditions in the early stages of infection, the conditionally compromised metXW mutants are able to grow and establish large populations. The IVET selection regimen is subsequently started by transferring the plants to dry conditions. Therefore, the name habitat-inducible rescue of survival was introduced (159).
The green fluorescent protein (GFP)-based IVET leaf array for identification of plant-upregulated genes in Erwinia chrysanthemi, described by Yang et al. (299), does not involve positive in planta selection using an essential growth factor gene. Selection of induced promoters is based upon differences in fluorescence intensity during plant infection and during growth on a general growth medium.
Benefits and Shortcomings of IVET Strategies
A major advantage of IVET is that the genes of interest are isolated from the fusion library by a powerful positive selection strategy (6). This is not possible with STM, for instance. Moreover, with STM there is no detection of virulence factors that are essential for survival in vitro because knocking out these genes results in defective growth (10).
Since the early IVET studies of animal infection by Salmonella enterica serovar Typhimurium and Pseudomonas aeruginosa, IVET has been adapted and applied to study a wide variety of microorganisms. It is clear from Table 1 that the various IVET selection strategies extended its use to study differential gene expression not only in gram-positive bacteria but also in eukaryotic microorganisms such as Candida albicans (262) and Histoplasma capsulatum (226).
IVET is not technically demanding and can be applied using standard molecular biology techniques. This means that in contrast to DFI or microarrays, no expensive equipment is required. Another major advantage of IVET is that no extensive knowledge of the genome of the microorganism under study is required to apply the technique. For example, Yersinia ruckeri and Pseudomonas stutzeri A15 are bacteria for which only a few DNA sequences were analyzed in the past, but IVET proved a useful technique to analyze gene expression in their host environments (65, 224). However, the availability of a (draft) genome sequence of the target microorganisms or close relatives certainly speeds up subsequent characterization of the trapped promoters.
Variations on the original IVET theme (antibiotic resistance-based IVET, RIVET, and DFI) have enabled the study of microorganisms for which straightforward genetic analysis, such as construction of defined mutants, is not readily available. For instance, in eukaryotes the presence of two alleles for each gene hampers mutational analysis, but IVET techniques enabled gene expression analysis of pathogenic fungi in their natural habitat (226, 262).
IVET can be applied to microorganisms residing in ecological niches that are very different in nature. IVET has been successfully applied to study microorganisms residing in animal hosts (ranging from fish, pigs, and chinchillas to mice), in macrophages, in plants, in the rhizosphere, and even in bacteria colonizing an oomycete (Table 1). When the microorganism under study is able to colonize two different host organisms, host-triggered gene expression can be assessed using the same promoter trap library. Comparing the two (different) subsets of host-induced genes provides information about the differences and similarities in the microenvironment of the two host organisms.
IVET is not limited to studying interactions with animal, fungal, or plant hosts, but can be extended for use in other complex environments. IVET was, for instance, used to study P. aeruginosa in biofilms with the so-called in-biofilm expression technology (67), or to study differential gene expression of P. aeruginosa during infection of burned mice tissues (92). A dapB-based IVET system was used to explore the genetic needs for survival of Pseudomonas fluorescens Pf0-1 in bulk soil (245). An IVET technique is also being developed to study gene expression of the oil-degrading marine bacterium Alcanivorax borkumensis in response to key environmental signals in order to study the bacterial determinants involved in biodegradation of hydrocarbons (82). In addition, an IVET-like strategy has been used to study differential gene expression in different genetic backgrounds. With the so-called identification of transcriptional regulator-activated promoters, the dependence of the transcription of Mycobacterium tuberculosis genes on various transcriptional regulators such as sigma factor σE could be analyzed by monitoring the reporter gene activity in a σE-overexpressing and a σE knockout strain of Mycobacterium smegmatis (173).
IVET has many attractive features, but some possible drawbacks have to be considered in the interpretation of the resulting data. First, IVET is not designed to isolate repressed promoters. Second, the subset of genes that are identified depends on the strength of the selection regimen in the wild. In each experimental system, the strength and the method of selection in the wild have to be chosen with consideration. If the selection is too strong, weakly or transiently expressed promoters will not be identified and highly expressed genes will be favored in the screening. On the other hand, a too weak selection in the wild will lead to false positive results. Third, proteins that are expressed constitutively but only activated in the wild (for example, by phosphorylation) are not detected. Fourth, the sets of genes defined as specifically induced in vivo are partially dependent on the “in vitro” growth conditions used to assess whether the reporter gene fusion is inactive outside the environment under investigation. For instance, the composition of the growth medium can significantly impact the expression of genes involved in nutrient acquisition and metabolism. Finally, mutants affected in genes that are isolated with IVET have to be constructed and phenotypically characterized. Only for a limited number of model microorganisms are ordered mutant libraries available that cover the entire sequenced genome.
As IVET can, in principle, be applied to study virtually all culturable microorganisms in their complex environments, it is clear that many researchers benefit from using the IVET strategy to study their favorite bug. The choice of selection strategy is facilitated by the development of the different IVET modifications. However, each specific selection strategy comes with its own advantages and disadvantages.
The major disadvantage of autotrophy-based selection is the need to construct an auxotrophic mutant, and for some microorganisms the tools to achieve this are not (yet) available. However, the nature of the auxotrophic mutation can determine in part the strength of the selection in the wild. When the generated auxotrophy is lethal for actively growing cells but does not impair cell survival, auxotrophy-based selection becomes a very powerful tool since the strength of selection in the wild can be easily adjusted by altering the time lapse between infection and reisolation (220). Whether low or transiently expressed genes will be detected depends on the strength of the selection regimen.
Switching to antibiotic selection avoids the construction of a mutant strain, thereby increasing the applicability of IVET. However, drug administration to the host might interfere with the complex process of interaction, e.g., with the immune defense of the host. Due to the presence of antibiotics, the composition of the natural ecological niche of which the microorganism is part might be altered. Furthermore, antibiotic administration to the host is not always possible, as the host organism itself might be affected by antibiotic treatments, as is often the case with plants (190). Moreover, to study microorganisms that reside within plant tissues, this selection strategy is scarcely suitable since several antibiotics are not translocated to all plant tissues. Once a suitable antibiotic for selection is found, it is important to evaluate the proper dose of antibiotic administration to allow selection of promoters that drive expression of the antibiotic resistance gene. The selection regimen in the wild can be modified easily. Variation of the antibiotic concentration allows isolation of genes that are expressed at different levels. Changing the time of drug administration permits isolation of genes that are expressed at different time points.
The main disadvantage of RIVET is the loss of a positive selection strategy after reisolation from the environment. This screening is rather laborious since isolated microorganisms are tested for antibiotic sensitivity by replica plating. However, Merrell and Camilli (175) solved this problem by inserting, together with an antibiotic resistance gene, a second reporter gene, sacB, into the excisable cassette. Its gene product, levansucrase, catalyzes conversion of sucrose into levan, which is toxic for most gram-negative and some gram-positive bacteria and results in defective growth in media containing sucrose. The sacB reporter gene can be used for a positive selection because bacteria that contain promoters induced in the wild have lost the reporter gene cassette, thereby enabling growth on media containing sucrose. Another strategy to avoid negative selection with RIVET is the use of a cat resistance gene which is disrupted by a tet resistance gene flanked by res sites (147). With this so-called selectable in vivo expression technology, the nonresolved strains remain resistant to tetracycline, while the resolved strains become resistant to chloramphenicol.
The major advantage of RIVET compared to antibiotic- and auxotrophy-based selection consists in the isolation of weakly or transiently expressed genes. However, the sensitivity of RIVET could also turn into a disadvantage, as genes important in the wild but expressed in vitro at a moderate to high basal level will not be isolated. The gene of study must be transcriptionally silent during strain construction and propagation in vitro. Otherwise, the antibiotic cassette is spliced out during the library construction, and consequently, the bacteria cannot survive the antibiotic selection. For this reason, fine tuning of the RIVET selection strategy was achieved by modulating the ribosome binding site of the promoterless recombinase gene (139). As a result of mutations in this ribosome binding site, translation efficiency at any transcriptional level is decreased, resulting in less sensitive selection.
Promoter traps consisting of tnpR alleles with different translation efficiencies render different pools of isolated genes that are induced to some level in the wild due to the lowered sensitivity of RIVET (139). Recently, a tunable RIVET system to study Vibrio cholerae infection was also achieved using resolvable cassettes with different efficiencies of excision (191). However, for each tnpR allele or resolvable cassette, a separate fusion library has to be constructed, which multiplies the manipulations.
RIVET permits the analysis of spatial and temporal gene expression (139). The expression patterns of the genes of interest can be investigated at different time points of the interaction, at different anatomic sites of the host organism, and even in different hosts or different genetic backgrounds simply by determining the proportion of resistant bacteria versus those sensitive to the antibiotic.
DIFFERENTIAL FLUORESCENCE INDUCTION
Development and Applications
DFI is a promoter-trapping technique that utilizes the green fluorescent protein (GFP) as a selectable marker to monitor promoter activity. In combination with fluorescence-activated cell sorting (FACS), DFI allows high-throughput screening of gene expression in microorganisms in a semiautomated way. Subsequent cycles of FACS screening result in the enrichment of clones containing genes specifically induced in the conditions under study (278).
DFI was originally designed to isolate acid-inducible genes in Salmonella enterica serovar Typhimurium (278, 280). This technique was subsequently adapted to study induction of Staphylococcus aureus and Streptococcus pneumoniae gene expression by in vitro stimuli that mimic the host environment, such as temperature shift, increased osmolarity, iron limitation, increased acidity, presence of competence stimulatory peptide, and cation starvation (13, 160, 237).
DFI studies of Salmonella enterica serovar Typhimurium, Mycobacterium marinum, and Listeria monocytogenes showed that DFI is not limited to studies of the effect of in vitro stimuli mimicking the host, but also enables analysis of gene expression during infection of macrophages (11, 221, 279, 295). Although these studies demonstrated successful sorting of macrophages based on the fluorescence of infecting bacteria, it is worth noting that the bacterial population within an infected macrophage is heterogeneous, which might lead to false positive results. However, it was possible to apply DFI to study Streptococcus pneumoniae infection when the pathogen was isolated from host body fluids, such as blood (160). Using two-color flow cytometry, Bumann (30) successfully analyzed gene expression of Salmonella organisms isolated from mouse Peyer's patches. Recently, the use of DFI was extended to explore differential gene expression of plant-associated bacteria such as Rhizobium leguminosarum (3), Pseudomonas syringae (35), and Bacillus cereus (57).
Benefits and Shortcomings of DFI
The benefits of DFI include semiautomated screening of large populations and the ability to change the sensitivity of the selection by simply altering the fluorescence threshold (278). Moreover, DFI is highly reproducible and enables integration of high-throughput screening and genomics (265). The use of GFP enables visualization of gene induction and the analysis of promoter activity on the single-cell level, which can be useful since heterogeneity of gene expression in a population increases with the complexity of the environment (18, 281).
In contrast to IVET, transcriptional fusions are not integrated into the chromosome but are provided on plasmids because single-copy gfp expression results in sufficiently intense fluorescence for accurate measurement only when driven by a strong promoter. In addition, the use of plasmids facilitates the isolation of in vivo-induced promoters. However, the use of multicopy plasmids prevents detection of context-dependent or topology-dependent effects of gene regulation.
DFI shares with IVET the caveats that are inherent to promoter trap approaches, such as the inability to detect genes that are regulated posttranscriptionally and the need to construct and analyze mutated target genes to assess their role in the wild (154). However, DFI shows additional disadvantages intrinsic to the technology. Flow cytometric analysis and sorting can be hampered by aggregation of bacteria or macrophages. Additional problems arise with the isolation and fluorescence quantification of bacteria that are isolated from infected host tissues because of the prevalence of background fluorescent particles (138, 278).
Other disadvantages are associated with the use of GFP, such as restrictions to the pH range in the studied environment, oxygen requirement for fluorophore development, and the absence of signal amplification. Due to the nonlinearity of fluorescent signals, it is necessary to calibrate and determine the linear range of the signal for each experiment to allow quantification of gene expression (180). However, most of these problems have been solved by the technological advances made concerning maturation, fluorophore development, fluorescence intensity, and spectral properties of GFP (42, 43, 258, 276).
OVERVIEW OF IVET- AND DFI-ISOLATED GENES
The application of IVET and DFI promoter-trapping techniques has allowed isolation of the promoters of many microbial genes that are specifically induced in complex environments. Identification of such genes is instrumental to unraveling microbial life in its natural habitat. Most IVET studies reported to date are unlikely to reflect a comprehensive view of the genes specifically transcribed in the wild. Nevertheless, the reported studies are a significant step forward in understanding how microorganisms respond to diverse environmental niches and provide clues as to the determinants of ecological success.
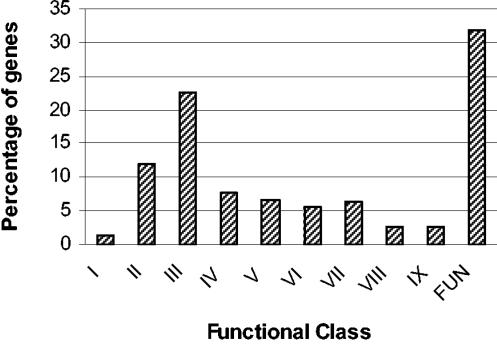
In Table 2, host-induced genes identified with IVET or DFI are classified in 9 functional groups. Most of the data in Table 2 were obtained from IVET studies of bacterial pathogens during infection of a mammalian host. In most cases a murine infection model was used to explore host-induced gene expression, but the interaction of animal pathogens with fish, pigs, rabbits, and chinchillas was also studied with promoter traps. In recent years, however, the IVET technique has found wider application for exploration of in planta gene expression of phytopathogenic bacteria as well as in nonpathogenic systems of bacterial interaction with crop plants (alfalfa, sugar beet, rice, and maize). In the majority of the studies, γ-proteobacterial members (17 species) were covered, mainly enterobacteria (seven species) and Pseudomonas (five species). Among the gram-positive bacteria, most data originate from members of the firmicutes (six species), whereas the use of IVET to study actinomycetes has only been reported for Mycobacterium tuberculosis. The data of 11 DFI studies of microbe-host interactions are incorporated in Table 2. DFI was used almost exclusively to study animal pathogens, mostly belonging to the γ-Proteobacteria (three species), firmicutes (three species), and mycobacteria (two species).
TABLE 2.
Promoters of genes that are upregulated in microorganisms during interaction with a eukaryotic hosta
| Class | Function and gene or fusion | Protein function; possible role in host interactions | Organism | Host or environment | Selection | Reference |
|---|---|---|---|---|---|---|
| Class I: genes involved in motility or chemotaxis | ||||||
| Flagellum/pilus biosynthesis | ||||||
| fliI | Flagellum-specific ATP synthase | V. cholerae | Mouse | RIVET | 191 | |
| fliF | Flagellar M-ring protein | P. fluorescens | Sugar beet | dapB | 78 | |
| fliF | Flagellar M-ring protein | S. suis | Pig | erm | 254 | |
| α-flaA | Antisense transcript—subunit flagellin | V. cholerae | Mouse | RIVET | 32 | |
| α-fliM | Antisense transcript—flagellar C-ring protein (switch complex) | P. stutzeri | Rice | dapB | a | |
| Rsc0726 | Type IV fimbrial biogenesis (PilW-related protein) | R. solanacearum | Tomato | trpEG | 26 | |
| Chemotaxis | ||||||
| iviIV | Chemotaxis receptor protein (MCP) | V. cholerae | Mouse | RIVET | 32 | |
| vca0773 | Chemotaxis receptor protein (MCP) | V. cholerae | Mouse | RIVET | 191 | |
| vc1535 | Chemotaxis receptor protein (MCP) | V. cholerae | Mouse | RIVET | 191 | |
| trg | Chemotaxis receptor protein (MCP) | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| cheR | Chemotaxis protein (methyltransferase) | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| cheY | Two-component response regulator | P. aeruginosa | Mouse | purEK | 290 | |
| cheY | Two-component response regulator | R. solanacearum | Tomato | trpEG | 26 | |
| α-cheV | Antisense transcript—chemotaxis protein | V. cholerae | Mouse | RIVET | 32 | |
| α-mcp | Antisense transcript—chemotaxis receptor protein (MCP) | P. stutzeri | Rice | dapB | 224 | |
| Class II: genes involved in nutrient scavenging | ||||||
| Metal ion acquisition | ||||||
| Siderophore synthesis | ||||||
| irp2 | Yersiniabactin synthetase subunit HMWP2 | Y. enterocolitica | Mouse | cat | 300 | |
| iucA | Aerobactin synthesis | K. pneumoniae | Mouse | galU | 133 | |
| entF | Enterobactin synthesis | S. enterica | Mouse | cat | 98 | |
| rucC | Ruckerbactin synthesis | Y. ruckeri | Fish | cat | 65 | |
| pvdD | Pyoverdine synthetase | P. aeruginosa | Mouse, rat | purA | 93 | |
| pvsA | Pyoverdine synthetase | P. fluorescens | Sugar beet | dapB | 78 | |
| Siderophore receptor and uptake | ||||||
| fepA | Ferrienterobactin receptor | K. pneumoniae | Mouse | galU | 133 | |
| fptA | Pyochelin receptor | P. aeruginosa | Mouse | purEK | 290 | |
| fyuA | Yersiniabactin receptor | Y. enterocolitica | Mouse | cat | 300 | |
| ufrA | Siderophore receptor | P. fluorescens | Sugar beet | dapB | 78 | |
| foxA | Siderophore receptor | Y. enterocolitica | Mouse | cat | 300 | |
| fhuA | Ferric hydroxamate receptor | S. enterica | Mouse | purA | 98 | |
| fhuA | Ferric hydroxamate receptor | S. flexneri | Epithelial cells | DFI | 233 | |
| ivi10 | Putative siderophore receptor | P. gingivalis | Mouse | tet | 298 | |
| rupA | Ruckerbactin receptor | Y. ruckeri | Fish | cat | 65 | |
| iviVII | TonB complex protein (ExbB-like) | Y. ruckeri | Fish | cat | 65 | |
| rupDGC | Siderophore ABC transporter protein | Y. ruckeri | Fish | cat | 65 | |
| Siderophore-independent iron transport | ||||||
| fbpA | Periplasmic iron binding protein of ABC transporter | B. abortus | Macrophage | DFI | 62 | |
| vc0202 | Periplasmic iron binding protein of ABC transporter | V. cholerae | Mouse | RIVET | 191 | |
| yfeA | Periplasmic (chelated) iron-binding protein of ABC transporter | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| hemT | Periplasmic hemin binding protein of ABC transporter | B. pseudomallei | Macrophage | cat | b | |
| hmuT | Periplasmic hemin binding protein of ABC transporter | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| vc0201 | ATP binding protein of ABC transporter | V. cholerae | Mouse | RIVET | 191 | |
| vca0687 | ATP binding protein of ABC transporter | V. cholerae | Mouse | RIVET | 191 | |
| yfuB | Permease of ABC transporter | Y. enterocolitica | Mouse | cat | 300 | |
| vca0203 | Permease of ABC transporter | V. cholerae | Mouse | RIVET | 191 | |
| hmuU | Permease of hemin ABC transporter | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| hmuS | Hemin degrading protein | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| sitABCD | ABC transporter (Fe2+/Mn2+ uptake) | S. enterica | Mouse | purA | 114, 156 | |
| sitA | Periplasmic binding protein of ABC transporter (Fe2+/Mn2+ uptake) | S. flexneri | Epithelial cells | DFI | 233, 234 | |
| sitC | Permease of ABC transporter (Fe2+/Mn2+ uptake) | Y. enterocolitica | Mouse | cat | 83 | |
| Regulation of iron uptake | ||||||
| np20 | Ferric uptake regulator (Fur) | P. aeruginosa | Mouse | purEK | 290 | |
| Other | ||||||
| nex10 | Putative K+ channel | S. meliloti | Alfalfa nodules | bacA | 188 | |
| Rv3237c | K+ channel | M. tuberculosis | Macrophage | inhA | 56 | |
| trkH | K+ transport system | B. abortus | Macrophage | DFI | 62 | |
| kup | K+ uptake protein | V. cholerae | Mouse | RIVET | 191 | |
| psaBCA | Mn2+ uptake system | S. pneumoniae | Mouse | DFI | 160, 161 | |
| mntH | Mn2+ transporter | B. pseudomallei | Macrophage | cat | b | |
| mgtA | Mg2+ transporter | S. enterica | Mouse | cat | 98 | |
| mgtB | Mg2+ transporter | S. enterica | Mouse | cat | 98 | |
| ntpJ | Na+ translocating ATPase | S. aureus | Mouse | RIVET | 152 | |
| Rsc1951 | Solute/Na+ symporter | R. solanacearum | Tomato | trpEG | 26 | |
| vca2705 | Solute/Na+ symporter | V. cholerae | Mouse | RIVET | 191 | |
| hoxQ | Ni2+ transport/hydrogenase activity | Y. enterocolitica | Mouse | cat | 300 | |
| Phosphate acquisition | ||||||
| phoA | Alkaline phosphatase | S. flexneri | Epithelial cells | DFI | 233 | |
| phoB | Response regulator of two-component system | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| phoS | Periplasmic binding protein | S. enterica | Macrophages | DFI | 279 | |
| phoU | Regulator of transport system | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| pstS | Periplasmic binding protein of high-affinity ABC transporter | S. flexneri | Epithelial cells | DFI | 233 | |
| pstB | ATP-binding protein of high-affinity ABC transporter | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| phnC | ATP-binding protein of ABC transporter | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| phnD | Periplasmic protein of ABC transporter | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| vc0721 | Periplasmic phosphate-binding protein | V. cholerae | Mouse | RIVET | 191 | |
| ppk | Polyphosphate kinase | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| Sulfate acquisition | ||||||
| cysA | Permease of ABC transporter | V. cholerae | Mouse | RIVET | 32 | |
| ssuBAC | ABC transporter | B. abortus | Macrophage | DFI | 62 | |
| cl52 | Transporter | L. monocytogenes | Macrophage | DFI | 295 | |
| cysD | Sulfate adenylate transferase | M. tuberculosis | Macrophages | DFI | 275 | |
| Amino acid acquisition | ||||||
| Rsp1575 | Periplasmic binding protein of ABC transporter | R. solanacearum | Tomato | trpEG | 26 | |
| ipx46 | ATP-binding component of ABC transporter | P. syringae | A. thaliana | hrcC | 16 | |
| livMH | Permease for high-affinity transport of branched amino acids | P. fluorescens | Sugar beet | panB | 218 | |
| hutT | Permease for histidine uptake | P. fluorescens | Sugar beet | panB | 218 | |
| iviD | Amino acid transporter | S. gordonii | Rabbit | cat | 127 | |
| sdaC2 | Serine transporter | V. cholerae | Mouse | RIVET | 191 | |
| Sugar uptake | ||||||
| Phosphotransferase systems | ||||||
| celB | Cellobiose PTS, II | L. monocytogenes | Mouse | hly | 77 | |
| pts14C | Cellobiose PTS, IIC | L. plantarum | Mouse | RIVET | 24 | |
| ptsIBCA | Sucrose PTS, EIIBCA | L. plantarum | Mouse | RIVET | 24 | |
| pts32BC | Sucrose PTS, EIIBC | L. plantarum | Mouse | RIVET | 24 | |
| vc0207 | Sucrose PTS, EIIBC | V. cholerae | Mouse | RIVET | 191 | |
| orf5-iviL | Cellobiose PTS | S. gordonii | Rabbit | cat | 127 | |
| ptfA | Fructose PTS | K. pneumoniae | Mouse | galU | 133 | |
| ptnA | Mannose PTS | L. monocytogenes | Macrophage | DFI | 295 | |
| pts37A | Sorbitol PTS, EIIA | L. plantarum | Mouse | RIVET | 24 | |
| pts19A | N-Acetylglucosamine/galactosamine PTS, IIA | L. plantarum | Mouse | RIVET | 24 | |
| nagE | N-Acetylglucosamine PTS, IIABC | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| Other sugar transporters | ||||||
| malA | Permease of ABC transporter for maltose uptake | S. aureus | Mouse | RIVET | 152 | |
| rbsC | Permease of ABC transporter for ribose uptake | H. influenzae | Chinchilla | DFI | 164 | |
| rbsD | Cytoplasmic ribose-binding protein | L. plantarum | Mouse | RIVET | 24 | |
| Rsp0536 | Transmembrane sugar-proton symporter | R. solanacearum | Tomato | trpEG | 26 | |
| uhpT | Hexose phosphate transporter | S. flexneri | Epithelial cells | DFI | 233 | |
| rhiT | Rhamnogalacturonide transporter | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| yicJ | Sodium galactoside symporter | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| Uptake of organic acids | ||||||
| dctD | Two-component response regulator of C4-dicarboxylate transport | P. syringae | Bean | metXW | 159 | |
| dctS | Two-component sensor of C4-dicarboxylate transport | P. fluorescens | Sugar beet | panB | 218 | |
| Rsc1598 | Two-component sensor (DctS-homologue) | R. solanacearum | Tomato | trpEG | 26 | |
| lldP | L-lactate permease | P. fluorescens | Sugar beet | dapB | 78 | |
| tctC | Tricarboxylate transport | Y. ruckeri | Fish | cat | 65 | |
| kgtP2 | α-ketoglutarate permease | R. solanacearum | Tomato | trpEG | 26 | |
| Uptake of miscellaneous compounds | ||||||
| sapA | Peptide ABC transporter | H. influenzae | Chinchilla | DFI | 164 | |
| cirA | Colicin I receptor | S. enterica | Macrophage | cat | 98 | |
| RT1006 | ATP binding component of ABC transporter | S. pneumoniae | Mouse | DFI | 160 | |
| comE | Competence protein (DNA uptake) | S. suis | Pig | erm | 254 | |
| potF2 | Putrescine transport protein | P. fluorescens | Sugar beet | dapB | 78 | |
| Transport of unknown substrates | ||||||
| Structural components of ABC transporters | ||||||
| ivil | S. gordonii | Rabbit | cat | 127 | ||
| ivs-6 | S. suis | Pig | erm | 254 | ||
| orf2 | P. putida | Phytophthora parasitica | pyrB | 140 | ||
| orfU | S. suis | Pig | erm | 254 | ||
| Rsc1376 | R. solanacearum | Tomato | trpEG | 26 | ||
| SPIV013 | S. pneumoniae | Mouse | DFI | 160 | ||
| uup | K. pneumoniae | Mouse | galU | 133 | ||
| pup-31 | E. chrysanthemi | Spinach | gfp-LA | 299 | ||
| lp_0299 | L. plantarum | Mouse | RIVET | 24 | ||
| rhi-37 | P. fluorescens | Sugar beet | dapB | 78 | ||
| yoaE | S. suis | Pig | erm | 254 | ||
| Periplasmic-binding proteins | ||||||
| pup-59 | E. chrysanthemi | Spinach | gfp-LA | 299 | ||
| p39 | B. abortus | Macrophage | DFI | 62 | ||
| Rsc0044 | R. solanacearum | Tomato | trpEG | 26 | ||
| Components of TRAP-T | ||||||
| vc0488 | Solute-binding protein | V. cholerae | Mouse | RIVET | 191 | |
| vc1275 | Constituent of TRAP-T carrier | V. cholerae | Mouse | RIVET | 191 | |
| Class III: genes of central intracellular metabolism | ||||||
| Intermediary metabolism | ||||||
| Glyoxylate pathway | ||||||
| aceA | Isocitrate lyase | M. tuberculosis | Macrophage | inhA | 56 | |
| aceB | Malate synthase | Y. enterocolitica | Mouse | cat | 300 | |
| aceE | Pyruvate dehydrogenase subunit | P. syringae | A. thaliana | hrcC | 16 | |
| pdhC | Pyruvate dehydrogenase subunit | M. marinum | Macrophage | DFI | 11 | |
| TCA cycle | ||||||
| sucA | Subunit of α-ketoglutarate dehydrogenase | V. cholerae | Mouse | RIVET | 32 | |
| fumC | Fumarase | R. solanacearum | Tomato | trpEG | 26 | |
| fumC | Fumarase | L. monocytogenes | Mouse | hly | 77 | |
| frdB | Fumarate reductase | Y. enterocolitica | Mouse | cat | 83 | |
| ppc | Phosphoenolpyruvate carboxylase | S. flexneri | HeLa monolayer | cat | 14 | |
| ppc | Phosphoenolpyruvate carboxylase | V. cholerae | Mouse | RIVET | 191 | |
| ppc | Phosphoenolpyruvate carboxylase | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| acnA | Aconitase | V. cholerae | Mouse | RIVET | 191 | |
| Glycolysis | ||||||
| hre-21 | Pyruvate kinase | Y. enterocolitica | Mouse | cat | 300 | |
| Gluconeogenesis | ||||||
| pckA | Phosphoenolpyruvate carboxykinase | M. tuberculosis | Macrophage | inhA | 56 | |
| pckA | Phosphoenolpyruvate carboxykinase | S. meliloti | Alfalfa nodules | bacA | 188 | |
| Pentose phosphate pathway | ||||||
| tal3 | Transaldolase | L. plantarum | Mouse | RIVET | 24 | |
| tkt | Transketolase | M. tuberculosis | Macrophages | DFI | 275 | |
| tkt | Deoxyxylulose 5′-phosphate synthase/transketolase | B. abortus | Macrophage | DFI | 62 | |
| tktI-N | Transketolase | L. plantarum | Mouse | RIVET | 24 | |
| Other | ||||||
| eno | Enolase | M. tuberculosis | Macrophage | inhA | 56 | |
| Lipid/fatty acid synthesis | ||||||
| dgk | Diacylglycerol kinase (diacylglycerol recycling) | P. putida | P. parasitica | pyrB | 140 | |
| accBC | Biotin carboxyl carrier protein | S. flexneri | HeLa monolayer | cat | 14 | |
| phbA | Acetyl-CoA acetyltransferase | R. solanacearum | Tomato | trpEG | 26 | |
| fadA4 | Acetyl-CoA acetyltransferase | M. tuberculosis | Macrophage | inhA | 56 | |
| ephF | Epoxide hydrolase | M. tuberculosis | Macrophage | inhA | 56 | |
| cfa | Cyclopropane fatty acid synthase (membrane modification) | S. enterica | Mouse | cat | 98 | |
| Lipid/fatty acid degradation | ||||||
| fadB | Fatty acid oxidation complex (alpha-subunit) | S. enterica | Mouse | cat | 157 | |
| fadB1 | Enoyl-CoA hydratase | B. abortus | Macrophage | DFI | 62 | |
| echA19 | Enoyl-CoA hydratase | M. tuberculosis | Macrophage | inhA | 56 | |
| fadB4 | 3-Hydroxyacyl-CoA dehydrogenase | M. tuberculosis | Macrophage | DFI | 275 | |
| Rv1144 | 3-Hydroxyacyl-CoA dehydrogenase | M. tuberculosis | Macrophage | inhA | 56 | |
| fadD | Long-chain fatty acid CoA ligase | P. syringae | A. thaliana | hrcC | 16 | |
| fadE | Probable acyl CoA dehydrogenase | P. fluorescens | Sugar beet | dapB | 78 | |
| est2 | Acetylesterase | L. plantarum | Mouse | RIVET | 24 | |
| lipA | Secreted triacylglyceride-specific lipase | V. cholerae | Mouse | RIVET | 32 | |
| lip | Glycerolester hydrolase | S. aureus | Mouse | RIVET | 152 | |
| Phospholipid metabolism | ||||||
| aas | Acyl acylglycerol phosphoethanolamine acyl | S. enterica | Macrophages | DFI | 279 | |
| transferase | ||||||
| licC | Phosphocholine cytidyltransferase | H. influenzae | Chinchilla | DFI | 164 | |
| glpQ | Protein D (lipoprotein) | P. multocida | Mouse | kan | 108 | |
| eutR | Ethanolamine operon regulator | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| Sugar metabolism | ||||||
| cl 143 | UDP-galactose epimerase | L. monocytogenes | Macrophage | DFI | 295 | |
| rbsK3 | Ribokinase | L. plantarum | Mouse | RIVET | 24 | |
| rbsR | Transcriptional repressor of ribose operon (LacI family) | K. pneumoniae | Mouse | galU | 133 | |
| xylA | Xylose isomerase | P. fluorescens | Sugar beet | panB | 218 | |
| xylA | Xylose isomerase | L. reuteri | Mouse | erm | 285 | |
| xylR | Xylose operon regulator (AraC family) | L. monocytogenes | Macrophage | DFI | 295 | |
| xylR | Xylose operon regulator (AraC family) | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| srlD | Sorbitol-6-phosphate dehydrogenase | P. multocida | Mouse | kan | 108 | |
| yoxD | Ribitol dehydrogenase | S. aureus | Mouse | RIVET | 152 | |
| uxuA | Mannonate dehydratase | H. influenzae | Chinchilla | DFI | 164 | |
| ram2 | α-l-Rhamnosidase | L. plantarum | Mouse | RIVET | 24 | |
| pbg10 | 6-Phospho-β-glucosidase | L. plantarum | Mouse | RIVET | 24 | |
| thgA1 | Galactoside O-acetyltransferase | L. plantarum | Mouse | RIVET | 24 | |
| vca0242 | Hexulose-6-phosphate synthase | V. cholerae | Mouse | RIVET | 191 | |
| glgA | Glycogen synthase | R. solanacearum | Tomato | trpEG | 26 | |
| glgB | 1,4-α-Glucan branching enzyme | V. cholerae | Mouse | RIVET | 191 | |
| glgX | Glycogen operon protein | R. solanacearum | Tomato | trpEG | 26 | |
| malQ | 4-α-Glucanotransferase | V. cholerae | Mouse | RIVET | 191 | |
| iviE | Exo-1,4-β-cellobiohydrolase | S. gordonii | Rabbit | cat | 127 | |
| iviH | Endo-1,3-β-glucanase | S. gordonii | Rabbit | cat | 127 | |
| Amino acid synthesis | ||||||
| Arginine biosynthesis | ||||||
| argA | N-Acetyl glutamate synthase | V. cholerae | Mouse | RIVET | 32 | |
| argF | Ornithine carbamoyl transferase | H. influenzae | Chinchilla | DFI | 164 | |
| argG | Argininosuccinate synthase | R. solanacearum | Tomato | trpEG | 26 | |
| argG | Argininosuccinate synthase | L. plantarum | Mouse | RIVET | 24 | |
| argH | Argininosuccinate lyase | V. cholerae | Mouse | RIVET | 191 | |
| carAB | Carbamoylphosphate synthetase (arginine/pyrimidine nucleotide biosynthesis) | S. enterica | Mouse | purA | 156 | |
| α-carA | Antisense transcript—carbamoylphosphate synthetase | P. gingivalis | Mouse | tet | 298 | |
| Aromatic amino acid biosynthesis | ||||||
| aroQ | Chorismate mutase | S. enterica | Mouse | DFI | 30 | |
| trpD3 | Anthranilate phosphoribosyl transferase (tryptophan biosynthesis) | R. solanacearum | Tomato | trpEG | 26 | |
| trpG | Anthranilate synthase β subunit (tryptophan biosynthesis) | V. cholerae | Mouse | RIVET | 191 | |
| trpR | Central regulator of tryptophan-related operons | S. flexneri | HeLa monolayer | cat | 14 | |
| pheA | Chorismate mutase-P/prephenate dehydratase (phenylalanine/tyrosine biosynthesis) | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| Cysteine biosynthesis | ||||||
| cysI | Sulfite reductase subunit | V. cholerae | Mouse | RIVET | 32 | |
| sseA | Thiosulfate sulfurtransferase | M. tuberculosis | Macrophages | DFI | 275 | |
| Histidine biosynthesis | ||||||
| hisA | Phosphoribosylformimino-5-aminoimidazole carboxamide | V. cholerae | Mouse | RIVET | 191 | |
| ribotide isomerase | ||||||
| hisB | Imidazole glycerol phosphate dehydratase | P. aeruginosa | Mouse | purEK | 290 | |
| hisB | Imidazole glycerol phosphate dehydratase | H. influenzae | Chinchilla | DFI | 164 | |
| Branched amino acid biosynthesis | ||||||
| ilvA | Threonine deaminase (isoleucine biosynthesis) | A. pleuropneumoniae | Pig | ribBAH | 76 | |
| ilvA | Threonine deaminase (isoleucine biosynthesis) | P. aeruginosa | Mouse | purEK | 290 | |
| ilvI | Acetolactate synthase subunit | A. pleuropneumoniae | Pig | ribBAH | 76 | |
| ilvI | Acetolactate synthase subunit | P. fluorescens | Sugar beet | dapB | 78 | |
| ilvl | Acetolactate synthase subunit | P. syringae | A. thaliana | hrcC | 16 | |
| Lysine biosynthesis | ||||||
| lysA | Diaminopimelate decarboxylase | K. pneumoniae | Mouse | galU | 133 | |
| lysA | Diaminopimelate decarboxylase | S. flexneri | Epithelial cells | DFI | 233 | |
| dcdA | Diaminopimelate decarboxylase (LysA homologue) | P. syringae | A. thaliana | hrcC | 16 | |
| dapC | N-Succinyldiaminopimelate aminotransferase | R. solanacearum | Tomato | trpEG | 26 | |
| Methionine biosynthesis | ||||||
| metL | Aspartokinase/homoserine reductase (homoserine biosynthesis) | Y. enterocolitica | Mouse | cat | 83 | |
| 50-55S3 | Homoserine-O-acetyltransferase | H. capsulatum | Macrophage | URA5 | 226 | |
| cysC | Homoserine-O-acetyltransferase | H. capsulatum | Mouse | URA5 | 226 | |
| metR | Transcription regulator (LysR family) | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| Proline biosynthesis | ||||||
| proC | Pyrroline-5-carboxylate reductase | M. tuberculosis | Macrophage | inhA | 56 | |
| proA | Glutamate-5-semialdehyde dehydrogenase | L. plantarum | Mouse | RIVET | 24 | |
| Threonine biosynthesis | ||||||
| thrC | Threonine synthase | S. suis | Pig | erm | 254 | |
| tdcA | Transcriptional activator of tdc operon | K. pneumoniae | Mouse | galU | 133 | |
| Glutamate/aspartate biosynthesis | ||||||
| gltB1 | Glutamate synthase (large subunit) | V. cholerae | Mouse | RIVET | 191 | |
| gdhA | Glutamate dehydrogenase | R. solanacearum | Tomato | trpEG | 26 | |
| ansA | Cytoplasmic l-asparaginase I | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| Amino acid degradation | ||||||
| gcvP | Glycine cleavage system (P protein) | R. solanacearum | Tomato | trpEG | 26 | |
| gcvH1 | Glycine cleavage system (H protein) | P. syringae | A. thaliana | hrcC | 16 | |
| gcsH1 | Glycine cleavage system (H protein) | L. plantarum | Mouse | RIVET | 24 | |
| Nucleotide synthesis | ||||||
| De novo synthesis of pyrimidine nucleotides | ||||||
| pyrG | CTP synthase | R. solanacearum | Tomato | trpEG | 26 | |
| dut | Deoxyuridine triphosphatase | M. tuberculosis | Macrophage | inhA | 56 | |
| rsuA | 16S rRNA pseudouridylate synthase | L. plantarum | Mouse | RIVET | 24 | |
| De novo synthesis of purine nucleotides | ||||||
| purF | Amidophosphoribosyltransferase | R. solanacearum | Tomato | trpEG | 26 | |
| purC | Phosphoribosylaminoimidazole-Succinocarboxamide synthase | S. pneumoniae | Mouse | DFI | 160 | |
| purE | Phosphoribosylaminoimidazole carboxylase subunit | H. influenzae | Chinchilla | DFI | 164 | |
| adk | Adenylate kinase | L. plantarum | Mouse | RIVET | 24 | |
| α-ivi12 | Antisense transcript— phosphoribosylglycinamide formyltransferase | P. gingivalis | Mouse | tet | 298 | |
| Salvage pathways | ||||||
| upp | Uracil phosphoribosyltransferase | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| Rsc0204 | Thymidine/pyrimidine-nucleoside phosphorylase | R. solanacearum | Tomato | trpEG | 26 | |
| udp-1 | Uridine phosphorylase | V. cholerae | Mouse | RIVET | 191 | |
| udk-dcd | Uridine kinase/dCTP deaminase | P. multocida | Mouse | kan | 108 | |
| xdhA | Xanthine dehydrogenase | R. solanacearum | Tomato | trpEG | 26 | |
| lp_0696 | Cytosine/adenosine deaminase | L. plantarum | Mouse | RIVET | 24 | |
| Peptide and protein synthesis | ||||||
| Nonribosomal peptide synthesis | ||||||
| P163 | Peptide synthetase | M. marinum | Macrophage | DFI | 11 | |
| Rsp1419 | Peptide synthetase | R. solanacearum | Tomato | trpEG | 26 | |
| Ribosomal synthesis | ||||||
| fusA2 | Elongation factor G | M. tuberculosis | Macrophage | inhA | 56 | |
| EF-Tu | Elongation factor Tu | L. monocytogenes | Macrophage | DFI | 295 | |
| rrf | Ribosome recycling factor | S. aureus | Mouse | RIVET | 152 | |
| prfB-N | Peptide chain release factor 2 (N-terminal fragment) | L. plantarum | Mouse | RIVET | 24 | |
| prfB-C | Peptide chain release factor 2 (C-terminal fragment) | L. plantarum | Mouse | RIVET | 24 | |
| α-prfC | Antisense transcript—peptide chain release factor 3 | R. solanacearum | Tomato | trpEG | 26 | |
| dbpA | DEAD-type RNA helicase | P. aeruginosa | Mouse | purEK | 290 | |
| tgt | Queuine-tRNA ribosyltransferase | Y. enterocolitica | Mouse | cat | 300 | |
| α-cii61 | Antisense transcript—16S rRNA gene | P. stutzeri | Rice | dapB | a | |
| Amino acid tRNA synthetases | ||||||
| alaS | Alanyl-tRNA synthetase | R. solanacearum | Tomato | trpEG | 26 | |
| argS | Arginyl-tRNA synthetase | M. marinum | Macrophage | DFI | 11 | |
| cysS | Cysteinyl-tRNA synthetase | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| glnS | Glutaminyl-tRNA synthetase | R. solanacearum | Tomato | trpEG | 26 | |
| pheST | Phenylalanyl-tRNA synthetase | S. enterica | Mouse | purA | 156 | |
| tyrS | Tyrosyl-tRNA synthetase | R. solanacearum | Tomato | trpEG | 26 | |
| gatA | Glu-tRNA (gln) amidotransferase (subunit A) | R. solanacearum | Tomato | trpEG | 26 | |
| Protein folding | ||||||
| ppiA | Peptidyl prolyl cis-trans isomerase A | K. pneumoniae | Mouse | galU | 133 | |
| groEL5 | Chaperonine | S. meliloti | Alfalfa nodules | bacA | 188 | |
| dsbB | Disulfide oxidoreductase | H. influenzae | Chinchilla | DFI | 164 | |
| dsbD | Thiol-disulfide interchange protein | P. multocida | Mouse | kan | 108 | |
| Protein degradation | ||||||
| pepN | Aminopeptidase N | R. solanacearum | Tomato | trpEG | 26 | |
| Rsp0196 | Prolyl aminopeptidase | R. solanacearum | Tomato | trpEG | 26 | |
| ipx41 | Carboxypeptidase | P. syringae | A. thaliana | hrcC | 16 | |
| Rsc1476 | Carboxypeptidase | R. solanacearum | Tomato | trpEG | 26 | |
| pepD1 | Dipeptidase | L. plantarum | Mouse | RIVET | 24 | |
| P238 | Zinc metalloprotease | M. marinum | Macrophage | DFI | 11 | |
| ipc017 | Zinc metalloprotease | S. pneumoniae | Mouse | DFI | 160 | |
| hreP | Protease | Y. enterocolitica | Mouse | cat | 300 | |
| IPC001 | Serine protease | S. pneumoniae | Mouse | DFI | 160 | |
| Rsp0603 | Serine protease | R. solanacearum | Tomato | trpEG | 26 | |
| Rsc3101 | Serine protease | R. solanacearum | Tomato | trpEG | 26 | |
| α-Rsc2654 | Antisense transcript—serine protease | R. solanacearum | Tomato | trpEG | 26 | |
| Rsc3101 | Serine protease | R. solanacearum | Tomato | trpEG | 26 | |
| lon | ATP-dependent protease | R. solanacearum | Tomato | trpEG | 26 | |
| clpC | ATP-dependent protease Clp (ATPase subunit) | L. plantarum | Mouse | RIVET | 24 | |
| Cofactor biosynthesis | ||||||
| Biotin biosynthesis | ||||||
| bioA | S-Adenosylmethionine-8-amino-7-oxononanoate aminotransferase | S. flexneri | Epithelial cells | DFI | 233 | |
| bioA | S-Adenosylmethionine-8-amino-7-oxononanoate aminotransferase | V. cholerae | Mouse | RIVET | 191 | |
| Rsc0082 | S-Adenosylmethionine-8-amino-7-oxononanoate aminotransferase | R. solanacearum | Tomato | trpEG | 26 | |
| bioH | Biotin synthase | Y. enterocolitica | Mouse | cat | 83 | |
| birA2 | Repressor of biotin ligase and biotin operon | L. plantarum | Mouse | RIVET | 24 | |
| Fe-S cluster biosynthesis | ||||||
| sufA | Fe-S cluster maturation protein | S. flexneri | Epithelial cells | DFI | 233 | |
| nifS | Cysteine desulfurase (synthesis nitrogenase metallocluster) | S. meliloti | Alfalfa nodules | bacA | 188 | |
| Nucleotide cofactor biosynthesis | ||||||
| nadE | NH3-dependent NAD+ synthetase | P. gingivalis | Mouse | tet | 298 | |
| Rsc2193 | Nicotinate nucleotide adenylyltransferase | R. solanacearum | Tomato | trpEG | 26 | |
| ribB | 3,4-Dihydroxy-2-butanone 4-phosphate synthase | H. influenzae | Chinchilla | DFI | 164 | |
| ribC2 | Bifunctional riboflavin kinase and FMN adenylyltransferae | L. plantarum | Mouse | RIVET | 24 | |
| pncA | Pyrazinamidase/nicotinamidase | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| Tetrapyrrole synthesis | ||||||
| hemA | Heme synthesis | S. enterica | Mouse | purA | 98 | |
| hemB | δ-Aminolevulinic acid dehydratase | R. solanacearum | Tomato | trpEG | 26 | |
| hemD | Heme synthesis | Y. enterocolitica | Mouse | cat | 300 | |
| cii-11 | Putative tetrapyrrole methylase (PA4422 homologue) | P. stutzeri | Rice | dapB | 224 | |
| Thiamine biosynthesis | ||||||
| thiE | Thiamine phosphate pyrophosphatase | R. leguminosarum | Pea nut | DFI | 3 | |
| thiF | Adenylation of ThiS | V. cholerae | Mouse | RIVET | 191 | |
| ipx45 | Lipoprotein | P. syringae | A. thaliana | hrcC | 16 | |
| Biosynthesis of other cofactors | ||||||
| moeZ | Putative molybdopterin biosynthetic enzyme | M. tuberculosis | Macrophages | DFI | 275 | |
| nadB2 | l-Aspartate oxidase (quinolinate biosynthesis) | R. solanacearum | Tomato | trpEG | 26 | |
| HI1647 | Pyridoxine biosynthesis protein | H. influenzae | Chinchilla | DFI | 164 | |
| folB | Dihydroneopterin aldolase (folate biosynthesis) | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| SPIV021 | Flavodoxin | S. pneumoniae | Mouse | DFI | 160 | |
| Conversion of miscellaneous or unknown compounds | ||||||
| dhaT | 1,3-Propanediol dehydrogenase | L. plantarum | Mouse | RIVET | 24 | |
| mdcA | Malonate decarboxylase | P. fluorescens | Sugar beet | dapB | 78 | |
| glcF | Glycolate oxidase (Fe-S subunit) | R. solanacearum | Tomato | trpEG | 26 | |
| gph | Phosphoglycolate phosphatase | R. solanacearum | Tomato | trpEG | 26 | |
| rhi-4 | MorB-like reductase (complex N-compounds) | P. fluorescens | Sugar beet | panB | 218 | |
| adhE | Bifunctional alcohol and acetaldehyde dehydrogenase | L. plantarum | Mouse | RIVET | 24 | |
| vanR | Transcriptional regulator of GntR family (aromatic compounds) | P. fluorescens | Sugar beet | dapB | 78 | |
| pcaC | Carboxymuconolactone decarboxylase (aromatic compounds) | P. stutzeri | Rice | dapB | 224 | |
| gabD1 | Succinate semialdehyde dehydrogenase (putrescine degradation) | R. solanacearum | Tomato | trpEG | 26 | |
| SPIV022 | 4-Oxalocrotonate tautomerase | S. pneumoniae | Mouse | DFI | 160 | |
| ipx 39 | Haloacid dehalogenase-like hydrolase | P. syringae | A. thaliana | hrcC | 16 | |
| pup-28 | Dioxygenase | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| yiaK | Putative dehydrogenase | P. multocida | Mouse | kan | 108 | |
| rhi-74 | Putative amidohydrolase (plant nitrilase-like) | P. fluorescens | Sugar beet | dapB | 78 | |
| 39B6 | MocC-like oxidoreductase (opine-like compounds) | B. abortus | Macrophage | DFI | 62 | |
| Energy metabolism | ||||||
| Rsc0087 | NADH dehydrogenase | R. solanacearum | Tomato | trpEG | 26 | |
| nex8 | NADH-ubiquinone oxidoreductase subunit | S. meliloti | Alfalfa nodules | bacA | 188 | |
| ivs-18 | NADH oxidase | S. suis | Pig | erm | 254 | |
| Rsc1280 | Transmembrane 4Fe-S ferredoxin | R. solanacearum | Tomato | trpEG | 26 | |
| α-Rsc0329 | Antisense transcript—ferredoxin 2Fe-S | R. solanacearum | Tomato | trpEG | 26 | |
| atpD | ATP synthase subunit | S. aureus | Mouse | RIVET | 152 | |
| phaZ | PHB depolymerase | R. solanacearum | Tomato | trpEG | 26 | |
| Class IV: genes involved in stress response and adaptation | ||||||
| Oxidative stress | ||||||
| Glutathione and thioredoxin metabolism | ||||||
| gsh | Glutathione synthetase | Y. enterocolitica | Mouse | cat | 300 | |
| Rsc2501 | γ-Glutamyltranspeptidase | R. solanacearum | Tomato | trpEG | 26 | |
| lsfA | Glutathione peroxidase | P. fluorescens | Sugar beet | panB | 218 | |
| orf6-iviM | Glutathione reductase | S. gordonii | Rabbit | cat | 127 | |
| ydhD | Glutaredoxin | Y. enterocolitica | Mouse | cat | 300 | |
| trxC | Thioredoxin II | V. cholerae | Mouse | RIVET | 191 | |
| ykmA | Glutathione peroxidase | P. fluorescens | Sugar beet | panB | 218 | |
| Peroxidases/catalases | ||||||
| bcp | Bacterioferritin co-migratory protein (peroxidase) | R. solanacearum | Tomato | trpEG | 26 | |
| bcp | Peroxidase | P. stutzeri | Rice | dapB | 224 | |
| Rsc2800 | Peroxidase | R. solanacearum | Tomato | trpEG | 26 | |
| nex1 | Peroxiredoxin | S. meliloti | Alfalfa nodules | bacA | 188 | |
| ohr | Organic hydroperoxide reductase | A. pleuropneumoniae | Pig | ribBAH | 76 | |
| catF | Catalase | P. syringae | A. thaliana | hrcC | 16 | |
| Other | ||||||
| Rsp1530 | l-Ascorbate oxidase | R. solanacearum | Tomato | trpEG | 26 | |
| msrA | Peptide methionine sulfoxide reductase | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| msrB | Peptide methionine sulfoxide reductase | L. reuteri | Mouse | erm | 285 | |
| orf4-iviK | Peptide methionine sulfoxide reductase | S. gordonii | Rabbit | cat | 127 | |
| vc1500 | PqiA family protein | V. cholerae | Mouse | RIVET | 191 | |
| pqiB | Paraquat-inducible protein | P. fluorescens | Sugar beet | dapB | 78 | |
| oxyR | Regulator (LysR family) | S. suis | Pig | erm | 254 | |
| soxR | Two-component response regulator | P. aeruginosa | Burned mouse | purEK | 92 | |
| indA | Indigoidine biosynthesis protein IndA | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| Acid tolerance | ||||||
| cadA | Lysine decarboxylase; cadaverine synthesis | V. cholerae | Rabbit, mouse | RIVET | 32,174 | |
| cadC | Transcriptional regulator of cadaverine synthesis (OmpR family) | S. enterica | Mouse | purA | 98 | |
| speF | Ornithine decarboxylase (polyamine synthesis) | P. multocida | Mouse | kan | 108 | |
| spe2 | S-Adenosylmethionine decarboxylase (polyamine synthesis) | H. capsulatum | Mouse | URA5 | 226 | |
| ureH | Urease accessory protein | H. influenzae | Chinchilla | DFI | 164 | |
| Osmoregulation | ||||||
| mdoG | Synthesis of periplasmic β-glucans | R. solanacearum | Tomato | trpEG | 26 | |
| mdoH | Synthesis of periplasmic β-glucans | Y. enterocolitica | Mouse | cat | 300 | |
| ndvB | Cyclic β-(1,2) glucan synthase | R. leguminosarum | Peanut | DFI | 3 | |
| otsA | Trehalose synthetase | S. enterica | Mouse | purA | 98 | |
| Low oxygen | ||||||
| nrfE | Formate-dependent nitrite reduction protein | P. multocida | Mouse | kan | 19,108 | |
| hemB | Porphobilinogen synthase | S. flexneri | HeLa monolayer | cat | 14 | |
| nirT | Respiratory nitrite reductase | V. cholerae | Mouse | RIVET | 32 | |
| Rsc2859 | Putative formate dehydrogenase | R. solanacearum | Tomato | trpEG | 26 | |
| Detoxification | ||||||
| Metal ion efflux and homeostasis | ||||||
| cutC | Copper homeostasis protein | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| copA | Copper transporting ATPase | L. plantarum | Mouse | RIVET | 24 | |
| copA | Copper transporting ATPase | S. aureus | Mouse | RIVET | 152 | |
| copRS | Two-component response regulator of copper resistance | P. fluorescens | Sugar beet | panB | 218 | |
| ragC | Cation efflux protein | P. fluorescens | Sugar beet | panB | 218 | |
| lp_3288 | Cation efflux protein | L. plantarum | Mouse | RIVET | 24 | |
| Rsp1597 | Putative cation efflux pump | R. solanacearum | Tomato | trpEG | 26 | |
| czcS | Two-component sensor kinase for regulation of Zn, Co, Cd resistance | R. solanacearum | Tomato | trpEG | 26 | |
| iviX | Heavy metal transport | S. enterica | Mouse | cat | 98 | |
| ipx33 | Metal transporting P-type ATPase | P. syringae | A. thaliana | hrcC | 16 | |
| Antibiotic resistance | ||||||
| Rsc1323 | Multidrug resistance protein | R. solanacearum | Tomato | trpEG | 26 | |
| P155 | Drug efflux pump | M. marinum | Macrophage | DFI | 11 | |
| lp_3303 | Multidrug transport protein | L. plantarum | Mouse | RIVET | 24 | |
| acrA | Periplasmic component of RND-type efflux pump | R. solanacearum | Tomato | trpEG | 26 | |
| acrF | Transmembrane protein of RND-type efflux pump | P. fluorescens | Sugar beet | dapB | 78 | |
| acrR | Transcriptional regulator (TetR) | Y. enterocolitica | Mouse | cat | 300 | |
| rosA | Transmembrane protein (fosmidomycin resistance) | P. fluorescens | Sugar beet | panB | 218 | |
| α-Rsc3205 | Antisense transcript—transmembrane protein | R. solanacearum | Tomato | trpEG | 26 | |
| aph | Aminoglycoside 3′ phosphotransferase | M. tuberculosis | Macrophage | inhA | 56 | |
| ydhC | Drug resistance protein | Y. ruckeri | Fish | cat | 65 | |
| pmrB | Regulator (polymyxin resistance) | S. enterica | Mouse | cat | 90,98 | |
| Methylglyoxal detoxification | ||||||
| ycbL | Glyoxylase II enzym family | P. fluorescens | Sugar beet | panB | 218 | |
| glxI | Lactoylglutathione lyase | B. abortus | Macrophage | DFI | 62 | |
| Bacteriocin autoimmunity | ||||||
| plnI | Bacteriocin (plantaricin) immunity protein | L. plantarum | Mouse | RIVET | 24 | |
| Other stress-related proteins | ||||||
| cspD | Cold shock-like protein | H. influenzae | Chinchilla | DFI | 164 | |
| hslU | Stress-inducible protease (ATPase component) | S. enterica | Macrophages | DFI | 279 | |
| hslU | Stress-inducible protease (ATPase component) | R. solanacearum | Tomato | trpEG | 26 | |
| grpE | Heat shock protein 24 | R. solanacearum | Tomato | trpEG | 26 | |
| yhbH | RpoN modulator protein; nutritional adaptation | P. stutzeri | Rice | dapB | 224 | |
| ynaF | Universal stress protein | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| ndk | Nucleoside diphosphate kinase (alarmone synthesis) | S. enterica | Mouse | purA | 98 | |
| dinF | DNA damage-inducible transmembrane protein (SOS response) | R. solanacearum | Tomato | trpEG | 26 | |
| Class V: genes involved in regulation | ||||||
| Two-component regulatory systems | ||||||
| vsrB | Regulator | R. solanacearum | Tomato | trpEG | 26 | |
| vieB | Regulator | V. cholerae | Mouse | RIVET | 32 | |
| phoP | Regulator | S. enterica | Mouse | purA | 98 | |
| ivs-25 | Regulator | S. suis | Pig | erm | 254 | |
| pehR | Regulator | R. solanacearum | Tomato | trpEG | 26 | |
| sapR | Regulator | S. suis | Pig | erm | 254 | |
| gacA | Regulator | Y. enterocolitica | Mouse | cat | 83 | |
| Rsc1597 | Regulator (LuxR-like) | R. solanacearum | Tomato | trpEG | 26 | |
| vsrD | Regulator (LuxR-like) | R. solanacearum | Tomato | trpEG | 26 | |
| ipx48 | Sensor | P. syringae | A. thaliana | hrcC | 16 | |
| ipx49 | Sensor | P. syringae | A. thaliana | hrcC | 16 | |
| vc0622 | Sensor | V. cholerae | Mouse | RIVET | 191 | |
| α-phoR | Antisense transcript—sensor (virulence) | L. monocytogenes | Mouse | hly | 55 | |
| Transcriptional regulators | ||||||
| argR | Repressor (AraC family) | H. influenzae | Chinchilla | DFI | 164 | |
| iviG | Regulator (AraC family) | S. gordonii | Rabbit | cat | 127 | |
| lp_3646 | Regulator (AraC family) | L. plantarum | Mouse | RIVET | 24 | |
| glpR | Repressor (DeoR family) | H. influenzae | Chinchilla | DFI | 164 | |
| cpsY | Regulator (LysR family) | S. suis | Pig | erm | 254 | |
| Rsc2094 | Regulator (LysR family) | R. solanacearum | Tomato | trpEG | 26 | |
| Rsp1574 | Regulator (LysR family) | R. solanacearum | Tomato | trpEG | 26 | |
| yeiE | Regulator (LysR family) | Y. enterocolitica | Mouse | cat | 300 | |
| tfdT | Regulator (LysR family) | P. aeruginosa | Mouse | purEK | 290 | |
| Rsp1644 | Regulator (MarR family) | R. solanacearum | Tomato | trpEG | 26 | |
| srpS | Regulator (Crp family) | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| pup-24 | Regulator (Cro/CI family) | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| crp | CRP regulator | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| relB | RelB protein | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| nex7 | Regulator (TspO/MBR family) | S. meliloti | Alfalfa nodules | bacA | 188 | |
| rhi-1 | Putative repressor (DnrO-like) | P. fluorescens | Sugar beet | panB | 218 | |
| Rv0549c | Putative regulator | M. tuberculosis | Macrophage | inhA | 56 | |
| orf1-iviB | Regulator (Rgg-like) | S. gordonii | Rabbit | cat | 127 | |
| Rsc0280 | Regulator (MerR family) | R. solanacearum | Tomato | trpEG | 26 | |
| Rsc1997 | Regulator (GntR family) | R. solanacearum | Tomato | trpEG | 26 | |
| Serine/threonine protein kinases | ||||||
| iviJ | S. gordonii | Rabbit | cat | 127 | ||
| ppkA | P. aeruginosa | Mouse | purEK | 288, 290 | ||
| pkn2 | L. plantarum | Mouse | RIVET | 24 | ||
| α-lu1 | Antisense transcript | H. capsulatum | Mouse | URA5 | 226 | |
| Transcription factors | ||||||
| greA | Transcription elongation factor | R. solanacearum | Tomato | trpEG | 26 | |
| hrpA | ATP-dependent RNA helicase | R. solanacearum | Tomato | trpEG | 26 | |
| infC | Initiation factor 3 | M. tuberculosis | Macrophage | inhA | 56 | |
| rpoS | Sigma factor σ54 | R. solanacearum | Tomato | trpEG | 26 | |
| Rsp1668 | σ54-interacting protein | R. solanacearum | Tomato | trpEG | 26 | |
| himA | IHF subunit | S. enterica | Macrophages | DFI | 279 | |
| himA | IHF subunit | S. enterica | Macrophages | purA | 156 | |
| ihfB | IHF subunit | H. influenzae | Chinchilla | DFI | 164 | |
| nusG | Antitermination and rho-dependent termination | A. pleuropneumoniae | Pig | ribBAH | 76 | |
| bglG4 | Antitermination | L. plantarum | Mouse | RIVET | 24 | |
| map 25 | WhiB-like putative transcription factor | M. marinum | Macrophage | DFI | 221 | |
| Other | ||||||
| hfq | Global regulator | P. stutzeri | Rice | dapB | 224 | |
| hflX | Located in mutL-hfq superoperon | Y. enterocolitica | Mouse | cat | 300 | |
| ack-pta | Acetate kinase/phosphotransacetylase | P. multocida | Mouse | kan | 108 | |
| pta | Phosphotransacetylase | P. stutzeri | Rice | dapB | 224 | |
| agrA | Accessory gene regulator; virulence regulator | S. aureus | Mouse | RIVET | 152 | |
| aldR | Inhibitor of protein synthesis (cleavage of mRNA) | S. suis | Pig | erm | 254 | |
| cpdP | 3′-5′ cAMP phosphodiesterase | Y. enterocolitica | Mouse | cat | 300 | |
| vc0130 | c-di-GMP metabolism | V. cholerae | Mouse | RIVET | 191 | |
| pup-44 | Zn-finger-containing protein | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| Class VI: genes involved in cell envelope structure and modification | ||||||
| Peptidoglycan | ||||||
| sltY | Lytic transglycosylase | S. flexneri | HeLa monolayer | cat | 14 | |
| ipx10 | Lytic transglycosylase | P. syringae | A. thaliana | hrcC | 16 | |
| mtgA | Transglycosylase | B. abortus | Macrophage | DFI | 62 | |
| vc2487 | Transglycosylase | V. cholerae | Mouse | RIVET | 191 | |
| pup-36 | Membrane protein with C-terminal transglycosylase domain | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| yvyH | N-Acetyl glucosamine epimerase | L. monocytogenes | Mouse | hly | 55 | |
| murC | UDP-N-acetylmuramoyl-L-alanine synthetase | S. aureus | Mouse | RIVET | 152 | |
| atl | N-Acetyl-muramoyl-L-alanine amidase | S. aureus | Mouse | RIVET | 152 | |
| Rv3717 | N-Acetyl-muramoyl-L-alanine amidase | M. tuberculosis | Macrophage | inhA | 56 | |
| ampG | Muropeptide transporter | P. syringae | A. thaliana | hrcC | 16 | |
| pup-33 | Periplasmic murein peptide-binding protein of ABC transporter | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| pbp2 | Penicilline-binding protein 2 | S. aureus | Mouse | RIVET | 152 | |
| yrbH | Putative sugar isomerase | S. flexneri | HeLa monolayer | cat | 14 | |
| LPS/EPS biosynthesis | ||||||
| orf3-iviF | ADP-l-glycero-d-mannoheptose-6-epimerase | S. gordonii | Rabbit | cat | 127 | |
| rffG | dTDP-glucose 4,6-dehydratase | Y. enterocolitica | Mouse | cat | 83 | |
| manB | Phosphomannomutase | Y. enterocolitica | Mouse | cat | 83 | |
| mrp | Putative ATPase | A. pleuropneumoniae | Pig | ribBAH | 76 | |
| rfb | Lipopolysaccharide synthesis | S. enterica | Mouse | purA | 156 | |
| algA | Alginate biosynthesis | P. syringae | A. thaliana | hrcC | 16 | |
| Adhesion/invasion | ||||||
| hag2 | Hemagglutinin | P. aeruginosa | Mouse, rat | purA | 93 | |
| hagB | Hemagglutinin | P. gingivalis | Mouse | tet | 141 | |
| hagC | Hemagglutinin | P. gingivalis | Mouse | tet | 141 | |
| iviIV | Putative hemagglutinin | Y. ruckeri | Fish | cat | 65 | |
| iviVI-A | Tia-like protein | S. enterica | Mouse | cat | 98 | |
| iviVI-B | PfEMP1-like protein | S. enterica | Mouse | cat | 98 | |
| flpA | Fibronectin/fibrinogen-binding protein | S. suis | Pig | erm | 254 | |
| nex18 | Putative fasciclin-like adhesion molecule | S. meliloti | Alfalfa nodules | bacA | 188 | |
| iviXIII | TadD-like protein | Y. ruckeri | Fish | cat | 65 | |
| tcpA | Pilin subunit of toxin-coregulated pilus | V. cholerae | Mouse | RIVET | 139 | |
| Outer membrane protein (gram-negative bacteria) | ||||||
| lolA | Outer membrane lipoprotein carrier protein | H. influenzae | Chinchilla | DFI | 164 | |
| pcp | Lipoprotein | P. multocida | Mouse | kan | 108 | |
| lppM | Lipoprotein | M. tuberculosis | Macrophage | inhA | 56 | |
| lppB | Lipoprotein | H. influenzae | Chinchilla | DFI | 164 | |
| Rsc1627 | Lipoprotein | R. solanacearum | Tomato | trpEG | 26 | |
| sif15 | Outer membrane protein | Y. enterocolitica | Mouse | cat | 83,300 | |
| ompW | Outer membrane protein | R. solanacearum | Tomato | trpEG | 26 | |
| pup-38 | Outer membrane protein | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| wssE | Cellulose synthase subunit | P. fluorescens | Sugar beet | dapB | 78 | |
| vca1008 | Porin | V. cholerae | Mouse | RIVET | 32,192 | |
| oprD2 | Porin | P. putida | P. parasitica | pyrB | 140 | |
| α-oprE | Antisense transcript—porin | P. aeruginosa | Mouse | purEK | 290 | |
| Cell surface proteins (gram-positive bacteria) | ||||||
| lp_0800 | L. plantarum | Mouse | RIVET | 24 | ||
| lp_2940 | L. plantarum | Mouse | RIVET | 24 | ||
| lp_1403 | L. plantarum | Mouse | RIVET | 24 | ||
| lp_0141 | L. plantarum | Mouse | RIVET | 24 | ||
| Class VII: genes involved in virulence and secretion | ||||||
| Secretion | ||||||
| SRP pathway | ||||||
| tig | Trigger factor | P. fluorescens | Sugar beet | panB | 218 | |
| General secretory pathway | ||||||
| ftsY | Docking protein | P. aeruginosa | Mouse, rat | purA | 93 | |
| secA | Translocation ATPase | S. meliloti | Alfalfa nodules | bacA | 188 | |
| secE | Cytoplasmic membrane protein | A. pleuropneumoniae | Pig | ribBAH | 76 | |
| outFG | Component of Out system | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| gspK | Type II secretory protein | R. solanacearum | Tomato | trpEG | 26 | |
| spsAB | Signal peptidase | S. aureus | Mouse | RIVET | 152 | |
| orf104 | Signal peptidase | L. monocytogenes | Mouse | hly | 55 | |
| α-cg316 | Antisense transcript—signal peptidase | L. monocytogenes | Mouse | hly | 55 | |
| TTSS structural components | ||||||
| ssaH | S. enterica | Macrophages | DFI | 279 | ||
| hrcC | R. solanacearum | Tomato | trpEG | 26 | ||
| hrpK | P. syringae | A. thaliana | hrcC | 16 | ||
| hrpJ | P. syringae | A. thaliana | hrcC | 16 | ||
| hrpG | P. syringae | A. thaliana | hrcC | 16 | ||
| hrcQb | P. syringae | A. thaliana | hrcC | 16 | ||
| hrpA | P. syringae | A. thaliana | hrcC | 16 | ||
| hrpA | E. chrysanthemi | Spinach | gfp-LA | 299 | ||
| hrpB | E. chrysanthemi | Spinach | gfp-LA | 299 | ||
| hrcC | P. fluorescens | Sugar beet | panB | 218 | ||
| TTSS effector proteins | ||||||
| pipB | Glycolipid biosynthesis | S. enterica | Mouse | DFI | 30 | |
| sifA | Essential virulence factor | S. enterica | Mouse | DFI | 30 | |
| avrPphD | P. syringae | A. thaliana | hrcC | 16 | ||
| avrPpiB | P. syringae | A. thaliana | hrcC | 16 | ||
| virPphA | P. syringae | A. thaliana | hrcC | 16 | ||
| dspA | E. chrysanthemi | Spinach | gfp-LA | 299 | ||
| dspE | E. chrysanthemi | Spinach | gfp-LA | 299 | ||
| TTSS regulatory proteins | ||||||
| ptrA | Inhibitor of transcriptional activator ExsA | P. aeruginosa | Burned mouse | purEK | 91, 92 | |
| Virulence factors | ||||||
| yhdP | Hemolysin-like protein (erythrocyte lysis) | L. monocytogenes | Macrophage | DFI | 295 | |
| shlB | Hemolysin activator protein | Y. ruckeri | Fish | cat | 65 | |
| plcA | Phosphatidylinositol phospholipase C | L. monocytogenes | Mouse | hly | 55 | |
| ispA | Hemolysin A homologue | L. plantarum | Mouse | RIVET | 24 | |
| ctxA | Catalytical subunit of CT (toxine) synthesis | V. cholerae | Mouse | RIVET | 139 | |
| spvB | Virulence factor | S. enterica | Mouse | cat | 98 | |
| mig-5 | Virulence plasmid lipoprotein | S. enterica | Macrophages | DFI | 279 | |
| α-mviM | Antisense transcript—virulence factor | L. monocytogenes | Mouse | hly | 55 | |
| actA | Actin recruitement and polymerisation protein | L. monocytogenes | Macrophage | DFI | 295 | |
| exc | Plasmid extrusion protein (TraT-like) | S. enterica | Macrophages | DFI | 279 | |
| ivi11 | Immunoreactive antigen | P. gingivalis | Mouse | tet | 298 | |
| lpxA | Acyltransferase | Y. enterocolitica | Mouse | cat | 300 | |
| plyD | Pectin lyase | P. syringae | A. thaliana | hrcC | 16 | |
| pme | Membrane-bound pectinesterase | R. solanacearum | Tomato | trpEG | 26 | |
| ogl | Oligogalacturonate lyase | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| syrE | Syringomycin synthetase (phytotoxin production) | P. syringae | A. thaliana | hrcC | 16 | |
| cfl-cfa | Coronatine biosynthesis (phytotoxin production) | P. syringae | A. thaliana | hrcC | 16 | |
| pup-29 | MsgA-like virulence protein | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| ivs-21 | Extracellular protein | S. suis | Pig | erm | 254 | |
| SAP2 | Secreted protease | C. albicans | Mouse | RIVET | 262 | |
| Rv2224c | Secreted protease | M. tuberculosis | Macrophage | inhA | 56 | |
| map 24 | PE-PGRS virulence protein | M. marinum | Macrophage | DFI | 221 | |
| map 85 | PE-PGRS protein | M. marinum | Macrophage | DFI | 221 | |
| vc2621 | Extracellular nuclease-related protein | V. cholerae | Mouse | RIVET | 191 | |
| iaaM | Tryptophan 2-monooxygenase (auxin biosynthesis) | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| vc1619.1 | Putative RTX toxin | V. cholerae | Mouse | RIVET | 191 | |
| Regulation | ||||||
| bvrA | Antiterminator of bvrBC, which encodes β-glucoside-specific permease | L. monocytogenes | Macrophage | DFI | 295 | |
| vacB | RNA processing | S. enterica | Mouse | purA | 98 | |
| vacC | RNA processing | S. enterica | Mouse | purA | 98 | |
| vacC | RNA processing | Y. enterocolitica | Mouse | cat | 300 | |
| chvD | Two-component response regulator | S. enterica | Mouse | purA | 98 | |
| virB | Regulator virulence genes (ParB-type) | S. flexneri | HeLa monolayer | cat | 14 | |
| Class VIII: genes involved in nucleic acid metabolism | ||||||
| DNA synthesis | ||||||
| iviC | Subunit of DNA polymerase III | S. gordonii | Rabbit | cat | 127 | |
| dnaN | Subunit of DNA polymerase III | R. solanacearum | Tomato | trpEG | 26 | |
| dnaX | Subunit of DNA polymerase III | L. plantarum | Mouse | RIVET | 24 | |
| traI | Relaxase | Y. ruckeri | Fish | cat | 65 | |
| DNA topology | ||||||
| gyrA | Subunit of DNA gyrase | K. pneumoniae | Mouse | galU | 133 | |
| topB | DNA topoisomerase III | L. monocytogenes | Mouse | hly | 77 | |
| RTI004 | DNA topoisomerase IV | S. pneumoniae | Mouse | DFI | 160 | |
| rhlB | Helicase | Y. enterocolitica | Mouse | cat | 83 | |
| helA | Helicase | P. fluorescens | Sugar beet | dapB | 302 | |
| helB | Helicase | P. fluorescens | Sugar beet | dapB | 302 | |
| helC | Helicase | P. fluorescens | Sugar beet | dapB | 302 | |
| DNA repair | ||||||
| alkA | Methyladenine DNA glycosylase | S. flexneri | HeLa monolayer | asd | 244 | |
| udg | Uracil DNA glycosylase | L. monocytogenes | Macrophage | DFI | 295 | |
| mutL | DNA mismatch repair protein | Y. enterocolitica | Mouse | cat | 300 | |
| uvrA | Subunit exonuclease ABC | R. solanacearum | Tomato | trpEG | 26 | |
| uvrB | Subunit exonuclease ABC | P. gingivalis | Mouse | tet | 298 | |
| uvrC | Subunit exonuclease ABC | M. tuberculosis | Macrophage | inhA | 56 | |
| recB | Subunit of exodeoxyribonuclease | Y. enterocolitica | Mouse | cat | 300 | |
| recD | Subunit of exodeoxyribonuclease | S. enterica | Mouse | cat | 98 | |
| recG | DNA helicase | H. influenzae | Chinchilla | DFI | 164 | |
| DNA methylation | ||||||
| met | Methyltransferase | L. monocytogenes | Mouse | hly | 77 | |
| cl 136 | Methyltransferase | L. monocytogenes | Macrophage | DFI | 295 | |
| mtpS | DNA methylase | Y. enterocolitica | Mouse | cat | 300 | |
| RNA degradation | ||||||
| orn | Oligoribonuclease | P. fluorescens | Sugar beet | dapB | 303 | |
| rbn | RNase BN | V. cholerae | Mouse | RIVET | 191 | |
| Cell division | ||||||
| SPSpoJ | Chromosome segregation protein | S. pneumoniae | Mouse | DFI | 160 | |
| ispZ | Septation protein | H. influenzae | Chinchilla | DFI | 164 | |
| mukF | Chromosome partition protein | H. influenzae | Chinchilla | DFI | 164 | |
| crcB | Chromosome condensation protein | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| Class IX: genes involved in transposition and site-specific recombination | ||||||
| Mobile DNA elements | ||||||
| IS600 | Transposase (family 8) | S. flexneri | Epithelial cells | DFI | 233 | |
| tnpA | Transposase (family 11; IS4-like) | P. syringae | A. thaliana | hrcC | 16 | |
| ivs-8 | Transposase (family 11) | S. suis | Pig | erm | 254 | |
| tnpA | Transposase (family 17; IS200-like) | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| tnp | Transposase (Tn5-like) | Y. enterocolitica | Mouse | cat | 300 | |
| ivs-1 | Putative transposase | S. suis | Pig | erm | 254 | |
| iviA | Putative transposase | S. gordonii | Rabbit | cat | 127 | |
| issfl4 | Insertion sequence | S. flexneri | HeLa monolayer | cat | 14 | |
| tnpF | IS2-like | S. flexneri | HeLa monolayer | cat | 14 | |
| ivi5 | IS195-like | P. gingivalis | Mouse | tet | 298 | |
| gipA | IS891-like | S. enterica | Mouse | purA | 264 | |
| vgrG | Vgr-like protein (located on multicopy genetic elements) | E. chrysanthemi | Spinach | gfp-LA | 299 | |
| vgrG | Vgr-like protein (located on multicopy genetic elements) | B. pseudomallei | Macrophage | cat | b | |
| Integrases/recombinases | ||||||
| Rsp1303 | Integrase | R. solanacearum | Tomato | trpEG | 26 | |
| rinA | Integrase gene activator | S. aureus | Mouse | RIVET | 152 | |
| redF | Resolvase | P. aeruginosa | Mouse | purEK | 290 | |
| xerD | Site-specific DNA recombinase | P. syringae | Bean | metXW | 159 | |
| xerD | Site-specific DNA recombinase | B. abortus | Macrophage | DFI | 62 | |
| xerD | Site-specific DNA recombinase | V. cholerae | Mouse | RIVET | 191 | |
| codV | Integrase/recombinase | L. plantarum | Mouse | RIVET | 24 |
Overview of promoters of genes or operons that are upregulated in microorganisms during their interaction with a eukaryotic host, isolated with the promoter-trapping techniques IVET and DFI. The (predicted) cellular function of the corresponding microbial gene product(s) in the respective environmental niche is indicated. For each IVET study, the selection strategy is specified. Most assignments of genes and gene product functions are based on similarity to known genes as described in the original papers. Some of these assignments were further verified or updated through homology searches with available gene sequences. Genes subsequently shown to be required for interaction with a host are indicated in bold. Abbreviations: ABC, ATP-binding cassette; CRP, cAMP receptor protein; gfp-LA, GFP-based IVET leaf array; IHF, integration host factor; MCP, methyl-accepting chemotaxis protein; PHB, polyhydroxybutyrate; PTS, phosphotransferase system; SRP, signal recognition particle; TRAP-T, tripartite ATP-independent periplasmic transporter; TTSS, type III secretion system. a, H. Rediers, unpublished results; b, M. S. Thomas, unpublished results.
When interpreting the data from Table 2, one should be aware that (i) although the listed genes are upregulated in the wild, ecological significance has been unequivocally demonstrated by mutant analysis for relatively few, and (ii) most of the listed gene assignments are tentative identifications based on homology or the presence of characteristic domains in the putative gene products. However, these assignments are valuable to group the large number of promoter-trapped genes in functional categories relevant to bacterial physiology. Such classification allows identification of commonalities as well as differences in gene expression patterns for these widely different experimental systems, involving phylogenetically diverse pathogenic and nonpathogenic microorganisms residing in diverse complex environments, in particular plant or animal hosts. In the following, these functional classes are discussed in more detail, with emphasis on new insights that resulted from IVET or DFI studies.
Genes Involved in Chemotaxis and Motility
Table 2 shows that several chemotaxis- and motility-related genes are upregulated in various plant- and animal-colonizing bacteria during interaction with their host. For instance, the fliF gene is expressed in Streptococcus suis infecting piglets (254) and in Pseudomonas fluorescens colonizing sugar beet rhizosphere (78). Multiple copies (≈25) of the FliF protein are assembled into the cytoplasmic membrane-embedded MS-ring, which constitutes the core of the flagellar motor (268). The isolation of these fliF genes indicates that the flagellar machinery is important for gram-positive as well as for gram-negative bacteria to establish interactions with both animal and plant hosts. This conclusion from IVET studies is in line with the results from other studies. For efficient colonization of tomato roots by nonpathogenic Pseudomonas fluorescens, flagellum-driven chemotaxis is required (48). In several other cases where competition between several bacterial species exists, flagellum-mediated motility is shown to provide a specific advantage for bacteria (181). For instance, for Vibrio cholerae, it was shown that mutants with knockouts in a flagellar subunit gene (flaA) or in genes encoding flagellar motor proteins (motAB and motY) are severely affected in colonization of the mouse small intestine (137).
Besides providing motility, flagella are important for bacterial attachment to surfaces and are thus generally considered important virulence factors (45, 181). Therefore, it is not surprising that the flagellar subunit is recognized by the innate immune system in organisms as diverse as plants (306) and mammals (256). This implies that for a successful host infection to occur, a bacterial pathogen might need to suppress flagellar assembly after the initial interaction stage. This requires strictly coordinated temporal gene expression during the different stages of interaction. Antisense transcripts of target genes may be involved in this. A putative antisense α-flaA was identified in Vibrio cholerae by RIVET (32). Likewise, a putative antisense transcript of fliM, encoding the flagellar C-ring, is upregulated in Pseudomonas stutzeri A15 during rice root colonization (H. Rediers, unpublished data). It is known that some bacteria adapt their flagellation pattern in response to the environmental conditions they encounter (reviewed in references 73 and 170).
The apparent temporal expression/repression of flagellin synthesis may be coordinated with the expression/repression of the cognate chemosensory machinery (Che system). A cheY-containing transcriptional fusion was isolated by an IVET screening in Pseudomonas aeruginosa infecting mice (290) and Ralstonia solanacearum infecting tomato plants (26). CheY is the response regulator protein that, in its phosphorylated form, interacts with the switch machinery of the flagellar motor to change the direction or speed of rotation (269). In addition to the temporal regulation of flagellin genes, chemotaxis may be fine-tuned throughout the infection process by differential expression of methyl-accepting chemotaxis protein subsets and multiple motility-linked chemosensory systems that are present in many bacteria, such as Vibrio spp. (169), Pseudomonas spp. (66), and Rhodobacter spp. (163). In two IVET studies, putative antisense transcripts of chemotaxis-related genes, encoding both sensory (α-mcp) and signal transduction (α-cheV) proteins were identified in rice-colonizing Pseudomonas stutzeri (224) and Vibrio cholerae infecting mice, respectively (32). CheV encodes a chimeric protein containing a CheY-homologous domain. It has been shown that a Vibrio cholerae cheV mutant colonizes mice better than the wild type (32). This is in agreement with the reciprocal regulation of motility and virulence genes in Vibrio cholerae (80). Downregulating chemotaxis genes might increase infection efficiency by favoring the formation and maintenance of microcolonies (175).
IVET also revealed that a gene involved in type IV pilus biogenesis is upregulated in Ralstonia solanacearum during tomato infection (26). Type IV pili enable twitching motility, a pilus-based form of translocation used by pathogens to spread over the host tissue surface, and are therefore recognized as important virulence factors for a wide range of plant and animal pathogens. In addition, type IV pili are important for the formation of biofilms and fruiting bodies (165).
Genes Involved in Nutrient Scavenging
Homeostasis of iron and other metal ions.
In numerous promoter-trapping studies, irrespective of the chosen selection strategy, genes involved in siderophore-dependent and siderophore-independent iron uptake as well as other genes involved in metal ion scavenging were found to be induced in both gram-negative and gram-positive bacteria (Table 2). Siderophores are secreted to bind Fe(III) with high affinity (reviewed in reference 222). Genes involved in the biogenesis of different types of siderophores (aerobactin, enterobactin, pyoverdin, ruckerbactin, and yersiniabactin) display elevated expression levels during the life of different γ-proteobacterial species in a plant or animal host environment.
After iron chelation, ferrisiderophores are captured at the cell surface by specific high-affinity siderophore receptors. Consistent with the induction of siderophore biosynthesis, several genes encoding such receptors have been identified by IVET or DFI. The ferric hydroxamate receptor, encoded by fhuA, is induced in both Salmonella enterica serovar Typhimurium infecting mice (98) and Shigella flexneri infecting monolayers of human epithelial cells (233). In Porphyromonas gingivalis, a putative siderophore receptor encoded by ivi10 is specifically induced during infection of mice. Wu et al. (298) demonstrated that a Porphyromonas gingivalis ivi10 knockout mutant is outcompeted by the wild type during survival in the host and displays a reduced ability to cause infection. The active translocation of ferrisiderophores is Ton dependent and is driven by the proton motive force. Following TonB-dependent translocation, ferrisiderophores are finally transported into the cytoplasm by an ATP-binding cassette (ABC) transporter. Genes encoding the RupDGC transporter as well as other genes involved in ruckerbactin-mediated iron uptake (synthesis of siderophore and receptor and TonB-dependent translocation) were identified by IVET in Yersinia ruckeri infecting fish (65).
Upon infection, several pathogenic bacteria display upregulation of genes encoding siderophore-independent iron uptake systems, such as the Salmonella enterica serovar Typhimurium SitABCD transporter (305). Application of IVET revealed that the sitABCD operon is specifically induced during infection of mice. It was subsequently demonstrated that a Salmonella enterica serovar Typhimurium sit mutant is severely attenuated in infection of mice (114). The sitABCD operon encodes an ABC transport system that mediates iron and probably also manganese uptake (124). Erwinia chrysanthemi yfeA, which encodes a component of a Sit-homologous transport system, is upregulated during plant infection (299). Other promoter trap studies demonstrated that sitA and sitC homologues are specifically expressed in Shigella flexneri (233) and Yersinia enterocolitica (83), respectively, during infection of mice. Although a sitA mutation does not affect plaque formation by Shigella flexneri on monolayers of human intestinal epithelial cells, a sitA mutation in combination with other iron acquisition mutations shows additive effects in these plaque assays (234).
Some bacteria possess mechanisms for the uptake of siderophores that are produced by other species or for uptake of iron-containing host proteins such as transferrin or heme (232, 251). IVET revealed the upregulation of genes involved in hemin uptake (hmuS, hmuT, and hmuU) in spinach-infecting Erwinia chrysanthemi (299). The Burkholderia pseudomallei hemT gene, encoding a periplasmic hemin binding protein, was also shown to be induced during macrophage infection (M. S. Thomas, personal communication).
In Pseudomonas aeruginosa, the np20 gene, encoding a homologue of the ferric uptake regulator (Fur), was also found to be specifically expressed during infection of mice. Mutational analysis revealed that np20 is not essential for growth in vitro. However, compared to the wild type, the mutant strain is required in a much higher dose to cause similar lethality in mice (290). Besides regulation of iron uptake, the housekeeping Fur protein is also directly or indirectly involved in the regulation of a substantial number of other genes encoding proteins with remarkably diverse functions, including other regulators, proteins involved in adaptation to oxidative stress, and virulence factors such as exotoxin A (284). Likewise, it was shown that in the plant pathogen Erwinia chrysanthemi, besides expression of two high-affinity iron uptake systems, pectate lyase-mediated cell wall degradation is also under control of the Fur regulator (72).
The frequent isolation of genes related to iron homeostasis reflects the importance of iron for microbial growth. Animal pathogens reside in an environment low in iron ions because host proteins such as transferrin and lactoferrin bind iron with high affinity (236). These proteins also play a role in host protection against microbial infection at the mucosal surface by depletion of the available iron (291). Likewise, in certain soils, the plant rhizosphere is scarce in ferrous iron (149, 195). Because iron is essential for microbial growth, the animal pathogens and plant-associated bacteria deploy dedicated systems for high-affinity iron uptake to capture the available iron (287). Furthermore, in several plant and animal pathogens, expression of pathogenesis-related genes is linked to iron availability (72, 206, 228, 283, 284).
Several genes involved in scavenging other metal ions, such as Cu2+, Mn2+, Mg2+, K+ and Na+ were identified with IVET. Using DFI, the Streptococcus pneumoniae psaBCA operon, encoding a manganese uptake system, was identified as being specifically expressed in mice (160). The psa promoter is induced more than 10-fold, suggesting an important role during survival within the host. Moreover, psaB, psaC, and psaA mutants are not only growth retarded in medium low in manganese, but are also completely attenuated in infection of mice (161). The Streptococcus pneumoniae Psa permease also plays an important role in resistance to hydrogen peroxide and superoxide, in systemic infections, and in nasopharyngeal mouse colonization (168).
In Sinorhizobium meliloti, a potassium channel, encoded by nex10, is specifically induced in the nodules during alfalfa symbiosis. A nex10 mutant strain is less efficient in symbiotic nitrogen fixation (188). The nex10 gene product resembles the regulatory β-subunit of the eukaryotic voltage-gated potassium channels, but the exact function of this type of channel in prokaryotes is unknown. Possible roles in osmoregulation or pH adaptation during symbiosis have been suggested (177, 188).
Amino acid uptake.
Although most IVET and DFI studies focused on animal infection systems, genes involved in amino acid acquisition were predominantly isolated from plant-associated bacteria, suggesting that amino acids are available for uptake in the plant environment but much less so in animal hosts. The host-induced expression of genes for amino acid uptake systems was reported for pathogenic bacteria upon plant infection (16, 26) as well as for beneficial bacteria colonizing the plant rhizosphere (218). Part of the amino acids synthesized by plants is exuded into the rhizosphere (113). Although the amount of amino acids in tomato root exudates is not sufficient to sustain rapid growth of plant root-colonizing microorganisms, the amino acids are likely to be taken up and utilized during colonization (249). This is supported by the observation that Pseudomonas fluorescens shows chemotaxis towards amino acids present in these root exudates (48).
Acquisition of phosphorus.
IVET and DFI studies have revealed host-induced expression of systems for uptake of phosphorus in both animal- and plant-pathogenic bacteria. For instance, the Shigella flexneri pstS encodes an ABC transporter for high-affinity phosphate uptake that is specifically expressed during infection of mice. The Shigella flexneri pstS mutant strain constructed shows no growth difference in low-phosphate media but causes smaller plaques on macrophage monolayers, suggesting that the loss of pstS results in lower infection efficiency (233).
Uptake of sugars and carbohydrates.
Several bacterial sugar uptake systems, mostly sugar permeases and sugar-specific phosphotransferase systems (PTS) for monosaccharides (fructose, mannose, and ribose) and disaccharides (sucrose, cellobiose, and maltose), were found to be induced during interaction with several eukaryotic hosts. During passage through the mouse gastrointestinal tract, Lactobacillus plantarum seems to deploy a diverse set of PTS systems for uptake of sugars (24). Sugar uptake systems specifically associated with plant infection were revealed for Erwinia chrysanthemi (299). Mutation of rhiT, encoding a rhamnogalacturonide transporter, compromises the systemic invasion capability of this phytopathogen (299).
Three genes (dctS, dctD, and the dctS homologue Rsc1598) involved in the regulation of C4-dicarboxylate uptake were isolated with independent IVET screenings for the rhizosphere-colonizing Pseudomonas fluorescens (218) and from the phytopathogens Pseudomonas syringae (159) and Ralstonia solanacearum (26), respectively. C4-dicarboxylate metabolism is induced in the presence of dicarboxylates and is under the control of regulatory sensor mechanisms. The DctSR two-component regulatory system, which shows high similarity with the FixLJ oxygen sensor system in rhizobia, is necessary for aerobic growth on C4-dicarboxylates. The DctBD two-component regulatory system is functionally similar to the NtrBC regulatory system that activates expression from σ54-dependent promoters (115). In a similar, ATP-dependent way, DctBD activates expression of dctA, encoding a C4-dicarboxylate:cation (H+ or Na+) symporter, which is essential for symbiotic nitrogen fixation (115). C4-dicarboxylates such as malate and succinate are present in plants and root exudates (9) and are major carbon and energy sources for nitrogen-fixing symbionts (301). IVET studies highlight the significance of this catabolic pathway for other plant-associated bacteria as well.
Miscellaneous nutrients.
IVET studies have revealed a variety of other genes specifically expressed in the wild, encoding ABC transporters, porins, and permeases for uptake of diverse components, such as lactate, peptides, choline, and undefined molecules. The potential role of an ABC transporter (ATP-binding subunit RTI006) of Streptococcus pneumoniae was revealed by DFI (160). This transporter mediates choline transport and subsequent analysis showed that the Streptococcus pneumoniae RTI006 mutant strain shows decreased respiratory tract infection (160). In Staphylococcus aureus, choline and its degradation product, glycine betaine, constitute potent osmoprotectants (87, 231). Besides their involvement in uptake of nutrients, substrate-binding components of ABC transporters have also been shown to be implicated in the infection process of some pathogens by facilitating adhesion to host cells, as unequivocally demonstrated in Streptococcus gordonii (117) and Campylobacter jejuni (205).
Genes Involved in Central Intracellular Metabolism
Intermediary metabolic pathways.
As might be anticipated, IVET and DFI screenings revealed the host-induced expression of several genes involved in intermediary metabolic pathways such as the tricarboxylic acid (TCA) and glyoxylate cycles. The glyoxylate pathway enables bacteria to grow on acetate. Interestingly, two genes, aceA and aceB, of which the corresponding gene products catalyze subsequent steps of the glyoxylate pathway, were isolated with IVET from the animal pathogens Mycobacterium tuberculosis (56) and Yersinia enterocolitica (300), respectively. An independent SCOTS experiment equally showed that Mycobacterium tuberculosis cells contain more aceA transcript during macrophage infection (86). Expression of aceA and aceB may be linked to degradation of host lipids through fatty acid β-oxidation, generating acetyl-coenzyme A for subsequent use as a carbon source (39). Acetyl-coenzyme A entering the TCA or glyoxylate cycle is also generated from pyruvate by the pyruvate dehydrogenase complex. The genes encoding the subunits of this enzyme complex (aceE and pdhC) have been identified by IVET in Pseudomonas syringae upon Arabidopsis thaliana infection (16) and by DFI in macrophages infecting Mycobacterium marinum (11).
IVET also demonstrated that some TCA cycle genes are upregulated in the host environment. The TCA cycle is a major degradation pathway for generation of ATP but also provides intermediates for biosyntheses. For instance, fumC, encoding the fumarase enzyme, showed elevated expression in Listeria monocytogenes during infection of mice (77). Analysis of a Listeria monocytogenes fumC mutant strain revealed defective growth in phagocytes (77). It is worth noting that fumC was also identified in the IVET screening of Ralstonia solanacearum upon infection of tomato plants (26).
Reduced coenzymes produced by oxidative metabolism, such as the TCA cycle and fatty acid degradation, can be used to drive ATP synthesis via oxidative phosphorylation. The ATP synthase subunit gene atpD was isolated with IVET in a Staphylococcus aureus mouse infection model (152). Another gene involved in energy metabolism, pckA, was found to be up-regulated during Sinorhizobium meliloti-alfalfa symbiosis (188). The phosphoenolpyruvate carboxykinase PckA catalyzes the formation of phosphoenolpyruvate from oxaloacetate. It was previously shown that rhizobial pckA is strongly induced at the onset of stationary phase or during growth on succinate or arabinose as the sole carbon source, but pckA expression is also induced by host root exudates (193). A Rhizobium pckA mutant strain revealed a host-dependent symbiotic phenotype, as it lost its ability to establish nitrogen-fixing nodules only in some leguminous plants (194). Using IVET, pckA was also found to be induced in Mycobacterium tuberculosis during macrophage infection. The host-induced expression of pckA was subsequently confirmed with reverse transcription-PCR (56).
Lipid and fatty acid metabolism.
IVET, RIVET, and DFI screenings revealed that several microbial genes involved in lipid and fatty acid metabolism are upregulated in animal and plant host environments. Degradation of lipids from host immune cells might protect the pathogen against the host immune response, but, as outlined above, lipid degradation and subsequent utilization of the released fatty acids may also fulfill a nutritional role (32, 157). It was also proposed that pathogen lipases, in combination with fatty acid-modifying enzymes, could inactivate the bactericidal lipids that are produced in host tissue abscesses, thereby increasing survival in this niche (121). This is in agreement with the isolation of the Staphylococcus aureus lip gene, encoding a glycerol ester hydrolase, which is specifically induced in host tissue (152).
The upregulation of genes encoding a 3-hydroxyl-coenzyme A dehydrogenase in Mycobacterium tuberculosis infecting macrophages was demonstrated independently with IVET (56) and DFI (275). An unexpectedly large number of host-induced genes that are involved in fatty acid metabolism were identified by IVET in Mycobacterium tuberculosis, suggesting that fatty acid metabolism is extremely important during life in the host environment. Mycobacterium tuberculosis has an astonishingly large number of genes presumed to be involved in β-oxidation of fatty acids (39). It has been suggested that Mycobacterium tuberculosis is able to utilize fatty acids as a major energy source during infection, but it is also possible that fatty acid metabolism is necessary for remodeling the cell envelope upon macrophage infection, thereby evading the host immune response (56). Likewise, Mahan and coworkers found that fadB, which is required for β-oxidation of fatty acids, is upregulated in Salmonella enterica serovar Typhimurium upon infection of mice and ascribed this to the high concentration of fatty acids encountered by the pathogen during infection (157). FadB homologues were also found to be specifically expressed in Brucella abortus and Mycobacterium tuberculosis (62, 275).
In the plant pathogen Erwinia chrysanthemi, the transcriptional regulator EutR was isolated using IVET (299). This regulator controls the eut operon, which is required for ethanolamine utilization (243). Ethanolamine utilization is an important trait for efficient plant infection, since an Erwinia chrysanthemi eutR mutant displayed a decreased ability to cause systemic invasion in African violets (299). Ethanolamine, released during degradation of phospholipids, is also a carbon and energy source in the intestinal tract, and the eut operon is coregulated with expression of virulence genes (125, 135). In Salmonella enterica serovar Typhimurium, the operon involved in ethanolamine utilization is coregulated with genes involved in motility and with genes encoding a TTSS (125).
Carbohydrate metabolism.
Several of the genes listed in Table 2 are involved in sugar metabolism. One of these genes, xylA, encoding xylose isomerase, was identified in the sugar beet colonizer Pseudomonas fluorescens (218). Xylose is a typical plant-derived sugar commonly present in the plant rhizosphere. Xylan is the main carbohydrate found in the hemicellulosic fraction of plant tissues and is hydrolyzed by xylanases into xylose monomers that can be utilized by many bacteria and fungi as a primary carbon source. Xylanase producers are found in all ecological niches where plant material is deposited (210). It is therefore plausible that genes enabling Pseudomonas fluorescens to utilize xylose are switched on when the organism is residing in the rhizosphere. It is known that the XylR transcriptional regulator activates xylA expression in the presence of xylose (257). The IVET isolation of xylR in the plant pathogen Erwinia chrysanthemi indicates that xylose metabolism is also activated in planta (299). Notably, xylA was also isolated in the mouse gut colonizer Lactobacillus reuteri (285). We speculate that Lactobacillus reuteri xylA might be induced during survival in the gut by xylose originating from plant material in the mouse feed.
It was mentioned above that promoter traps enabled the isolation of several sugar uptake systems, such as the ribose transport proteins RbsD and RbsC, from Lactobacillus plantarum during passage in the gastrointestinal tract of mice (24) and Haemophilus influenzae infecting chinchilla, respectively (164). Likewise, other members of the ribose (rbs) operon, rbsR and rbsK, were isolated with IVET from Lactobacillus plantarum (24) and from Klebsiella pneumoniae (133), respectively, during life in the mouse host. RbsR is a transcriptional repressor of the rbs operon, which enables uptake and utilization of ribose. The rbsR gene is also upregulated in Salmonella enterica serovar Typhi during macrophage infection, as demonstrated with SCOTS (44). An rbsR mutant exhibits decreased survival in macrophages compared to the wild type, confirming the importance of rbsR induction during macrophage infection (44). Differences in the regulation (repression versus activation) of ribose metabolism in the different host environments might be explained by different sugar contents present in the spleen and intestine or experienced inside macrophages.
Amino acid synthesis.
The importance of amino acid uptake for bacterial life in the plant environment was discussed. Likewise, amino acid synthesis seems to be a key trait for interaction and/or survival in the host environment. Members of this class were found to be induced in microorganisms as diverse as the fungal pathogen Histoplasma capsulatum and gram-positive and various gram-negative bacteria during interactions with either plant or animal hosts. The overall requirement for amino acids is reflected in the independent isolation of functional homologues of biosynthetic genes, which is the case for argG, hisB, ilvA, ilvI, and lysA (Table 2).
The importance of amino acid metabolism is illustrated by the mutational analysis of the Vibrio cholerae argA gene, initially traced by RIVET, which encodes the N-acetyl glutamate synthase involved in de novo synthesis of arginine and proline. Camilli and Mekalanos showed that an argA mutant was severely outcompeted by the wild type during infection of mice (32). In an independent RIVET study, the Vibrio cholerae argH gene was also shown to be upregulated during infection of mice (191). On the other hand, a Vibrio cholerae cysI mutant did not show attenuated pathogenicity, suggesting that arginine biosynthesis plays a more important role in survival of Vibrio cholerae during infection of mice than sulfate assimilation for cysteine biosynthesis (32). Another promoter identified with IVET drives expression of the Salmonella enterica serovar Typhimurium carAB operon, which encodes carbamoylphosphate synthetase, involved in arginine and pyrimidine synthesis (156). Construction of a carAB mutant strain revealed that expression of this operon is critical for full virulence (156). SCOTS analysis of macrophage-infecting Mycobacterium avium also revealed carA expression (105). However, a putative antisense α-carA transcript was identified with IVET in Porphyromonas gingivalis (298).
Amino acid catabolism.
In contrast to the genes involved in amino acid synthesis that were isolated ubiquitously, only a few genes involved in amino acid degradation were found to be induced in the wild. Three genes (gcvP, gcvH1, and gcsH1) involved in glycine cleavage were specifically induced during plant infection by Ralstonia solanacearum (26) and Pseudomonas syringae (16) and during gastrointestinal tract transit of Lactobacillus plantarum (24), respectively.
Pseudomonas bacteria colonizing the rhizosphere of plants apparently also adapt their metabolism by activating specific catabolic pathways, as illustrated by the IVET identification of genes probably involved in the degradation of plant-derived compounds (78, 218, 224). In Ralstonia solanacearum, two genes involved in the metabolism of glycolate were found to be upregulated during tomato infection (26). Glycolate is produced during photorespiration (28), and it appears that this phytopathogen activates a catabolic pathway for using this plant-derived compound as a carbon source.
Nucleotide synthesis.
In several IVET studies with auxotrophy-based selection, knockouts in nucleotide biosynthesis genes (purA, purEK, pyrB, ura5, and thyA) were used as genetic background because the corresponding mutants were demonstrated to be severely attenuated in growth in the host environment (Table 1) (92, 93, 98, 140, 156, 226, 264, 290). It is therefore not surprising that application of promoter traps showed elevated expression of several genes involved in nucleotide metabolism in plant- or animal-colonizing or -infecting bacteria. These include genes involved in de novo nucleotide biosynthesis as well as genes involved in salvage pathways for pyrimidine or purine nucleotides.
Protein synthesis and degradation.
In class III, a subgroup of genes involved in protein synthesis can be distinguished. The repeated isolation of several genes encoding amino acid tRNA synthetases together with the gene for ribosomal recycling factor (Rrf) drew attention to the importance of the translation process. For instance, using DFI, the arginyl tRNA synthetase encoded by argS was shown to be upregulated in Mycobacterium marinum during macrophage infection (11). The same gene was isolated in Mycobacterium avium in a SCOTS screen (105).
In diverse microorganisms, genes involved in protein folding were identified. A thiol-disulfide interchange protein, encoded by dsbD, was isolated with IVET from Pasteurella multocida (108). DsbD is a membrane-bound enzyme that is responsible for maintaining DsbC in the reduced state by translocating electrons from thioredoxins in the cytoplasm. DsbC is a periplasmic disulfide bond isomerase/reductase that catalyzes the rearrangement of disulfide bonds. In this way, improper protein folding resulting from inaccurate disulfide bonds is corrected (119). The dsbD gene was also isolated by STM screening, demonstrating that a Pasteurella multocida dsbD mutant strain is attenuated in virulence (75). Interestingly, the dsbB gene of Haemophilus influenzae was also isolated by means of IVET (164). The membrane-bound DsbB transfers electrons to the respiratory chain to keep DsbA in the oxidized form. DsbA is a strong thiol oxidant that catalyzes disulfide bond formation of proteins that are exported to the periplasm (119). As proper folding, stability, or activity of extracellular proteins often relies on the formation of disulfide bonds, dsbB and dsbD may be involved in maturation and stability of virulence factors or secreted toxins and thereby play a role in pathogenesis. It is therefore not surprising that dsbA mutants of various pathogens display avirulent phenotypes (119, 204).
Conversely, IVET and DFI enabled the isolation of several genes involved in protein degradation. In Yersinia enterocolitica, a protease encoded by hreP was found to be upregulated during infection of mice. Mutational analysis revealed a strong reduction of competitiveness and virulence of the hreP mutant (300). The HreP protease shows significant similarity with eukaryotic subtilisin/kexin-like proprotein convertases and was probably acquired by horizontal gene transfer (100). Although mutational studies unequivocally demonstrated the importance of HreP in virulence, its precise role has yet to be elucidated. In several bacterial pathogens, proteases were identified as virulence factors (274).
Cofactor biosynthesis.
Another subgroup comprises the genes involved in the biosynthesis of several cofactors, such as biotin, ubiquinone, nucleotide cofactors, thiamine, and iron-containing cofactors such as heme and Fe-S clusters. For instance, three host-induced genes that are involved in heme biosynthesis (hemA, hemB, and hemD) were isolated with IVET from Salmonella enterica serovar Typhimurium (98), Ralstonia solanacearum (26), and Yersinia enterocolitica (300), respectively. It has been postulated that heme synthesis may add to protection against oxidative stress by serving as a catalase cofactor (98). The IVET-identified Sinorhizobium meliloti nifS gene encodes a cysteine desulfurase known to be required for nodulation (188). NifS supplies the inorganic sulfide for the formation of the Fe-S clusters in the nitrogenase enzyme, which catalyzes nitrogen fixation during symbiosis. Expression of the nitrogenase enzyme is tightly regulated and known to be specifically induced during symbiosis.
Genes Involved in Adaptation to Environmental Stresses
Oxidative stress.
IVET studies exploring bacterial life in various host environments have revealed a variety of mechanisms to cope with oxidative stress. Life in the presence of oxygen poses a permanent threat due to the generation of reactive oxygen species in biological systems (110). Furthermore, plants and animals harnessed this toxic potential as a biological weapon against invading microorganisms. During host infection, animal pathogens are frequently exposed to reactive oxygen species, such as superoxides, hydrogen peroxides, or organic peroxides, as a result of the release of lysosomal contents within inflammatory cells (29). It is also known that plant cells induce a series of defense responses against pathogens, including the generation of reactive oxygen species such as superoxide and hydrogen peroxide, also known as the oxidative burst (134).
Several IVET screens revealed genes playing a role in glutathione synthesis or glutathione reduction that are upregulated during interaction with plant or animal hosts. Glutathione is an important biomarker for oxidative stress. It plays a major role in protection against oxidative stress by dismantling free radicals but is also important for protection against other stresses, such as detoxification of hazardous chemicals or heavy metals (97, 107, 162).
Thiol-specific antioxidants comprise a broad class of antioxidant enzymes that are involved in protection against oxidative stress, and several members of this class were identified with promoter traps. Peroxiredoxins are widespread in the Archaea, Bacteria, and Eukarya and function as antioxidants by reducing peroxides, thereby preventing damage to biomolecules (297). The bacterioferritin comigratory protein encoded by bcp is a peroxiredoxin (118) that was found to be upregulated in both Ralstonia solanacearum during tomato infection (26) and Pseudomonas stutzeri during rice colonization (224). In Sinorhizobium meliloti, a peroxiredoxin, encoded by nex1, was shown to be involved in the symbiotic interaction with alfalfa, since nitrogen fixation is slightly impaired in a nex1 mutant strain. Using the gusA reporter contained in the promoter trap, Oke and Long (188) also showed that nex1 is expressed in a restricted zone of the nodule, more specifically in the nodule tip. This constituted the first evidence of bacterial antioxidant activity in nodules. Recently it was also shown that in Rhizobium etli, the peroxiredoxin PrxS is expressed during the symbiotic interaction with its host, thereby protecting Rhizobium etli cells in the nodules (51). The frequent IVET isolation of genes required for inactivation of reactive oxygen species indicates that for bacteria interacting with plants, oxidative damage of biomolecules represents a major form of stress. It can be seen from Table 2 that plant colonizers frequently express peroxidases or catalases for direct inactivation of peroxides, whereas only one example was found in an animal pathogen. The Actinobacillus pleuropneumoniae ohr gene, encoding an organic hydroperoxide reductase, is induced during pig infection. It was shown that ohr expression could be induced by organic peroxides such as cumene hydroperoxide but not by paraquat or hydrogen peroxide (240).
Peptide methionine sulfoxide reductases, encoded by msrA and msrB, are important antioxidant enzymes that mediate the repair of proteins damaged by sulfoxidation of methionine residues (1, 292). The genes are upregulated in both spinach-infecting Erwinia chrysanthemi (299) and mouse-infecting Lactobacillus reuteri (285).
Acid stress.
Gastrointestinal pathogens have to cope with acid stress during transit of the gastric acid barrier before colonizing the intestine. Polyamines, such as cadaverine and spermidine, play a role in the physiological adaptation process known as the acid tolerance response (71, 200). The transcriptional regulator cadC was isolated with IVET from Salmonella enterica serovar Typhimurium following intragastric infection (98). CadC controls expression of the cadaverine-generating lysine decarboxylase CadA, which is an important component of the acid tolerance response (200). Decarboxylation of lysine produces cadaverine and carbon dioxide, thereby consuming a cytoplasmic proton. Cadaverine is subsequently excreted and exchanged with lysine by the cadB-encoded lysine/cadaverine antiporter. The CadC-mediated upregulation of Salmonella enterica serovar Typhimurium cadA is in line with results from SCOTS which also showed that cadA is specifically expressed during macrophage infection by Salmonella enterica serovar Typhi. Analysis of a Salmonella enterica serovar Typhi cadA mutant exhibited decreased survival in macrophages, especially in the early stage of infection (44).
In addition, RIVET enabled the identification of Vibrio cholerae cadA as a gene induced during infection of mice (174). Vibrio cholerae is only weakly resistant to acid stress, and it was originally thought not to possess an acid tolerance response. However, using IVET, it was shown for the first time that cadA is upregulated in Vibrio cholerae during infection of mice. CadA was subsequently demonstrated to play an important role in the acid tolerance response in Vibrio cholerae. However, mutational analysis revealed that cadA is not essential for full virulence (174), probably because other amino acid decarboxylases are still functional and because amino acid decarboxylases represent only part of the overall acid tolerance response (70).
The speF gene encodes ornithine decarboxylase, which catalyzes synthesis of putrescine, thereby consuming a cytoplasmic proton. Expression of this gene in Pasteurella multocida during infection of mice was detected with IVET (108). Likewise, the spe2 gene was captured with IVET from mouse-infecting Haemophilus influenzae (226). This gene encodes a putative S-adenosylmethionine decarboxylase and is probably involved in increasing polyamine synthesis during infection.
Some polyamines, such as putrescine, are found in plant root exudates. During plant root colonization, Pseudomonas fluorescens upregulates a gene involved in putrescine uptake (potF2) (218). This observation is consistent with the ability of Pseudomonas fluorescens to utilize putrescine as sole nitrogen source (132). Degradation of putrescine during infection of tomato plants by Ralstonia solanacearum is suggested by the upregulation of a gabD1 homologue, encoding a putative succinate semialdehyde dehydrogenase (26). This phytopathogen may use this plant-derived compound as a carbon and nitrogen source, but at the same time, interference with the plant's defense system may be exerted. Putrescine is a precursor in the synthesis of spermine that has been implicated in the induction of the hypersensitive response (286).
Osmotic stress.
The Yersinia enterocolitica mdoH gene was found to be specifically induced during infection of mice (300). MdoH is involved in the synthesis of periplasmic β-glucans and might protect Yersinia enterocolitica against osmotic stress. It is known that periplasmic β-glucans, which form intrinsic components of the gram-negative bacterial envelope, improve survival in low-osmolarity environments (179). Periplasmic β-glucans are also required for full virulence of the plant pathogens Erwinia chrysanthemi (198) and Pseudomonas syringae (150).
A Ralstonia solanacearum mdoG homologue encoding a putative glucosyl transferase for β-1,2-glucan synthesis was identified during an IVET study of tomato infection (26). In Rhizobium leguminosarum it was demonstrated that the ndvB gene, which is involved in the synthesis of cyclic β-glucans, is specifically expressed during symbiosis (3). These low-molecular-weight cell surface carbohydrates are known to be required for root infection and are thought to function as suppressors of a host defense response. ndvB homologues are found almost exclusively in members of the Rhizobiaceae, such as Sinorhizobium meliloti, Agrobacterium tumefaciens, and Brucella abortus, and transcription of the ndvB gene is usually repressed in conditions of high osmolarity (21, 46). An ndvB homologue was recently identified in Pseudomonas aeruginosa, and it was demonstrated that a Pseudomonas aeruginosa ndvB mutant was not able to develop high-level biofilm-specific antibiotic resistance. Mah and coworkers (153) discovered that periplasmic glucans can interact with antibiotics and suggested that the antibiotics may thereby be arrested in the periplasm, preventing them from reaching their sites of action.
Detoxification by efflux systems.
Several host-induced genes are involved in divalent metal transport. Cation efflux pumps putatively involved in detoxification of cations and heavy metals were isolated independently with IVET in several bacteria during interaction with plant and animal hosts. For instance, a copper-transporting ATPase (CopA) is specifically expressed in Lactobacillus plantarum during colonization of the gastrointestinal tract (24) and Staphylococcus aureus during infection of mice (152). Although the exact biological role during interactions with plant or animal hosts is not well known, bacterial cation efflux pumps are widespread and allow detoxification when the heavy metal concentration in the cells reaches toxic levels (248).
Using IVET, homologues of genes encoding components of a resistance-nodulation-cell division (RND)-type multidrug efflux pump, acrF and acrA, were isolated from plant-associated Pseudomonas fluorescens (78) and Ralstonia solanacearum (26), respectively. In Escherichia coli, the acr genes are involved in acriflavine resistance. In Yersinia enterocolitica, the transcriptional regulator (acrR) of the acriflavine efflux pump was also shown to be expressed during infection of mice (300). In addition, drug efflux pumps and other proteins involved in antibiotic resistance were shown to be up-regulated in the host environment.
Finally, quite a few genes, encoding heat shock proteins and proteins involved in general stress response, were isolated as well.
Regulatory Genes
The promoter trapping of numerous regulatory genes reflects the ability to respond to environmental challenges through changes in the gene expression profile during interaction with a host. In Table 2, regulatory systems that control specific and well-defined processes are listed together with their target genes, and some are discussed in the corresponding sections. This class comprises genes encoding global regulators, regulatory genes that control expression of genes with unknown function or have unknown target genes.
Several of the IVET-identified regulatory genes encode two-component regulatory systems. These regulatory systems permit organisms to respond to changes in their environment and are often associated with global regulatory systems as well as with regulation of virulence. Sensor kinases are able to perceive external stimuli and can activate the response regulator that subsequently acts as a transcriptional regulator (reviewed in reference 293). However, response regulators are not uniquely activated by the corresponding sensor kinase. For instance, it has been shown that several response regulator proteins can be phosphorylated by acetyl phosphate, a significant secondary source of phosphoryl groups (128, 171, 266). Therefore, the two key enzymes of acetyl phosphate metabolism, acetate kinase (encoded by ack) and phosphotransacetylase (encoded by pta), can indirectly modulate some regulatory pathways. IVET isolation suggests a possible role for pta-encoded phosphotransacetylase during rice root colonization by Pseudomonas stutzeri (224). Also, in Pasteurella multocida, an ack-pta operon was shown to be specifically expressed during infection of mice (108). Interestingly, the Vibrio cholerae pta gene was previously isolated using the STM screening for genes required for colonization of the host intestine, implying that a Vibrio cholerae pta mutant strain is significantly reduced in virulence efficiency (36). Acetyl phosphate can also serve as an energy source. In Bradyrhizobium japonicum bacteroids (208) and in Azotobacter vinelandii (22), acetyl phosphate is used as an energy source to support nitrogen fixation.
Vibrio cholerae vieB is a well-characterized example of an IVET-isolated two-component response regulator (32). Since no tested signals have been able to induce vieB expression in vitro, traditional in vitro studies would probably not have revealed this gene, underscoring the potential of IVET as a tool to explore gene expression in complex conditions that are not easy to mimic in vitro. vieB is part of the vieSAB cluster with vieS, encoding the constitutively expressed sensor kinase, and vieA and vieB, encoding two distinct response regulators (136, 175). Although Camilli et al. (32) found that a Vibrio cholerae vieB mutant exhibited small but reproducible colonization defects, Lee et al. (136) demonstrated that the wild-type strain had no competitive advantage during colonization.
VieB is an atypical response regulator, because, in contrast to VieA, it lacks the C-terminal DNA binding domain. It is therefore hypothesized that VieB might modulate the phosphorylation state of VieA by competing for phosphate of phosphorylated VieS. RIVET was used to demonstrate that vieB is only expressed during the infection and enabled analysis of spatial and temporal vieB expression patterns for further elucidation of the possible role of vieB during infection (136). In addition, it was shown with a modified RIVET technique, designed to isolate regulators of ctxA (encoding a subunit of the cholera toxin) or toxT (encoding an important virulence regulator), that the vieSAB regulatory system is required for full expression of cholera toxin during infection as well as in in vitro conditions (137, 139, 272).
The Yersinia enterocolitica yeiE gene, encoding a LysR-like transcriptional regulator, was also found to be up-regulated during infection of mice, suggesting a possible role in virulence by either positive or negative gene regulation (300). Knocking out yeiE apparently resulted in a more aggressive pathogen in the initial stage of infection. However, the Vibrio cholerae yeiE mutant strain caused less mortality than the wild type in later stages of infection, suggesting that fast spread and aggressive infection are not necessarily linked to high virulence (300).
Some global regulators, such as himA and hfq, are found to be upregulated in the host environment. The Salmonella enterica serovar Typhimurium himA gene encodes the α-subunit of the integration host factor and was identified as a host-induced gene with both IVET (156) and DFI (279). Application of DFI revealed a 15-fold induction of himA during macrophage infection (279). The integration host factor is an auxiliary DNA-binding protein that is involved in gene regulation, DNA replication, and recombination (74). In addition, it was observed with DFI that the Haemophilus influenzae ihfB gene, encoding the β-subunit of the integration host factor, was induced during chinchilla infection. Subsequent reverse transcription-PCR analysis confirmed a 2.5-fold upregulation (164).
The hfq gene encodes the so-called host factor I (HF-I, Hfq) and was shown to be specifically induced in Pseudomonas stutzeri during rice colonization (224). Moreover, the equivalent DNA region in Yersinia enterocolitica, containing the open reading frames mutL, miaA, hfq, and hflX, was also isolated with IVET (300). Hfq probably has a general regulatory role, because it is necessary for efficient translation of the alternative sigma factor RpoS, which in turn controls the expression of several genes in the stationary phase. But Hfq is also involved in regulation by affecting the stability of mRNAs that are involved in DNA damage repair (mutS) and modification of the outer membrane (ompA) (253). Furthermore, Hfq plays an important role in the interaction of certain pathogenic bacteria with their host. The Hfq homologue of Yersinia enterocolitica positively regulates expression of an enterotoxin gene (yst), involved in the expression regulation of virulence factors (186). It was also shown that an hfq homologue (brg) in the phytopathogen Erwinia carotovora is necessary for synthesis of low-molecular-weight bacteriocins (38), again suggesting an important role of hfq in bacterial survival and enhanced competitiveness inthe rhizosphere. Hfq is also essential for the persistence of spleen infection by species of the animal pathogen Brucella. Brucella mutants that lack the hfq gene are more sensitive to acidic conditions and oxidative stress (230).
Besides transcriptional gene regulation, some host-induced genes are involved in posttranslational regulation. IVET enabled the identification of serine/threonine protein kinases that are specifically expressed in Pseudomonas aeruginosa (290), Lactobacillus plantarum (24), and Streptococcus gordonii (127) during interaction with mouse and rabbit hosts. Serine/threonine kinases are ubiquitous in both prokaryotic and eukaryotic cells and are thought to play a central role in signal transduction. Moreover, some serine/threonine kinases are known to be indispensable for virulence (79). The Pseudomonas aeruginosa serine/threonine protein kinase encoded by the IVET-traced ppkA gene was subjected to mutational analysis. Compared to the wild type, a ppkA null mutant revealed similar growth in vitro, but a clear difference in virulence was observed. A time delay in animal death was observed when mice were infected with the ppkA mutant and a 10-fold-higher initial inoculum was required to cause similar disease effects in mice compared to wild-type Pseudomonas aeruginosa (288). However, since the nature of the substrates of PpkA kinase activity is unknown, no exact function for ppkA in virulence regulation can yet be assigned (185).
Genes Involved in Cell Envelope Structure and Modification
Peptidoglycan layer.
Genes involved in modification or recycling of the peptidoglycan structure have been isolated repeatedly with IVET or DFI (Table 2), not only from several mammalian pathogens but also from plant-pathogenic bacteria. One such example is Pseudomonas syringae ampG, which is involved in peptidoglycan recycling and is induced during Arabidopsis thaliana infection (16). This is in agreement with the finding in Ralstonia solanacearum that a knockout of ampD, involved in peptidoglycan synthesis, results in decreased plant infection (271). STM showed that Neisseria meningitidis AmpD is necessary during rat infection (267).
Another gene involved in peptidoglycan synthesis encodes the N-acetylmuramoyl-l-alanine amidase and was isolated with IVET and RIVET from Mycobacterium tuberculosis (56) and Staphylococcus aureus (152). These results indicate that the bacterial cell envelope is an important determinant for the establishment of a bacterium-host interaction. In line with this, it was observed that Salmonella enterica serovar Typhimurium peptidoglycan undergoes structural alterations that probably add to its fitness when it resides in mammalian cells (17, 216).
Another enzyme specifically expressed during plant and animal pathogenesis is the lytic transglycosylase, of which homologues were isolated with IVET or DFI in Shigella flexneri (14), Pseudomonas syringae (16), Brucella abortus (62), and Erwinia chrysanthemi (299). The lytic transglycosylase, involved in recycling and remodeling of the peptidoglycan (14), is thought to be required for the integration of various macromolecular transport systems in the cell wall (130). It was shown that the lytic transglycosylase encoded by the Vibrio cholerae sltA gene is in some way involved in the regulation of toxT in Vibrio cholerae. ToxT is a transcriptional regulator belonging to the virulence gene regulatory cascade. It was subsequently shown that a mutation in sltA affects colonization fitness (137). This is in line with the observation in Shigella flexneri that a lytic transglycosylase encoded by sltY is specifically expressed during infection of HeLa cells but not during survival in macrophage cells. The subsequently constructed Shigella flexneri sltY mutant exhibited attenuated virulence (14).
In contrast to the Shigella flexneri transglycosylase, the peptidoglycan transglycosylase MtgA in Brucella abortus is upregulated during macrophage infection (62). By mutational analysis, an IVET-identified lytic transglycosylase gene, ipx10, encoding a homologue of Escherichia coli MetD, was also shown to be implicated in the virulence of a plant pathogen, Pseudomonas syringae pv. tomato, on Arabidopsis thaliana (16). The host-induced Pseudomonas syringae ipx10 gene is located in the so-called conserved effector locus. It is thought to play a role in facilitating the assembly of the TTSS into the peptidoglycan. Expression analysis showed that ipx10 exhibited sixfold induction during infection. Decreased virulence was observed in a Pseudomonas syringae ipx10 mutant strain (16). Notably, the ipx10-encoded transglycosylase shares the soluble lytic transglycosylase (SLT) domain with the SltY transglycosylase, which was also shown to be required for Shigella flexneri virulence (14), as mentioned above.
Surface-exposed components.
Several genes involved in lipopolysaccharide biosynthesis were found to be induced in animal pathogens during survival within the host. Lipopolysaccharides are well-defined virulence factors, and the structure and decoration of lipopolysaccharides are strictly regulated (144). Expression of genes involved in the biosynthesis of lipid A, a major constituent of lipopolysaccharides, is induced by the transcriptional regulator PhoP (223). Interestingly, the Salmonella enterica serovar Typhimurium phoP gene was found to be upregulated during mouse infection. The activity of the PmrA-PmrB two-component system, that is equally upregulated during infection (98), is modulated by the PhoP-PhoQ two-component system (122). Activation of PmrA-PmrB leads to modification of lipopolysaccharide and thereby confers resistance to cationic antibiotic polypeptides, which may allow bacteria to survive within macrophages (90).
In several bacteria, adhesion molecules are induced during interaction with the host. For example, in Porphyromonas gingivalis, the hagB and hagC genes, both encoding hemagglutinins, were identified with IVET (141, 298). Furthermore, in Sinorhizobium meliloti, the putative adhesion molecule encoded by the host-induced nex18 gene proved important for symbiosis, as disruption of nex18 resulted in reduced nitrogen fixation (188). Nex18 is a fasciclin I-like protein containing a predicted signal peptide, suggesting that nex18 is secreted. Fasciclin I is an adhesion molecule found in some eukaryotes (58).
Outer membrane proteins.
Several IVET and DFI studies demonstrated the importance of lipoproteins and other outer membrane proteins during life in the host environment (Table 2). For instance, IVET demonstrated the upregulation of two genes (pcp and glpQ), encoding membrane-associated lipoproteins, in Pasteurella multocida during infection of mice (108). Although no enzymatic function could be assigned to Pcp, it was demonstrated that Pcp is surface exposed (148). Surface-exposed proteins probably contribute to the modulation of bacterial cell surface properties during interaction with the host, thus playing a role in evading the host immune response. As GlpQ is not surface exposed, it is probably not involved in host immunity but might still play a role in pathogenesis. Based on its glycerophosphodiester phosphodiesterase activity, GlpQ is predicted to play a role in the utilization of deacylated phospholipids originating from mucosal secretions, thereby enabling the bacteria to multiply in the mucus layer (148). Another outer membrane protein, encoded by sif15, displays higher expression in Yersinia enterocolitica during systemic infection of mice (83). The sif15 mutant is drastically attenuated in virulence compared to the wild type after intraperitoneal coinoculation (83).
One of the isolated host-induced porin genes, the Vibrio cholerae-encoded vca1008 gene (32), was studied in more detail to assess its role in mouse virulence. The close relationship with another Vibrio cholerae porin, OmpU, suggests that OmpU and Vca1008 are paralogues and might have overlapping functions. This can explain why an ompU mutant strain does not show defective growth or virulence (214). Likewise, a Vibrio cholerae vca1008 mutant shows no defective growth in vitro. However, during infection of mice, the vca1008 mutant strain is severely outcompeted by the wild-type strain, indicating that the Vca1008 porin is necessary and sufficient for virulence, while OmpU is dispensable (192).
IVET enabled the identification of the Pseudomonas fluorescens wssE gene as being specifically induced in sugar beet rhizosphere (78). wssE encodes a cellulose synthase subunit and is part of the wss operon for synthesis of acetylated cellulose polymers (259, 260). It is thought that this polymer functions in colony development and bacterial cell-cell contact rather than mediating adhesion to the plant surface (78, 259). It was also demonstrated that the wss operon contributes to ecological fitness on the leaf surface and, to a minor extent, to ecological fitness in the sugar beet rhizosphere (78).
The cell envelope is crucial for communication and interaction with host cells. Surface-exposed proteins, such as adhesins, can mediate cell-cell contact by anchoring the bacterium to the surface of the host tissue. Alterations of the cell wall during the interaction might be critical for adaptation to the different stresses encountered during life in the host environment. In addition, by changing the cell surface topology, pathogens can escape the host immune response.
The frequent isolation of cell envelope genes by IVET and DFI reflects the important role played by the bacterial cell surface in the interaction with other microorganisms. This is in agreement with microarray data showing that approximately 70% of the Salmonella enterica serovar Typhimurium genes that are involved in cell surface structure and over 20% of the genes involved in cell envelope biogenesis and outer membrane proteins are differentially expressed during macrophage infection (60).
Genes Involved in Virulence and Secretion
Several genes encoding components of different types of secretion machineries were isolated with promoter traps. In Erwinia chrysanthemi, genes involved in the type II secretion pathway, outF and outG, are induced during spinach infection (299). Pectinases, which are major virulence factors in the soft rot-causing Erwinia chrysanthemi, are secreted through the Out system (123, 273). It was also demonstrated with IVET that genes involved in pectin degradation, plyD and pme, are upregulated during plant infection by the plant pathogens Pseudomonas syringae (16) and Ralstonia solanacearum (26), respectively. In tomato-infecting Ralstonia solanacearum, a gene encoding GspK, a component of the type II secretion system, is also induced. This secretion system is required for full virulence of this phytopathogen (120).
Type III secretion system.
The majority of genes involved in secretion, identified through IVET and DFI in several animal and plant pathogens, encode components of a TTSS. Many bacterial pathogens of plants and animals use this specialized machinery to deliver virulence effector proteins across the bacterial cell envelope into host cells (reviewed in references 2, 31, 40, and 106). Besides translocating effector molecules, it was demonstrated in Escherichia coli that TTSS also mediates the secretion of adhesion molecules such as intimin (187).
In Salmonella enterica serovar Typhimurium, DFI enabled the isolation of a macrophage-inducible TTSS component encoded by ssaH (279). ssaH exhibited a 400-fold induction during growth in macrophages compared to in vitro growth. An ssaH mutant strain was severely outcompeted by the wild type in a mouse infection model, pointing to the importance of a functional TTSS during macrophage infection (99, 279). Interestingly, application of DFI also revealed the host-induced expression of an effector protein encoded by sifA in Salmonella enterica serovar Typhimurium, suggesting a coordinated expression pattern of the secreted protein and its secretion system (30). sifA appeared to be essential for Salmonella enterica serovar Typhimurium virulence as well, since an sifA mutant was severely outcompeted by the wild type in a mouse infection model (15).
DFI analysis of Salmonella enterica serovar Typhimurium infection revealed that another TTSS effector (PipB) is only expressed during mouse infection (30). pipB is part of a Salmonella pathogenicity island and is thought to be involved in glycolipid biosynthesis (296). It was demonstrated that PipB associates with host intracellular membranes, thereby possibly allowing specific interactions with host membrane molecules (129). This DFI application confirmed an earlier observation of Pfeifer et al. (209), who demonstrated, by random insertion of a lux reporter gene into the genome, that pipB is upregulated during mouse infection. The pipB mutant strain that resulted from the lux insertion is slightly attenuated in infection of mice, demonstrating a specific virulence function of the host-induced pipB gene (209).
One of the Pseudomonas aeruginosa open reading frames identified through IVET using the burned-mouse infection model (92), PA2808, was shown to encode a novel TTSS-regulatory protein, PtrA, inhibiting the activity of the transcriptional activator ExsA through a direct interaction (91). Although highly conserved among Pseudomonas aeruginosa strains, no ptrA homologues have been found in other bacteria, including other TTSS-containing Pseudomonas species.
In the plant pathogens Pseudomonas syringae (16) and Erwinia chrysanthemi (299), several components of the TTSS along with effector proteins were shown to be upregulated during plant infection. The Erwinia chrysanthemi hrpB gene plays a vital role in plant infection, since a mutation in hrpB severely reduces plant pathogenesis. Reduced virulence was also observed when two other hrpB-linked genes, hrcJ and hrpD, were knocked out. Notably, in the same cluster, a gene encoding a lytic murein transglycosylase was found, but unlike Pseudomonas syringae ipx10, the pup2D gene is not essential for virulence (299). DFI trapping of HrpL-regulated promoters has been implemented in genomewide screens for TTSS effector proteins in two Pseudomonas syringae pathovars (35).
Unexpectedly, an hrcC homologue, rscC, was also isolated with IVET from the nonpathogenic Pseudomonas fluorescens SBW25 (218). The rscC gene is specifically expressed in the sugar beet rhizosphere, but in contrast to Pseudomonas syringae hrcC, it was not induced upon leaf colonization. Although a Pseudomonas fluorescens hrcC mutant is not attenuated in sugar beet root colonization, the gene cluster encoding the TTSS in Pseudomonas fluorescens is functional. The biological significance of TTSS upregulation in the rhizosphere in this particular case remains to be determined (212). Among plant-associated bacteria, TTSSs were previously only reported in pathogenic bacteria and symbiotic rhizobia. However, DNA hybridization and PCR amplification experiments indicate that TTSSs are more widespread than originally thought (167, 212, 215, 227).
Virulence factors.
Another subgroup of class VII includes major virulence factors, such as the Salmonella enterica serovar Typhimurium spvB gene (98). SpvB is predicted to be a secreted protein containing a carboxy-terminal ADP-ribosyltransferase domain (84, 196). ADP-ribosylation activity is a well-known feature of certain bacterial toxins by which target proteins in their animal hosts can be inactivated. The virulence function of the clustered spvABCD genes is not known, but these genes were shown to be involved in systemic infection by increasing the replication rate of the bacteria in host tissues beyond the intestines (88, 89). Although an spvB mutation does not result in a significant virulence defect in the early stages of infection (98), it was shown that spv genes are essential for systemic infection at a later time of infection. A clear decrease in intracellular proliferation and in epithelial cell apoptosis was observed during infection by the spv mutants (15, 197).
In addition, DFI identified the mig5 gene, encoding a putative lipoprotein, which is located on the Salmonella enterica serovar Typhimurium virulence plasmid, approximately 2 kb upstream of the spv virulence operon. mig5 exhibits 24-fold induction during growth in macrophages, and a Salmonella enterica serovar Typhimurium mig5 mutant shows decreased infection ability (279). Another DFI study revealed granuloma-specific expression of genes encoding members of the proline glutamic acid-polymorphic GC-rich repetitive sequence (PE-PGRS) family in Mycobacterium marinum infecting frogs (221). Mutations in these genes prevented replication in macrophages and impaired persistence in granulomas.
The Porphyromonas gingivalis ivi11 gene, encoding an immunoreactive antigen, is another example of a novel IVET-isolated gene that was subsequently shown by mutational analysis to contribute to infection efficiency and survival in the host. IVET confirmed its significance during Porphyromonas gingivalis infection of mice (298).
The hemolysin homologues ispA and yhdP were also shown to be specifically expressed in Lactobacillus plantarum and Listeria monocytogenes, respectively, during their interaction with murine hosts (24, 295). A Listeria monocytogenes yhdP mutant exhibited decreased survival in murine spleens, indicating an important role for the yhdP-encoded hemolysin homologue in the infection process (295). In Yersinia ruckeri, the hemolysin activator protein (ShlB) is also specifically expressed during fish pathogenesis (65). Notably, listeriolysin-encoding hlyC was used as a selection tool in the Listeria monocytogenes IVET screen (77) (Table 1). Expression of hemolysins causes lysis of red blood cells. The released hemoglobins can be dissociated into heme, which can subsequently be utilized as an iron source by many pathogenic bacteria (232). Therefore, secreted hemolysins represent important virulence factors in several animal pathogens, but they also provide a target for vaccine development (50).
Using RIVET, expression of ctxA, encoding a subunit of cholera toxin during Vibrio cholerae infection of mice, was confirmed (32). This represents an example of a prophage-encoded virulence factor. Upregulation of several phage-derived genes in Escherichia coli during chicken infection was also demonstrated using SCOTS (54). This phenomenon is not restricted to animal pathogens, as expression of several phage-derived genes was also detected with the Ralstonia solanacearum/tomato IVET system (26). It has been proposed that phage-encoded factors can contribute to the fitness of their lysogenic host in different ecological niches (27).
Genes Involved in Nucleic Acid Metabolism
This class of host-induced genes contains several DNA synthesis and modification genes from various microorganisms. For instance, the Streptococcus pneumoniae DNA topoisomerase IV, encoded on the DFI-identified transcriptional fusion RTI004, is upregulated during macrophage infection (160). Topoisomerases are required during every step in the replication process. Topoisomerase, together with gyrase, influences the superhelicity of DNA (145). Other genes of this class might be involved in DNA supercoiling, which has been reported to regulate virulence gene expression in Shigella flexneri (52). Helicases, also involved in modifying DNA topology, were upregulated in Yersinia enterocolitica infecting mice (83) and in Pseudomonas fluorescens colonizing sugar beets (302). It was shown that a Pseudomonas fluorescens helA mutant was significantly attenuated in root and shoot colonization of sugar beets (302).
A second subgroup consists of genes encoding the DNA-modifying enzymes DNA methylases. DNA methylation can play a role in gene regulation by inhibiting the interaction between regulatory proteins and their target DNA sequences (151). In several pathogens, altered DNA methylation patterns result in avirulent strains. Alteration of DNA methylation enables bacteria to control temporal gene expression in response to environmental stimuli and provides a mechanism by which information about environmental parameters experienced by parental cells can be inherited by the progeny cells (151).
Several genes are involved in DNA repair. Genes encoding the three subunits of the excision nuclease ABC (uvrA, uvrB, and uvrC) were isolated from three unrelated bacteria, Ralstonia solanacearum (26), Porphyromonas gingivalis (298), and Mycobacterium tuberculosis (56), respectively. Upregulated expression of the excision nuclease ABC during bacterial life in both animal and plant hosts suggests a major role for this repair enzyme during bacterial life in the host environment. Similarly, recB and recD, encoding subunits of exonuclease V, were identified through IVET in Yersinia enterocolitica (300), and Salmonella enterica serovar Typhimurium (98), respectively. RecBCD is known to participate in the repair of double-strand breaks but was recently also implicated in the virulence of Salmonella enterica (33). It was demonstrated that RecBCD is required for infection of mice, since mutants lacking a functional RecBCD are avirulent in mice and are unable to grow in macrophages. It was suggested that systemic infection by Salmonella enterica may require RecBCD-mediated recombinational repair to prime DNA replication inside phagocytes (33).
In addition, it was demonstrated with IVET that the Shigella flexneri alkA gene is specifically upregulated during infection of mice (244). AlkA is a 3-methyladenine DNA glycosylase and is mainly involved in DNA damage repair but also functions in hypoxanthine excision and DNA demethylation (158, 213). In both mice and HeLa cell infection, a Shigella flexneri alkA mutant was significantly outcompeted by the wild type, suggesting a vital role for alkA in pathogenesis (244). The uracil DNA glycosylase encoded by udg, which was isolated from Listeria monocytogenes (295), might have a similar significance for macrophage infection. Udg is critical for removal of uracil resulting from cytosine deamination from DNA (203)
Genes Involved in Transposition and Site-Specific Recombination
Table 2 shows that several transposases linked with insertion sequence elements, resolvases, and recombinases were found to be induced in host environments. For example, the Salmonella enterica serovar Typhimurium gipA gene, which shares homology with the IS891-like insertion element, was isolated with IVET (264). This family of insertion sequence elements is widely distributed among prokaryotes. Expression analysis showed that gipA is specifically induced in the early stages of infection. Consistent with the expression profile, it was demonstrated that a gipA mutant strain exhibits decreased survival in Peyer's patches after oral infection but not during infection of epithelial cells or spleen (264). This study clearly demonstrates that insertion sequence elements can play an important role in pathogenesis.
Insertion sequence elements are known to play a role in bacterial microevolution, but they might also play a general role in the modulation of gene expression of adjacent genes (183). We speculate that the genes belonging to this class might contribute to genetic variability for better adaptation and survival of bacterial species in a particular niche. In line with this, it was shown that transposition of the catabolic transposon Tn4652 in nutrient-starved Pseudomonas putida (104) is triggered by environmental cues through a two-component system, ColR-ColS. This signal relay system was previously identified as an important root colonization factor in Pseudomonas fluorescens (49).
FUN Genes
Besides the extensive list of host-induced genes in Table 2, a considerable number of genes encoding putative proteins with unknown function or genes without significant homology to known proteins, referred to as FUN genes (101), were isolated with IVET and DFI. These FUN genes constitute almost a third of the total number of host-induced genes (Fig. 3), indicating that there is still a lot to be learned about bacterial life in natural environments.
FIG. 3.
Distribution of promoter trap-isolated host-induced genes among different functional classes. The percentages of genes involved in chemotaxis and motility (class I), nutrient scavenging (class II), central metabolism (class III), adaptation to environmental stresses (class IV), regulation (class V), cell envelope structure and modification (class VI), virulence and secretion (class VII), nucleic acid metabolism (class VIII), and transposition and site-specific recombination (class IX) and FUN genes (genes with unknown function or without significant similarity with known genes) are presented in the diagram.
Follow-up studies of some IVET- and DFI-isolated genes unequivocally demonstrated that FUN genes can be very important for bacterial life in the host environment. For instance, it was confirmed by mutational analysis that the DFI-isolated Salmonella enterica serovar Typhimurium mig14 gene (279) is required for lethal infection of mice (277). Recently, it was shown that mig14 encodes an inner membrane-associated protein that promotes persistent infection, survival within macrophages, and resistance to an antimicrobial peptide (23). IVET enabled the isolation of two other genes with unknown function, of which the corresponding mutants showed attenuated virulence in a mouse infection model: the Vibrio cholerae iviXIII gene and the Staphylococcus aureus ivi17 gene (32, 152). FUN genes involved in interaction with plants have also been identified. A transcriptional fusion to the Sinorhizobium meliloti nex4 gene, which revealed no similarity to known genes, was expressed in a restricted zone (nodule tip) of the alfalfa nodule. The nex4 mutant was significantly attenuated in nodule formation and in its overall nitrogen-fixing capacity (188).
A considerable fraction of promoter trap-isolated FUN genes are predicted to be (integral) inner membrane proteins. DFI revealed the up-regulation of ipc009 during Streptococcus pneumoniae infection of the murine respiratory tract. The ipc009 gene encodes a predicted integral inner membrane protein. Analysis of a Streptococcus pneumoniae ipc009 mutant revealed that a slightly higher dose was required to achieve the same lethality during infection of mice (160).
The frequent isolation of FUN genes that are specifically expressed in the host environment indicates that knowledge about the function of many genes in growth and survival in complex niches is still lacking and points to the need for further functional analysis of these genes to explore their exact role. Several attempts are being made to unravel the function of FUN genes that are upregulated in the host environment.
CONCLUSIONS AND PERSPECTIVES
The establishment of a microorganism in a particular environment is a complex process requiring coordinated expression of many genes (Fig. 4). Gene expression needs to be modulated in response to changes in osmolarity and pH and availability of nutrients, just to mention a few of the environmental parameters that can change drastically and are experienced by the microorganism at various stages during niche colonization. This spatial and temporal fine-tuning implies the concomitant up- or downregulation of specific subsets of genes. Superimposed on this, for microbes interacting with a (eukaryotic) host, active communication to sustain the interaction also requires differential gene expression.
FIG. 4.
Schematic overview of bacterial genes that are induced during life in the host environment. The host environment (black box) is a complex system of environmental parameters that activate or repress expression of several microbial genes. Activated genes can be isolated with promoter-trapping techniques such as IVET and DFI. Genes involved in chemotaxis and motility (1) are specifically expressed during (early stages of) interaction with the host. IVET and DFI studies of plant- and animal-associated microorganisms reveal several parallels and dissimilarities. Genes involved in amino acid uptake (2) and in direct inactivation of reactive oxygen species (3) were predominantly isolated from plant-associated microorganisms, while genes involved in suppression of chemotaxis and motility (4) and the acid stress response (5) were predominantly found in animal-associated microorganisms. In both ecological niches, genes involved in TTSS (6), DNA modification (7), cell surface modification (8), nutrient scavenging (9), and more specifically, iron acquisition (10) are upregulated. A significant number of the genes that are specifically induced in the wild have so far unknown functions (indicated with a question mark).
IVET: a Powerful and Flexible Tool
In vitro studies have their limitations in the study of host-microbe interactions. Many virulence genes are likely to remain unidentified because in vitro conditions cannot mimic all environmental cues that control gene expression. Therefore, a variety of genetic approaches (e.g., IVET, DFI, microarray, and SCOTS) have been developed. Opportunities to study microbial behavior in complex environments have thereby increased and have enabled the identification of novel traits that respond to environment-derived signals and may also contribute to ecological performance. Genes whose expression cannot be induced in vitro can be isolated with in vivo expression techniques.
Two main genetic approaches are used to study microbes in their natural habitat. One approach (exemplified by STM) is based upon inactivation of genes and subsequent phenotypic characterization of the mutants. However, relevant mutations may be overlooked during phenotypic screening because microbial physiological versatility often compensates for these defects. Furthermore, ecological performance is the result of coordinated expression of an ensemble of genes rather than being determined by single genes (143, 218). In a second approach, techniques are used that rely on analysis of gene expression patterns. The use of promoter traps (such as IVET and DFI) is based on the assumption that a major fraction of the genes that are important for bacterial life in complex environments are likely to be upregulated in these conditions.
Our survey indicates that subsets of STM- and IVET-isolated genes show little overlap, suggesting that these techniques are complementary (10). On the other hand, many genes isolated with SCOTS have also been identified with IVET or vice versa (44, 105). This is not surprising, as both techniques rely on the detection of differential gene expression, in contrast to STM.
With IVET, SCOTS, DFI, and STM, it is a laborious task to produce complete coverage of a genome, in contrast to the use of microarrays. Microarrays have the additional advantage that gene expression can be quantified. However, all techniques that are based upon isolation of microbial mRNA from a natural habitat (such as SCOTS or microarrays) experience difficulties due to the instability of RNA. Contamination originating from other microorganisms in the environment of interest raises additional problems. Also, the number of isolated microorganisms is often too small to isolate a sufficient amount of quality RNA (102). Microarrays are also limited because they measure the average of gene expression of the total bacterial population. Differences in gene expression occurring within a population residing in a heterogeneous environment may therefore be masked (20), whereas with IVET an individual bacterium expressing a gene in such an environment can be recovered. Furthermore, the construction of microarrays requires the availability of the annotated genome sequence of the organism under study. These requirements explain why microarray technology is mostly used to study differential gene expression of microorganisms cultured in defined media and in well-controlled environmental conditions. Nevertheless, microarrays were applied successfully to study bacterial gene expression in relatively simple habitats for which environmental parameters are easy to control on a laboratory scale (102, 241, 250), such as Mycobacterium tuberculosis-infected macrophages (270).
Global patterns of gene expression can reveal members of gene regulons. Cluster analysis of in vitro transcriptome profiles often proved useful to assign a possible function to genes of unknown function or to reveal regulatory networks (81). Valuable information about gene expression profiles in the wild can also be obtained through IVET. For instance, it was shown that approximately one third of all IVET-identified Pseudomonas syringae genes that are upregulated during Arabidopsis thaliana infection is under the control of the alternative sigma factor HrpL (16).
The power and the usefulness of IVET are apparent from the following: (i) many microbial IVET-isolated genes were already known to be involved in interaction with a host; (ii) induction of several host-induced genes was confirmed by independent IVET isolation of homologues in more than one microorganism; (iii) the upregulation of a variety of IVET-isolated genes was demonstrated with other techniques (SCOTS, reverse transcription-PCR, or microarray); (iv) in several cases, IVET isolation of a transcriptional fusion was followed by a spatiotemporal expression analysis by means of the reporter gene provided on the promoter trap, confirming the induction of gene expression in the wild; and (v) several IVET-isolated genes that were not known previously to be expressed in the conditions under study were analyzed further, and subsequent mutational analysis unequivocally demonstrated their ecological significance in the microbe's natural habitat.
Once the promoter trap library is constructed, it can be applied to study that particular microorganism in all sorts of complex environments. For instance, the IVET library constructed in Streptococcus gordonii was originally used to study gene expression during infection of mice (127). The same library was subsequently used to analyze gene expression during growth on saliva-coated hydroxyapatite (142) and during biofilm formation on polystyrene surfaces (304). Likewise, the IVET library constructed for the study of Pseudomonas aeruginosa infecting mice was subsequently used to study biofilm formation (67) and infection of burned tissues (92).
The same IVET library was used to identify Yersinia enterocolitica hre (host-responsive element) genes (300) and sif (systemic infection factor) genes (83). By altering the time of reisolating bacteria, virulence factors that are specifically expressed during the early stage of infection (hre) and genes required for systemic infection (sif) were isolated. When both subsets of IVET genes were compared, only fyuA (encoding the yersiniabactin siderophore receptor) was isolated in both screens, indicating that different subsets of genes are required at different stages of infection. Likewise, altering the mode of infection (intraperitoneal, ear cavity, or respiratory tract) revealed dissimilarities in the subsets of DFI-identified genes during Streptococcus pneumoniae infection of mice. Some genes were isolated with the three different infection methods, but various genes showed tissue-specific gene expression (160). This again points to the complexity of bacterial gene expression during interaction with a host and the need for appropriate tools to study this.
In addition to the isolation of genes induced in the wild, promoter trap strategies make it possible to subsequently investigate the expression of the captured genes in this complex environment by using the reporter gene provided on the promoter trap. In particular, with promoter trap strategies such as RIVET and DFI, it is possible to quantify the temporal and spatial expression of the genes of interest and analyze microenvironments in the host. For instance, RIVET enabled an in depth spatiotemporal expression analysis of secreted aspartic proteinases in Candida albicans (131, 261, 263). RIVET was also adapted to explore the complex gene expression patterns of known virulence factors (TcpA and CtxA) (7, 136, 139). RIVET application in Vibrio cholerae demonstrated that tcpA (encoding a pilin subunit of the toxin-coregulated pilus) and ctxA (encoding the enzymatic subunit of the cholera toxin) are not coregulated in the wild, in contrast to what was generally accepted (175). The versatility of RIVET is further illustrated by the design of a variant designated SIVET (for selectable in vivo expression technology) that enabled the quantitative analysis of dependence of prophage induction on host cellular physiology (147).
The knowledge gathered by IVET and DFI about the genes that are required for establishment of a pathogen in its host environment can be exploited for identifying new targets to direct new antimicrobial drug development or the construction of live attenuated vaccines (4, 235, 252). The subset of bacterial antigens that are highly expressed during infection can be rapidly evaluated for use in vaccine development (229). Hemolysin A and listeriolysin, for instance, are IVET-isolated genes which are highly suitable for the elicitation of cell-mediated immunity and might be used as tools for vaccine delivery (50).
The promoters that are isolated with IVET or DFI can also be used to drive spatially and/or temporally controlled heterologous gene expression, e.g., when the recombinant gene product is only desired at a specific stage during interaction with a host. Promoters that are specifically active in the rhizosphere can, for instance, be used to drive the conditional expression of genes involved in biocontrol.
The identification of genes induced in a particular environment provides information about the nature of the environment in which the organism functions and how the organism perceives its environment. For instance, genes involved in resistance to acid stress were only isolated from animal pathogens, indicating that plant-associated bacteria are less exposed to this type of stress.
Concluding Remarks
In many studies, the impetus for the development and application of promoter trap technologies has been to obtain insights into the mechanistic bases of bacterial fitness in complex environments: genes specifically activated in the wild are likely to contribute to the ecological success of the organism in that environment. Explicit tests of this hypothesis, which involves the construction of mutants and analysis of their performance in the environment of interest, are relatively few compared to the large number of genes identified. To cite only a few representative studies, Camilli and Mekalanos (32), Valdivia and Falkow (279), and Gal et al. (78) constructed mutations in genes showing elevated levels of expression in the mouse intestine for Vibrio cholerae, in macrophages for Salmonella enterica serovar Typhimurium, and in the sugar beet rhizosphere for Pseudomonas fluorescens, respectively, and found that these mutants were compromised in their ability to grow in their host environments. However, in several other instances where mutants defective in IVET- or DFI-identified genes were constructed, such phenotypes were not revealed. This was, for instance, the case with a TTSS mutant of sugar beet-colonizing Pseudomonas fluorescens (212).
The reasons for nondefective phenotypes are numerous and could indicate that (i) the gene of interest (and the trait toward which it contributes) has no ecological significance; (ii) there is genetic redundancy; (iii) the gene is ecologically significant but it contributes subtly, in a quantitative manner, to the trait in question, and as a consequence defective phenotypes are not generated; and (iv) the gene does contribute significantly to ecological performance but assays of fitness inadequately account for environmental complexity and thus return spurious results.
To expand on this last point, imagine the following hypothetical scenario. Gene X contributes significantly to ecological performance but only in niches where the inducer of gene X is present. In the absence of knowledge about the spatial and temporal distribution of the inducing signal, a gene X mutant is made to compete against the wild type. After a period of time the ratio of mutant to wild type is determined, and no significant difference is found. Unbeknownst to the experimenters, though, is that only 10% of the bacteria colonized niches where the inducing signal was present, and despite a significant reduction in fitness, the effect was masked by the 90% of cells that grew in niches where gene X was not induced. This plausible scenario draws attention to the fact that a valid measure of the fitness effects of specific genes is dependent upon an understanding of the spatial and temporal distribution of inducing signals in the environment of interest.
The importance of the ability to adapt to the changing nutritional environment is reflected in the high proportion of trapped genes that are involved in metabolism (Fig. 3). These genes are often considered of less interest because they are linked to housekeeping functions and do not encode “genuine” colonization or virulence factors. However, since metabolic genes are isolated frequently in practically all IVET studies, their increased expression tells us something: getting food matters! The identification of metabolic genes specifically expressed in these conditions can render new information about the metabolism of the microbe that may, in the case of animal pathogens, prove useful for vaccine development.
Our overview reveals that microorganisms display niche-specific gene expression rather than an expression pattern that is common to phylogenetically related species. Niche-specific gene expression is not surprising, since several microorganisms seem to possess similar mechanism for acclimation, protection, and survival in the host environment. Moreover, they are exposed to similar environmental challenges and have to change their gene expression in response to the same environmental stimuli.
Evidence has accumulated in recent years that plant and animal pathogens share more features than previously recognized. For instance, gram-negative bacterial pathogens use a common machinery (TTSS) to deliver effector proteins into eukaryotic cells (31). The legume symbiont genus Rhizobium, the phytopathogen genus Agrobacterium, and the animal pathogen genus Brucella use similar strategies to achieve host infection (202). Certain bacteria, such as Pseudomonas aeruginosa, are capable of infecting plants and animals using overlapping virulence mechanisms (217). More such parallels have appeared from studies of gene expression in the wild, such as iron acquisition, nutrient metabolism, stress response, and glutathione-mediated protection against oxidative stress. This reflects the use of antimicrobial protection strategies that are shared by animal and plant hosts, and these conserved defense mechanisms apparently elicit similar responses in the invading microorganisms (12, 103).
IVET also revealed unexpected parallels between pathogens and nonpathogens, such as the expression of a TTSS in the nonpathogenic Pseudomonas fluorescens during sugar beet colonization (212, 218) and the IVET identification of an Agrobacterium tumefaciens ChvD homologue in Salmonella enterica serovar Typhimurium which is normally required for plant pathogenesis. Promoter traps also revealed that a common set of genes are upregulated in animal pathogens and in bacteria that are assumed to be beneficia; to the animal hosts, such as some Lactobacillus species. Such information should be taken into account when developing new antibiotics or therapies in order to avoid collateral damage to beneficial bacteria.
Camilli and Mekalanos (32) were the first to discuss the isolation of transcriptional fusions from Vibrio cholerae that are apparently oriented in the wrong direction to drive reporter gene expression. Since then, several apparent antisense transcripts were identified with IVET from Pseudomonas aeruginosa (290), Pseudomonas fluorescens (218), Pseudomonas stutzeri (224), Ralstonia solanacearum (26), Histoplasma capsulatum (226), Porphyromonas gingivalis (298), Listeria monocytogenes (55), and Staphylococcus aureus (152). At present, very little is known about these putative antisense transcripts, but considering the frequency at which antisense transcripts are identified with promoter traps, it is likely that they have a real function rather than being artifacts of the technique. They might be involved in down-regulating gene expression (32, 175, 246).
The corresponding host-induced promoters might drive the expression of small noncoding RNA molecules (246). Recent genomewide screens resulted in the identification of approximately 50 small RNAs in Escherichia coli, suggesting that they occur more abundantly than expected (225). Small RNAs are involved in the regulation of protein synthesis by affecting transcription (by pairing with their target mRNAs), translation, and stability. In addition, by binding to proteins, small RNAs can alter their activity. Many noncoding RNAs require the RNA-binding protein Hfq for activity. Interestingly, the Pseudomonas stutzeri A15 hfq gene was also found to be induced in the rice rhizosphere (224). These observations are in agreement with the idea that small noncoding RNA molecules are important for environmental adaptation (225). Notably, IVET enabled the identification of an RNase acting specifically on small oligoribonucleotides that is upregulated in Pseudomonas fluorescens during sugar beet colonization (303).
In recent years, DNA sequence data for entire microbial genomes have accumulated rapidly. But since it is impossible to assign a biochemical and biological function for many genes based purely on annotation and comparative genomics, the need to perform additional mutational and expression analyses, that is, functional genomics, remains. However, these experiments are hampered by the complexity of the natural environment of the microorganisms. Promoter traps, IVET in particular, are powerful tools for high-throughput screening for genes that are specifically expressed in these complex environments. Considering the continuous flow of new IVET papers, it appears that the IVET star is still rising and will continue to render new insights into the secret lives of bacteria.
Acknowledgments
H. Rediers is indebted to the Instituut voor de Aanmoediging van Innovatie door Wetenschap en Technologie in Vlaanderen for a predoctoral fellowship.
We thank U. Bonas (Martin Luther University, Germany) and M. S. Thomas (University of Sheffield, United Kingdom) for communicating unpublished data. We thank J. Handelsman (University of Wisconsin-Madison) for critical reading of the manuscript.
REFERENCES
- 1.Alamuri, P., and R. J. Maier. 2004. Methionine sulphoxide reductase is an important antioxidant enzyme in the gastric pathogen Helicobacter pylori. Mol. Microbiol. 53:1397-1406. [DOI] [PubMed] [Google Scholar]
- 2.Alfano, J. R., and A. Collmer. 2004. Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu. Rev. Phytopathol. 42:385-414. [DOI] [PubMed] [Google Scholar]
- 3.Allaway, D., N. A. Schofield, M. E. Leonard, L. Gilardoni, T. M. Finan, and P. S. Poole. 2001. Use of differential fluorescence induction and optical trapping to isolate environmentally induced genes. Environ. Microbiol. 3:397-406. [DOI] [PubMed] [Google Scholar]
- 4.Allsop, A. E. 1998. New antibiotic discovery, novel screens, novel targets and impact of microbial genomics. Curr. Opin. Microbiol. 1:530-534. [DOI] [PubMed] [Google Scholar]
- 5.Altier, C., and M. Suyemoto. 1999. A recombinase-based selection of differentially expressed bacterial genes. Gene 240:99-106. [DOI] [PubMed] [Google Scholar]
- 6.Angelichio, M. J., and A. Camilli. 2002. In vivo expression technology. Infect. Immun. 70:6518-6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelichio, M. J., D. S. Merrell, and A. Camilli. 2004. Spatiotemporal analysis of acid adaptation-mediated Vibrio cholerae hyperinfectivity. Infect. Immun. 72:2405-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angelini, F., A. Menard, C. Asencio, A. Marais, and F. Megraud. 2004. Construction of replicative and integrative plasmids for setting up the in vivo expression technology in Helicobacter pylori. Plasmid 51:101-107. [DOI] [PubMed] [Google Scholar]
- 9.Aulakh, M. S., R. Wassmann, C. Bueno, J. Kreuzwieser, and H. Rennenberg. 2001. Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol. 3:139-148. [Google Scholar]
- 10.Autret, N., and A. Charbit. Lessons from signature-tagged mutagenesis on the infectious mechanisms of pathogenic bacteria. FEMS Microbiol. Rev., in press. [DOI] [PubMed]
- 11.Barker, L. P., D. M. Brooks, and P. L. Small. 1998. The identification of Mycobacterium marinum genes differentially expressed in macrophage phagosomes using promoter fusions to green fluorescent protein. Mol. Microbiol. 29:1167-1177. [DOI] [PubMed] [Google Scholar]
- 12.Baron, C., and P. C. Zambryski. 1995. The plant response in pathogenesis, symbiosis, and wounding: variations on a common theme? Annu. Rev. Genet. 29:107-129. [DOI] [PubMed] [Google Scholar]
- 13.Bartilson, M., A. Marra, J. Christine, J. S. Asundi, W. P. Schneider, and A. E. Hromockyj. 2001. Differential fluorescence induction reveals Streptococcus pneumoniae loci regulated by competence stimulatory peptide. Mol. Microbiol. 39:126-135. [DOI] [PubMed] [Google Scholar]
- 14.Bartoleschi, C., M. C. Pardini, C. Scaringi, M. C. Martino, C. Pazzani, and M. L. Bernardini. 2002. Selection of Shigella flexneri candidate virulence genes specifically induced in bacteria resident in host cell cytoplasm. Cell. Microbiol. 4:613-626. [DOI] [PubMed] [Google Scholar]
- 15.Beuzon, C. R., and D. W. Holden. 2001. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 3:1345-1352. [DOI] [PubMed] [Google Scholar]
- 16.Boch, J., V. Joardar, L. Gao, T. L. Robertson, M. Lim, and B. N. Kunkel. 2002. Identification of Pseudomonas syringae pv. tomato genes induced during infection of Arabidopsis thaliana. Mol. Microbiol. 44:73-88. [DOI] [PubMed] [Google Scholar]
- 17.Boneca, I. G. 2005. The role of peptidoglycan in pathogenesis. Curr. Opin. Microbiol. 8:46-53. [DOI] [PubMed] [Google Scholar]
- 18.Bongaerts, R. J., I. Hautefort, J. M. Sidebotham, and J. C. Hinton. 2002. Green fluorescent protein as a marker for conditional gene expression in bacterial cells. Methods Enzymol. 358:43-66. [DOI] [PubMed] [Google Scholar]
- 19.Boucher, D. J., B. Adler, and J. D. Boyce. 2005. The Pasteurella multocida nrfE gene is upregulated during infection and is essential for nitrite reduction but not for virulence. J. Bacteriol. 187:2278-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyce, J. D., I. Wilkie, M. Harper, M. L. Paustian, V. Kapur, and B. Adler. 2002. Genomic scale analysis of Pasteurella multocida gene expression during growth within the natural chicken host. Infect. Immun. 70:6871-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breedveld, M. W. and K. J. Miller. 1994. Cyclic β-glucans of members of the family Rhizobiaceae. Microbiol. Rev. 58:145-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bresters, T. W., J. Krul, P. C. Scheepens, and C. Veeger. 1972. Phosphotransacetylase associated with the pyruvate dehydrogenase complex from the nitrogen fixing Azotobacter vinelandii. FEBS Lett. 22:305-309. [DOI] [PubMed] [Google Scholar]
- 23.Brodsky, I. E., N. Ghori, S. Falkow, and D. Monack. 2005. Mig-14 is an inner membrane-associated protein that promotes Salmonella typhimurium resistance to CRAMP, survival within activated macrophages and persistent infection. Mol. Microbiol. 55:954-972. [DOI] [PubMed] [Google Scholar]
- 24.Bron, P. A., C. Grangette, A. Mercenier, W. M. de Vos, and M. Kleerebezem. 2004. Identification of Lactobacillus plantarum genes that are induced in the gastrointestinal tract of mice. J. Bacteriol. 186:5721-5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broughton, W. J., S. Jabbouri, and X. Perret. 2000. Keys to symbiotic harmony. J. Bacteriol. 182:5641-5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown, D. G., and C. Allen. 2004. Ralstonia solanacearum genes induced during growth in tomato: an inside view of bacterial wilt. Mol. Microbiol. 53:1641-1660. [DOI] [PubMed] [Google Scholar]
- 27.Brussow, H., C. Canchaya, and W. D. Hardt. 2004. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68:560-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchanan, B., W. Gruissem,, and R. L. Jones. 2000. Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, Md.
- 29.Buettner, G. R. 1993. The pecking order of free radicals and antioxidants: lipid peroxidation, α-tocopherol, and ascorbate. Arch. Biochem. Biophys. 300:535-543. [DOI] [PubMed] [Google Scholar]
- 30.Bumann, D. 2002. Examination of Salmonella gene expression in an infected mammalian host using the green fluorescent protein and two-colour flow cytometry. Mol. Microbiol. 43:1269-1283. [DOI] [PubMed] [Google Scholar]
- 31.Buttner, D., and U. Bonas. 2003. Common infection strategies of plant and animal pathogenic bacteria. Curr. Opin. Plant Biol. 6:312-319. [DOI] [PubMed] [Google Scholar]
- 32.Camilli, A., and J. J. Mekalanos. 1995. Use of recombinase gene fusions to identify Vibrio cholerae genes induced during infection. Mol. Microbiol. 18:671-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cano, D. A., M. G. Pucciarelli, F. Garcia-del Portillo, and J. Casadesus. 2002. Role of the RecBCD recombination pathway in Salmonella virulence. J. Bacteriol. 184:592-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalfie, M., Y. Tu, G. Euskirchen, W. W. Ward, and D. C. Prasher. 1994. Green fluorescent protein as a marker for gene expression. Science 263:802-805. [DOI] [PubMed] [Google Scholar]
- 35.Chang, J. H., J. M. Urbach, T. F. Law, L. W. Arnold, A. Hu, S. Gombar, S. R. Grant, F. M. Ausubel, and J. L. Dangl. 2005. A high-throughput, near-saturating screen for type III effector genes from Pseudomonas syringae. Proc. Natl. Acad. Sci. USA 102:2549-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiang, S. L., and J. J. Mekalanos. 1998. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol. Microbiol. 27:797-805. [DOI] [PubMed] [Google Scholar]
- 37.Chiang, S. L., J. J. Mekalanos, and D. W. Holden. 1999. In vivo genetic analysis of bacterial virulence. Annu. Rev. Microbiol. 53:129-154. [DOI] [PubMed] [Google Scholar]
- 38.Chuang, D. Y., A. G. Kyeremeh, Y. Gunji, Y. Takahara, Y. Ehara, and T. Kikumoto. 1999. Identification and cloning of an Erwinia carotovora subsp. carotovora bacteriocin regulator gene by insertional mutagenesis. J. Bacteriol. 181:1953-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 40.Collmer, A., M. Lindeberg, T. Petnicki-Ocwieja, D. J. Schneider, and J. R. Alfano. 2002. Genomic mining type III secretion system effectors in Pseudomonas syringae yields new picks for all TTSS prospectors. Trends Microbiol. 10:462-469. [DOI] [PubMed] [Google Scholar]
- 41.Contag, C. H., and M. H. Bachmann. 2002. Advances in in vivo bioluminescence imaging of gene expression. Annu. Rev. Biomed. Eng. 4:235-260. [DOI] [PubMed] [Google Scholar]
- 42.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 43.Cubitt, A. B., R. Heim, S. R. Adams, A. E. Boyd, L. A. Gross, and R. Y. Tsien. 1995. Understanding, improving and using green fluorescent proteins. Trends Biochem. Sci. 20:448-455. [DOI] [PubMed] [Google Scholar]
- 44.Daigle, F., J. E. Graham, and R. Curtiss III. 2001. Identification of Salmonella typhi genes expressed within macrophages by selective capture of transcribed sequences (SCOTS). Mol. Microbiol. 41:1211-1222. [DOI] [PubMed] [Google Scholar]
- 45.Dalton, H. M., and P. E. March. 1998. Molecular genetics of bacterial attachment and biofouling. Curr. Opin. Biotechnol. 9:252-255. [DOI] [PubMed] [Google Scholar]
- 46.de Iannino, N. I., G. Briones, F. Iannino, and R. A. Ugalde. 2000. Osmotic regulation of cyclic 1,2-β-glucan synthesis. Microbiology 146:1735-1742. [DOI] [PubMed] [Google Scholar]
- 47.de Lorenzo, V., and J. M. Fernandez. 2000. Expression vectors and delivery systems. Playing alien genes in remote theaters. Curr. Opin. Biotechnol. 11:427-428. [DOI] [PubMed] [Google Scholar]
- 48.de Weert, S., H. Vermeiren, I. H. M. Mulders, I. Kuiper, N. Hendrickx, G. V. Bloemberg, J. Vanderleyden, R. De Mot, and B. J. J. Lugtenberg. 2002. Flagella-driven chemotaxis towards exudate components is an important trait for tomato root colonization by Pseudomonas fluorescens. Mol. Plant-Microbe Interact. 15:1173-1180. [DOI] [PubMed] [Google Scholar]
- 49.Dekkers, L. C., C. J. Bloemendaal, L. A. de Weger, C. A. Wijffelman, H. P. Spaink, and B. J. J. Lugtenberg. 1998. A two-component system plays an important role in the root-colonizing ability of Pseudomonas fluorescens strain WCS365. Mol. Plant-Microbe Interact. 11:45-56. [DOI] [PubMed] [Google Scholar]
- 50.Dietrich, G., J. F. Viret, and I. Gentschev. 2003. Haemolysin A and listeriolysin-two vaccine delivery tools for the induction of cell-mediated immunity. Int. J. Parasitol. 33:495-505. [DOI] [PubMed] [Google Scholar]
- 51.Dombrecht, B., C. Heusdens, S. Beullens, C. Verreth, E. Mulkers, P. Proost, J. Vanderleyden, and J. Michiels. 2005. Defence of Rhizobium etli bacteroids against oxidative stress involves a complexly regulated atypical 2-Cys peroxiredoxin. Mol. Microbiol. 55:1207-1221. [DOI] [PubMed] [Google Scholar]
- 52.Dorman, C. J., N. N. Bhriain, and C. F. Higgins. 1990. DNA supercoiling and environmental regulation of virulence gene expression in Shigella flexneri. Nature 344:789-792. [DOI] [PubMed] [Google Scholar]
- 53.Doyle, T. C., S. M. Burns, and C. H. Contag. 2004. In vivo bioluminescence imaging for integrated studies of infection. Cell. Microbiol. 6:303-317. [DOI] [PubMed] [Google Scholar]
- 54.Dozois, C. M., F. Daigle, and R. Curtiss III. 2003. Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proc. Natl. Acad. Sci. USA 100:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dubail, I., P. Berche, and A. Charbit. 2000. Listeriolysin O as a reporter to identify constitutive and in vivo-inducible promoters in the pathogen Listeria monocytogenes. Infect. Immun. 68:3242-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dubnau, E., P. Fontan, R. Manganelli, S. Soares-Appel, and I. Smith. 2002. Mycobacterium tuberculosis genes induced during infection of human macrophages. Infect. Immun. 70:2787-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dunn, A. K., A. K. Klimowicz, and J. Handelsman. 2003. Use of a promoter trap to identify Bacillus cereus genes regulated by tomato seed exudate and a rhizosphere resident, Pseudomonas aureofaciens. Appl. Environ. Microbiol. 69:1197-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elkins, T., M. Hortsch, A. J. Bieber, P. M. Snow, and C. S. Goodman. 1990. Drosophila fasciclin I is a novel homophilic adhesion molecule that along with fasciclin III can mediate cell sorting. J. Cell Biol. 110:1825-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engstrom, P., P. Zambryski, M. Van Montagu, and S. Stachel. 1987. Characterization of Agrobacterium tumefaciens virulence proteins induced by the plant factor acetosyringone. J. Mol. Biol. 197:635-645. [DOI] [PubMed] [Google Scholar]
- 60.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47:103-118. [DOI] [PubMed] [Google Scholar]
- 61.Escobar, M. A., and A. M. Dandekar. 2003. Agrobacterium tumefaciens as an agent of disease. Trends Plant Sci. 8:380-386. [DOI] [PubMed] [Google Scholar]
- 62.Eskra, L., A. Canavessi, M. Carey, and G. Splitter. 2001. Brucella abortus genes identified following constitutive growth and macrophage infection. Infect. Immun. 69:7736-7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Espinosa-Urgel, M., and M. I. Ramos-Gonzalez. 2004. In vivo gene expression: the IVET system, p. 351-366. In J. L. Ramos (ed.), Pseudomonas, vol. I: genomics, life style and molecular architecture. Kluwer Publishing, New York, N.Y.
- 64.Ferguson, G. P., A. Datta, J. Baumgartner, R. M. Roop, R. W. Carlson, and G. C. Walker. 2004. Similarity to peroxisomal-membrane protein family reveals that Sinorhizobium and Brucella BacA affect lipid-A fatty acids. Proc. Natl. Acad. Sci. USA 101:5012-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernandez, L., I. Marquez, and J. A. Guijarro. 2004. Identification of specific in vivo-induced (ivi) genes in Yersinia ruckeri and analysis of ruckerbactin, a catecholate siderophore iron acquisition system. Appl. Environ. Microbiol. 70:5199-5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferrandez, A., A. C. Hawkins, D. T. Summerfield, and C. S. Harwood. 2002. Cluster II che genes from Pseudomonas aeruginosa are required for an optimal chemotactic response. J. Bacteriol. 184:4374-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finelli, A., C. V. Gallant, K. Jarvi, and L. L. Burrows. 2003. Use of in-biofilm expression technology to identify genes involved in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 185:2700-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fischer, S. E., M. J. Miguel, and G. B. Mori. 2003. Effect of root exudates on the exopolysaccharide composition and the lipopolysaccharide profile of Azospirillum brasilense Cd under saline stress. FEMS Microbiol. Lett. 219:53-62. [DOI] [PubMed] [Google Scholar]
- 69.Fislage, R. 1998. Differential display approach to quantitation of environmental stimuli on bacterial gene expression. Electrophoresis 19:613-616. [DOI] [PubMed] [Google Scholar]
- 70.Foster, J. W. 1999. When protons attack: microbial strategies of acid adaptation. Curr. Opin. Microbiol. 2:170-174. [DOI] [PubMed] [Google Scholar]
- 71.Foster, J. W. 2004. Escherichia coli acid resistance: tales of an amateur acidophile. Nat. Rev. Microbiol. 2:898-907. [DOI] [PubMed] [Google Scholar]
- 72.Franza, T., I. Michaud-Soret, P. Piquerel, and D. Expert. 2002. Coupling of iron assimilation and pectinolysis in Erwinia chrysanthemi 3937. Mol. Plant-Microbe Interact. 15:1181-1191. [DOI] [PubMed] [Google Scholar]
- 73.Fraser, G. M., and C. Hughes. 1999. Swarming motility. Curr. Opin. Microbiol. 2:630-635. [DOI] [PubMed] [Google Scholar]
- 74.Friedman, D. I. 1988. Integration host factor: a protein for all reasons. Cell 55:545-554. [DOI] [PubMed] [Google Scholar]
- 75.Fuller, T. E., M. J. Kennedy, and D. E. Lowery. 2000. Identification of Pasteurella multocida virulence genes in a septicemic mouse model using signature-tagged mutagenesis. Microb. Pathog. 29:25-38. [DOI] [PubMed] [Google Scholar]
- 76.Fuller, T. E., R. J. Shea, B. J. Thacker, and M. H. Mulks. 1999. Identification of in vivo induced genes in Actinobacillus pleuropneumoniae. Microb. Pathog. 27:311-327. [DOI] [PubMed] [Google Scholar]
- 77.Gahan, C. G., and C. Hill. 2000. The use of listeriolysin to identify in vivo induced genes in the Gram-positive intracellular pathogen Listeria monocytogenes. Mol. Microbiol. 36:498-507. [DOI] [PubMed] [Google Scholar]
- 78.Gal, M., G. M. Preston, R. C. Massey, A. J. Spiers, and P. B. Rainey. 2003. Genes encoding a cullulosic polymer contribute toward ecological succes of Pseudomonas fluorescens SBW25 on plant surfaces. Mol. Ecol. 12:3109-3121. [DOI] [PubMed] [Google Scholar]
- 79.Galyov, E. E., S. Hakansson, A. Forsberg, and H. Wolf-Watz. 1993. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature 361:730-732. [DOI] [PubMed] [Google Scholar]
- 80.Gardel, C. L., and J. J. Mekalanos. 1996. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect. Immun. 64:2246-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gat-Viks, I., R. Sharan, and R. Shamir. 2003. Scoring clustering solutions by their biological relevance. Bioinformatics 19:2381-2389. [DOI] [PubMed] [Google Scholar]
- 82.Golyshin, P. N., D. S. Martins, V. O. Kaiser, M. Ferrer, Y. S. Sabirova, H. Lünsdorf, T. N. Chernikova, O. V. Golyshina, M. M. Yakimov, A. Pühler, and K. N. Timmis. 2003. Genome sequence completed of Alcanivorax borkumensis, a hydrocarbon-degrading bacterium that plays a global role in oil removal from marine systems. J. Biotechnol. 106:215-220. [DOI] [PubMed] [Google Scholar]
- 83.Gort, A. S., and V. L. Miller. 2000. Identification and characterization of Yersinia enterocolitica genes induced during systemic infection. Infect. Immun. 68:6633-6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gotoh, H., N. Okada, Y. G. Kim, K. Shiraishi, N. Hirami, T. Haneda, A. Kurita, Y. Kikuchi, and H. Danbara. 2003. Extracellular secretion of the virulence plasmid-encoded ADP-ribosyltransferase SpvB in Salmonella. Microb. Pathog. 34:227-238. [DOI] [PubMed] [Google Scholar]
- 85.Gould, S. J., and S. Subramani. 1988. Firefly luciferase as a tool in molecular and cell biology. Anal. Biochem. 175:5-13. [DOI] [PubMed] [Google Scholar]
- 86.Graham, J. E., and J. E. Clark-Curtiss. 1999. Identification of Mycobacterium tuberculosis RNAs synthesized in response to phagocytosis by human macrophages by selective capture of transcribed sequences (SCOTS). Proc. Natl. Acad. Sci. USA 96:11554-11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Graham, J. E., and B. J. Wilkinson. 1992. Staphylococcus aureus osmoregulation: roles for choline, glycine betaine, proline, and taurine. J. Bacteriol. 174:2711-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gulig, P. A., H. Danbara, D. G. Guiney, A. J. Lax, F. Norel, and M. Rhen. 1993. Molecular analysis of spv virulence genes of the Salmonella virulence plasmids. Mol. Microbiol. 7:825-830. [DOI] [PubMed] [Google Scholar]
- 89.Gulig, P. A., T. J. Doyle, M. J. Clare-Salzler, R. L. Maiese, and H. Matsui. 1997. Systemic infection of mice by wild-type but not Spv− Salmonella typhimurium is enhanced by neutralization of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 65:5191-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gunn, J. S., S. S. Ryan, J. C. Van Velkinburgh, R. K. Ernst, and S. I. Miller. 2000. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 68:6139-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ha, U., J. Kim, H. Badrane, J. Jia, H. V. Baker, D. Wu, and S. Jin. 2004. An in vivo inducible gene of Pseudomonas aeruginosa encodes an anti-ExsA to suppress the type III secretion system. Mol. Microbiol. 54:307-320. [DOI] [PubMed] [Google Scholar]
- 92.Ha, U., and S. Jin. 1999. Expression of the soxR gene of Pseudomonas aeruginosa is inducible during infection of burn wounds in mice and is required to cause efficient bacteremia. Infect. Immun. 67:5324-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Handfield, M., D. E. Lehoux, F. Sanschagrin, M. J. Mahan, D. E. Woods, and R. C. Levesque. 2000. In vivo-induced genes in Pseudomonas aeruginosa. Infect. Immun. 68:2359-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Handfield, M., and R. C. Levesque. 1999. Strategies for isolation of in vivo expressed genes from bacteria. FEMS Microbiol. Rev. 23:69-91. [DOI] [PubMed] [Google Scholar]
- 95.Handfield, M., H. P. Schweizer, M. J. Mahan, F. Sanschagrin, T. Hoang, and R. C. Levesque. 1998. ASD-GFP vectors for in vivo expression technology in Pseudomonas aeruginosa and other Gram-negative bacteria. BioTechniques 24:261-264. [DOI] [PubMed] [Google Scholar]
- 96.Hautefort, I., and J. C. Hinton. 2000. Measurement of bacterial gene expression in vivo. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:601-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hayes, J. D., J. U. Flanagan, and I. R. Jowsey. 2005. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 45:51-88. [DOI] [PubMed]
- 98.Heithoff, D. M., C. P. Conner, P. C. Hanna, S. M. Julio, U. Hentschel, and M. J. Mahan. 1997. Bacterial infection as assessed by in vivo gene expression. Proc. Natl. Acad. Sci. USA 94:934-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hensel, M., J. E. Shea, M. D. Jones, E. Dalton, C. Gleeson, and D. W. Holden. 1995. Simultaneous identification of bacterial virulence genes by negative selection. Science 269:400-403. [DOI] [PubMed] [Google Scholar]
- 100.Heusipp, G., G. M. Young, and V. L. Miller. 2001. HreP, an in vivo-expressed protease of Yersinia enterocolitica, is a new member of the family of subtilisin/kexin-like proteases. J. Bacteriol. 183:3556-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hinton, J. C. 1997. The Escherichia coli genome sequence: the end of an era or the start of the FUN? Mol. Microbiol. 26:417-422. [DOI] [PubMed] [Google Scholar]
- 102.Hinton, J. C., I. Hautefort, S. Eriksson, A. Thompson, and M. Rhen. 2004. Benefits and pitfalls of using microarrays to monitor bacterial gene expression during infection. Curr. Opin. Microbiol. 7:277-282. [DOI] [PubMed] [Google Scholar]
- 103.Hoffmann, J. A., F. C. Kafatos, C. A. Janeway, and R. A. Ezekowitz. 1999. Phylogenetic perspectives in innate immunity. Science 284:1313-1318. [DOI] [PubMed] [Google Scholar]
- 104.Horak, R., H. Ilves, P. Pruunsild, M. Kuljus, and M. Kivisaar. 2004. The ColR-ColS two-component signal transduction system is involved in regulation of Tn4652 transposition in Pseudomonas putida under starvation conditions. Mol. Microbiol. 54:795-807. [DOI] [PubMed] [Google Scholar]
- 105.Hou, J. Y., J. E. Graham, and J. E. Clark-Curtiss. 2002. Mycobacterium avium genes expressed during growth in human macrophages detected by selective capture of transcribed sequences. Infect. Immun. 70:3714-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hultberg, M. 1998. Rhizobacterial glutathione levels as affected by starvation and cadmium exposure. Curr. Microbiol. 37:301-305. [DOI] [PubMed] [Google Scholar]
- 108.Hunt, M. L., D. J. Boucher, J. D. Boyce, and B. Adler. 2001. In vivo-expressed genes of Pasteurella multocida. Infect. Immun. 69:3004-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ichige, A., and G. C. Walker. 1997. Genetic analysis of the Rhizobium meliloti bacA gene: functional interchangeability with the Escherichia coli sbmA gene and phenotypes of mutants. J. Bacteriol. 179:209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Imlay, J. A. 2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57:395-418. [DOI] [PubMed] [Google Scholar]
- 111.Ito, T., and Y. Sakaki. 1996. Toward genome-wide scanning of gene expression: a functional aspect of the Genome Project. Essays Biochem. 31:11-21. [PubMed] [Google Scholar]
- 112.Ito, T., and Y. Sakaki. 1997. Fluorescent differential display. Methods Mol. Biol. 85:37-44. [DOI] [PubMed] [Google Scholar]
- 113.Jaeger, C. H., III, S. E. Lindow, W. Miller, E. Clark, and M. K. Firestone. 1999. Mapping of sugar and amino acid availability in soil around roots with bacterial sensors of sucrose and tryptophan. Appl. Environ. Microbiol. 65:2685-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Janakiraman, A., and J. M. Slauch. 2000. The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium. Mol. Microbiol. 35:1146-1155. [DOI] [PubMed] [Google Scholar]
- 115.Janausch, I. G., E. Zientz, Q. H. Tran, A. Kroger, and G. Unden. 2002. C4-dicarboxylate carriers and sensors in bacteria. Biochim. Biophys. Acta 1553:39-56. [DOI] [PubMed] [Google Scholar]
- 116.Jefferson, R. A. 1989. The GUS reporter gene system. Nature 342:837-838. [DOI] [PubMed] [Google Scholar]
- 117.Jenkinson, H. F. 1992. Adherence, coaggregation, and hydrophobicity of Streptococcus gordonii associated with expression of cell surface lipoproteins. Infect. Immun. 60:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jeong, W., M. K. Cha, and I. H. Kim. 2000. Thioredoxin-dependent hydroperoxide peroxidase activity of bacterioferritin comigratory protein (BCP) as a new member of the thiol-specific antioxidant protein (TSA)/Alkyl hydroperoxide peroxidase C (AhpC) family. J. Biol. Chem. 275:2924-2930. [DOI] [PubMed] [Google Scholar]
- 119.Kadokura, H., F. Katzen, and J. Beckwith. 2003. Protein disulfide bond formation in prokaryotes. Annu. Rev. Biochem. 72:111-135. [DOI] [PubMed] [Google Scholar]
- 120.Kang, Y. W., J. Z. Huang, G. Z. Mao, L. Y. He, and M. A. Schell. 1994. Dramatically reduced virulence of mutants of Pseudomonas solanacearum defective in export of extracellular proteins across the outer membrane. Mol. Plant-Microbe Interact. 7:370-377. [Google Scholar]
- 121.Kapral, F. A., S. Smith, and D. Lal. 1992. The esterification of fatty acids by Staphylococcus aureus fatty acid modifying enzyme (FAME) and its inhibition by glycerides. J. Med. Microbiol. 37:235-237. [DOI] [PubMed] [Google Scholar]
- 122.Kato, A., and E. A. Groisman. 2004. Connecting two-component regulatory systems by a protein that protects a response regulator from dephosphorylation by its cognate sensor. Genes Dev. 18:2302-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kazemi-Pour, N., G. Condemine, and N. Hugouvieux-Cotte-Pattat. 2004. The secretome of the plant pathogenic bacterium Erwinia chrysanthemi. Proteomics 4:3177-3186. [DOI] [PubMed] [Google Scholar]
- 124.Kehres, D. G., A. Janakiraman, J. M. Slauch, and M. E. Maguire. 2002. SitABCD is the alkaline Mn2+ transporter of Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:3159-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kelly, A., M. D. Goldberg, R. K. Carroll, V. Danino, J. C. Hinton, and C. J. Dorman. 2004. A global role for Fis in the transcriptional control of metabolism and type III secretion in Salmonella enterica serovar Typhimurium. Microbiology 150:2037-2053. [DOI] [PubMed] [Google Scholar]
- 126.Khan, M. A., and R. E. Isaacson. 2002. Identification of Escherichia coli genes that are specifically expressed in a murine model of septicemic infection. Infect. Immun. 70:3404-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kiliç, A. O., M. C. Herzberg, M. W. Meyer, X. Zhao, and L. Tao. 1999. Streptococcal reporter gene-fusion vector for identification of in vivo expressed genes. Plasmid 42:67-72. [DOI] [PubMed] [Google Scholar]
- 128.Kim, S. B., B. S. Shin, S. K. Choi, C. K. Kim, and S. H. Park. 2001. Involvement of acetyl phosphate in the in vivo activation of the response regulator ComA in Bacillus subtilis. FEMS Microbiol. Lett. 195:179-183. [DOI] [PubMed] [Google Scholar]
- 129.Knodler, L. A., B. A. Vallance, M. Hensel, D. Jackel, B. B. Finlay, and O. Steele-Mortimer. 2003. Salmonella type III effectors PipB and PipB2 are targeted to detergent-resistant microdomains on internal host cell membranes. Mol. Microbiol. 49:685-704. [DOI] [PubMed] [Google Scholar]
- 130.Koraimann, G. 2003. Lytic transglycosylases in macromolecular transport systems of Gram-negative bacteria. Cell. Mol. Life Sci. 60:2371-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kretschmar, M., A. Felk, P. Staib, M. Schaller, D. Hess, M. Callapina, J. Morschhauser, W. Schafer, H. C. Korting, H. Hof, B. Hube, and T. Nichterlein. 2002. Individual acid aspartic proteinases (Saps) 1-6 of Candida albicans are not essential for invasion and colonization of the gastrointestinal tract in mice. Microb. Pathog. 32:61-70. [DOI] [PubMed] [Google Scholar]
- 132.Kuiper, I., G. V. Bloemberg, S. Noreen, J. E. Thomas-Oates, and B. J. J. Lugtenberg. 2001. Increased uptake of putrescine in the rhizosphere inhibits competitive root colonization by Pseudomonas fluorescens strain WCS365. Mol. Plant-Microbe Interact. 14:1096-1104. [DOI] [PubMed] [Google Scholar]
- 133.Lai, Y. C., H. L. Peng, and H. Y. Chang. 2001. Identification of genes induced in vivo during Klebsiella pneumoniae CG43 infection. Infect. Immun. 69:7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lamb, C., and R. A. Dixon. 1997. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48:251-275. [DOI] [PubMed] [Google Scholar]
- 135.Lawhon, S. D., J. G. Frye, M. Suyemoto, S. Porwollik, M. McClelland, and C. Altier. 2003. Global regulation by CsrA in Salmonella typhimurium. Mol. Microbiol. 48:1633-1645. [DOI] [PubMed] [Google Scholar]
- 136.Lee, S. H., M. J. Angelichio, J. J. Mekalanos, and A. Camilli. 1998. Nucleotide sequence and spatiotemporal expression of the Vibrio cholerae vieSAB genes during infection. J. Bacteriol. 180:2298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee, S. H., S. M. Butler, and A. Camilli. 2001. Selection for in vivo regulators of bacterial virulence. Proc. Natl. Acad. Sci. USA 98:6889-6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee, S. H., and A. Camilli. 2000. Novel approaches to monitor bacterial gene expression in infected tissue and host. Curr. Opin. Microbiol. 3:97-101. [DOI] [PubMed] [Google Scholar]
- 139.Lee, S. H., D. L. Hava, M. K. Waldor, and A. Camilli. 1999. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell 99:625-634. [DOI] [PubMed] [Google Scholar]
- 140.Lee, S. W., and D. A. Cooksey. 2000. Genes expressed in Pseudomonas putida during colonization of a plant-pathogenic fungus. Appl. Environ. Microbiol. 66:2764-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee, S. W., J. D. Hillman, and A. Progulske-Fox. 1996. The hemagglutinin genes hagB and hagC of Porphyromonas gingivalis are transcribed in vivo as shown by use of a new expression vector. Infect. Immun. 64:4802-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lei, Y., Y. Zhang, A. O. Kiliç, and M. C. Herzberg. 2002. Streptococcus gordonii gene expression during adhesion and biofilm formation in an in vitro model. J. Dent. Res. 81:A117. [Google Scholar]
- 143.Lenski, R. E., and P. D. Sniegowski. 1995. “Adaptive mutation”: the debate goes on. Science 269:285-288. [DOI] [PubMed] [Google Scholar]
- 144.Lerouge, I., and J. Vanderleyden. 2002. O-antigen structural variation: mechanisms and possible roles in animal/plant-microbe interactions. FEMS Microbiol. Lett. 26:17-47. [DOI] [PubMed] [Google Scholar]
- 145.Levine, C., H. Hiasa, and K. J. Marians. 1998. DNA gyrase and topoisomerase IV: biochemical activities, physiological roles during chromosome replication, and drug sensitivities. Biochim. Biophys. Acta 1400:29-43. [DOI] [PubMed] [Google Scholar]
- 146.Litwin, C. M., and S. B. Calderwood. 1993. Role of iron in regulation of virulence genes. Clin. Microbiol. Rev. 6:137-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Livny, J., and D. I. Friedman. 2004. Characterizing spontaneous induction of Stx encoding phages using a selectable reporter system. Mol. Microbiol. 51:1691-1704. [DOI] [PubMed] [Google Scholar]
- 148.Lo, M., J. D. Boyce, I. W. Wilkie, and B. Adler. 2004. Characterization of two lipoproteins in Pasteurella multocida. Microbes Infect. 6:58-67. [DOI] [PubMed] [Google Scholar]
- 149.Loper, J. E., and M. D. Henkels. 1999. Utilization of heterologous siderophores enhances levels of iron available to Pseudomonas putida in the rhizosphere. Appl. Environ. Microbiol. 65:5357-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Loubens, I., L. Debarbieux, A. Bohin, J. M. Lacroix, and J. P. Bohin. 1993. Homology between a genetic locus (mdoA) involved in the osmoregulated biosynthesis of periplasmic glucans in Escherichia coli and a genetic locus (hrpM) controlling pathogenicity of Pseudomonas syringae. Mol. Microbiol. 10:329-340. [DOI] [PubMed] [Google Scholar]
- 151.Low, D. A., N. J. Weyand, and M. J. Mahan. 2001. Roles of DNA adenine methylation in regulating bacterial gene expression and virulence. Infect. Immun. 69:7197-7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lowe, A. M., D. T. Beattie, and R. L. Deresiewicz. 1998. Identification of novel staphylococcal virulence genes by in vivo expression technology. Mol. Microbiol. 27:967-976. [DOI] [PubMed] [Google Scholar]
- 153.Mah, T. F., B. Pitts, B. Pellock, G. C. Walker, P. S. Stewart, and G. A. O'Toole. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306-310. [DOI] [PubMed] [Google Scholar]
- 154.Mahan, M. J., D. M. Heithoff, R. L. Sinsheimer, and D. A. Low. 2000. Assessment of bacterial pathogenesis by analysis of gene expression in the host. Annu. Rev. Genet. 34:139-164. [DOI] [PubMed] [Google Scholar]
- 155.Mahan, M. J., J. M. Slauch, and J. J. Mekalanos. 1993. Bacteriophage P22 transduction of integrated plasmids: single-step cloning of Salmonella typhimurium gene fusions. J. Bacteriol. 175:7086-7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mahan, M. J., J. M. Slauch, and J. J. Mekalanos. 1993. Selection of bacterial virulence genes that are specifically induced in host tissues. Science 259:686-688. [DOI] [PubMed] [Google Scholar]
- 157.Mahan, M. J., J. W. Tobias, J. M. Slauch, P. C. Hanna, R. J. Collier, and J. J. Mekalanos. 1995. Antibiotic-based selection for bacterial genes that are specifically induced during infection of a host. Proc. Natl. Acad. Sci. USA 92:669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mansfield, C., S. M. Kerins, and T. V. McCarthy. 2003. Characterisation of Archaeglobus fulgidus AlkA hypoxanthine DNA glycosylase activity. FEBS Lett. 540:171-175. [DOI] [PubMed] [Google Scholar]
- 159.Marco, M. L., J. Legac, and S. E. Lindow. 2003. Conditional survival as a selection strategy to identify plant-inducible genes of Pseudomonas syringae. Appl. Environ. Microbiol. 69:5793-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Marra, A., J. Asundi, M. Bartilson, S. Lawson, F. Fang, J. Christine, C. Wiesner, D. Brigham, W. P. Schneider, and A. E. Hromockyj. 2002. Differential fluorescence induction analysis of Streptococcus pneumoniae identifies genes involved in pathogenesis. Infect. Immun. 70:1422-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Marra, A., S. Lawson, J. S. Asundi, D. Brigham, and A. E. Hromockyj. 2002. In vivo characterization of the psa genes from Streptococcus pneumoniae in multiple models of infection. Microbiology 148:1483-1491. [DOI] [PubMed] [Google Scholar]
- 162.Marrs, K. A. 1996. The functions and regulation of glutathione S-transferases in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47:127-158. [DOI] [PubMed] [Google Scholar]
- 163.Martin, A. C., G. H. Wadhams, and J. P. Armitage. 2001. The roles of the multiple CheW and CheA homologues in chemotaxis and in chemoreceptor localization in Rhodobacter sphaeroides. Mol. Microbiol. 40:1261-1272. [DOI] [PubMed] [Google Scholar]
- 164.Mason, K. M., R. S. Munson, Jr., and L. O. Bakaletz. 2003. Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect. Immun. 71:3454-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mattick, J. S. 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56:289-314. [DOI] [PubMed] [Google Scholar]
- 166.Maurelli, A. T. 1989. Temperature regulation of virulence genes in pathogenic bacteria: a general strategy for human pathogens? Microb. Pathog. 7:1-10. [DOI] [PubMed] [Google Scholar]
- 167.Mazurier, S., M. Lemeunier, S. Siblot, C. Mougel, and P. Lemanceau. 2004. Distribution and diversity of type three secretion system-like genes in saprophytic and phytopathogenic fluorescent pseudomonads. FEMS Microbiol. Ecol. 49:455-467. [DOI] [PubMed] [Google Scholar]
- 168.McAllister, L. J., H. J. Tseng, A. D. Ogunniyi, M. P. Jennings, A. G. McEwan, and J. C. Paton. 2004. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol. Microbiol. 53:889-901. [DOI] [PubMed] [Google Scholar]
- 169.McCarter, L. L. 2001. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 65:445-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McCarter, L. L. 2004. Dual flagellar systems enable motility under different circumstances. J. Mol. Microbiol. Biotechnol. 7:18-29. [DOI] [PubMed] [Google Scholar]
- 171.McCleary, W. R., and J. B. Stock. 1994. Acetyl phosphate and the activation of two-component response regulators. J. Biol. Chem. 269:31567-31572. [PubMed] [Google Scholar]
- 172.McClelland, M., F. Mathieu-Daude, and J. Welsh. 1995. RNA fingerprinting and differential display using arbitrarily primed PCR. Trends Genet. 11:242-246. [DOI] [PubMed] [Google Scholar]
- 173.McLendon, M. M., and T. M. Shinnick. 2003. I-TRAP: a method to identify transcriptional regulator activated promoters. BMC Infect. Dis. 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Merrell, D. S., and A. Camilli. 1999. The cadA gene of Vibrio cholerae is induced during infection and plays a role in acid tolerance. Mol. Microbiol. 34:836-849. [DOI] [PubMed] [Google Scholar]
- 175.Merrell, D. S., and A. Camilli. 2000. Detection and analysis of gene expression during infection by in vivo expression technology. Phil. Trans. R. Soc. Lond. B Biol. Sci. 355:587-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Miché, L., S. Belkin, R. Rozen, and J. Balandreau. 2003. Rice seedling whole exudates and extracted alkylresorcinols induce stress-response in Escherichia coli biosensors. Environ. Microbiol. 5:403-411. [DOI] [PubMed] [Google Scholar]
- 177.Milkman, R. 1994. An Escherichia coli homologue of eukaryotic potassium channel proteins. Proc. Natl. Acad. Sci. USA 91:3510-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Miller, J. F., J. J. Mekalanos, and S. Falkow. 1989. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science 243:916-922. [DOI] [PubMed] [Google Scholar]
- 179.Miller, K. J., E. P. Kennedy, and V. N. Reinhold. 1986. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science 231:48-51. [DOI] [PubMed] [Google Scholar]
- 180.Misteli, T., and D. L. Spector. 1997. Applications of the green fluorescent protein in cell biology and biotechnology. Nat. Biotechnol. 15:961-964. [DOI] [PubMed] [Google Scholar]
- 181.Moens, S., and J. Vanderleyden. 1996. Functions of bacterial flagella. Crit. Rev. Microbiol. 22:67-100. [DOI] [PubMed] [Google Scholar]
- 182.Morrissey, J. A., A. Cockayne, K. Brummell, and P. Williams. 2004. The staphylococcal ferritins are differentially regulated in response to iron and manganese and via PerR and Fur. Infect. Immun. 72:972-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Morschhauser, J., G. Kohler, W. Ziebuhr, G. Blum-Oehler, U. Dobrindt, and J. Hacker. 2000. Evolution of microbial pathogens. Phil. Trans. R. Soc. Lond. B Biol. Sci. 355:695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mostertz, J., C. Scharf, M. Hecker, and G. Homuth. 2004. Transcriptome and proteome analysis of Bacillus subtilis gene expression in response to superoxide and peroxide stress. Microbiology 150:497-512. [DOI] [PubMed] [Google Scholar]
- 185.Motley, S. T., and S. Lory. 1999. Functional characterization of a serine/threonine protein kinase of Pseudomonas aeruginosa. Infect. Immun. 67:5386-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nakao, H., H. Watanabe, S. Nakayama, and T. Takeda. 1995. yst gene expression in Yersinia enterocolitica is positively regulated by a chromosomal region that is highly homologous to Escherichia coli host factor 1 gene (hfq). Mol. Microbiol. 18:859-865. [DOI] [PubMed] [Google Scholar]
- 187.Nougayrede, J. P., P. J. Fernandes, and M. S. Donnenberg. 2003. Adhesion of enteropathogenic Escherichia coli to host cells. Cell. Microbiol. 5:359-372. [DOI] [PubMed] [Google Scholar]
- 188.Oke, V., and S. R. Long. 1999. Bacterial genes induced within the nodule during the Rhizobium-legume symbiosis. Mol. Microbiol. 32:837-849. [DOI] [PubMed] [Google Scholar]
- 189.Olson, E. R. 1993. Influence of pH on bacterial gene expression. Mol. Microbiol. 8:5-14. [DOI] [PubMed] [Google Scholar]
- 190.Osbourn, A. E., C. E. Barber, and M. J. Daniels. 1987. Identification of plant-induced genes of the bacterial pathogen Xanthomonas campestris pathovar campestris using a promoter-probe plasmid. EMBO J. 6:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Osorio, C. G., J. A. Crawford, J. Michalski, H. Martinez-Wilson, J. B. Kaper, and A. Camilli. 2005. Second-generation recombination-based in vivo expression technology for large-scale screening for Vibrio cholerae genes induced during infection of the mouse small intestine. Infect. Immun. 73:972-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Osorio, C. G., H. Martinez-Wilson, and A. Camilli. 2004. The ompU paralogue vca1008 is required for virulence of Vibrio cholerae. J. Bacteriol. 186:5167-5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Osteras, M., B. T. Driscoll, and T. M. Finan. 1995. Molecular and expression analysis of the Rhizobium meliloti phosphoenolpyruvate carboxykinase (pckA) gene. J. Bacteriol. 177:1452-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Osteras, M., T. M. Finan, and J. Stanley. 1991. Site-directed mutagenesis and DNA sequence of pckA of Rhizobium NGR234, encoding phosphoenolpyruvate carboxykinase: gluconeogenesis and host-dependent symbiotic phenotype. Mol. Gen. Genet. 230:257-269. [DOI] [PubMed] [Google Scholar]
- 195.O'Sullivan, D. J., and F. O'Gara. 1992. Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol. Rev. 56:662-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Otto, H., D. Tezcan-Merdol, R. Girisch, F. Haag, M. Rhen, and F. Koch-Nolte. 2000. The spvB gene-product of the Salmonella enterica virulence plasmid is a mono(ADP-ribosyl)transferase. Mol. Microbiol. 37:1106-1115. [DOI] [PubMed] [Google Scholar]
- 197.Paesold, G., D. G. Guiney, L. Eckmann, and M. F. Kagnoff. 2002. Genes in the Salmonella pathogenicity island 2 and the Salmonella virulence plasmid are essential for Salmonella-induced apoptosis in intestinal epithelial cells. Cell. Microbiol. 4:771-781. [DOI] [PubMed] [Google Scholar]
- 198.Page, F., S. Altabe, N. Hugouvieux-Cotte-Pattat, J. M. Lacroix, J. Robert-Baudouy, and J. P. Bohin. 2001. Osmoregulated periplasmic glucan synthesis is required for Erwinia chrysanthemi pathogenicity. J. Bacteriol. 183:3134-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Palma, M., D. DeLuca, S. Worgall, and L. E. Quadri. 2004. Transcriptome analysis of the response of Pseudomonas aeruginosa to hydrogen peroxide. J. Bacteriol. 186:248-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Park, Y. K., B. Bearson, S. H. Bang, I. S. Bang, and J. W. Foster. 1996. Internal pH crisis, lysine decarboxylase and the acid tolerance response of Salmonella typhimurium. Mol. Microbiol. 20:605-611. [DOI] [PubMed] [Google Scholar]
- 201.Parkinson, T. 2002. The impact of genomics on anti-infectives drug discovery and development. Trends Microbiol. 10:S22-S26. [DOI] [PubMed] [Google Scholar]
- 202.Paulsen, I. T., R. Seshadri, K. E. Nelson, J. A. Eisen, J. F. Heidelberg, T. D. Read, R. J. Dodson, L. Umayam, L. M. Brinkac, M. J. Beanan, S. C. Daugherty, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, W. C. Nelson, B. Ayodeji, M. Kraul, J. Shetty, J. Malek, S. E. Van Aken, S. Riedmuller, H. Tettelin, S. R. Gill, O. White, S. L. Salzberg, D. L. Hoover, L. E. Lindler, S. M. Halling, S. M. Boyle, and C. M. Fraser. 2002. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc. Natl. Acad. Sci. USA 99:13148-13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pearl, L. H. 2000. Structure and function in the uracil-DNA glycosylase superfamily. Mutat. Res. 460:165-181. [DOI] [PubMed] [Google Scholar]
- 204.Peek, J. A., and R. K. Taylor. 1992. Characterization of a periplasmic thiol:disulfide interchange protein required for the functional maturation of secreted virulence factors of Vibrio cholerae. Proc. Natl. Acad. Sci. USA 89:6210-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pei, Z., and M. J. Blaser. 1993. PEB1, the major cell-binding factor of Campylobacter jejuni, is a homologue of the binding component in Gram-negative nutrient transport systems. J. Biol. Chem. 268:18717-18725. [PubMed] [Google Scholar]
- 206.Perkins-Balding, D., M. Ratliff-Griffin, and I. Stojiljkovic. 2004. Iron transport systems in Neisseria meningitidis. Microbiol. Mol. Biol. Rev. 68:154-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Peters, N. K., J. W. Frost, and S. R. Long. 1986. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 233:977-980. [DOI] [PubMed] [Google Scholar]
- 208.Peterson, J. B., and T. A. LaRue. 1982. Soluble aldehyde dehydrogenase and metabolism of aldehydes by soybean bacteroids. J. Bacteriol. 151:1473-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pfeifer, C. G., S. L. Marcus, O. Steele-Mortimer, L. A. Knodler, and B. B. Finlay. 1999. Salmonella typhimurium virulence genes are induced upon bacterial invasion into phagocytic and nonphagocytic cells. Infect. Immun. 67:5690-5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Prade, R. A. 1996. Xylanases: from biology to biotechnology. Biotechnol. Genet. Eng. Rev. 13:101-131. [DOI] [PubMed] [Google Scholar]
- 211.Pragai, Z., N. E. Allenby, N. O'Connor, S. Dubrac, G. Rapoport, T. Msadek, and C. R. Harwood. 2004. Transcriptional regulation of the phoPR operon in Bacillus subtilis. J. Bacteriol. 186:1182-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Preston, G. M., N. Bertrand, and P. B. Rainey. 2001. Type III secretion in plant growth-promoting Pseudomonas fluorescens SBW25. Mol. Microbiol. 41:999-1014. [DOI] [PubMed] [Google Scholar]
- 213.Privezentzev, C. V., M. Saparbaev, A. Sambandam, M. M. Greenberg, and J. Laval. 2000. AlkA protein is the third Escherichia coli DNA repair protein excising a ring fragmentation product of thymine. Biochemistry 39:14263-14268. [DOI] [PubMed] [Google Scholar]
- 214.Provenzano, D., C. M. Lauriano, and K. E. Klose. 2001. Characterization of the role of the ToxR-modulated outer membrane porins OmpU and OmpT in Vibrio cholerae virulence. J. Bacteriol. 183:3652-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Puhler, A., M. Arlat, A. Becker, M. Gottfert, J. P. Morrissey, and F. O'Gara. 2004. What can bacterial genome research teach us about bacteria-plant interactions? Curr. Opin. Plant Biol. 7:137-147. [DOI] [PubMed] [Google Scholar]
- 216.Quintela, J. C., M. A. de Pedro, P. Zollner, G. Allmaier, and F. Garcia-del Portillo. 1997. Peptidoglycan structure of Salmonella typhimurium growing within cultured mammalian cells. Mol. Microbiol. 23:693-704. [DOI] [PubMed] [Google Scholar]
- 217.Rahme, L. G., F. M. Ausubel, H. Cao, E. Drenkard, B. C. Goumnerov, G. W. Lau, S. Mahajan-Miklos, J. Plotnikova, M. W. Tan, J. Tsongalis, C. L. Walendziewicz, and R. G. Tompkins. 2000. Plants and animals share functionally common bacterial virulence factors. Proc. Natl. Acad. Sci. USA 97:8815-8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rainey, P. B. 1999. Adaptation of Pseudomonas fluorescens to the plant rhizosphere. Environ. Microbiol. 1:243-257. [DOI] [PubMed] [Google Scholar]
- 219.Rainey, P. B., D. M. Heithoff, and M. J. Mahan. 1997. Single-step conjugative cloning of bacterial gene fusions involved in microbe-host interactions. Mol. Gen. Genet. 256:84-87. [DOI] [PubMed] [Google Scholar]
- 220.Rainey, P. B., and G. M. Preston. 2000. In vivo expression technology strategies: valuable tools for biotechnology. Curr. Opin. Biotechnol. 11:440-444. [DOI] [PubMed] [Google Scholar]
- 221.Ramakrishnan, L., N. A. Federspiel, and S. Falkow. 2000. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science 288:1436-1439. [DOI] [PubMed] [Google Scholar]
- 222.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 223.Rebeil, R., R. K. Ernst, B. B. Gowen, S. I. Miller, and B. J. Hinnebusch. 2004. Variation in lipid A structure in the pathogenic yersiniae. Mol. Microbiol. 52:1363-1373. [DOI] [PubMed] [Google Scholar]
- 224.Rediers, H., V. Bonnecarrère, P. B. Rainey, K. Hamonts, J. Vanderleyden, and R. De Mot. 2003. Development and application of a dapB-based in vivo expression technology system to study colonization of rice by the endophytic nitrogen-fixing bacterium Pseudomonas stutzeri A15. Appl. Environ. Microbiol. 69:6864-6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Repoila, F., N. Majdalani, and S. Gottesman. 2003. Small non-coding RNAs, coordinators of adaptation processes in Escherichia coli: the RpoS paradigm. Mol. Microbiol. 48:855-861. [DOI] [PubMed] [Google Scholar]
- 226.Retallack, D. M., G. S. Deepe, Jr., and J. P. Woods. 2000. Applying in vivo expression technology (IVET) to the fungal pathogen Histoplasma capsulatum. Microb. Pathog. 28:169-182. [DOI] [PubMed] [Google Scholar]
- 227.Rezzonico, F., G. Défago, and Y. Moënne-Loccoz. 2004. Comparison of ATPase-encoding type III secretion system hrcN genes in biocontrol fluorescent Pseudomonads and in phytopathogenic Proteobacteria. Appl. Environ. Microbiol. 70:5119-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rodriguez, G. M., and I. Smith. 2003. Mechanisms of iron regulation in mycobacteria: role in physiology and virulence. Mol. Microbiol. 47:1485-1494. [DOI] [PubMed] [Google Scholar]
- 229.Rollenhagen, C., M. Sorensen, K. Rizos, R. Hurvitz, and D. Bumann. 2004. Antigen selection based on expression levels during infection facilitates vaccine development for an intracellular pathogen. Proc. Natl. Acad. Sci. USA 101:8739-8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Roop, R. M., G. T. Robertson, G. P. Ferguson, L. E. Milford, M. E. Winkler, and G. C. Walker. 2002. Seeking a niche: putative contributions of the hfq and bacA gene products to the successful adaptation of the brucellae to their intracellular home. Vet. Microbiol. 90:349-363. [DOI] [PubMed] [Google Scholar]
- 231.Rosenstein, R., D. Futter-Bryniok, and F. Gotz. 1999. The choline-converting pathway in Staphylococcus xylosus C2A: genetic and physiological characterization. J. Bacteriol. 181:2273-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rouault, T. A. 2004. Microbiology. Pathogenic bacteria prefer heme. Science 305:1577-1578. [DOI] [PubMed] [Google Scholar]
- 233.Runyen-Janecky, L. J., and S. M. Payne. 2002. Identification of chromosomal Shigella flexneri genes induced by the eukaryotic intracellular environment. Infect. Immun. 70:4379-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Runyen-Janecky, L. J., S. A. Reeves, E. G. Gonzales, and S. M. Payne. 2003. Contribution of the Shigella flexneri Sit, Iuc, and Feo iron acquisition systems to iron acquisition in vitro and in cultured cells. Infect. Immun. 71:1919-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Saunders, N. J., and E. R. Moxon. 1998. Implications of sequencing bacterial genomes for pathogenesis and vaccine development. Curr. Opin. Biotechnol. 9:618-623. [DOI] [PubMed] [Google Scholar]
- 236.Schaible, U. E., and S. H. Kaufmann. 2004. Iron and microbial infection. Nat. Rev. Microbiol. 2:946-953. [DOI] [PubMed] [Google Scholar]
- 237.Schneider, W. P., S. K. Ho, J. Christine, M. Yao, A. Marra, and A. E. Hromockyj. 2002. Virulence gene identification by differential fluorescence induction analysis of Staphylococcus aureus gene expression during infection-simulating culture. Infect. Immun. 70:1326-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Schoolnik, G. K. 2002. Microarray analysis of bacterial pathogenicity. Adv. Microb. Physiol. 46:1-45. [DOI] [PubMed] [Google Scholar]
- 239.Shalom, G., J. G. Shaw, and M. S. Thomas. 2000. pGSTp: an IVET-compatible promoter probe vector conferring resistance to trimethoprim. BioTechniques 29:954-958. [DOI] [PubMed] [Google Scholar]
- 240.Shea, R. J., and M. H. Mulks. 2002. ohr, encoding an organic hydroperoxide reductase, is an in vivo-induced gene in Actinobacillus pleuropneumoniae. Infect. Immun. 70:794-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shelburne, S. A., and J. M. Musser. 2004. Virulence gene expression in vivo. Curr. Opin. Microbiol. 7:283-289. [DOI] [PubMed] [Google Scholar]
- 242.Shen, H., S. E. Gold, S. J. Tamaki, and N. T. Keen. 1992. Construction of a Tn7-lux system for gene expression studies in Gram-negative bacteria. Gene 122:27-34. [DOI] [PubMed] [Google Scholar]
- 243.Sheppard, D. E., and J. R. Roth. 1994. A rationale for autoinduction of a transcriptional activator: ethanolamine ammonia-lyase (EutBC) and the operon activator (EutR) compete for adenosyl-cobalamin in Salmonella typhimurium. J. Bacteriol. 176:1287-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Shi, Z. X., H. L. Wang, K. Hu, E. L. Feng, X. Yao, G. F. Su, P. T. Huang, and L. Y. Huang. 2003. Identification of alkA gene related to virulence of Shigella flexneri 2a by mutational analysis. World J. Gastroenterol. 9:2720-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Silby, M. W., and S. B. Levy. 2004. Use of in vivo expression technology to identify genes important in growth and survival of Pseudomonas fluorescens Pf0-1 in soil: discovery of expressed sequences with novel genetic organization. J. Bacteriol. 186:7411-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Silby, M. W., P. B. Rainey, and S. B. Levy. 2004. IVET experiments in Pseudomonas fluorescens reveal cryptic promoters at loci associated with recognizable overlapping genes. Microbiology 150:518-520. [DOI] [PubMed] [Google Scholar]
- 247.Silhavy, T. J., and J. R. Beckwith. 1985. Uses of lac fusions for the study of biological problems. Microbiol. Rev. 49:398-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Silver, S., and L. T. Phung. 1996. Bacterial heavy metal resistance: new surprises. Annu. Rev. Microbiol. 50:753-789. [DOI] [PubMed] [Google Scholar]
- 249.Simons, M., Permentier, H. P., de Weger, L. A., Wijffelman, C. A., and B. J. J. Lugtenberg. 1997. Amino acid synthesis is necessary for tomato root colonization by Pseudomonas fluorescens strain WCS365. Mol. Plant-Microbe Interact. 10:102-106. [Google Scholar]
- 250.Singh, A. K., L. M. McIntyre, and L. A. Sherman. 2003. Microarray analysis of the genome-wide response to iron deficiency and iron reconstitution in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 132:1825-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Skaar, E. P., M. Humayun, T. Bae, K. L. DeBord, and O. Schneewind. 2004. Iron-source preference of Staphylococcus aureus infections. Science 305:1626-1628. [DOI] [PubMed] [Google Scholar]
- 252.Slauch, J. M., and A. Camilli. 2000. IVET and RIVET: use of gene fusions to identify bacterial virulence factors specifically induced in host tissues. Methods Enzymol. 326:73-96. [DOI] [PubMed] [Google Scholar]
- 253.Sledjeski, D. D., C. Whitman, and A. Zhang. 2001. Hfq is necessary for regulation by the untranslated RNA DsrA. J. Bacteriol. 183:1997-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Smith, H. E., H. Buijs, R. R. de Vries, H. J. Wisselink, N. Stockhofe-Zurwieden, and M. A. Smits. 2001. Environmentally regulated genes of Streptococcus suis: identification by the use of iron-restricted conditions in vitro and by experimental infection of piglets. Microbiology 147:271-280. [DOI] [PubMed] [Google Scholar]
- 255.Smith, H. E., H. Buijs, H. J. Wisselink, N. Stockhofe-Zurwieden, and M. A. Smits. 2001. Selection of virulence-associated determinants of Streptococcus suis serotype 2 by in vivo complementation. Infect. Immun. 69:1961-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Smith, K. D., E. Andersen-Nissen, F. Hayashi, K. Strobe, M. A. Bergman, S. L. Barrett, B. T. Cookson, and A. Aderem. 2003. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 4:1247-1253. [DOI] [PubMed] [Google Scholar]
- 257.Song, S., and C. Park. 1997. Organization and regulation of the D-xylose operons in Escherichia coli K-12: XylR acts as a transcriptional activator. J. Bacteriol. 179:7025-7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Southward, C. M., and M. G. Surette. 2002. The dynamic microbe: green fluorescent protein brings bacteria to light. Mol. Microbiol. 45:1191-1196. [DOI] [PubMed] [Google Scholar]
- 259.Spiers, A. J., J. Bohannon, S. M. Gehrig, and P. B. Rainey. 2003. Biofilm formation at the air-liquid interface by the Pseudomonas fluorescens SBW25 wrinkly spreader requires an acetylated form of cellulose. Mol. Microbiol. 50:15-27. [DOI] [PubMed] [Google Scholar]
- 260.Spiers, A. J., S. G. Kahn, J. Bohannon, M. Travisano, and P. B. Rainey. 2002. Adaptive divergence in experimental populations of Pseudomonas fluorescens. I. Genetic and phenotypic bases of wrinkly spreader fitness. Genetics 161:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Staib, P., M. Kretschmar, T. Nichterlein, H. Hof, and J. Morschhauser. 2002. Host versus in vitro signals and intrastrain allelic differences in the expression of a Candida albicans virulence gene. Mol. Microbiol. 44:1351-1366. [DOI] [PubMed] [Google Scholar]
- 262.Staib, P., M. Kretschmar, T. Nichterlein, G. Kohler, S. Michel, H. Hof, J. Hacker, and J. Morschhauser. 1999. Host-induced, stage-specific virulence gene activation in Candida albicans during infection. Mol. Microbiol. 32:533-546. [DOI] [PubMed] [Google Scholar]
- 263.Staib, P., M. Kretschmar, T. Nichterlein, G. Kohler, and J. Morschhauser. Expression of virulence genes in Candida albicans. Adv. Exp. Med. Biol. 485:167-176, 2000. [DOI] [PubMed] [Google Scholar]
- 264.Stanley, T. L., C. D. Ellermeier, and J. M. Slauch. 2000. Tissue-specific gene expression identifies a gene in the lysogenic phage Gifsy-1 that affects Salmonella enterica serovar Typhimurium survival in Peyer's patches. J. Bacteriol. 182:4406-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Strauss, E. J., and S. Falkow. 1997. Microbial pathogenesis: genomics and beyond. Science 276:707-712. [DOI] [PubMed] [Google Scholar]
- 266.Summers, M. L., M. C. Denton, and T. R. McDermott. 1999. Genes coding for phosphotransacetylase and acetate kinase in Sinorhizobium meliloti are in an operon that is inducible by phosphate stress and controlled by phoB. J. Bacteriol. 181:2217-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Sun, Y. H., S. Bakshi, R. Chalmers, and C. M. Tang. 2000. Functional genomics of Neisseria meningitidis pathogenesis. Nat. Med. 6:1269-1273. [DOI] [PubMed] [Google Scholar]
- 268.Suzuki, H., K. Yonekura, and K. Namba. 2004. Structure of the rotor of the bacterial flagellar motor revealed by electron cryomicroscopy and single-particle image analysis. J. Mol. Biol. 337:105-113. [DOI] [PubMed] [Google Scholar]
- 269.Szurmant, H., and G. W. Ordal. 2004. Diversity in chemotaxis mechanisms among the Bacteria and Archaea. Microbiol. Mol. Biol. Rev. 68:301-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Talaat, A. M., R. Lyons, S. T. Howard, and S. A. Johnston. 2004. The temporal expression profile of Mycobacterium tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 101:4602-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Tans-Kersten, J., J. Gay, and C. Allen. 2000. Ralstonia solanacearum ampD is required for wild type bacterial wilt virulence. Mol. Plant Pathol. 1:179-185. [DOI] [PubMed] [Google Scholar]
- 272.Tischler, A. D., S. H. Lee, and A. Camilli. 2002. The Vibrio cholerae vieSAB locus encodes a pathway contributing to cholera toxin production. J. Bacteriol. 184:4104-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Toth, I. K., K. S. Bell, M. C. Holeva, and R. J. Birch. 2003. Soft rot erwiniae: from genes to genomes. Mol. Plant Pathol. 4:17-30. [DOI] [PubMed] [Google Scholar]
- 274.Travis, J., J. Potempa, and H. Maeda. 1995. Are bacterial proteinases pathogenic factors? Trends Microbiol. 3:405-407. [DOI] [PubMed] [Google Scholar]
- 275.Triccas, J. A., F. X. Berthet, V. Pelicic, and B. Gicquel. 1999. Use of fluorescence induction and sucrose counterselection to identify Mycobacterium tuberculosis genes expressed within host cells. Microbiology 145:2923-2930. [DOI] [PubMed] [Google Scholar]
- 276.Tsien, R. Y. 1998. The green fluorescent protein. Annu. Rev. Biochem. 67:509-544. [DOI] [PubMed] [Google Scholar]
- 277.Valdivia, R. H., D. M. Cirillo, A. K. Lee, D. M. Bouley, and S. Falkow. 2000. mig-14 is a horizontally acquired, host-induced gene required for Salmonella enterica lethal infection in the murine model of typhoid fever. Infect. Immun. 68:7126-7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 279.Valdivia, R. H., and S. Falkow. 1997. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 277:2007-2011. [DOI] [PubMed] [Google Scholar]
- 280.Valdivia, R. H., and S. Falkow. 1997. Probing bacterial gene expression within host cells. Trends Microbiol. 5:360-363. [DOI] [PubMed] [Google Scholar]
- 281.Valdivia, R. H., and S. Falkow. 1998. Flow cytometry and bacterial pathogenesis. Curr. Opin. Microbiol. 1:359-363. [DOI] [PubMed] [Google Scholar]
- 282.van der Straaten, T., L. Zulianello, A. van Diepen, D. L. Granger, R. Janssen, and J. T. van Dissel. 2004. Salmonella enterica serovar Typhimurium RamA, intracellular oxidative stress response, and bacterial virulence. Infect. Immun. 72:996-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.van Vliet, A. H., J. M. Ketley, S. F. Park, and C. W. Penn. 2002. The role of iron in Campylobacter gene regulation, metabolism and oxidative stress defense. FEMS Microbiol. Rev. 26:173-186. [DOI] [PubMed] [Google Scholar]
- 284.Vasil, M. L., and U. A. Ochsner. 1999. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol. Microbiol. 34:399-413. [DOI] [PubMed] [Google Scholar]
- 285.Walter, J., N. C. Heng, W. P. Hammes, D. M. Loach, G. W. Tannock, and C. Hertel. 2003. Identification of Lactobacillus reuteri genes specifically induced in the mouse gastrointestinal tract. Appl. Environ. Microbiol. 69:2044-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Walters, D. R. 2003. Polyamines and plant disease. Phytochemistry. 64:97-107. [DOI] [PubMed] [Google Scholar]
- 287.Wandersman, C., and P. Delepelaire. 2004. Bacterial iron sources: from siderophores to hemophores. Annu. Rev. Microbiol. 58:611-647. [DOI] [PubMed] [Google Scholar]
- 288.Wang, J., C. Li, H. Yang, A. Mushegian, and S. Jin. 1998. A novel serine/threonine protein kinase homologue of Pseudomonas aeruginosa is specifically inducible within the host infection site and is required for full virulence in neutropenic mice. J. Bacteriol. 180:6764-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wang, J., S. Lory, R. Ramphal, and S. Jin. 1996. Isolation and characterization of Pseudomonas aeruginosa genes inducible by respiratory mucus derived from cystic fibrosis patients. Mol. Microbiol. 22:1005-1012. [DOI] [PubMed] [Google Scholar]
- 290.Wang, J., A. Mushegian, S. Lory, and S. Jin. 1996. Large-scale isolation of candidate virulence genes of Pseudomonas aeruginosa by in vivo selection. Proc. Natl. Acad. Sci. USA 93:10434-10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ward, P. P., and O. M. Conneely. 2004. Lactoferrin: role in iron homeostasis and host defense against microbial infection. Biometals 17:203-208. [DOI] [PubMed] [Google Scholar]
- 292.Weissbach, H., F. Etienne, T. Hoshi, S. H. Heinemann, W. T. Lowther, B. Matthews, G. St John, C. Nathan, and N. Brot. 2002. Peptide methionine sulfoxide reductase: structure, mechanism of action, and biological function. Arch. Biochem. Biophys. 397:172-178. [DOI] [PubMed] [Google Scholar]
- 293.West, A. H., and A. M. Stock. 2001. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26:369-376. [DOI] [PubMed] [Google Scholar]
- 294.Whipps, J. M. 2001. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 52:487-511. [DOI] [PubMed] [Google Scholar]
- 295.Wilson, R. L., A. R. Tvinnereim, B. D. Jones, and J. T. Harty. 2001. Identification of Listeria monocytogenes in vivo induced genes by fluorescence-activated cell sorting. Infect. Immun. 69:5016-5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wood, M. W., M. A. Jones, P. R. Watson, S. Hedges, T. S. Wallis, and E. E. Galyov. 1998. Identification of a pathogenicity island required for Salmonella enteropathogenicity. Mol. Microbiol. 29:883-891. [DOI] [PubMed] [Google Scholar]
- 297.Wood, Z. A., E. Schroder, H. J. Robin, and L. B. Poole. 2003. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 28:32-40. [DOI] [PubMed] [Google Scholar]
- 298.Wu, Y., S. W. Lee, J. D. Hillman, and A. Progulske-Fox. 2002. Identification and testing of Porphyromonas gingivalis virulence genes with a pPGIVET system. Infect. Immun. 70:928-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Yang, S., N. T. Perna, D. A. Cooksey, Y. Okinaka, S. E. Lindow, A. M. Ibekwe, N. T. Keen, and C. H. Yang. 2004. Genome-wide identification of plant-upregulated genes of Erwinia chrysanthemi 3937 using a GFP-based IVET leaf array. Mol. Plant-Microbe Interact. 17:999-1008. [DOI] [PubMed] [Google Scholar]
- 300.Young, G. M., and V. L. Miller. 1997. Identification of novel chromosomal loci affecting Yersinia enterocolitica pathogenesis. Mol. Microbiol. 25:319-328. [DOI] [PubMed] [Google Scholar]
- 301.Yurgel, S. N., and M. L. Kahn. 2004. Dicarboxylate transport by rhizobia. FEMS Microbiol. Rev. 28:489-501. [DOI] [PubMed] [Google Scholar]
- 302.Zang, X. X., A. K. Lilley, M. Bailey, and P. B. Rainey. 2004. The indigenous Pseudomonas plasmid pQBR103 encodes plant-inducible genes, including three putative helicases. FEMS Microbiol. Ecol. 51:9-17. [DOI] [PubMed] [Google Scholar]
- 303.Zhang, X. X., A. K. Lilley, M. J. Bailey, and P. B. Rainey. 2004. Functional and phylogenetic analysis of a plant-inducible oligoribonuclease (orn) gene from an indigenous Pseudomonas plasmid. Microbiology 150:2889-2898. [DOI] [PubMed] [Google Scholar]
- 304.Zhang, Y., Y. Lei, A. Khammanivong, and M. C. Herzberg. 2004. Identification of a novel two-component system in Streptococcus gordonii V288 involved in biofilm formation. Infect. Immun. 72:3489-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Zhou, D., W. D. Hardt, and J. E. Galan. 1999. Salmonella typhimurium encodes a putative iron transport system within the centisome 63 pathogenicity island. Infect. Immun. 67:1974-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zipfel, C., S. Robatzek, L. Navarro, E. J. Oakeley, J. D. Jones, G. Felix, and T. Boller. 2004. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428:764-767. [DOI] [PubMed] [Google Scholar]