Abstract
Background:
The selection of sheep with high genetic resistance to gastrointestinal nematodes is a sustainable alternative for parasite control.
Aim:
This study was performed to categorize three breeds of hair sheep according to their resistance to gastrointestinal nematodes during the peripartum period using hematocrit (HCT) and to compare these results with categorizations derived from the nematode eggs per gram of feces (EPG).
Methods:
Parasitological records from two studies involving 46 Katahdin × Pelibuey and 25 Blackbelly ewes were used, along with information from pregnancy (week 22) to lactation (week 13) of a flock of 31 Pelibuey ewes. All ewes of the three breeds were naturally infected by grazing. The ewes were categorized as resistant, intermediate, or susceptible in each breed and by physiological stage (gestation or lactation) using the EPG ± three standard errors. We also categorized ewes based on their HCT ± one standard deviation.
Results:
During pregnancy, resistant ewes were those with less than 257, 148, and 96 EPG for the Blackbelly, Katahdin, and Pelibuey breeds, respectively, while in lactation, resistant ewes had less than 1,587, 912, and 310 EPG, respectively. In the classification by HCT, Blackbelly ewes had values lower than 31.0%; therefore, only intermediate (HCT of 24.4%–31.0%) and susceptible ewes (HCT < 24.4%) were identified. Among the Katahdin, the resistant ewes had only 149 EPGs recorded during lactation, thereby making the classification by lactation-HTC (94 EPG) comparable to the classification by EPG. In Pelibuey ewes, classification by HCT during early lactation (week 1–4) allowed the selection of resistant ewes with higher EPG (379 EPG) compared with the EPG classification (80 EPG), but intermediate and resistant ewes had similar EPG.
Conclusion:
Classification by HCT and nematode eggs per gram of feces allows the selection of ewes with resistance to gastrointestinal nematodes at the beginning of lactation.
Keywords: Genetic selection, Haemonchus contortus, Pelibuey, Peripartum, Resistance
Introduction
The high prevalence of gastrointestinal nematodes in sheep farms poses a significant health problem in many countries (Torres-Acosta et al., 2019). The most important nematode species in tropical areas of Mexico, due to their pathogenicity and high prevalence, are Haemonchus contortus, Cooperia curticei, and Trichostrongylus colubriformis (Candy et al., 2018; González-Garduño et al., 2018; Lalramhluna et al., 2020). These parasites negatively affect the productivity of small ruminants in Mexico (Olivas- Salazar et al., 2018). Anemia in small ruminants becomes serious in the presence of H. contortus, the primary gastrointestinal nematode. This blood-sucking parasite causes acute hemorrhagic anemia, leading to loss of protein along with sudden drops in hematocrit (HCT). In the host, this manifests as pale mucous membranes, submandibular edema, and ascites (Moosa et al., 2022), which facilitate the appearance of other secondary diseases (Casanova et al., 2018). Infections by gastrointestinal nematodes are particularly important during the peripartum and lactation periods of ewes. During these stages, ewes develop immunosuppression due to hormonal changes, in addition to other physiological, immunological, nutritional, and genetic factors that make them more vulnerable (Gasparina et al., 2019; David et al., 2020; Pereira et al., 2020; González-Garduño et al., 2021).
The traditional method for controlling gastrointestinal nematodes is the use of anthelmintics. However, there has been an increase in anthelmintic resistance, which has become a serious global problem as drugs have increasingly proven ineffective (Pawar et al., 2019; Claerebout et al., 2020; Dey et al., 2020). Therefore, alternative control measures have been sought to reduce the negative impact of nematodes on animal health (Sayers and Sweeney, 2005). Recently, several combined options have been implemented to create an integrative approach to parasite control (Calvete et al., 2020). One sustainable tool for studying host genetic resistance to nematodes is the identification of genetic markers associated with phenotypic resistance are identified (Guo et al., 2016). This method has been used to select individuals capable of regulating gastrointestinal nematode infection (Dominik, 2005; Gonçalves et al., 2018) through an understanding of the immune response of breeds and individuals with resistance (Alba-Hurtado and Muñoz-Guzmán, 2013; Maza-Lopez et al., 2020; Cruz-Tamayo et al., 2021; Machín et al., 2021).
Nematode eggs per gram of feces (EPG) is the main variable for determining the genetic resistance of sheep to gastrointestinal nematodes. This trait has long been the main phenotypic trait used to select resistant sheep and compare them with susceptible (Woolaston, 1992). The variability of this characteristic necessitates fecal sampling and coproparasitoscopic examination in some physiological stages of ewes (Zaragoza-Vera et al., 2019).
Packed cell volume (HCT) is negatively correlated with EPG (Vanimisseti et al.,2004), which in turn is the result of parasite burden (degree of infection) of hematophagous species and blood parameters (David et al., 2020), resulting in anemia in some ewes. Therefore, it is important to consider HCT as a variable when selecting sheep with genetic resistance to gastrointestinal nematodes, specifically H. contortus (Zaragoza-Vera et al., 2022). Understanding these relationships is crucial for identifying resistant animals and breeds.
It has been hypothesized that during lactation, the EPG of resistant ewes classified by HCT will be small and comparable to that of ewes classified by EPG. In the present study, we compared HCT and EPG as measures for categorizing hair ewes of three breeds according to their resistance to gastrointestinal nematodes during the peripartum period. We also aimed to identify the ideal physiological stage for the selection of ewes with resistance to gastrointestinal nematodes.
Materials and Methods
To compare the classification between EPG and HTC within each breed, parasitological and hematological information from two previously published studies was used: one involving Katahdin × Pelibuey ewes (González-Garduño et al., 2014c; Torres-Acosta et al., 2014) and another involving Blackbelly ewes (González Garduño et al., 2017). In addition, an unpublished database was formed using the parasitological and hematological information obtained in 2021 from a Pelibuey flock selected for genetic resistance (Zaragoza- Vera et al., 2023) from within a flock belonging to the Center for Training and Reproduction of small species of the government of Tabasco, Mexico.
Location
All experiments were carried out on the same farm located in the municipality of Salto de Agua, Chiapas, Mexico (17° 33’ 20” north latitude and 92° 20’ 02 west longitude) at an altitude of 20 m above sea level. The climate is classified as type equatorial rainforest, fully humid (Kottek et al., 2006) characterized by warm, humid conditions with rainfall throughout the year. The average annual temperature is 26.6°C, and the average annual precipitation is 3,289 mm (SMN, 2021).
Animal management
The three flocks described were kept in the same grazing area in a rotational scheme in paddocks of wiregrass (Paspalum notatum), bittercress (Paspalum conjugatum), stargrass (Cynodon plectostachyus), and humidicola grass (Urochloa humidicola) at a carrying capacity of 15 sheep per hectare. Ewes did not receive anthelmintic treatment during the study period, except for those at risk, and once treated, they were removed from the database so as not to affect the group’s response. Reproductive management in the three flocks (2013, 2016, and 2021) was based on an accelerated lambing model with three breeding seasons in the months of March, July, and November and therefore three months of lambing in August, December, and April, respectively. Each breeding season lasted 35 days.
Katahdin sheep flock
Information from 46 KT ewes of reproductive age (2–6 years) was used. The ewes were grazing in the morning (10 hours) and housed in the afternoon for protection. Only lactating ewes were supplemented with 150 g of a mixture of different ingredients (ground sorghum, corn, soybeans, or sugar cane) according to availability. The ewes were vaccinated annually with triple bacterin (Chinoin®; Chinoin Pharmaceuticals, Mexico City, Mexico) against Clostridium and Pasteurella multocida and administered A/D/E vitamins (González-Garduño et al., 2014c). Fecal and blood samples and live weight were obtained every 14 days during pregnancy and lactation.
Blackbelly sheep flock
From a flock of 200 Blackbelly ewes, 25 ewes with an average age of two and a half years old that were in their second lambing were selected. The ewes were moved to the same area as the Kathadin flock and received similar management. During the study period, they grazed for 10 hours (7:00–17:00 H) and were therefore naturally infected with gastrointestinal nematodes. Fecal and blood samples and live weight were obtained every 14 days (González Garduño et al., 2017). The sheep received vaccinations with Biobac 11 vias (Bio Zoo, Zapopan, Jalisco, Mexico) against six strains of Clostridium, two strains of P. multocida, Mannheimia haemolytica, and Histophilus somni. In addition, ewes were supplemented with 150 g of a mixture of different ingredients during lactation.
Pelibuey sheep flock
This group comprised 31 Pelibuey ewes aged 3 years. They were naturally infected with GINs during grazing. This flock was also located in the same area as the Katahdin and Blackbelly flocks, and management was similar to daytime grazing for 10 hours. The weight, date, and type of lambing (single or double) of each sheep were recorded. The ewes were vaccinated with Biobac 11 vias and supplemented during lactation.
Fecal egg count
Fecal samples were collected from each ewe during the study period, with consideration of the physiological stage (gestation or lactation). Sampling was performed monthly during pregnancy and weekly during peripartum and lactation. To quantify the EPG of each ewe, 5–10 g of feces was taken directly from the animal’s rectum using polyethylene bags, which were labeled and placed in containers with refrigerant for transport to the laboratory. The samples were processed using a modified McMaster technique (Cringoli et al., 2004). Two grams of feces were mixed with 28 ml of a sodium chloride solution, and two compartments of the McMaster chamber were filled and observed at 10× (Thienpont et al., 2003). Each egg represents 50 EPGs.
Hematocrit
Blood samples were obtained from each ewe by venipuncture of the jugular vein using ethylenediaminetetraacetic acid tubes (Vacutainer; BD Biosciences, Franklin Lakes, NJ). The HCT was determined from whole blood using the microhematocrit method (Huerta Aragonés and Cela de Julián, 2018).
Physiological stages
The data from the three flocks were entered into an Excel database, and then thoroughly reviewed, and homogenized to classify the ewes. The data were grouped by breed and physiological stage (pregnancy and lactation). Pregnancy was divided into three phases: the initial phase (weeks 1–6 of gestation), the middle phase (weeks 7–14), and the late phase (weeks 15–22). Lactation was also divided into three phases: the initial phase (weeks 1–4 postpartum), the middle phase (weeks 5–9), and the late phase (weeks 10–13). The number of ewes in each phase was determined, and the average HCT and EPG values of each classification group were calculated. The data per ewe at lambing were rearranged from the sampling date to the day relative to the lambing date of each ewe; after this, the data were grouped according to the described phases.
Ewe classification based on egg count per gram of feces
The average EPG during pregnancy was determined for each ewe, and the average EPG during lactation was calculated. The resistant and susceptible ewes were separated based on the methodology described by (Morteo-Gómez et al., 2004). Specifically, resistant ewes were those below the average EPG minus three standard errors, which was the threshold. Susceptible ewes were those above average EPG plus three standard errors (upper limit of segregation). Ewes with an EPG between these two thresholds were considered intermediate.
With the EPG, two classifications of the ewes were created, and with the HCT, four classifications of the ewes were created (Table 1). The first classification involved comparing the average EPG of each ewe during gestation with the upper and lower limits of the EPG ± three standard errors obtained from the ewe group during gestation (pregnancy-EPG). The second classification involved contrasting the average EPG of each ewe during lactation with the upper and lower limits of the EPG ± three standard errors of the group of lactating ewes (lactation-EPG). This procedure was also applied to the Blackbelly, Pelibuey, and Katahdin breeds.
Table 1. Classification of the three breeds of hair sheep (Pelibuey, Blackbelly, and Katahdin × Pelibuey) into resistant and susceptible groups.
| Classification | Nematode fecal egg count | HCT, % |
|---|---|---|
| Pregnancy-EPG | Lower limit (Resistant) | |
| μEPG - three SE during pregnancy | ||
| Upper Limit (Susceptible) | ||
| μEPG + three SE during pregnancy | ||
| Lactation- EPG | Lower limit (Resistant) | |
| μEPG - three SE during lactation | ||
| Upper Limit (Susceptible) | ||
| μEPG + three SE during lactation | ||
| Pregnancy- HCT | Lower limit (Susceptible) | |
| μHCT- one SD during pregnancy | ||
| Upper Limit (Resistant) | ||
| μHCT + one SD during pregnancy | ||
| Initial lactation -HCT | Lower limit (Susceptible) | |
| μHCT -one SD in lactation 1-4 w | ||
| Upper Limit (Resistant) | ||
| μHCT +one SD in lactation 1-4 w | ||
| Mid lactation - HCT | Lower limit (Susceptible) | |
| μHCT -one SD in lactation 5-9 w | ||
| Upper Limit (Resistant) | ||
| μHCT +one SD in lactation 5-9 w | ||
| Late lactation - HCT | Lower limit (Susceptible) | |
| μHCT-one SD in lactation 10-13 w | ||
| Upper Limit (Resistant) | ||
| μHCT+one SD in lactation 10-13 w |
eggs per gram of feces; w = week; HCT = Hematocrit.
Ewe classification based on HCT content
To classify the ewes as either resistant or susceptible to HCT in each breed, the HCT indicative of anemia (HCT = 24%) was taken as the threshold. This coincided with the overall average of the three breeds (27.7%) minus one standard deviation (3.3); thus, sheep with an HCT of <24.4% were considered susceptible and those with an HCT of >31.0% were considered resistant. Sheep with an HCT between these thresholds were considered intermediate.
The third classification of ewes during pregnancy was based on the average HCT. Ewes were classified as susceptible when their HCT was <24.4% and as resistant if their HCT was >31.0%. The fourth classification compared the average HCT of ewes during the initial phase of lactation (1–4 weeks postpartum) with the established limits. The fifth classification of ewes was based on average HCT during midlactation using the same thresholds. The sixth classification of ewes during late lactation (weeks 10–13), in which ewes with an HCT of <24.4% were classified as susceptible, and those with an HCT of >31.0% were classified as resistant (Table 1).
Two classifications were generated using the EPG and four classifications using the HCT, and in each one, the confidence intervals were obtained in the two physiological stages (gestation and lactation), with which the ewes were classified as resistant or susceptible.
Statistical analysis
The parasitological and hematological data of the ewes according to breed (Blackbelly, Katahdin, and Pelibuey) and physiological stage were analyzed using SAS software (SAS, 2017). The EPG value was log- transformed (EPG + 1) to correct for heterogeneity of variance and approximate a normal distribution. The following model was used:
yijkl = μ + γi + γτi(j) + δγτi(k) + εijkl
where yijkl = variable (HCT or EPG), μ = general mean, γi = effect of the i-th breed (Blackbelly, Katahdin, and Pelibuey), γτi(j) = effect of breed nested in the j-th physiological stage (pregnancy or lactation), δγτi(k) = effect of breed nested in the physiological stage and k-th phase (initial, middle, or late), and εijkl= experimental error. A comparison of means was performed using Duncan’s test.
Level of concordance
To determine the concordance between the ewe classification using the gold standard test (EPG) and the HCT classification within the same group of individuals, Cohen’s kappa method was used. This step determines whether HCT is a suitable diagnostic tool. The following decision rules were applied to interpret the kappa values: If the kappa is 0.0, the agreement is poor. If the kappa is between 0.0 and 0.2 (0%–20%), the agreement is very small. If the kappa is between 0.2 and 0.4 (20%–40%), the agreement is slight. If the kappa is between 0.4 and 0.6 (40%–60%), the agreement is moderate. If the kappa is between 0.6 and 0.8 (60%–80%), the agreement is substantial. If the kappa is between 0.8 and 1.0 (80%–100%), the agreement is almost perfect (Landis and Koch, 1977). The concordance value was determined using the following categories: Ewes showing positive results with both methods (concordance in positives). The ewes obtained negative results with the EPG method and positive results with the HCT method (discordance). Ewes obtained positive results using the EPG method and negative results using the HCT method (discordance). Ewes show negative results with both methods (concordance in negatives). The level of concordance was determined by calculating the concordance rate (%) as follows: (a + b) / N, where a is the number of ewes showing concordance in positives, b is the number of ewes showing concordance in negatives, and N is the total number of ewes.
Results
Ewe classification
The threshold values for the ewe classification (average ± three standard errors) of the Blackbelly, Katahdin, and Pelibuey breeds are shown in Table 2. The values of the Pelibuey and Blackbelly ewes showed clear resistance, whereas the Blackbelly ewes showed the highest EPG values. In the case of Katahdin, the EPG of the resistant and susceptible ewes were between the other two breeds.
Table 2. Lower and upper limits of eggs per gram of feces for classifying ewes as resistant and susceptible according to fecal egg counts during pregnancy and lactation. In parentheses the number of ewes after classification.
| Breed | Physiological stage | N | EPG Mean | SE | Resistant | Susceptible |
|---|---|---|---|---|---|---|
| Blackbelly | Pregnancy | 25 | 608 | 117 | <257 (1) | >959 (3) |
| Blackbelly | Lactation | 25 | 2,616 | 343 | <1,587 (3) | >3,645 (7) |
| Katahdin | Pregnancy | 46 | 232 | 28 | <148 (22) | >316 (9) |
| Katahdin | Lactation | 46 | 1,578 | 222 | <912 (9) | >2,244 (7) |
| Pelibuey | Pregnancy | 32 | 279 | 61 | <96 (8) | >462 (3) |
| Pelibuey | Lactation | 32 | 487 | 59 | <310(14) | >664 (8) |
RES = resistant; SUS = susceptible. Resistant ewes were those with values lower than the mean–two standard errors, and susceptible ewes were those with values higher than the mean + two standard errors. Number of ewes = N.
Response variables during pregnancy and lactation
In all three breeds, the highest EPG values occurred in lactation. However, in Blackbelly ewes, the mean EPG value (2,616 ± 2,422 EPG) exceeded that of Katahdin (1,578 ± 2,596 EPG), whereas in Pelibuey ewes, the EPG value during lactation was the lowest (487 ± 815 EPG). During pregnancy, the Blackbelly ewes had the highest EPG values, and similar values were observed between the Katahdin and Pelibuey ewes. Similar findings were observed for the HCT, which had the lowest lactation value among the three breeds (Table 3).
Table 3. Mean eggs per gram of feces and HCT of hair sheep during pregnancy and lactation.
| Breed | Physiological stage | EPG | HCT (%) | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| Blackbelly | Pregnancy | 59 | 608c | 900 | 58 | 25.1cd | 2.5 |
| Blackbelly | Lactation | 50 | 2,616a | 2,422 | 50 | 22.0e | 3.9 |
| Katahdin | Pregnancy | 364 | 232c | 541 | 367 | 27.8b | 3.2 |
| Katahdin | Lactation | 137 | 1,578b | 2,596 | 149 | 23.9d | 3.7 |
| Pelibuey | Pregnancy | 56 | 279c | 459 | 43 | 30.1a | 3.5 |
| Pelibuey | Lactation | 194 | 487c | 815 | 202 | 26.1c | 5.0 |
Different letters in each column represent statistical differences with p < 0.05. EPG = eggs per gram of feces, N: number of observations per stage, HCT = hematocrit.
Physiological stage
Blackbelly ewes had the highest EPG during midlactation (3,831 EPG) and then drastically decreased toward late lactation (Fig. 1). Katahdin ewes showed the highest EPG during early lactation (1,981 EPG), and Blackbelly ewes showed a similar dynamic, with a decrease in EPG toward the end of lactation. Pelibuey ewes had the lowest EPG during late gestation (279 EPG), with a slight increase from gestation to late lactation (up to 553 EPG).
Fig. 1. Performance of the fecal egg count according to breed and physiological stage. Each point represents the average EPG in early gestation (1–6 week), middle (7–14 week) or late (15–22 week), as well as in early lactation (1–4 week), middle (5–9 week) or late (10–13 week).
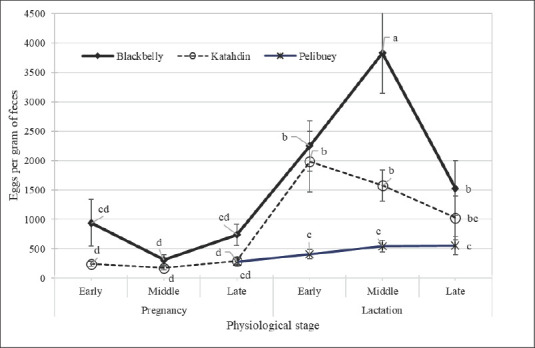
Ewe classification based on eggs per gram of feces and HCT
Very few Blackbelly ewes were classified as resistant based on EPG; their average values were 28 EPG in pregnancy and 129 EPG in lactation (Table 3). When classified based on HCT, no ewes were resistant to either pregnancy or lactation because the HCT values were <31.0%. The ewes were classified as intermediate (24.4%–31.0%) or susceptible (<24.4%).
In the Katahdin ewes, classification using the average HCT during pregnancy did not allow us to distinguish resistance because the EPG values were similar (resistant: 546 EPG, intermediate: 539 EPG, and susceptible: 499 EPG). However, when ewes were classified by HCT in early lactation, resistant ewes (HCT of >31.0%) had only 94 EPG, intermediate ewes (HCT of 24.4%–31.0%) had 593 EPG, and susceptible ewes (HCT of <24.4%) had 1,145 EPG. Therefore, this classification was very similar to the EPG classification. In Pelibuey ewes, classification by EPG during lactation showed the greatest variability among the three categories (resistant, intermediate, and susceptible). When classified based on pregnancy-HCT, none of the ewes were susceptible (all ewes had an HCT of >24.4%). When the average value during initial lactation was used to classify ewes, the values were comparable to those obtained by EPG classification during lactation (Table 4).
Table 4. Fecal egg count according to resistance (intermediate, resistant, and susceptible) to gastrointestinal nematodes in three breeds (Blackbelly, Katahdin and Pelibuey). The values represent the average of the group classified by physiological stage (the same ewes in gestation and lactation).
| Breed | Resistant | Intermediate | Susceptible | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Categorization | N | Mean | Std dev | N | Mean | Std dev | N | Mean | Std dev |
| Blackbelly | |||||||||
| Pregnancy - EPG | 9 | 28c | 51 | 62 | 1,835a | 2,449 | 25 | 1,684a | 1.288 |
| Lactation - EPG | 14 | 129d | 215 | 33 | 1,500ab | 1,328 | 62 | 1,861a | 2,402 |
| Pregnancy - HCT | 67 | 1,640a | 2,374 | 29 | 1,593a | 1,457 | |||
| Early lactation -HCT | 44 | 1,401ab | 2,367 | 56 | 1,769a | 1,859 | |||
| Middle lactation - HCT | 14 | 989b | 1,355 | 77 | 1,832a | 2,233 | |||
| Late lactation - HCT | 22 | 1,041b | 1,114 | 51 | 1,902a | 2,086 | |||
| Katahdin | |||||||||
| Pregnancy - EPG | 240 | 187c | 691 | 157 | 764bc | 1,746 | 96 | 1,034b | 1,770 |
| Lactation - EPG | 89 | 149d | 541 | 208 | 654c | 1,364 | 108 | 1,137b | 2,562 |
| Pregnancy - HCT | 56 | 546b | 1,178 | 392 | 539b | 1,475 | 45 | 499b | 705 |
| Early lactation -HCT | 31 | 94d | 238 | 203 | 593c | 1,509 | 116 | 1,145b | 2,312 |
| Middle lactation - HCT | 168 | 340c | 953 | 224 | 928b | 2,069 | |||
| Late lactation - HCT | 47 | 467c | 1,095 | 184 | 942b | 2,151 | |||
| Pelibuey | |||||||||
| Pregnancy - EPG | 55 | 151c | 365 | 128 | 493c | 888 | 29 | 894bc | 777 |
| Lactation - EPG | 107 | 80d | 152 | 71 | 361cd | 413 | 72 | 1,054b | 1,099 |
| Pregnancy - HCT | 70 | 227b | 382 | 111 | 546b | 1,005 | |||
| Early lactation -HCT | 43 | 379cd | 523 | 172 | 361d | 753 | 29 | 1,002bc | 882 |
| Middle lactation - HCT | 84 | 863b | 1,060 | 33 | 148c | 268 | |||
| Late lactation - HCT | 50 | 360c | 426 | 53 | 708bc | 1,184 | |||
Different letters represent significant differences (p < 0.05).
Initial lactation-HCT = resistance based on average HCT of initial lactation; Lactation-EPG = resistance based on average EPG of lactation; Late lactation-HCT = resistance based on average HCT of late lactation; Mid-lactation-HCT = resistance based on average HCT of mid-lactation; N = number of samples; Pregnancy-EPG = resistance based on average EPG of pregnancy; Pregnancy-HCT = resistance based on average HCT of pregnancy.
Hematological variations
During pregnancy, the three breeds maintained a high HCT despite the differences between them. However, from the beginning of lactation, all ewes showed a reduction in HCT. The Blackbelly ewes had the lowest values (HCT of <20%) in mid-lactation, but the HCT then slightly increased to 23% in late lactation. The Katahdin ewes had intermediate values between those of the Pelibuey and Blackbelly ewes, but a consistent reduction was maintained until late lactation (Fig. 2).
Fig. 2. Performance of HCT in three breeds (Blackbelly, Kathadin and Pelibuey) and physiological stage. Each point represents the average HCT in early gestation (week 1–6), middle (week 7–14), or late (week 15–22), as well as in early lactation (week 1–4), middle (week 5–9), or late (week 10–13).
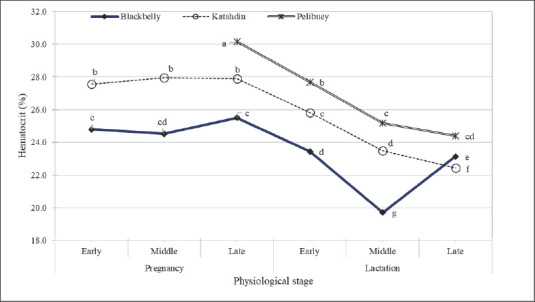
Hematological indices in ewe classification
In the Katahdin and Blackbelly ewes, classification using the average EPG in pregnancy and lactation showed that the HCT values were similar among the three categories of ewes (resistant, intermediate, and susceptible). With classification using the HCT, all Blackbelly ewes had an HCT of <31.0% during pregnancy and lactation; thus, no resistant ewes were detected at any stage.
When the Katahdin ewes were classified using the HCT during gestation and initial lactation, the resistant ewes showed higher HCT values, whereas the susceptible ewes showed the lowest values. Therefore, classification during these two stages using the HCT could serve as an alternative selection method. During midlactation and late lactation, no resistant ewes were detected because none had an HCT > 31.0% (Table 5). In the Pelibuey ewes, classification based on the average EPG in pregnancy and lactation showed that the resistant ewes had the highest HCT (27.8% and 28.9%, respectively), whereas the susceptible ewes had the lowest HCT (22.0% and 24.0%, respectively). When classified by HCT during gestation, many ewes had HCT values >24.4%; therefore, no susceptible ewes were identified. In early and mid-lactation, the HCT values were comparable to those of the EPG classification. In late lactation, however, the HCT decreased to <31%; therefore, no resistant ewes were identified.
Table 5. HCT according to classification (resistant, intermediate, or susceptible) to gastrointestinal nematodes in three breeds (Blackbelly, Katahdin and Pelibuey). The values represent the average of the group classified by physiological stage (the same ewes in gestation and lactation).
| Breed | Resistant | Intermediate | Susceptible | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Categorization | N | Mean | Std dev | N | Mean | Std dev | N | Mean | Std dev |
| Blackbelly | |||||||||
| Pregnancy - EPG | 9 | 26.9a | 1.8 | 62 | 23.6c | 4.0 | 24 | 22.3c | 2.4 |
| Lactation - EPG | 14 | 25.7bc | 2.9 | 32 | 22.5d | 2.8 | 62 | 23.8c | 3.9 |
| Pregnancy - HCT | 67 | 24.1d | 4.0 | 28 | 22.4e | 2.3 | |||
| Early lactation - HCT | 44 | 24.8c | 3.6 | 55 | 22.8d | 3.5 | |||
| Middle lactation - HCT | 14 | 22.9d | 3.2 | 76 | 23.7d | 3.7 | |||
| Late lactation - HCT | 22 | 23.7b | 2.9 | 50 | 23.2c | 3.8 | |||
| Katahdin | |||||||||
| Pregnancy - EPG | 250 | 27.5a | 3.1 | 157 | 26.2b | 3.8 | 99 | 26.1b | 4.3 |
| Lactation - EPG | 91 | 26.5b | 3.2 | 214 | 26.2b | 4.0 | 113 | 26.0b | 3.8 |
| Pregnancy - HCT | 60 | 30.2a | 3.6 | 401 | 26.7c | 3.3 | 45 | 23.6de | 2.7 |
| Early lactation - HCT | 32 | 28.9a | 1.9 | 210 | 26.9b | 3.7 | 120 | 25.0c | 4.1 |
| Middle lactation - HCT | 177 | 28.2b | 3.2 | 230 | 24.6c | 3.4 | |||
| Late lactation - HCT | 54 | 28.5a | 3.2 | 188 | 24.3b | 3.4 | |||
| Pelibuey | |||||||||
| Pregnancy - EPG | 55 | 27.8a | 3.9 | 124 | 26.6ab | 5.1 | 26 | 22.0c | 5.2 |
| Lactation - EPG | 105 | 28.9a | 3.7 | 72 | 26.4b | 5.2 | 68 | 24.1c | 5.2 |
| Pregnancy - HCT | 72 | 28.2b | 4.9 | 107 | 26.0c | 4.4 | |||
| Early lactation - HCT | 46 | 29.0a | 4.3 | 167 | 26.6b | 4.7 | 26 | 23.7cd | 6.3 |
| Middle lactation - HCT | 35 | 30.4a | 3.5 | 131 | 28.2b | 3.7 | 79 | 23.1d | 5.3 |
| Late lactation - HCT | 51 | 28.2a | 4.4 | 53 | 24.7b | 5.4 | |||
Pregnancy-HPG: Resistance based on the average EPG of the pregnancy; Lactation-EPG: Resistance based on the average EPG of lactation; Pregnancy-HCT: Resistance based on the average hematocrit of pregnancy; Initial lactation-HCT: Resistance based on the average hematocrit of initial lactation; Mid-lactation HCT: Resistance based on the average hematocrit of mid-lactation; Late lactation-HCT: Resistance based on the average hematocrit of late lactation. Different letters represent significant differences (p < 0.05).
Concordance rate
A comparison of the number of resistant and susceptible ewes obtained using the gold standard method (EPG) versus the HCT classification indicated that the Katahdin breed had the highest concordance (moderate, 56.3%) during lactation. In the Blackbelly and Pelibuey breeds, the highest rate of concordance occurred between the classification by pregnancy-EPG and pregnancy-HCT, and in the Blackbelly breed, the concordance was substantial in early lactation (weeks 1–4) (Table 6).
Table 6. Concordance rate between the numbers of resistant and susceptible ewes based on eggs per gram of feces and HCT.
| Breed | EPG-pregnancy | EPG -Lactation | ||
|---|---|---|---|---|
| HCT-pregnancy | HCT week 1–4 | HCT week 5–9 | HCT week 10–13 | |
| Blackbelly | 75.0 | 60.0 | 70.0 | 60.0 |
| Kathadin × Pelibuey | 35.5 | 56.3 | 43.8 | 43.8 |
| Pelibuey | 72.7 | 40.9 | 54.5 | 27.3 |
EPG = eggs per gram of feces.
Discussion
Peripartum rise, defined as an increase in EPG during lactation, is a widely known phenomenon in both wool and hair sheep. Affected ewes develop reduced immunity, and the immunoglobulin level and eosinophil count markedly decrease (González-Garduño et al., 2021). The low presence of antibodies hinders infection control; during lactation, the EPG increases and ewes become more susceptible to infections by other microorganisms (Hamer et al., 2019). The ewes recovered immunity at the end of lactation, and the EPG was markedly reduced. In the present study, Blackbelly and Katahdin ewes showed an increase in EPG at weeks 1–4 post-lambing, similar to other study (Notter et al., 2017), and the highest excretion of gastrointestinal nematode eggs was observed during mid-lactation (weeks 5–9). In contrast, Pelibuey ewes did not show an increase in EPG, possibly because this breed was previously selected for its high resistance to gastrointestinal nematodes, as observed in a study that concluded that the Pelibuey breed is considered resistant to nematodes (Zaragoza-Vera et al., 2019). The Katahdin breed showed intermediate behavior between Pelibuey ewes (more resistant) and Blackbelly ewes (more susceptible), consistent with the findings of another study in Yucatan, Mexico (Palomo-Couoh et al., 2016). These three breeds of hair sheep are considered more resistant to gastrointestinal nematode infection than wool breeds (Notter et al., 2003). However, despite this resistance, it was possible to carry out selection because all three breeds showed high genetic variability to infection, as also indicated in other studies (Zvinorova et al., 2016; Berton et al., 2019). Additionally, it has previously been considered that the inheritance rate can reach 40%.
Genetic resistance to gastrointestinal nematodes has been observed in many sheep breeds, including Florida, Blackbelly, Pelibuey, and Santa Cruz. Some animals in the flock show a natural ability to resist H. contortus infection, reducing the burden, length, and fecundity of females (Rowe et al., 2008; González et al., 2011) and can even maintain acceptable levels of productivity without showing signs of infection (i.e., they show resilience) (Karrow et al., 2014). The immune response to these parasites is crucial for resistance against gastrointestinal nematodes (Ortolani et al., 2013; Machín et al., 2021). This resistance trait can be transmitted to lambs, contributing to natural selection in wild populations (Guo et al., 2016). Therefore, determining the EPG as a correlated variable of parasite burden is essential for selecting animals with natural resistance to gastrointestinal nematodes (Muñoz-Guzmán et al., 2006).
Based on the information generated by the average EPGs in pregnancy and lactation, the optimal time to select ewes appears to be during lactation. During pregnancy, there were some inconsistencies in the Blackbelly ewes: when segregated by the average EPG of pregnancy, intermediate ewes had a higher EPG (1,835 EPG) than susceptible ewes (1684 EPG). However, this pattern did not occur in the Katahdin breed, in which intermediate ewes had 764 EPG and susceptible ewes had 1,034 EPG. During lactation, the Katahdin ewes showed 654 EPG in the intermediate group and 1,137 EPG in the susceptible group. The Pelibuey ewes exhibited a similar trend, with the intermediate group showing 361 EPG and the susceptible group showing 1,054 EPG. Categorizations were also performed during lactation in other studies involving similar breeds (Palomo-Couoh et al., 2016; Zaragoza-Vera et al., 2019), and our results coincide with these previous studies.
A decrease in HCT is associated with the presence of blood-sucking nematodes, such as H. contortus. Therefore, the relationship between HCT and EPG has been studied for many years (Vanimisetti et al., 2004; Figueroa Castillo et al., 2011), and a correlation coefficient of –0.5 to –0.6 has been established. Based on this relationship between EPG and HCT, genetic resistance variables were identified (Bell et al., 2019). According to the HCT classification, resistant ewes should be selected during the initial lactation phase. This is based on our finding that both Katahdin and Pelibuey ewes showed similar EPG values among resistant ewes, allowing for the selection of these sheep by both EPG and HCT during this stage. However, in mid-lactation and late lactation, the HCT values decrease because of the presence of gastrointestinal nematode infections, and selection is not possible in these stages (David et al., 2020). Naturally, the priority during lactation is the production of milk to maintain the offspring; this requires increased water and nutrient mobilization to the mammary gland through the vascular system (Soliman, 2014). Using the HCT categorization, none of the Blackbelly ewes were resistant to lactation. Additionally, none of the Katahdin ewes were resistant in the classification during midlactation and late lactation. In Pelibuey ewes, no resistant ewes were observed in late lactation because of the gradual reduction in HCT. Therefore, the ideal stage for ewe selection is during initial lactation using both EPG and HCT.
Hematological variations in the ewes were caused by the high prevalence of H. contortus. This parasite can cause different degrees of anemia and hypoproteinemia in ewes and lambs (Casanova et al., 2018). According to previous studies and a coproculture performed in the most recent study, the main species of nematodes were H. contortus with a prevalence of 55%, C. curticei with 13%, T. colubriformis with 30%, and Oesophagostomum columbianum with 2% (González-Garduño et al., 2014a, 2014b; Herrera-Manzanilla et al., 2017).
Haemonchus contortus infections are highly pathogenic, mainly due to the hematophagous action of adult parasites, which locally produce small ulcers in the mucosa (Moosa et al., 2022). At a systemic level, acute hemorrhagic anemia with protein loss occurs along with sudden drops in HCT, manifesting in animals as pale mucous membranes, submandibular edema, and ascites. These phenomena put the health of the sheep at risk (Torres-Chable et al., 2020).
The Blackbelly ewes in this study showed greater susceptibility to parasites; however, this result contradicts reports by other authors (Terefe et al., 2007), who described Blackbelly ewes as a resistant breed. This discrepancy could be attributed to the inadequate selection process of the parents in the flock and the type of productive and reproductive management because environmental factors play an important role in the presence of gastrointestinal nematodes. Over time, some breeds may improve their genetic characteristics, whereas others may lose them. Consequently, the value of the inheritance index and the repeatability of the characteristic are intrinsic to the flock. Nevertheless, evaluating the HCT can accurately indicate blood loss due to H. contortus (Andronicos et al., 2014), making it a useful selection tool. This conclusion was found in the Katahdin and Pelibuey ewes. The ewes classified using the average HCT during initial lactation coincided with those classified using the EPG classification. The susceptible Katahdin ewes had an average EPG of 1,145, which was closely aligned with the 1,137 EPG determined by the average EPG during lactation. Similarly, the Pelibuey ewes showed comparable EPG values when classified by either HCT or EPG.
Although the EPG and HCT values were similar for the Katahdin ewes when using the EPG-lactation and initial lactation HCT methods, the concordance between the two methods was 56.3%. In the Pelibuey breed, the EPG values during lactation-EPG and initial lactation-HCT were also very similar, but only in the intermediate and susceptible groups. The HCT results obtained by both classification methods were similar. However, the concordance observed based on the number of ewes showed that 72.7% of resistant and susceptible ewes were consistently classified using both methods. During midlactation (weeks 5–9), the concordance rate was moderate (54.5%). For the Blackbelly ewes, similar EPG values were observed during lactation-EPG for the intermediate and susceptible ewes, and the HCT values were also very similar between the lactation EPG and mid- lactation-HCT classifications. The concordance rate was high during mid-lactation, reaching a substantial value of 70.0%. A study classifying resistance in sheep showed that susceptible lines had significant decreases in red blood cells, hemoglobin, and HCT, but an increase in reticulocytes compared with resistant sheep (Andronicos et al., 2014). In general, studies that aim to classify sheep as resistant or susceptible rely on EPG (Palomo-Couoh et al., 2016). Although the negative correlation between EPG and HCT is well known in the presence of H. contortus (Vanimisetti et al., 2004), only a few studies have indicated that HCT might be used as a selection marker for resistance in sheep in tropical regions (Zaragoza-Vera et al., 2019, 2022)
Conclusions
It is possible to categorize ewes based on HCT levels together with fecal egg counts, both of which are indicators of resistance in sheep when H. contortus is the main nematode in gastrointestinal nematode infection.
Pelibuey breed showed resistance to infection by gastrointestinal nematodes, as indicated by the highest HCT, lowest fecal egg count, and the highest number of resistant sheep within the breed.
In Pelibuey ewes, classification by HCT during early lactation (weeks 1–4) allowed the selection of resistant ewes. Overall, HCT may be a useful parameter for selecting ewes with resistance to gastrointestinal nematodes at the beginning of lactation.
The increase in the excretion of nematode eggs during early lactation (peripartum rise) allows ewes to be classified into resistant, susceptible, and intermediate in this stage based on the fecal egg count. The ewes with the lowest fecal nematode egg count represent the resistant ewes and the highest number of nematode eggs per gram during early lactation corresponds to susceptible ewes.
The concordance rate between fecal egg counts and HCT classification allowed us to conclude that it is possible to categorize ewes based on their HCT levels during early lactation.
Acknowledgments
We would like to thank the General Directorate of Research and Postgraduate Studies of the Autonomous University of Chapingo for its support under project 24041-C-92.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
This research did not receive external funding.
Author contributions
MGPL: Conceptualization, data curation, formal analysis, investigation. RGG. Data curation, project administration, writing –original draft. GJP: Validation, visualization, writing –review, and editing, GTH: Writing –review and editing. MZV: Writing –review and editing. JJRH: validation visualization, writing –review and editing. MGZV: validation visualization, writing –review and editing.
Data availability
Data are available from the authors upon reasonable request.
References
- Alba-Hurtado F., Muñoz-Guzmán M.A. Immune responses associated with resistance to haemonchosis in sheep. Biomed. Res. Int. 2013;11:162158. doi: 10.1155/2013/162158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andronicos N.M., Henshall J.M., Le Jambre L.F., Hunt P.W., Ingham A.B. A one shot blood phenotype can identify sheep that resist Haemonchus contortus challenge. Vet. Parasitol. 2013;205:595–605. doi: 10.1016/j.vetpar.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Bell A., McNally J., Smith D.V., Rahman A., Hunt P., Kotze A.C., Dominik S., Ingham A. Quantification of differences in resistance to gastrointestinal nematode infections in sheep using a multivariate blood parameter. Vet. Parasitol. 2019;270:31–39. doi: 10.1016/j.vetpar.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Berton M.P., Silva R.P., Carvalho F.E., Chiaia H.L.J., Oliveira P.S., Eler J.P., Banchero G., Ferraz J.B.S., Baldi F. Genetic parameter estimates for gastrointestinal nematode parasite resistance and maternal efficiency indicator traits in Santa Inês breed. J. Anim. Breed. Genet. 2019;136:495–504. doi: 10.1111/jbg.12424. [DOI] [PubMed] [Google Scholar]
- Calvete C., González J.M., Ferrer L.M., Ramos J.J., Lacasta D., Delgado I., Uriarte J. Assessment of targeted selective treatment criteria to control subclinical gastrointestinal nematode infections on sheep farms. Vet. Parasitol. 2020;277:109018. doi: 10.1016/j.vetpar.2019.109018. [DOI] [PubMed] [Google Scholar]
- Candy P.M., Waghorn T.S., Miller C.M., Ganesh S., Leathwick D.M. The effect on liveweight gain of using anthelmintics with incomplete efficacy against resistant Cooperia oncophora in cattle. Vet. Parasitol. 2018;251:56–62. doi: 10.1016/j.vetpar.2017.12.023. [DOI] [PubMed] [Google Scholar]
- Casanova V.P., Aires A.R., Collet S.G., Krause A., Moresco R.N., Bochi G. V., Silva A.S., Leal M.L.R. Iron supplementation for lambs experimentally infected by Haemonchus contortus: response to anemia and iron store in the bone marrow. Pesq. Vet. Bras. 2018;38:1543–1548. doi: 10.1590/1678-5150-PVB-5490. [DOI] [Google Scholar]
- Claerebout E., De Wilde N., Van Mael E., Casaert S., Velde F.V., Roeber F., Geldhof P. Anthelmintic resistance and common worm control practices in sheep farms in Flanders, Belgium. Vet. Parasitol. Reg. Stud. Reports. 2020;20:100393. doi: 10.1016/j.vprsr.2020.100393. [DOI] [PubMed] [Google Scholar]
- Cringoli G., Rinaldi L., Veneziano V., Capelli G., Scala A. The influence of flotation solution, sample dilution and the choice of McMaster slide area (volume) on the reliability of the McMaster technique in estimating the faecal egg counts of gastrointestinal strongyles and Dicrocoelium dendriticum in sheep. Vet. Parasitol. 2004;123:121–131. doi: 10.1016/j.vetpar.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Cruz-Tamayo A.A., López-Arellano M.E., González-Garduño R., Torres-Hernández G., de la Mora-Valle A., Becerril-Pérez C., Hernández-Mendo O., Ramírez-Bribiesca E., Huchin-Cab M. Haemonchus contortus infection induces a variable immune response in resistant and susceptible Pelibuey sheep. Vet. Immunol. Immunopathol. 2021;234:110218. doi: 10.1016/j.vetimm.2021.110218. [DOI] [PubMed] [Google Scholar]
- David C.M.G., Costa R.L.D., Parren G.A.E., Rua M.A.S., Nordi E.C.P., Paz C.C.P., Quirino C.R., Figueiredo R.S., Bohland E. Hematological, parasitological and biochemical parameters in sheep during the peripartum period. Revista Colombiana de Ciencias Pecuarias. 2020;33:81–95. doi: 10.17533/udea.rccp.v33n1a04. [DOI] [Google Scholar]
- Dey A.R., Begum N., AnisuzzamanAlim Md. A., Alam M.Z. Multiple anthelmintic resistance in gastrointestinal nematodes of small ruminants in Bangladesh. Parasitol. Int. 2020;77:102105. doi: 10.1016/j.parint.2020.102105. [DOI] [PubMed] [Google Scholar]
- Dominik S. Quantitative trait loci for internal nematode resistance in sheep : a review. Genet. Sel. Evol. 2005;37:83–96. doi: 10.1051/gse:2004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa Castillo J.A., Medina R.D.M., Villalobos J.M.B., Gayosso-Vázquez A., Ulloa-Arvízu R., Rodríguez R.A., Ramírez H.P., Alonso Morales R.A. Association between major histocompatibility complex microsatellites, fecal egg count, blood packed cell volume and blood eosinophilia in Pelibuey sheep infected with Haemonchus contortus. Vet. Parasitol. 2011;177:339–344. doi: 10.1016/j.vetpar.2010.11.056. [DOI] [PubMed] [Google Scholar]
- Gasparina J.M., Fonseca L., Loddi M.M., de Souza Martins A., da Rocha R.A. Resistance of ewes to gastrointestinal nematode infections during the peripartum and dry periods and the performance of their lambs. Revista Brasileira de Saude e Producao Animal. 2019;20:1–11. doi: 10.1590/S1519-9940200282019. [DOI] [Google Scholar]
- Gonçalves T.C., Alencar M.M., Giglioti R., Bilhassi T.B., Oliveira H.N., Rabelo M.D., Esteves S.N., Oliveira M.C.S. Resistance of sheep from different genetic groups to gastrointestinal nematodes in the state of São Paulo, Brazil. Small Rumin. Res. 2018;166:7–11. doi: 10.1016/j.smallrumres.2018.07.003. [DOI] [Google Scholar]
- González Garduño R., López Arellano M.E., Conde Felipe M.M., Mendoza de Gives P., Aguilar Marcelino L., Jaso Díaz G. Immune and haematological parameters of Blackbelly ewes infected with gastrointestinal nematodes. Revista Colombiana de Ciencias Pecuarias. 2017;30:219–230. doi: 10.17533/udea.rccp.v30n3a05. [DOI] [Google Scholar]
- González J.F., Hernández Á., Meeusen E.N.T., Rodríguez F., Molina J., Jaber J., Raadsma H., PIedrafita D. Parasitology fecundity in adult Haemonchus contortus parasites is correlated with abomasal tissue eosinophils and T cells in resistant Canaria Hair Breed sheep. Vet. Parasitol. 2011;178:286–292. doi: 10.1016/j.vetpar.2011.01.005. [DOI] [PubMed] [Google Scholar]
- González-Garduño R., Arece-García J., Torres-Hernández G. Physiological, immunological and genetic factors in the resistance and susceptibility to gastrointestinal nematodes of sheep in the peripartum period: a review. Helminthologia (Poland) 2021;58:134–151. doi: 10.2478/helm-2021-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Garduño R., López-Arellano M.E., Mendoza-de-Gives P., Torres-Hernández G., Arece-García J. Immune response in Blackbelly lambs to Haemonchus contortus and Trichostrongylus colubriformis mixed. Trop. Biomed. 2018;35:696–708. [PubMed] [Google Scholar]
- González-Garduño R, Navarro-Martínez F., Arece- García J. 2014a Presence of Cooperia curticei, C. punctata and Trichostrongylus colubriformis, (Strongylida: Trichostrongylidae) in Tabasco, Mexico. Revista de Salud Animal. 36:159–163. [Google Scholar]
- González-Garduño R., Navarro-Martínez F., Arias-Julián J., Gutiérrez-Cruz S., Zaragoza Vera M., Zaragoza Vera C. Descripción morfológica de Haemonchus contortus y Mecistocirrus digitatus de ovinos y bovinos en Tabasco, México. Avances en Ciencias Veterinarias. 2014b;28:76–86. doi: 10.5354/0716-260x.2013.30208. [DOI] [Google Scholar]
- González-Garduño R., Torres-Acosta J.F.J., Chay-Canul A.J. Susceptibility of hair sheep ewes to nematode parasitism during pregnancy and lactation in a selective anthelmintic treatment scheme under tropical conditions. Res. Vet. Sci. 2014c;96:487–492. doi: 10.1016/j.rvsc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Guo Z., González J.F., Hernandez J.N., McNeilly T.N., Corripio-Miyar Y., Frew D., Morrison T., Yu P., Li R.W. Possible mechanisms of host resistance to Haemonchus contortus infection in sheep breeds native to the Canary Islands. Sci. Rep. 2016;6:1–14. doi: 10.1038/srep26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer K., McIntyre J., Morrison A.A., Jennings A., Kelly R.F., Leeson S., Bartley D.J., Chaudhry U., Busin V., Sargison N. The dynamics of ovine gastrointestinal nematode infections within ewe and lamb cohorts on three Scottish sheep farms. Prev. Vet. Med. 2019;171:104752. doi: 10.1016/j.prevetmed.2019.104752. [DOI] [PubMed] [Google Scholar]
- Herrera-Manzanilla F.A., Ojeda-Robertos N.F., González-Garduño R., Cámara-Sarmiento R., Torres-Acosta J.F.J. Gastrointestinal nematode populations with multiple anthelmintic resistance in sheep farms from the hot humid tropics of Mexico. Vet. Parasitol. Reg. Stud. Reports. 2017;9:29–33. doi: 10.1016/j.vprsr.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Huerta Aragonés J., Cela de Julián E. Hematología práctica : interpretación del hemograma y de las pruebas de coagulación. In: Lúa Ediciones., editor. In: AEPap. Madrid, España: 2018. pp. 507–526. [Google Scholar]
- Karrow N.A., Goliboski K., Stonos N., Schenkel F., Peregrine A. Review: Genetics of helminth resistance in sheep. Can. J. Anim. Sci. 2014;94:1–9. doi: 10.4141/CJAS2013-036. [DOI] [Google Scholar]
- Kottek M., Grieser J., Beck C., Rudolf B., Rubel F. World map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift. 2006;15:259–263. doi: 10.1127/0941-2948/2006/0130. [DOI] [Google Scholar]
- Lalramhluna M., Bordoloi G., Pandit S., Baidya S., Joardar S.N., Patra A.K., Jas R. Parasitological and immunological response to Haemonchus contortus infection: comparison between resistant Garole and susceptible Sahabadi sheep. Vet. Parasitol. Reg. Stud. Reports. 2020;22:100477. doi: 10.1016/j.vprsr.2020.100477. [DOI] [PubMed] [Google Scholar]
- Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Machín C., Corripio-Miyar Y., Hernández J.N., Pérez-Hernández T., Hayward A.D., Wright H.W., Price D.R.G., Matthews J.B., McNeilly T.N., Nisbet A.J., González J.F. Cellular and humoral immune responses associated with protection in sheep vaccinated against Teladorsagia circumcincta. Vet. Res. 2021;52:1–14. doi: 10.1186/s13567-021-00960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maza-Lopez J., Pacheco-Armenta M.J., Reyes-Guerrero D.E., Olmedo-Juárez A., González-Garduño R., Olazarán-Jenkins S., López-Arellano M.E. Immune response related to Pelibuey sheep naturally infected with gastrointestinal nematodes in a tropical region of Mexico. Vet. Parasitol. Reg. Stud. Reports. 2020;21:100422. doi: 10.1016/j.vprsr.2020.100422. [DOI] [PubMed] [Google Scholar]
- Moosa D.A., Hussien A.M., Hameed H.M., Hasan S.A. Diagnostic and hematological study in sheep infected with gastrointestinal nematode in Mosul city. Al-Anbar J Vet Sci. 2022;15:29–33. [Google Scholar]
- Morteo-Gómez R., González-Garduño R., Torres-Hernández G., Nuncio-Ochoa G., Becerril-Pérez C.M., Gallegos-Sánchez J., Aranda-Ibañez E. Effect of the phenotypic variation in the resistance of Pelibuey lambs to the infestation with gastrointestinal nematodes. Agrociencia. 2004;38:395–404. [Google Scholar]
- Muñoz-Guzmán M.A., Cuéllar-Ordaz J.A., Valdivia-Anda A.G., Buendía-Jiménez J.A., Alba-Hurtado F. Correlation of parasitological and immunological parameters in sheep with high and low resistance to haemonchosis. Can. J. Anim. Sci. 2006;86:363–371. doi: 10.4141/A06-010. [DOI] [Google Scholar]
- Notter D.R., Andrew S.A., Zajac A.M. Responses of hair and wool sheep to a single fixed dose of infective larvae of Haemonchus contortus. Small Rumin Res. 2003;47:221–225. doi: 10.1016/S0921-4488(02)00279-1. [DOI] [Google Scholar]
- Notter D.R., Burke J.M., Miller J.E., Morgan J.L.M. Factors affecting fecal egg counts in periparturient Katahdin ewes and their lambs. J. Anim. Sci. 2017;95:103–112. doi: 10.2527/jas2016.0955. [DOI] [PubMed] [Google Scholar]
- Olivas-Salazar R., Estrada-Angulo A., Mellado M., Aguilar-Caballero A.J., Castro-Pérez B.I., Gutiérrez-Blanco E., Ruiz-Zárate F. Prevalence of gastrointestinal nematode infections in goat flocks on semi-arid rangelands of northeastern Mexico. Trop. Anim. Health Prod. 2018;50:807–813. doi: 10.1007/s11250-017-1499-x. [DOI] [PubMed] [Google Scholar]
- Ortolani E.L., Leal M.L. do R., Minervino A.H.H., Aires A.R., Coop R.L., Jackson F., Suttle N.F. Effects of parasitism on cellular immune response in sheep experimentally infected with Haemonchus contortus. Vet. Parasitol. 2013;196:230–234. doi: 10.1016/j.vetpar.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Palomo-Couoh J.G., Aguilar-Caballero A.J., Torres- Acosta J.F. de J., Magaña-Monforte J.G. Evaluation of different models to segregate Pelibuey and Katahdin ewes into resistant or susceptible to gastrointestinal nematodes. Trop. Anim. Health. Prod. 2016;48:1517–1524. doi: 10.1007/s11250-016-1122-6. [DOI] [PubMed] [Google Scholar]
- Pawar P., Das Singla L., Kaur P., Bal M.S., Javed M. Evaluation and correlation of multiple anthelmintic resistances to gastrointestinal nematodes using different fecal egg count reduction methods in small ruminants of Punjab, India. Acta Parasitol. 2019;64:456–463. doi: 10.2478/s11686-019-00083-3. [DOI] [PubMed] [Google Scholar]
- Pereira F.C., Longo C., Castilho C., Leme D.P., Seugling J., Bassetto C.C., Amarante A.F.T., Bricarello P.A. Peripartum phenomenon in crioula lanada sheep susceptible and resistant to gastrointestinal nematodes. Front. Vet. Sci. 2020;7:1–8. doi: 10.3389/fvets.2020.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe A., McMaster K., Emery D., Sangster N. Haemonchus contortus infection in sheep: Parasite fecundity correlates with worm size and host lymphocyte counts. Vet. Parasitol. 2008;153:285–293. doi: 10.1016/j.vetpar.2008.01.040. [DOI] [PubMed] [Google Scholar]
- SAS . Cary, NC, USA: 2017. SAS/STAT User’s Guide. Release 6. [Google Scholar]
- Sayers G., Sweeney T. Gastrointestinal nematode infection in sheep–a review of the alternatives to anthelmintics in parasite control. Anim. Health. Res. Rev. 2005;6:159–171. doi: 10.1079/AHR2005108. [DOI] [PubMed] [Google Scholar]
- SMN Servicio Meteorológico Nacional. Normales climatológicas [WWW Document] 2021 Available via. http://smn.cna.gob.mx/climatologia/normales/estacion/tab/NORMAL27068.TXT. [Google Scholar]
- Soliman E.B. Effect of physiological status on some hematological and biochemical parameters of Ossimi sheep. Egyptian J. Sheep Goat Sci. 2014;9:33–42. [Google Scholar]
- Terefe G., Lacroux C., Andreoletti O., Grisez C., Prevot F., Bergeaud J.P., Penicaud J., Rouillon V., Gruner L., Brunel J.C., Francois D., Bouix J., Dorchies P., Jacquiet P. Immune response to Haemonchus contortus infection in susceptible (INRA 401) and resistant (Barbados Black Belly) breeds of lambs. Parasite Immunol. 2007;29:415–424. doi: 10.1111/j.1365-3024.2007.00958.x. [DOI] [PubMed] [Google Scholar]
- Thienpont D., Rochette F., Vanparijs O.F.J. Beerse, Belgium: Janssen Research Foundation; Diagnosing helminthiasis by coprological examination. 3rd. [Google Scholar]
- Torres-Acosta J.F., Hoste H., Sandoval-Castro C.A., Torres-Fajardo R.A., Ventura- J., González-pech P.G., Mancilla-montelongo M.G., Ojeda-Robertos N.F. The “Art of War ” against gastrointestinal nematodes in sheep and goat herds of the tropics. Revista Académica Ciéncia Animal. 2019;17:39–46. [Google Scholar]
- Torres-Chable O.M., García-Herrera R.A., González-Garduño R., Ojeda-Robertos N.F., Peralta-Torres J.A., Chay-Canul A.J. Relationships among body condition score, FAMACHA© score and haematological parameters in Pelibuey ewes. Trop. Anim. Health Prod. 2020;52:3403–3408. doi: 10.1007/s11250-020-02373-9. [DOI] [PubMed] [Google Scholar]
- Vanimisetti H.B., Andrew S.L., Zajac A.M., Notter D.R. Inheritance of fecal egg count and packed cell volume and their relationship with production traits in sheep infected with Haemonchus contortus. J. Anim. Sci. 82:1602–1611. doi: 10.2527/2004.8261602x. [DOI] [PubMed] [Google Scholar]
- Woolaston R.R. Selection of Merino sheep for increased and decreased resistance to Haemonchus contortus: peri-parturient effects on faecal egg counts. Int. J. Parasitol. Drugs Drug Resist. 1992;22:947–953. doi: 10.1016/0020-7519(92)90052-M. [DOI] [PubMed] [Google Scholar]
- Zaragoza-Vera C.V., González-Garduño R., Flores-Santiago E. del J., Chay-Canul A.J., Zaragoza-Vera M., Arjona-Jiménez G., Torres-Chablé O.M. Hematological changes during pregnancy and lactation in Pelibuey ewes infected with gastrointestinal nematodes. Comp. Clin. Path. 2022;31:827–838. doi: 10.1007/s00580-022-03386-6. [DOI] [Google Scholar]
- Zaragoza-Vera C.V., González-Garduño R., Zaragoza-Vera M., Arjona-Jimenez G., Ortega-Pacheco A., Torres-Chable O.M. Evaluation of Pelibuey Lambs Born to Mothers Phenotypically Segregated According to Resistance to Gastrointestinal Nematodes in the Humid Tropics of Mexico. J. Parasitol. 2023;109(1):1–8. doi: 10.1645/22-44. [DOI] [PubMed] [Google Scholar]
- Zaragoza-Vera C.V, Aguilar-Caballero A.J., González-Garduño R., Arjona-Jiménez G., Zaragoza-Vera M., Torres-Acosta J.F.J., Medina-Reynés J.U., Berumen-Alatorre A.C. Variation in phenotypic resistance to gastrointestinal nematodes in hair sheep in the humid tropics of Mexico. Parasitol. Res. 2019;118(2):567–573. doi: 10.1007/s00436-018-06201-w. [DOI] [PubMed] [Google Scholar]
- Zvinorova P.I., Halimani T.E., Muchadeyi F.C., Matika O., Riggio V., Dzama K. Breeding for resistance to gastrointestinal nematodes - the potential in low-input/output small ruminant production systems. Vet. Parasitol. 2016;225:19–28. doi: 10.1016/j.vetpar.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the authors upon reasonable request.


