Abstract
The Munc13/UNC-13 family protein Ync13 is essential for septum integrity and cytokinesis in fission yeast. To further explore the mechanism of Ync13 functions, spontaneous suppressors of ync13 mutants, which can suppress the colony-formation defects and lysis phenotype of ync13 mutant cells, are isolated and characterized. One of the suppressor mutants, bst1-s27, shows defects in the cytokinetic contractile ring constriction, septation, and daughter cell separation, similar to bst1Δ mutant. Bst1, a predicted GPI inositol deacylase, was an uncharacterized protein in fission yeast. It localizes to ER and puncta structures in the cytoplasm. The Bst1 puncta overlaps frequently with Anp1, which is a marker of endoplasmic reticulum (ER)-Golgi transport, but rarely with trans-Golgi marker Sec72. The nuclear ER signal of Anp1 increases in bst1Δ mutant, whereas Sec72 localization shows no obvious changes. In addition, more cytoplasmic puncta structures of COPII subunits, Sec13 and Sec24, are observed in bst1Δ mutant, and acid phosphatase secretion is compromised without Bst1. Consistently, the division site targeting of the β-glucanase Eng1 and α-glucanase Agn1 is reduced in bst1Δ and bst1Δ ync13Δ mutant. Taken together, our results suggest that Bst1 regulates ER-Golgi transport and is involved in cytokinesis through regulating the secretion of glucanases.
Plasma membrane deposition at the division site via membrane trafficking is an essential step to complete cytokinesis. However, it is still poorly understood including the roles of the essential protein Ync13 in Munc13/UNC-13 gene family.
The authors report the identification and characterization of mutations in GPI inositol deacylase Bst1 that suppress ync13 mutants. Bst1 regulates the ER-Golgi transport, COPII subunit distribution, and the secretion of glucanases for daughter cell separation.
This work provides an example of how early and late regulators of the secretory pathway functionally compensate during cytokinesis by regulating proteins at different steps of exocytosis.
INTRODUCTION
Cytokinesis partitions cytoplasm and organelles from a mother cell to two daughter cells as the last step of the cell-division cycle. It is essential for cell proliferation, cell differentiation, embryo development, and tissue homeostasis. Defects in cytokinesis can lead to cell death, genomic instability, or tumorigenesis (Lens and Medema, 2019; Petsalaki and Zachos, 2021; Darp et al., 2022; Rezig et al., 2023). In most eukaryotic cells from fungi to mammalian cells, cytokinesis relies on several crucial steps: cleavage site selection; actomyosin contractile ring assembly, maturation (or slow phase of constriction), constriction (fast phase); plasma membrane deposition and expansion at the division plane; and extracellular matrix (septum in a fungal cell) formation or remodeling (Wu et al., 2003; Vavylonis et al., 2008; Pollard and Wu, 2010; Meitinger and Palani, 2016; Gerien and Wu, 2018; Ramos et al., 2019; Rezig et al., 2023; Shi et al., 2023). These events are coordinated with cell cycle progression and regulated by the Septation Initiation Network, or Mitotic Exit Network, or Hippo pathways (McCollum and Gould, 2001; Lattmann et al., 2009; Foltman and Sanchez-Diaz, 2017; Zhang and Zhu, 2018). Cytokinesis also needs the cooperation of exocytosis and endocytosis to succeed (Karahara et al., 2010; Rybak et al., 2014; Gerien and Wu, 2018; Zhu et al., 2018; Singh et al., 2024). Thus, it is not surprising that >200 proteins are involved in cytokinesis.
Our previous study has identified and initially characterized one of the essential proteins in cytokinesis named Ync13 (Zhu et al., 2018), which is a member of UNC-13/Munc13 protein family. UNC-13/Munc13 proteins are important for vesicle tethering and SNARE complex assembly during exocytosis (Hata et al., 1993; Guan et al., 2008; Khodthong et al., 2011; Park et al., 2017; Rodarte et al., 2018; Shu et al., 2020). Ync13 dynamically localizes to cell tips during interphase and is relocated to the division site during early anaphase and remains there until daughter cell separation (Zhu et al., 2018). Ync13 is involved in both exocytosis and endocytosis to regulate the proper recruitment, maintenance, and distribution of cell wall enzymes including the glucan synthases Bgs1, Bgs4, Ags1, and the glucanase Eng1 during cytokinesis (Ribas et al., 1991; Cortes et al., 2002; Liu et al., 2002; Cortes et al., 2005; Cortes et al., 2007; Cortes et al., 2012; Cortés et al., 2016; Pérez et al., 2016; Zhu et al., 2018). Temperature-sensitive ync13 mutants at the restrictive temperature and ync13Δ are lethal due to defective septum and cell lysis. Interestingly, deletion of eng1 can partially rescue ync13Δ cells (Zhu et al., 2018). However, the mechanisms of Ync13 functions in cytokinesis remain poorly understood.
Many cell surface proteins anchor to the plasma membrane through the posttranslationally modified glycosylphosphatidylinositol (GPI) (Kinoshita and Fujita, 2016; Saha et al., 2016; Lebreton et al., 2018; Essen et al., 2020; Kinoshita, 2020). GPI-anchored proteins have been studied from plants, yeasts, to mammals, and play crucial roles in several cellular processes, including morphogenesis, cell growth, cell division, neurological development, and pathogenesis (Ueda et al., 2007; Chen et al., 2014; Cheung et al., 2014; Fujihara et al., 2014; Murakami et al., 2014; Bosch et al., 2015; Lebreton et al., 2018). GPI-anchor is synthesized in the endoplasmic reticulum (ER). After transferred to the target proteins, the GPI anchor remodels to properly sort and to help transport the GPI-anchored proteins (Eisenhaber et al., 2003; Kinoshita, 2020). Soon after the GPI transfers to proteins, the acyl-chain linked to inositol is usually removed by inositol deacylase (Chen et al., 1998). Defects in mammalian inositol deacylase, PGAP1, cause delay in the transport of GPI-anchored proteins from ER, although no significance effect on the cell surface attachment (Tanaka et al., 2004; Murakami et al., 2014). In budding yeast, the orthologue Bst1 localizes to ER and is required not only for efficient transport of GPI-anchored proteins from ER to Golgi, but also for the quality control of GPI-anchored proteins and other misfolded proteins (Elrod-Erickson and Kaiser, 1996; Vashist et al., 2001; Tanaka et al., 2004; Fujita et al., 2006). In addition, budding yeast Bst1 negatively regulates COPII vesicle function, and is required for proper sorting of resident ER proteins and Golgi-bound cargo molecules (Elrod-Erickson and Kaiser, 1996; Vashist et al., 2001; Tanaka et al., 2004; Fujita et al., 2006). Homologues of PGAP1 and Bst1 are also important for efficient transport and cell surface localization of GPI-anchored proteins in Arabidopsis thaliana and Candida albicans (Liu et al., 2016; Bernat-Silvestre et al., 2021). GPI inositol deacylase mediated remodeling is important for the recognition of p24 family cargo receptors, then facilitates correctly remodeled GPI-anchored proteins to be sorted into COPII vesicles (Castillon et al., 2009; Fujita et al., 2011). However, whether PGAP1 and Bst1 affect other normal cargoes transport is unknown.
Essentially all the ync13 temperature-sensitive (at 36°C) or ync13Δ mutant cells died from cell lysis without forming colonies in rich medium (Zhu et al., 2018). However, we observed that some ync13 mutant cells with spontaneous suppressors can form colonies after extended incubation. But the nature of the suppressors was unknown. In this study, we isolated and characterized five of the suppressor mutants, focusing on bst1-s27. Our results suggest that Bst1 regulates ER-Golgi transport and is involved in cytokinesis through regulating the secretion of glucanases Eng1 and Agn1.
RESULTS
Identification of bst1 mutant as a spontaneous suppressor of ync13 mutant
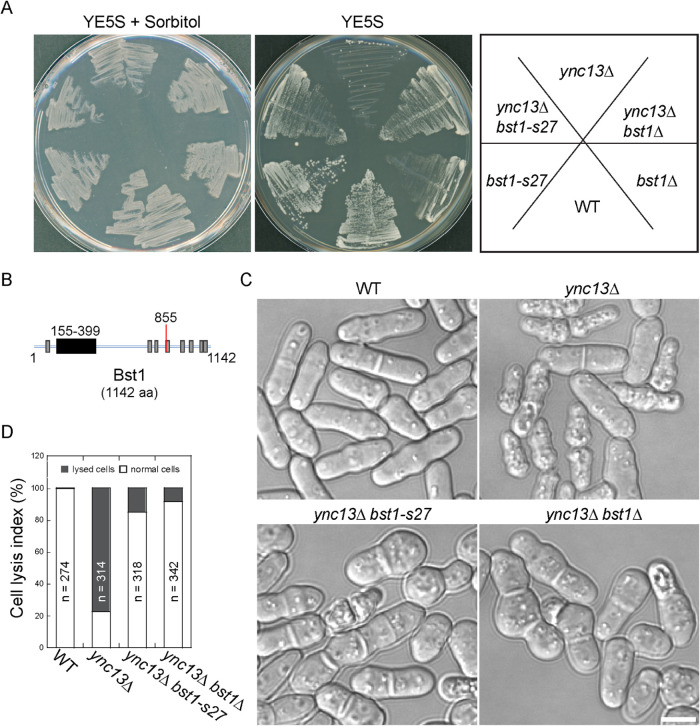
Ync13 is essential for the cell-wall integrity during cytokinesis (Zhu et al., 2018). Deletion of ync13 leads to cell lysis and difficulties in colony formation on YE5S-rich medium without osmotic stabilizer sorbitol (Figure 1A). To explore the mechanism on how Ync13 regulates cell integrity, the spontaneous suppressors of ync13 mutants were isolated from single colonies on YE5S agar plates. After approximately five rounds of backcrosses to wild type (WT), five strong suppressors (sdy13-1M, sdy13-8, ys4c, s3, and s27) were chosen for genetic mapping (Table 1). The genomic DNAs from the mapped loci were sequenced and the suppressors were identified as mutations in mid2, spn1, mid2, spn4, and bst1, respectively, which resulted in frame shift and/or premature stop codons and encoded truncated proteins (Table 2; Figure 1B).
FIGURE 1:
bst1 mutants suppress cell lysis in ync13Δ. (A) Growth test of bst1 and ync13 mutants. Cells of WT, ync13Δ, bst1-s27, bst1Δ, ync13Δ bst1-s27, and ync13Δ bst1Δ were grown on YE5S plate with 1.2 M sorbitol, then restreaked onto YE5S or YE5S + 1.2 M sorbitol plate and grown for 3 d at 25°C. (B) Domain structures of Bst1 derived from the PomBase database with numbers showing the amino acid residues. Dark and gray boxes indicate GPI inositol deacylase domain and transmembrane domains, respectively, the red line marks the position of bst1-s27 truncation. (C and D) Morphology (C) and quantification of cell lysis (D) in ync13 and bst1 ync13 mutants. Cells of WT, ync13Δ, ync13Δ bst1-s27 and ync13Δ bst1Δ were grown in YE5S + 1.2 M sorbitol plate, then cultured in YE5S liquid medium in log phase at 25°C for ∼18 h. Bar, 5 µm.
TABLE 1.
Tetrad analyses to map ync13 suppressors.
| Suppressor | sdy13-1M | sdy13-8 | ys4c | s27 | s3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene/locus | Chromosome, locus | PD | TT | NPDa | PD | TT | NPD | PD | TT | NPD | PD | TT | NPD | PD | TT | NPD |
| sdy13 | 11 | 0 | 0 | 19 | 1 | 0 | 6 | 0 | 0 | |||||||
| ys4c | 58 | 0 | 0 | 19 | 4 | 0 | ||||||||||
| sdy13-8 | 21 | 3 | 0 | |||||||||||||
| ade6 b | III, 1316337-1317995 | 5 | 2 | 11 | 1 | 0 | 2 | 7 | 0 | 4 | 4 | 0 | 4 | |||
| leu1b | II, 1974488-1975603 | 10 | 2 | 6 | 1 | 0 | 2 | 6 | 0 | 5 | 7 | 0 | 1 | |||
| lys1b | I, 3739162-3743421 | 16 | 2 | 0 | 3 | 0 | 0 | 11 | 0 | 0 | ||||||
| spn1 | I, 4852998-4855199 | 8 | 2 | 0 | 8 | 0 | 0 | 12 | 5 | 0 | ||||||
| mid2 | I, 4751456-4748861 | 23 | 0 | 0 | 36 | 0 | 0 | |||||||||
| rho5 | I, 2131895-2132615 | 13 | 9 | 4 | ||||||||||||
| spn4 | I, 1991787-1992929 | 28 | 14 | 3 | 39 | 0 | 0 | |||||||||
| sid1 | I, 1985757-1987228 | 12 | 15 | 1 | ||||||||||||
| cdc42 | I, 1916108-1917279 | 12 | 15 | 0 | ||||||||||||
| SPAPB1A10.16 | I, 1863972-1864457 | 16 | 16 | 1 | ||||||||||||
| rec12 | I, 1772925-1774203 | 24 | 2 | 0 | ||||||||||||
| SPAC824.03c | I, 1688902-1689645 | 54 | 0 | 0 | ||||||||||||
| ubx3 | I, 1654284-1655516 | 27 | 4 | 0 | ||||||||||||
| SPAC167.05 | I, 1551408-1553213 | 22 | 5 | 0 | ||||||||||||
| SPAC19E9.03 | I, 1362571-1363806 | 13 | 17 | 0 | ||||||||||||
| rst2 | I, 1310980-1312683 | 11 | 13 | 2 | ||||||||||||
| SPAC1565.02c | I, 1292617-1293803 | 10 | 11 | 4 | ||||||||||||
aPD: parental ditype; TT: tetratype; NPD: nonparental ditype.
bBackground of tetrad crosses is rec12Δ.
TABLE 2.
Sequence analyses of ync13 suppressors.
| Suppressor gene | sdy13-1M | sdy13-8 | ys4c | s27 | s3 |
|---|---|---|---|---|---|
| Gene identified | mid2 | spn1 | mid2 | bst1 | spn4 |
| Genomic sequence | AT1812TTTC | AG304GGGA | TA529 AAAAAAAAT | CC2474 AAAATCTTTCTGGAAAC | GA245AAAAAAT |
| Mutation in DNAa | AG1812TTTC | AG304GGA | TA529 AAAAAAAAAT | CC2474 AAAATCTTTCTGGAAACAAAATCTTTCTGGAAAC | GA245 AAAAAAAT |
| Resulted proteinb | FL706aa →N603aa | FL469aa → N111aa | FL706aa → N185aa | FL1142aa → N855aa | FL380aa → N93aa |
aMutation shown in nucleotide, numbers indicate the position within the ORFs.
bResulted protein shown in amino acids. FL, full-length protein; N, NH2 terminal.
The suppressors sdy13-1M, sdy13-8, and ys4c resembled the multi-septated mid2Δ and spn1Δ mutants (Berlin et al., 2003; Tasto et al., 2003; An et al., 2004; Wu et al., 2010), no obvious temperature sensitivity was observed (Supplemental Figure S1A). Isolation of known septation mutants validated our approach. Rescue of ync13 mutant by these suppressors and their deletion mutants was also confirmed by genetic crosses (Figure 1A and our unpublished data). Bst1 encodes a predicted GPI inositol deacylase (SPAC824.02) in the fission yeast Schizosaccharomyces pombe. To explore how bst1 rescues ync13 mutant, we created a deletion mutant of bst1. The intragenic noncomplementation test showed that the diploid cells of bst1+/s27 and bst1+/bst1Δ had almost normal septa and morphology as WT diploid. However, s27/bst1Δ cells showed similar phenotype as the haploid bst1 mutants, except cells were bigger, indicating that the mutation in s27 (shown as bst1-s27) is indeed located in the bst1 gene (Supplemental Figure S1B). Both bst1-s27 and bst1Δ restored growth and colony formation of ync13Δ cells on YE5S medium without sorbitol (Figure 1A), reduced cell lysis in ync13Δ mutant dramatically (from 77 to 15% and 9%, respectively; Figure 1, C and D). Thus, loss of function of bst1 mutation can suppress the cell-lysis phenotype in ync13 mutants.
Bst1 is required for cell polarity and cytokinesis
bst1-s27 and bst1Δ mutants showed similar defects in cell morphology and septation. Compared with the rod shape of WT cells, rounder, swollen, and more septating cells were observed in bst1-s27 and bst1Δ mutants (Figure 2A). About 23% WT cells contained one septum, in bst1-s27 and bst1Δ mutants, ∼52% cells had one septum, and ∼30% cells had ≥ 2 septa, which was not observed in WT cells (Figure 2B).
FIGURE 2:
bst1 mutants show defects in morphology and cytokinesis. (A and B) Morphology (A) and septum index (B) of bst1 mutants. Cells of WT, bst1-s27, and bst1Δ were grown in YE5S in log phase at 25°C for ∼36 h, then stained with Calcofluor before imaging. (C) Time lapse (in min) of GFP-Psy1 Rlc1-tdTomato mRFP-Atb2 in WT and bst1-s27 mutant. Time 0 indicates spindle breakdown. (D and E) Time course (D) and quantification (E) of the main events (Rlc1 appearance at the division site, contractile-ring formation, maturation, and constriction) of cytokinesis (in min) of GFP-Psy1 Rlc1-tdTomato Sad1-mEGFP in WT and bst1Δ mutant. Time 0 marks the start of SPBs separation (arrowheads). Only Rlc1 signal is shown before time 0 for clarity. Numbers of the cells analyzed (n) are shown above or below the bars in (E). **p < 0.001. (F) Kymographs showing Rlc1 ring maturation and constriction in WT and bst1Δ cells as in D. Time interval is 2 min. Bars, 5 µm.
To further explore the roles of Bst1 in septation and/or daughter cell separation, we used t-SNARE syntaxin Psy1 (Nakamura et al., 2001), α-tubulin Atb2 (Sato et al., 2009) or spindle-pole-body (SPB) protein Sad1 (Tatebe et al., 2001), and myosin-II regulatory light chain Rlc1 (Le Goff et al., 2000; Naqvi et al., 2000) to examine the plasma membrane, cell cycle stages, and contractile ring during cytokinesis, respectively (Figure 2, C–F). In WT cells, the mitotic spindle elongated toward two cell ends and broke down when reached to the poles (Figure 2C). Interestingly, the mitotic spindle curved and continued to elongate even when it reached to cell ends, and interphase microtubules were short and misoriented in bst1-s27 mutant (Figure 2C). Like in WT cells (Ramos et al., 2019), the contractile ring still formed from the precursor nodes and constricted to guide plasma membrane invagination and septum formation in bst1-s27 and bst1Δ cells (Figure 2, C and D). In WT cells and bst1Δ mutant, Rlc1-tdTomato nodes appeared at the division site ∼12 min before SPB separation, the compact-ring formation (from nodes appearance to condensation into a compact ring without lagging nodes) and ring maturation (from a compact ring to the start of fast phase of ring constriction) took ∼22 min (p = 0.05) and ∼14 min (p = 0.13), respectively, no significant difference was detected in these two strains. In contrast, the contractile ring constricted and disassembled (from the start of fast phase of ring constriction to ring disappearance at the division site) significantly slower with big variation in speed in bst1Δ (78.4 ± 31.5 min) than in WT cells (34.2 ± 3.5 min, p < 0.001) (Figure 2, E and F). These results suggest that Bst1 plays roles in cell morphogenesis and promotes the contractile ring constriction and cell septation during cytokinesis.
Bst1 plays a role in early ER transport
GPI inositol deacylase is an ER-localized integral membrane protein (Elrod-Erickson and Kaiser, 1996; Tanaka et al., 2004; Bernat-Silvestre et al., 2021). Consistently, endogenously expressed functional Bst1-mScarlet appeared to localize to both cortical and nuclear envelope associated ER, although the signals were weak (Figure 3A). In addition, we observed many punctate structures of Bst1-mScarlet in the cytoplasm as well (Figure 3, [A] showed the middle focal planes and [D] showed the maximum intensity projections of Z sections). Functional Bst1-mECitrine and Bst1-tdTomato exhibited similar localization patterns in ERs and cytosolic punctate structure (Figure 3B; Supplemental Figure S2). It seemed that different tags had no significant effect on the essential function of Bst1 according to growth test and cell morphology of tagged bst1 strains (Figure 3, A, B, and D; Supplemental Figures S2 and S3A), and Bst1-mECitrine could not rescue ync13Δ (Supplemental Figure S3B). As bst1 mutants suppressed ync13 mutants, we asked whether Ync13 and Bst1 depend on each other for localization. In WT cells, Ync13 localized to the cell tips in interphase and appeared at the division site before spindle break-down, and its intensity increased until the contractile ring started to constrict (Zhu et al., 2018). Although Ync13 still recruited to the division site before spindle break-down, its intensity was higher in bst1Δ cells (Figure 3, B and C; Supplemental Figure S3C). On the other hand, no obvious differences in Bst1 localization were observed between WT and ync13Δ cells (Figure 3B), suggesting that Bst1 is directly or indirectly involved in Ync13’s recruitment or maintenance at the division site.
FIGURE 3:
Bst1 contributes to ER-Golgi transport. (A) Localization of Bst1. Left, the single middle focal plane; Right, max intensity projection of the middle 4–8 Z focal planes. (B) Localization dependence of Ync13 and Bst1. For Bst1 localization, cells were grown in YE5S + 1.2 M sorbitol liquid medium in log phase at 25°C for ∼18 h, then washed (3x) into YE5S medium and grown for 2 h before imaging. Other strains were cultured as routinely. Arrowhead indicates appearance of Ync13-mECitrine signal at the division site. (C) Quantification of Ync13 intensity at the division site in cells imaged as in B. Total Ync13 intensity at the division site versus the ratio of Myo2 ring diameter/cell diameter was plotted. (D) Colocalization of Bst1 with Sec72 or Anp1. Arrowheads indicate overlapped signals of Bst1-mScarlet and Anp1-GFP. Percentage after Merge is the ratio of Bst1-mScarlet punctate structure that containing either Sec72-GFP or Anp1-GFP. (E) Localization of Sec72-GFP and Anp1-GFP in WT and bst1Δ mutant. The images showed the single middle focal plane. (F) Secretion of acid phosphatase into culture medium. Acid phosphatase secretion assays of WT, bst1-s27, and bst1Δ cells. The absorbance at 405 nm was normalized to cell density of OD595nm. Error bars are SEM of three experiments. Bars, 5 µm.
To address what structures that Bst1 puncta associated with, we tested the colocalization of Bst1 with Anp1 (Golgi mannan polymerase I complex subunit) as a cis-Golgi marker, and Sec72 (Arf GEF) as a trans-Golgi marker (Vjestica et al., 2008). Most (66%) punctate Bst1-mScarlet structures colocalized with Anp1-GFP, but colocalization with Sec72-GFP was rarer (12%, Figure 3D). Interestingly, although we found that Sec72-GFP showed similar distribution in WT and bst1Δ cells, bst1Δ cells had increased signal of Anp1-GFP at the nuclear ER (neER) besides the cytosolic puncta, unlike Anp1-GFP in WT cells (Figure 3E), suggesting that loss of bst1 leads to defect in early ER transport.
The previous studies show that Bst1 is required for sorting of misfolded proteins rather than the transport of many normal proteins in Saccharomyces cerevisiae (Elrod-Erickson and Kaiser, 1996; Vashist et al., 2001; Fujita et al., 2006), so we wondered whether S. pombe Bst1 affects the transport of normal cargo proteins. In acid phosphatase secretion assay, both bst1-s27 and bst1Δ mutants had decreased secretion of acid phosphatase compared with WT (Figure 3F). These data suggest that Bst1 localizes to ER and cis-Golgi and is involved in early ER transport.
Bst1 affects COPII subunits distribution
Based on the above data, we predicted that Bst1 may function with the COPII complex, which facilitates vesicle formation and mediates ER to Golgi trafficking of various cargoes (Van der Verren and Zanetti, 2023). First, we examined whether Bst1 colocalizes with the COPII complex. Sec13 is the outer coat subunit of the complex and localizes to neER, cell periphery, and some cytosolic puncta in WT cells (Pryer et al., 1993; Vjestica et al., 2008; Figure 4A). We defined the cytosolic puncta as the punctate strucutres in the cytoplasm that excluded the ones near the plasma membrane or neER as diagramed in Figure 4C. The inner coat subunit, Sec24 mainly localizes to cell periphery and some cytosolic puncta (Pryer et al., 1993; Vjestica et al., 2008). Besides overlapped with Sec13-GFP at neER, Bst1-mScarlet showed partial overlap at the cell periphery and punctate structure near the neER with Sec13-GFP and Sec24-GFP, but rarely colocalized at the cytosolic puncta, 18.4 and 7.3% of Bst1-mScarlet cytosolic puncta showed colocalization with Sec13-GFP and Sec24-GFP, respectively (Figure 4A; Supplemental Figure S4A). Next, we tested whether distribution or assembly of the COPII complex is affected in bst1 mutants (Figure 4, B and C). In WT cells, Sec13-GFP exhibited signal at neER and 2.1 ± 1.6 cytosolic punctate structures in one single middle focal plane. In contrast, bst1Δ cells showed significant more cytosolic punctate structures, 8.5 ± 4.7 per focal plane (p < 0.001). Similarly, Sec24-GFP exhibited the punctate structures around cell cortex and a few puncta in the cytosol in WT cells as reported (Vjestica et al., 2008). However, more punctate structures in the cytosol were detected in bst1Δ cells (6.2 ± 3.6) compared with in WT cells (2.8 ± 1.8; p < 0.001) (Figure 4, B and C), suggesting that bst1Δ caused COPII complex transport or turnover delay. In addition, more mCherry-AHDL (an artificial luminal ER marker) structures connecting peripheral and perinuclear ER were observed in bst1Δ cells (Figure 4B), implying increasing of tubular ER (Schuck et al., 2009). To further address whether the structures are ER tubules or sheets, we used reticulon Rtn1-GFP as tubular cortical ER marker (Schuck et al., 2009; Zhang et al., 2010). As reported (Schuck et al., 2009; Zhang et al., 2010), Rtn1-GFP almost exclusively localized to the cortical ER in WT cells (Figure 4D). In contrast, cytosolic networks of Rtn1-GFP were often observed in bst1Δ mutant and they overlapped well with the increased mCherry-AHDL structures (Figure 4D), indicating that these structures are likely to be ER tubules.
FIGURE 4:
Bst1 affects COPII distribution. (A) Colocalization of Bst1 and Sec13. The upper images show max intensity projection of nine continuous timepoints without delay (exposure time 300 ms for Bst1 and 200 ms for Sec13 at each timepoint) at middle focal plane, the lower images show the single middle focal plane. The boxes in the merged panels mark the zoomed regions. The graph (right) shows cytosolic punctated Bst1-mScarlet structure (defined as puncta in the cytosol that are not in the periphery of cortical or nuclear ER) colocalizes with Sec13-GFP or Sec24-GFP. (B) Distribution of Sec13 and Sec24 in WT and bst1Δ. mCherry-AHDL is an artificial luminal ER marker. Arrowheads mark extended AHDL. (C) The cytosolic distribution of Sec13 and Sec24 in WT and bst1Δ. Single middle focal plane images were used to quantify the numbers of puncta in cytosol excluding the green area. **p < 0.001. (D) The distribution of Rtn1 in WT and bst1Δ. The images shown are the middle focal plane. Arrowheads mark extended Rtn1. (E) The distribution of Sec13 after MBC treatment. WT and bst1Δ cells expressing sec13-GFP and mCherry-AHDL were treated with DMSO or MBC for 12 min before imaging. The images shown are the middle focal plane. Bars, 5 µm.
Because microtubules were defective in bst1 mutant (Figure 2C), we asked whether the increased tubular ER and defects in COPII distribution were due to microtubule defects. After methyl benzimidazole-2-yl carbamate (MBC) treatment, which depolymerized microtubule (Supplemental Figure S4B), neither tubular ER nor Sec13-GFP or Sec24-GFP distribution showed significant changes (Figure 4E; Supplemental Figure S4C). These results indicate that Bst1 coordinates with the COPII complex for vesicle trafficking independent of microtubules.
Bst1 is important for the secretion of glucanases
Ync13 is crucial for septum integrity by regulating the recruitment and distribution of glucan synthases and glucanases at the division site (Zhu et al., 2018). Our data show that cytokinesis and cell separation are impaired in bst1 mutants, therefore we hypothesized that bst1 mutants suppress ync13 mutant through glucan synthases and/or glucanases. First, we tested the distributions and fluorescence intensity of glucan synthases Bgs1, Bgs4, and Ags1, which synthesize a three-layer septum composed of mainly α- and β-glucan during cytokinesis (Cortes et al., 2005; Cortes et al., 2007; Cortes et al., 2012). Rlc1-tdTomato was used as the contractile-ring marker. GFP-Bgs1, GFP-Bgs4, and Ags1-GFP localized at secretory vesicles and the plasma membrane throughout the cell cycle and are recruited to the division site during the contractile ring maturation and constriction, and remained at the division site until cell separation as reported (Supplemental Figure S5A). In bst1Δ mutant, all three glucan synthases still localized to vesicles, cell tips, and the division site (Supplemental Figure S5A). For comparison, we measured the fluorescence intensity of the glucan synthases at the similar stage of cytokinesis when Rlc1-tdTomato constricted to a dot at the division site. The protein levels of GFP-Bgs4 and Ags1-GFP showed no obvious difference in bst1Δ mutant and WT cells. However, intensities of GFP-Bgs1 at the division site in bst1Δ mutant was slightly higher (p = ∼0.003) than that in WT (Supplemental Figure S5B). As expected, GFP-Bgs4 peak levels were similar in WT and bst1Δ cells in time-lapse movies, but GFP-Bgs4 stayed longer at the division site in bst1Δ cells (Supplemental Figure S5C).
Next, we examined the distribution and intensity of the endo-1,3-β-glucanase Eng1 and endo-1,3-α-glucanase Agn1 at the division site, which are involved in primary septum digestion for separating two daughter cells (Baladrón et al., 2002; Martin-Cuadrado et al., 2003; Dekker et al., 2004; Garcia et al., 2005; Martin-Cuadrado et al., 2008), in WT and bst1Δ cells. The Eng1 and Agn1 were concentrated to the division site during the late cytokinesis in WT cells as reported (Figure 5, A and B). We measured the intensities of Eng1-NeonGreen and Agn1-NeonGreen in cells at the final stage of ring constriction with Pmo25-tdTomato (a contractile ring marker) constricted to dot (Figure 5, A and B). In bst1Δ mutant, the levels of Eng1-NeonGreen and Agn1-NeonGreen at the division site were significantly lower than those in WT cells (Figure 5, C and D), which was confirmed by time-lapse microscopy (Figure 5E). These results indicate that Bst1 regulates the trafficking of glucanases Eng1 and Agn1 to the division site and is important for daughter cell separation.
FIGURE 5:
bst1 mutant compromises Eng1 and Agn1 targeting to the division site. (A and B) Localization of β-glucanase Eng1-mNeonGreen (A) and α-glucanase Agn1-mNeonGreen (B) in WT and bst1Δ mutant. Pmo25-tdTomato used as a marker for the contractile ring. (C and D) Eng1 (C) and Agn1 (D) levels at the division site. Fluorescence intensities (left, individual cells; Right, mean intensities) are from line scans across the division site using the sum intensity projections. Error bars: ± SEM. (E) Eng1 levels at the division site during cytokinesis in time-lapse movies. Total Eng1 fluorescence intensities across the division site are measured in sum intensity projections. Time 0 marks Eng1-mNeonGreen appearing at the division site. The last time point of WT is the start of daughter cell separation, while that of bst1Δ is the end of movie if cells have not separated yet. Bars, 5 µm.
Previously we reported that eng1Δ mutant can partially rescue ync13Δ mutant (Zhu et al., 2018), suggesting that Eng1 might be one of the factors that involves in bst1 mutants’ suppression of ync13 mutants. To test this hypothesis, Eng1’s recruitment to the division site in ync13Δ and ync13Δ bst1Δ mutants was examined (Figure 6). Compared with ync13Δ mutant, Eng1’s recruitment to the division site was impaired in ync13Δ bst1Δ mutant (Figures 5A and 6A). We measured the fluorescence intensity of Eng1 in the cells when Rlc1-tdTomato constricted to a dot at the division site or in the cells without Rlc1 signal (the ring disassembled) but with a visible septum. The Eng1 level at the division site in ync13Δ bst1Δ mutant was significantly lower than that in ync13Δ mutant (Figure 6B). In contrast, the concentration of the glucan synthase Bgs1 at the division site had no obvious difference in ync13Δ and ync13Δ bst1Δ mutants (our unpublished data). These results indicate that bst1 mutants rescue ync13 mutants by affecting the recruitment of the glucanase Eng1 to the division site.
FIGURE 6:
ync13 bst1 double mutant impairs Eng1 recruitment to the division site. Micrographs (A) and quantification (B) of Eng1 localization in ync13Δ and ync13Δ bst1Δ cells. Cells were grown in YE5S + 1.2 M sorbitol liquid medium in log phase at 25°C for ∼36 h, then washed (3x) into YE5S medium and grown for 2.5 h before imaging in a coverglass bottom dish covered with YE5S agar. (B) Cells at or after the final stage of ring constriction as judged by Rlc1-tdTomato signal or closed septa were quantified. **p < 0.001. Bar, 5 µm.
DISCUSSION
S. pombe GPI inositol deacylase Bst1 coordinates ER-Golgi trafficking
In this study, we identified and characterized bst1-s27 as a spontaneous suppressor of ync13 mutants, which are defective in both exocytosis and endocytosis during late stages of cytokinesis (Zhu et al., 2018). bst1-s27 encodes a truncated Bst1, a predicted GPI inositol deacylase, that lacks the five transmembrane domains at its COOH-terminus (Figure 1B). Bst1 localizes to the early Golgi compartment in addition to the ER (Figure 3, A and B), where is the main subcellular distribution organelle of PGAP1/Bst1 in budding yeast, Arabidopsis thaliana, and mammalian cells (Elrod-Erickson and Kaiser, 1996; Tanaka et al., 2004; Bernat-Silvestre et al., 2021), indicating that S. pombe Bst1 may have additional functions than those reported in other cell types (Elrod-Erickson and Kaiser, 1996; Tanaka et al., 2004; Bernat-Silvestre et al., 2021).
Several lines of evidence suggest that S. pombe Bst1 may regulate the general ER to Golgi transport. First, bst1Δ mutant causes the accumulation of Golgi mannan polymerase I complex subunit, a cis-Golgi marker Anp1 (Vjestica et al., 2008) but not Sec72, at the neER (Figure 3E). Consistently, Bst1 colocalizes more frequently with Anp1 than the trans-Golgi marker Sec72 (Vjestica et al., 2008). It will be interesting to observe the secretion routes of Bst1 and Anp1 containing vesicles, and to explore the roles of Bst1 in Golgi in S. pombe. As Anp1 was tagged with GFP, we cannot rule out the possibility that misfolding of some of the tagged protein led to the delayed transport from ER, which has been reported in budding yeast that Bst1 regulates transport of misfolded proteins (Vashist et al., 2001; Fujita et al., 2006). Second, our assay shows significant decrease of the acid phosphatase secretion to the culture medium in bst1 mutants, indicating the delayed transport for the normal cargoes (Figure 3F), which is consistent with the slower growth rate of bst1 mutants. Third, bst1 mutants accumulate more cytosolic puncta structures of the COPII subunits, Sec13 and Sec24 (Figure 4, A–C), we suspect that the turnover of COPII complex may be affected by bst1 mutations. Excepted for the rounder shape than WT cells, bst1 mutants show increased tubular ER structures in cytosol as marked by Rtn1-mEGFP, which overlaps well with general ER marker mCherry-AHDL in the cytosol (Figure 4D). Whether the excess tubular ER structures or ER expansion leading to the increased cytosolic puncta of COPII complex needs further investigation; and analyzing the dynamics of COPII complex in bst1 mutants by FRAP is necessary in the future. COPII distribution and tubular ER expansion in bst1 mutant cells suggest that Bst1 affects protein (such as GPI-anchored proteins) folding and accumulation, then indirectly affects ER expansion, or S. pombe Bst1 directly involves in ER organization. Fourth, deletion of bst1 decreases the secreted glucanases Eng1 and Agn1 at the septum, which causes delay in daughter cell separation. Eng1 is predicted to be GPI-anchor modified ( https://www.pombase.org/gene/SPAC821.09), indicating that S. pombe Bst1 may also regulate GPI-anchored protein for cell surface localization. Thus, S. pombe Bst1 regulates the transport of several different cargoes including Anp1, acid phosphatase, Eng1, Agn1, and Ync13, indicating that Bst1 may affect extensive cargoes sorting or overall ER transport in fission yeast. In other organisms, the known functions of GPI inositol deacylases are mainly involved in GPI-anchored proteins’ transport, misfolded protein quality control, or ER resident protein leaking (Elrod-Erickson and Kaiser, 1996; Vashist et al., 2001; Tanaka et al., 2004; Fujita et al., 2006).
Early and late regulators of the secretory pathway functionally compensate during cytokinesis
Exocytosis is essential for cytokinesis by delivering membrane and cargo proteins for plasma membrane expansion and septum formation or extracellular-matrix remodeling during cytokinesis.
In budding yeast, Bst1 was originally identified as a suppressor of mutation in COPII protein Sec13 and both proteins function in early secretory pathway (Elrod-Erickson and Kaiser, 1996). As stated in the Introduction, Ync13 is a member of the Munc13/UNC-13 family proteins, which are the priming factors for the SNARE complex assembly and are also important for vesicle tethering at the plasma membrane in late secretory pathway (Guan et al., 2008; Khodthong et al., 2011; Rodarte et al., 2018; Zhu et al., 2018; Shu et al., 2020). Ync13 localizes to the plasma membrane at the cell tips during interphase and to the division site during cytokinesis. It has no roles in actomyosin contractile-ring assembly or constriction (Zhu et al., 2018). However, it is important for exocytosis of glucan synthases and glucanases at the division site (Zhu et al., 2018). Although the exact functions of Ync13 on the plasma membrane remain obscure, it is clear that Ync13 is a late regulator of exocytosis. Mutations in anillin-like protein Mid2 and septins Spn1 and Spn4 are also identified as spontaneous suppressors of ync13 mutants (Table 2). Mid2 regulates organization of septin cytoskeleton and all of these proteins function at the plasma membrane at the division site and are involved in late stages of cytokinesis (Berlin et al., 2003; Tasto et al., 2003; An et al., 2004; Wu et al., 2010; Singh et al., 2024). Recently, we have found that septins and the vesicle tether exocyst physically interact to regulate the proper recruiting and targeting of Bgs1 and Eng1 during late cytokinesis (Singh et al., 2024). In this study, we identified Bst1 as an early regulator of secretory pathway that functions at ER and early Golgi and is required for the ER to Golgi transport and for the localization of putative GPI-anchored proteins and other proteins to cell surface. We find that bst1-s27 and bst1Δ mutants restore the colony formation and prevent lysis of ync13 mutants by slowing down constriction of the contractile ring and reducing the secretion of glucanases Eng1 and Agn1 to prevent premature cell separation with a defective septum.
This study will provide insights for future studies of UNC-13/Munc13 proteins and Bst1 homologs in other systems. The suppression of ync13 mutants by the bst1 mutants also provides an interesting example how early and late regulators of the secretory pathway functionally compensate during cytokinesis by regulating glucanases and glucan synthases at different steps of exocytosis.
MATERIALS AND METHODS
Strains, genetics, and cellular methods
Strains used in this study are listed in Supplemental Table S1. Gene targeting and crosses were performed using PCR-based and standard genetic methods (Moreno et al., 1991; Bähler et al., 1998). All tagged genes are expressed under endogenous promoters and integrated into the native chromosomal loci except where noted. The general ER lumen marker AHDL is expressed under the bip1 promoter.
To isolate ync13 spontaneous suppressors, ync13Δ and ync13-19 mutant cells were grown on YE5S plates at 25°C or 36°C, respectively. After 3 d, the larger isolated suppressor colonies were selected for back crosses and phenotype analysis. For genetic mapping (Table 1), the suppressors mutants were first crossed in rec12Δ mutant background, which dramatically reduces homologous recombination, to determine the chromosome in which the suppressors locate (Anders et al., 2008). Then we used different genetic markers to map the linked loci by tetrad analyses. The coding region, promoters, and untranslated region (UTRs) of the candidate genes in the linked loci were sequenced using Sanger sequencing to identify the mutations (Table 2).
For the acid phosphatase secretion assay (Wang et al., 2002; Tay et al., 2019; Gerien et al., 2020), cells were grown at log phase for ∼36 h in EMM5S (Edinburgh minimal medium plus five supplements) at 25°C, washed twice with EMM5S, and then inoculated in 15 ml new EMM5S medium at OD595 = ∼0.3 for the secretion assay sampling every hour for 5 h. Because bst1 mutant cells flocculate in the liquid culture and the cell shapes are variable, before OD measurement, the cell cultures were vortexed thoroughly. In addition, we also noticed that after centrifugation, bst1 cell pellets were almost twice the size of WT cell pellet with similar optical density (ODs). To measure the acid phosphatase secretion, 0.75 ml cell cultures were centrifuged at each timepoint, and 500 µl supernatant was mixed with 500 µl substrate (2 mM p-nitrophenyl phosphate, 0.1 M sodium acetate, pH 4.0), incubated at 30°C for 10 min. Then 500 µl of sodium hydroxide (1 M) was added to stop the reaction, and the acid phosphatase secretion was measured as the absorbance at 405 nm. The absorbance was subtracted by the 405 nm absorbance at time zero and then normalized to cell density (OD at 595 nm) at each timepoint.
Microscopy and data analysis
For microscopy imaging, cells were woken up from −80°C stocks and grown on YE5S plates at 25°C for ∼2 d, and then fresh cells were inoculated into YE5S liquid medium and grown at 25°C for ∼36 h at log phase (diluted twice daily) before imaging except where noted. Microscopy sample preparations were carried out as described (Ye et al., 2012; Gerien et al., 2020) except where noted. Briefly, cultured cells were collected by centrifugation at ∼3000 to 3500 rpm, washed once with EMM5S, and then washed with EMM5S containing 5 µM n-propyl-gallate to reduce autofluorescence and protect cells from free radicals during microscopy. For MBC treatment, 5 µl MBC (5 mg/ml in DMSO) or equal volume DMSO was added to the samples during the second EMM5S wash and incubated at room temperature in the dark for 12 min. Samples were loaded onto a pad of EMM5S with 20% gelatin and 0.1 mM n-propyl-gallate, and observed at ∼23°C.
For septum staining and quantification, samples were treated with 10 µg/ml Calcofluor for 2 min in dark and loaded directly onto slides, stained cells were imaged with a 100 × /1.4 numerical aperture (NA) Plan-Apo objective lens on a Nikon Eclipse Ti inverted microscope (Nikon, Melville, NY) equipped with a Nikon cooled digital camera DS-QI1 and a 4′,6-diamidino-2-phenylindole (DAPI) filter. All other microscopy was carried out on a PerkinElmer spinning disk confocal system (UltraVIEW Vox CSUX1 system; PerkinElmer, Waltham, MA) on a Nikon Ti-E microscope with Hamamatsu EMCCD camera C9100-23B and Plan-Apo 100x/1.45 NA objective; or on a Nikon CSU-W1 SoRa spinning disk confocal microscope with Hamamatsu ORCA Quest qCMOS camera C15550 on Nikon Eclipse Ti2 microscope and SR HP Apo TIRF 100x/1.49 NA oil or Plan Apo λD 100x/1.45 NA oil objectives.
Images and data were collected and analyzed by Volocity, NIS Elements, and Fiji software. The fluorescence intensity at the division site was measured in the images that were projected with sum intensity projections of 0.5 (single stack) or 0.75 µm (movie)-spaced Z-slices as described previously (Ye et al., 2012; Gerien et al., 2020; Longo et al., 2022). ROI (region of interest) covering the signal at the division site was drawn to measure the mean intensity, and ∼2 × ROI was used to measure and calculate background intensity (Ye et al., 2012; Zhu et al., 2018; Singh et al., 2024). Micrographs shown in the figures are maximum projections except where noted. Statistical tests were performed using a two-tailed Student's t test. Kymograph was generated from maximum-intensity projections of each timepoint in time-lapse movies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Juan Carlos, Snezhana Oliferenko, Beatriz Santos, Ken Sawin, and Yihua Zhu for yeast strains; Anthony Vetter from Nikon for microscopy technical support; Clara Sablak, Komal Khan, and Amirreza Sabzian in the lab for technical support; and current and former members of the Wu lab for helpful discussions and suggestions. The work was supported by the National Institute of General Medical Sciences of the National Institutes of Health (grant R01 GM118746 to J.-Q.W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used:
- COPII
coat protein complex II
- DIC
differential interference contrast
- ER
endoplasmic reticulum
- GPI
glycosylphosphatidylinositol
- MBC
methyl benzimidazole-2-yl carbamate
- neER
nuclear envelope ER
- SPB
spindle pole body
- tdTomato
tandem dimer Tomato
- WT
wild type.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E24-08-0375) on January 15, 2025.
REFERENCES
- An H, Morrell JL, Jennings JL, Link AJ, Gould KL (2004). Requirements of fission yeast septins for complex formation, localization, and function. Mol Biol Cell 15, 5551–5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders A, Watt S, Bähler J, Sawin KE (2008). Improved tools for efficient mapping of fission yeast genes: Identification of microtubule nucleation modifier mod22-1 as an allele of chromatin- remodelling factor gene swr1. Yeast 25, 913–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler J, Wu J-Q, Longtine MS, Shah NG, McKenzie A, III, Steever AB, Wach A, Philippsen P, Pringle JR (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951. [DOI] [PubMed] [Google Scholar]
- Baladrón V, Ufano S, Dueñas E, Martín-Cuadrado AB, del Rey F, Vázquez de Aldana CR (2002). Eng1p, an endo-1,3-β-glucanase localized at the daughter side of the septum, is involved in cell separation in Saccharomyces cerevisiae. Eukaryot Cell 1, 774–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin A, Paoletti A, Chang F (2003). Mid2p stabilizes septin rings during cytokinesis in fission yeast. J Cell Biol 160, 1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat-Silvestre C, Sanchez-Simarro J, Ma Y, Montero-Pau J, Johnson K, Aniento F, Marcote MJ (2021). AtPGAP1 functions as a GPI inositol-deacylase required for efficient transport of GPI-anchored proteins. Plant Physiol 187, 2156–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch DG, Boonstra FN, Kinoshita T, Jhangiani S, de Ligt J, Cremers FP, Lupski JR, Murakami Y, de Vries BB (2015). Cerebral visual impairment and intellectual disability caused by PGAP1 variants. Eur J Hum Genet 23, 1689–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillon GA, Watanabe R, Taylor M, Schwabe TM, Riezman H (2009). Concentration of GPI-anchored proteins upon ER exit in yeast. Traffic 10, 186–200. [DOI] [PubMed] [Google Scholar]
- Chen R, Walter EI, Parker G, Lapurga JP, Millan JL, Ikehara Y, Udenfriend S, Medof ME (1998). Mammalian glycophosphatidylinositol anchor transfer to proteins and posttransfer deacylation. Proc Natl Acad Sci U S A 95, 9512–9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhu J, Ying SH, Feng MG (2014). The GPI-anchored protein Ecm33 is vital for conidiation, cell wall integrity, and multi-stress tolerance of two filamentous entomopathogens but not for virulence. Appl Microbiol Biotechnol 98, 5517–5529. [DOI] [PubMed] [Google Scholar]
- Cheung AY, Li C, Zou YJ, Wu HM (2014). Glycosylphosphatidylinositol anchoring: Control through modification. Plant Physiol 166, 748–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés JC, Carnero E, Ishiguro J, Sanchez Y, Duran A, Ribas JC (2005). The novel fission yeast (1,3)β-D-glucan synthase catalytic subunit Bgs4p is essential during both cytokinesis and polarized growth. J Cell Sci 118, 157–174. [DOI] [PubMed] [Google Scholar]
- Cortés JC, Ishiguro J, Duran A, Ribas JC (2002). Localization of the (1,3) β-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J Cell Sci 115, 4081–4096. [DOI] [PubMed] [Google Scholar]
- Cortés JC, Konomi M, Martins IM, Munoz J, Moreno MB, Osumi M, Duran A, Ribas JC (2007). The (1,3) β-D-glucan synthase subunit Bgs1p is responsible for the fission yeast primary septum formation. Mol Microbiol 65, 201–217. [DOI] [PubMed] [Google Scholar]
- Cortés JC, Sato M, Munoz J, Moreno MB, Clemente-Ramos JA, Ramos M, Okada H, Osumi M, Duran A, Ribas JC (2012). Fission yeast Ags1 confers the essential septum strength needed for safe gradual cell abscission. J Cell Biol 198, 637–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés JCG, Ramos M, Osumi M, Pérez P, Ribas JC (2016). The cell biology of fission yeast septation. Microbiol Mol Biol Rev 80, 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darp R, Vittoria MA, Ganem NJ, Ceol CJ (2022). Oncogenic BRAF induces whole-genome doubling through suppression of cytokinesis. Nat Commun 13, 4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker N, Speijer D, Grun CH, van den Berg M, de Haan A, Hochstenbach F (2004). Role of the α-glucanase Agn1p in fission-yeast cell separation. Mol Biol Cell 15, 3903–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhaber B, Maurer-Stroh S, Novatchkova M, Schneider G, Eisenhaber F (2003). Enzymes and auxiliary factors for GPI lipid anchor biosynthesis and post-translational transfer to proteins. Bioessays 25, 367–385. [DOI] [PubMed] [Google Scholar]
- Elrod-Erickson MJ, Kaiser CA (1996). Genes that control the fidelity of endoplasmic reticulum to Golgi transport identified as suppressors of vesicle budding mutations. Mol Biol Cell 7, 1043–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essen LO, Vogt MS, Mosch HU (2020). Diversity of GPI-anchored fungal adhesins. Biol Chem 401, 1389–1405. [DOI] [PubMed] [Google Scholar]
- Foltman M, Sanchez-Diaz A (2017). Studying the role of the mitotic exit network in cytokinesis. Methods Mol Biol 1505, 245–262. [DOI] [PubMed] [Google Scholar]
- Fujihara Y, Okabe M, Ikawa M (2014). GPI-anchored protein complex, LY6K/TEX101, is required for sperm migration into the oviduct and male fertility in mice. Biol Reprod 90, 60. [DOI] [PubMed] [Google Scholar]
- Fujita M, Watanabe R, Jaensch N, Romanova-Michaelides M, Satoh T, Kato M, Riezman H, Yamaguchi Y, Maeda Y, Kinoshita T (2011). Sorting of GPI-anchored proteins into ER exit sites by p24 proteins is dependent on remodeled GPI. J Cell Biol 194, 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Yoko OT, Jigami Y (2006). Inositol deacylation by Bst1p is required for the quality control of glycosylphosphatidylinositol-anchored proteins. Mol Biol Cell 17, 834–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia I, Jimenez D, Martin V, Duran A, Sanchez Y (2005). The α-glucanase Agn1p is required for cell separation in Schizosaccharomyces pombe. Biol Cell 97, 569–576. [DOI] [PubMed] [Google Scholar]
- Gerien KS, Wu J-Q (2018). Molecular mechanisms of contractile-ring constriction and membrane trafficking in cytokinesis. Biophys Rev 10, 1649–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerien KS, Zhang S, Russell AC, Zhu Y-H, Purde V, Wu J-Q (2020). Roles of Mso1 and the SM protein Sec1 in efficient vesicle fusion during fission yeast cytokinesis. Mol Biol Cell 31, 1570–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, Dai H, Rizo J (2008). Binding of the Munc13-1 MUN domain to membrane-anchored SNARE complexes. Biochemistry 47, 1474–1481. [DOI] [PubMed] [Google Scholar]
- Hata Y, Slaughter CA, Sudhof TC (1993). Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature 366, 347–351. [DOI] [PubMed] [Google Scholar]
- Karahara I, Staehelin LA, Mineyuki Y (2010). A role of endocytosis in plant cytokinesis. Commun Integr Biol 3, 36–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodthong C, Kabachinski G, James DJ, Martin TF (2011). Munc13 homology domain-1 in CAPS/UNC31 mediates SNARE binding required for priming vesicle exocytosis. Cell Metab 14, 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T (2020). Biosynthesis and biology of mammalian GPI-anchored proteins. Open Biol 10, 190290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Fujita M (2016). Biosynthesis of GPI-anchored proteins: Special emphasis on GPI lipid remodeling. J Lipid Res 57, 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattmann E, Krapp A, Simanis V (2009). Cytokinesis: Closure resets your SIN. Curr Biol 19, R1040–R1042. [DOI] [PubMed] [Google Scholar]
- Le Goff X, Motegi F, Salimova E, Mabuchi I, Simanis V. (2000). The S. pombe rlc1 gene encodes a putative myosin regulatory light chain that binds the type II myosins Myo3p and Myo2p. J Cell Sci 113, 4157–4163. [DOI] [PubMed] [Google Scholar]
- Lebreton S, Zurzolo C, Paladino S (2018). Organization of GPI-anchored proteins at the cell surface and its physiopathological relevance. Crit Rev Biochem Mol Biol 53, 403–419. [DOI] [PubMed] [Google Scholar]
- Lens SMA, Medema RH (2019). Cytokinesis defects and cancer. Nat Rev Cancer 19, 32–45. [DOI] [PubMed] [Google Scholar]
- Liu J, Tang X, Wang H, Oliferenko S, Balasubramanian MK (2002). The localization of the integral membrane protein Cps1p to the cell division site is dependent on the actomyosin ring and the septation-inducing network in Schizosaccharomyces pombe. Mol Biol Cell 13, 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Zou Z, Huang X, Shen H, He LJ, Chen SM, Li LP, Yan L, Zhang SQ, Zhang JD, et al. (2016). Bst1 is required for Candida albicans infecting host via facilitating cell wall anchorage of Glycosylphosphatidyl inositol anchored proteins. Sci Rep 6, 34854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo LVG, Goodyear EG, Zhang S, Kudryashova E, Wu J-Q (2022). Involvement of Smi1 in cell wall integrity and glucan synthase Bgs4 localization during fission yeast cytokinesis. Mol Biol Cell 33, ar17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cuadrado AB, Duenas E, Sipiczki M, Vazquez de Aldana CR, del Rey F (2003). The endo-β-1,3-glucanase eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J Cell Sci 116, 1689–1698. [DOI] [PubMed] [Google Scholar]
- Martin-Cuadrado AB, Encinar del Dedo J, de Medina-Redondo M, Fontaine T, del Rey F, Latge JP, Vazquez de Aldana CR (2008). The Schizosaccharomyces pombe endo-1,3-β-glucanase Eng1 contains a novel carbohydrate binding module required for septum localization. Mol Microbiol 69, 188–200. [DOI] [PubMed] [Google Scholar]
- McCollum D, Gould KL (2001). Timing is everything: Regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol 11, 89–95. [DOI] [PubMed] [Google Scholar]
- Meitinger F, Palani S (2016). Actomyosin ring driven cytokinesis in budding yeast. Semin Cell Dev Biol 53, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P (1991). Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194, 795–823. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Tawamie H, Maeda Y, Buttner C, Buchert R, Radwan F, Schaffer S, Sticht H, Aigner M, Reis A, et al. (2014). Null mutation in PGAP1 impairing GPI-anchor maturation in patients with intellectual disability and encephalopathy. PLoS Genet 10, e1004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Nakamura-Kubo M, Hirata A, Shimoda C (2001). The Schizosaccharomyces pombe spo3+ gene is required for assembly of the forespore membrane and genetically interacts with psy1+-encoding syntaxin-like protein. Mol Biol Cell 12, 3955–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NI, Wong KC, Tang X, Balasubramanian MK (2000). Type II myosin regulatory light chain relieves auto-inhibition of myosin-heavy-chain function. Nat Cell Biol 2, 855–858. [DOI] [PubMed] [Google Scholar]
- Park S, Bin NR, Yu B, Wong R, Sitarska E, Sugita K, Ma K, Xu J, Tien CW, Algouneh A, et al. (2017). UNC-18 and Tomosyn antagonistically control synaptic vesicle priming downstream of UNC-13 in Caenorhabditis elegans. J Neurosci 37, 8797–8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez P, Cortés JCG, Martín-García R, Ribas JC (2016). Overview of fission yeast septation. Cell Microbiol 18, 1201–1207. [DOI] [PubMed] [Google Scholar]
- Petsalaki E, Zachos G (2021). The abscission checkpoint: A guardian of chromosomal stability. Cells 10, 3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Wu J-Q (2010). Understanding cytokinesis: Lessons from fission yeast. Nat Rev Mol Cell Biol 11, 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryer NK, Salama NR, Schekman R, Kaiser CA (1993). Cytosolic Sec13p complex is required for vesicle formation from the endoplasmic reticulum in vitro. J Cell Biol 120, 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos M, Cortes JCG, Sato M, Rincon SA, Moreno MB, Clemente-Ramos JA, Osumi M, Perez P, Ribas JC (2019). Two S. pombe septation phases differ in ingression rate, septum structure, and response to F-actin loss. J Cell Biol 218, 4171–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezig IM, Yaduma WG, Gould GW, McInerny CJ (2023). The role of anillin/Mid1p during medial division and cytokinesis: From fission yeast to cancer cells. Cell Cycle 22, 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas JC, Diaz M, Duran A, Perez P (1991). Isolation and characterization of Schizosaccharomyces pombe mutants defective in cell wall (1-3)β-D-glucan. J Bacteriol 173, 3456–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodarte EM, Ramos MA, Davalos AJ, Moreira DC, Moreno DS, Cardenas EI, Rodarte AI, Petrova Y, Molina S, Rendon LE, et al. (2018). Munc13 proteins control regulated exocytosis in mast cells. J Biol Chem 293, 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak K, Steiner A, Synek L, Klaeger S, Kulich I, Facher E, Wanner G, Kuster B, Zarsky V, Persson S, Assaad FF (2014). Plant cytokinesis is orchestrated by the sequential action of the TRAPPII and exocyst tethering complexes. Dev Cell 29, 607–620. [DOI] [PubMed] [Google Scholar]
- Saha S, Anilkumar AA, Mayor S (2016). GPI-anchored protein organization and dynamics at the cell surface. J Lipid Res 57, 159–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Toya M, Toda T (2009). Visualization of fluorescence-tagged proteins in fission yeast: The analysis of mitotic spindle dynamics using GFP-tubulin under the native promoter. Methods Mol Biol 545, 185–203. [DOI] [PubMed] [Google Scholar]
- Schuck S, Prinz WA, Thorn KS, Voss C, Walter P (2009). Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J Cell Biol 187, 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Luo C, Xiang Y, Qian D (2023). Rab GTPases, tethers, and SNAREs work together to regulate Arabidopsis cell plate formation. Front Plant Sci 14, 1120841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Jin H, Rothman JE, Zhang Y (2020). Munc13-1 MUN domain and Munc18-1 cooperatively chaperone SNARE assembly through a tetrameric complex. Proc Natl Acad Sci U S A 117, 1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Liu Y, Zhu Y-H, Zhang S, Naegele S, Wu J-Q (2024). Septins function in exocytosis via physical interactions with the exocyst complex in fission yeast cytokinesis. PMID: 39026698. https://elifesciences.org/reviewed-preprints/101113. [Google Scholar]
- Tanaka S, Maeda Y, Tashima Y, Kinoshita T (2004). Inositol deacylation of glycosylphosphatidylinositol-anchored proteins is mediated by mammalian PGAP1 and yeast Bst1p. J Biol Chem 279, 14256–14263. [DOI] [PubMed] [Google Scholar]
- Tasto JJ, Morrell JL, Gould KL (2003). An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J Cell Biol 160, 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebe H, Goshima G, Takeda K, Nakagawa T, Kinoshita K, Yanagida M (2001). Fission yeast living mitosis visualized by GFP-tagged gene products. Micron 32, 67–74. [DOI] [PubMed] [Google Scholar]
- Tay YD, Leda M, Spanos C, Rappsilber J, Goryachev AB, Sawin KE (2019). Fission yeast NDR/LATS kinase Orb6 regulates exocytosis via phosphorylation of the exocyst complex. Cell Rep 26, 1654–1667.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Yamaguchi R, Ikawa M, Okabe M, Morii E, Maeda Y, Kinoshita T (2007). PGAP1 knock-out mice show otocephaly and male infertility. J Biol Chem 282, 30373–30380. [DOI] [PubMed] [Google Scholar]
- Van der Verren SE, Zanetti G (2023). The small GTPase Sar1, control centre of COPII trafficking. FEBS Lett 597, 865–882. [DOI] [PubMed] [Google Scholar]
- Vashist S, Kim W, Belden WJ, Spear ED, Barlowe C, Ng DT (2001). Distinct retrieval and retention mechanisms are required for the quality control of endoplasmic reticulum protein folding. J Cell Biol 155, 355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavylonis D, Wu J-Q, Hao S, O'Shaughnessy B, Pollard TD (2008). Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science 319, 97–100. [DOI] [PubMed] [Google Scholar]
- Vjestica A, Tang XZ, Oliferenko S (2008). The actomyosin ring recruits early secretory compartments to the division site in fission yeast. Mol Biol Cell 19, 1125–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tang X, Liu J, Trautmann S, Balasundaram D, McCollum D, Balasubramanian MK (2002). The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol Biol Cell 13, 515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J-Q, Kuhn JR, Kovar DR, Pollard TD (2003). Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev Cell 5, 723–734. [DOI] [PubMed] [Google Scholar]
- Wu J-Q, Ye Y, Wang N, Pollard TD, Pringle JR (2010). Cooperation between the septins and the actomyosin ring and role of a cell-integrity pathway during cell division in fission yeast. Genetics 186, 897–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Lee I-J, Runge KW, Wu J-Q (2012). Roles of putative Rho-GEF Gef2 in division-site positioning and contractile-ring function in fission yeast cytokinesis. Mol Biol Cell 23, 1181–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Vjestica A, Oliferenko S (2010). The cortical ER network limits the permissive zone for actomyosin ring assembly. Curr Biol 20, 1029–1034. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhu H (2018). Cytokinesis and the Hippo pathway: New molecular links between intimate partners. Gastroenterology 155, 976–978. [DOI] [PubMed] [Google Scholar]
- Zhu Y-H, Hyun J, Pan YZ, Hopper JE, Rizo J, Wu J-Q (2018). Roles of the fission yeast UNC-13/Munc13 protein Ync13 in late stages of cytokinesis. Mol Biol Cell 29, 2259–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.