Abstract
Hydrogels have been widely studied for coating the surface of biomedical devices with the purpose of preventing thrombosis. The hydrogels can in principle be further functionalized with potent nanoparticles for imaging or drug delivery. However, using nanoparticles to functionalize the hydrogel coatings has received little attention. In addition, the performance of hydrogels under conditions characteristic to these devices, such as supraphysiological flow and associated turbulence, has yet to be sufficiently investigated. Thus, the purpose of this study was to use a hydrogel-coating nitinol surface as a model to understand the functions of hydrogels and the capture of nanoparticles. The hydrogels and nanoparticles were functionalized with a pair of oligonucleotides (ONs). Specifically, nitinol foils were silanized, hydrogel coated and functionalized with ONs. Fluorescent silica nanoparticles were functionalized with complementary ONs (CONs). The capture of nanoparticles on the ON-functionalized surfaces was studied under both static and dynamic flow conditions. Modified nitinol samples underwent flow treatment under both laminar and turbulent regimes and demonstrated increased nanoparticle retention under static conjugation, while permitting comparable functionalization by upstream injection in both laminar and turbulent flow. The results demonstrate promising potential of hydrogel-functionalized nitinol for capturing nanoparticles using nucleic acid hybridization to resist mechanical damage and loss of biomolecular functionalization by exposure to turbulence and warrant further investigation of drug delivery and antithrombogenic modification.
Keywords: aptamers, biomaterial, flow, surface, turbulence
Introduction
Implanted devices have been a staple of cardiovascular intervention for decades and have improved patient outcomes in a variety of clinical applications [31]. However, blood-contacting devices are subject to complications associated with adverse blood-surface interactions, namely an immunogenic response (biofouling) and thrombosis. Both of these phenomena are mediated by surface bioabsorption of bloodborne proteins onto the biomaterial surface of the device [12, 15, 26]. In the native state, blood-contacting surfaces can become prothrombogenic loci for occlusive thrombus formation, leading to myocardial infarction, stroke, and other severe pathologies [6, 8, 12]. In vitro studies have outlined a clear relationship between flow and platelet adhesion for both biomaterials and mechanical circulatory support devices [23, 32]. Flow conditions are a crucial component of predictive computational models of thrombosis [27, 31–32]. The intersection between flow conditions and surface properties is a critical topic in developing surface-modified biomaterials for implantation.
Modulation of surface properties to limit bioadhesion is an attractive and much explored strategy for thrombus prevention [5]. Surface modifications or coatings, such as with the hydrophilic molecule polyethylene glycol (PEG), have been demonstrated to successfully prevent the absorption of proteins to hydrophobic surfaces [19–21]. In vivo results have been less promising, as patients still require systemic oral anticoagulation to prevent thrombosis, despite the anti-thrombogenic surface modification of implanted devices [12]. Due to the well documented shortcomings of systemic anticoagulation [4, 6, 8, 12], incorporation of anticoagulant molecules into a functionalized bioabsorption-resistant surface is a potential next step in prohibiting device-induced thrombosis. Drug-eluting stents are a prime candidate for antithrombogenic surface modification, with drug release behaviors designed to prevent platelet deposition during long-term implantation. The modification of nitinol is an attractive strategy to reduce the incidence of thrombosis associated with other blood-contacting cardiovascular devices, such as pumps or mechanical valves. To demonstrate that antithrombogenic modification of a biomaterial is a feasible strategy, surface functionalization of a common blood-contacting material must be demonstrated, particularly in a manner that optimizes drug delivery strategies.
Specificity of ligand-receptor interactions is an important component of engineering optimal approaches for drug delivery but has yet to be completely implemented into the functionalization of blood-contacting devices. An absorption-resistant surface could be achieved by pairing the use of synthetic ligand-receptor pairs and transient release of anticoagulant molecules over an extended duration [1]. While hydrogels have previously been functionalized with heparin to permit chelation of free cations [18, 27–28], or peptides that delay protein release [3, 29], the Wang group has successfully made use of nucleic acids to control the surface properties of hydrogels [14, 16–17, 36]. While these studies have validated nucleic acids and hydrogels as a potential method of antithrombogenic functionalization of cardiovascular devices, most of these studies were performed under static conditions. In blood-contacting devices, flow conditions produce supraphysiological levels of shear stress that can mechanically influence surface coatings or alter biochemical processes that interact with a functionalized surface [4, 11–12]. These levels of shear stresses are associated with pockets of transitional and turbulent flow under certain anatomical conditions. While some studies have considered the effects of laminar flow on hydrogel performance [9, 25], little has been published on the behavior of modified-hydrogel surfaces in a turbulent flow regime. The retention of the hydrogel layer under higher than physiological glow is important for maintaining modified surface mediation of nanoparticle attachment. Our group has not only investigated blood-surface interactions as a result of flow [23, 32], but device performance and vascular patency as a result of circulatory turbulence in pathological or device-induced flow conditions [10, 34–35]. These and other recent studies have made it clear that blood-contacting devices are exposed to elements of turbulence within local hemodynamics, and that both thrombosis and performance of surface coatings might be significantly affected by flow.
The purpose of this study was to investigate hydrogel durability and functionalization under the aforementioned conditions, to demonstrate the utility of oligonucleotide-modified nanoparticles as a method of selective drug delivery. Nickel-titanium, or nitinol, a commonly used biomaterial for blood-contacting devices such as stents [25], was coated with oligonucleotides-functionalized hydrogel [2]. Variable flow conditions and a nitinol surface were chosen to examine relevant conditions for patients with stents and mechanically supported circulation. Nanoparticles were treated with biotinylated complementary oligonucleotides to permit binding to the hydrogel-coated nitinol surface. Retention of the nitinol’s hydrogel coating under both laminar and turbulent flow conditions were investigated to assess the stability of hydrogel coating under different flow conditions. Finally, absorption and retention of statically adhered or dynamically injected nanoparticles under both flow laminar and turbulent regimes was examined.
Materials and Methods
Materials
Nitinol foils were obtained from Kellogg’s Research Labs (Salem, NH). The acrylamide/bis-acrylamides solution (40% w/v; 37.5:1), phosphate buffered saline (PBS), and sodium hydroxide (NaOH) were purchased from Thermo Fisher Scientific (Waltham, MA). The Streptavidin-conjugated yellow silica nanoparticles (420 nm) were purchased from Spherotech (Lake Forest, IL). 3-(Methacryloyloxy) propyl trimethoxysilane (TMSPMA) and Irgacure 2959 were obtained from Sigma-Aldrich (ST Louis, MO). Oligonucleotides (Table S1) with different modifications were synthesized by Integrated DNA Technologies (Coralville, IA).
Nitinol surface modification
A high precision dicing machine (Kulicke and Soffa Industries, Inc., Singapore) was used to trim the nitinol foils. The 500 µm thick sheets were sectioned into small strips of 3.95×5 mm, then filed and polished to remove any defects from the machining process. Afterwards, a grid was notched onto each sample for use as a visual reference point during the imaging process (Figure 1A). The nitinol strips were treated according to previously published methods [2].
Figure 1.
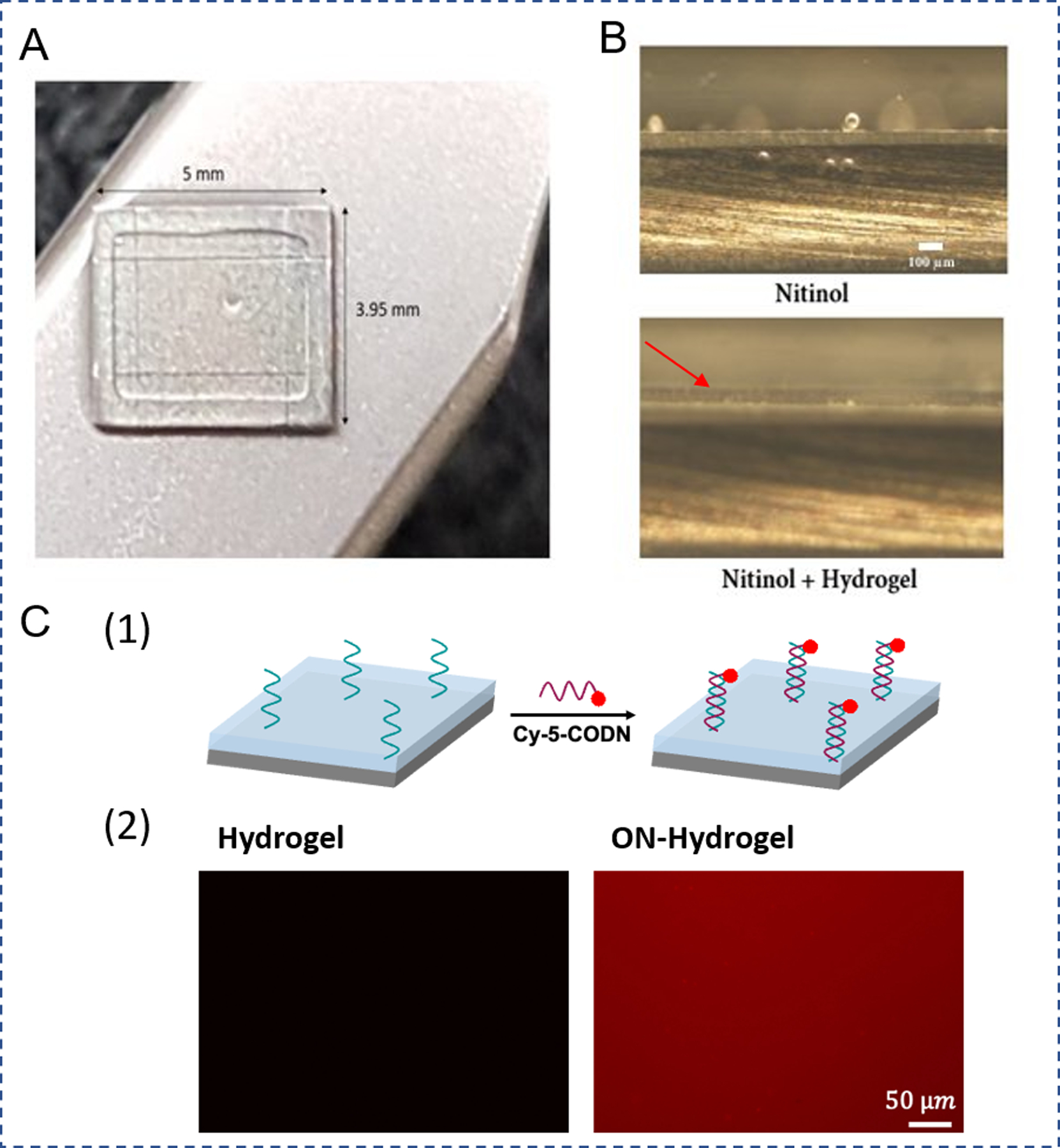
Characterization of ON-functionalized hydrogel coating. A. Photographic image of the hydrogel coating on nitinol surface. B. Inverted microscope image of the nitinol surface (side view) before and after hydrogel coating. Red arrow labels the hydrogel coating (scale bar 100 µm) C. ON- functionalized hydrogel surface was stained with Cy-5 labeled complementary ON sequence (CON). (1) Schematic illustration. (2) The hydrogel coated nitinol surface was examined under fluorescence microscope. Hydrogel: no ON (control sample) and ON-Hydrogel: ON-functionalized hydrogel (scale bar 50 µm).
Coating of hydrogel on nitinol surface
To coat the hydrogel film on the silanized nitinol strips, 0.1% photoinitiator (Irgacure 2959) and 40 % of acrylamide/ bis-acrylamide (37.5:1) were prepared and mixed in 1:1 v/v ratio. For oligonucleotide (ON) modified samples, acrydite-labeled ON (20 pmol) was added to the solution. ONs were chosen due to the stability and ease of modification in biological fluids [2]. The silanized nitinol strip was immersed with a pipetted pregel solution, covered with a cover slip, and treated under UV light at 365 nm for 3 minutes. After the polymerization, the coverslip was gently removed from the sample. The samples were washed in PBS overnight to remove all unreacted monomers and photoinitiator. The nitinol surface was examined before and after coating under an inverted microscope. To examine ON functionalization to the hydrogel, the hydrogel coated nitinol was incubated with a Cy-5 labeled complementary sequence of ON (CON) (40 pM) for 30 minutes at 4°C. The hydrogel was thoroughly washed with PBS and imaged under a fluorescent microscope. Images of hydrogel thickness were captured under both brightfield and fluorescent microscopy. Metalized mirrors (Opco labs, Fitchburg MA) were used to obtain an orthogonal side-view of the hydrogel. Thickness of hydrogel before and after flow treatments was then quantified in ImageJ.
Conjugation of CON to nanoparticles
Streptavidin and Biotin interaction was used to conjugate CON to nanoparticles. For the reaction, streptavidin coated nanoparticles were suspended in deionized water (1% w/v) and mixed with 3 nM of biotinylated ONs. The reaction was performed at room temperature on a shaker for two hrs. After the reaction, the nanoparticles were washed with PBS five times to remove unbound CON. The zeta-potential of the nanoparticles was measured before and after CON conjugation with Zetasizer Nano ZS (Malvern, United States) (Figure S1).
Passive attachment of nanoparticles to the nitinol surface
The ON-hydrogel coated nitinol strips were incubated with CON-functionalized nanoparticles (final concentration 0.001% w/v) overnight at 4°C. The nitinol strips were washed in PBS to remove unbound nanoparticles and, prior to any flow treatment, were imaged under fluorescence microscope. For imaging, strips were placed flat in a rectangular glass capillary tube (Vitrocom, Mountain Lakes NJ) while immersed in deionized water. The hydrogel was stained with dye-labeled CON to verify the uniform modification of the surface with ON. The nitinol strips were subsequently removed from the glass capillary imaging channel and placed in hydrated storage at 4°C until flow testing.
Dynamic injection of nanoparticles
In order to test the efficacy of nanoparticle attachment under both laminar and turbulent flow conditions, the hydrogel coated nitinol strips were placed in the testing chamber (Figure 3A, B). These conditions were chosen to approximate arterial shear stresses under both physiological and mechanically supported flow. An open flow loop (Figure 4A) was constructed with an upstream reservoir and a downstream waste sink, to prevent recirculation of nanoparticles and to ensure that nanoparticles contacted the hydrogel only once. The roller pump (Masterflex, Germany) was operated at both laminar and turbulent flow conditions to achieve a Reynolds number of 500 and 5000, respectively (Table 1). These flow conditions yielded shear stresses characteristic of arterial or device flow (Table 2) [4, 12–13]. Using a Pump 11 Elite programmable syringe pump (Harvard Apparatus, Holliston MA), the CON-modified nanoparticles at 0.01% w/v were injected into the flow loop upstream of the test channel. Nanoparticles were injected for 1 minute at a volumetric flow rate of 3 ml/min. The injected nanoparticle solution joined the main flow loop (Figure 3B, 4A), where flow was operating at either laminar or turbulent conditions (Table 1). After the injection, the pump was run for additional 30 seconds to clear the remaining nanoparticles in the chamber domain. Similarly, to the previous passive protocol, the nitinol strips were washed in PBS, imaged, and stored.
Figure 3.

Assessment of nanoparticle attachment under dynamic flow conditions. A. Image of the printed testing chamber with flow connectors and side-view mirrors (M-1 and M-2). B. Closed flow loop schematic. C. Hydrogel thickness before and after flow treatment. Data is presented as mean ± standard error of the mean.
Figure 4.

Nanoparticle attachment after injection under different dynamic flow conditions. A. Schematic of the open loop system for dynamic injection. B. Retention of statically-adhered NPs after laminar flow treatment (Re 500) and turbulent flow treatment (Re 5000). C. Attachment of nanoparticles under laminar flow (Re 500) and turbulent flow (Re 5000). Data is presented as mean ± standard error of the mean.
Table 1:
Description of flow conditions during both initial attachment method and subsequent flow treatments.
| NP Injection Rate | Loop Re# (During Injection) | Loop Re# (Flow Treatment) | Loop Q (During Injection) | Loop Q (Flow Treatment) | |
|---|---|---|---|---|---|
| Static 500 | 3 µL/min | N/A | 500 | N/A | 80 mL/min |
| Static 5000 | 3 µL/min | N/A | 5000 | N/A | 800 mL/min |
| Dynamic 500 | 3 µL/min | 500 | 500 | 80 mL/min | 80 mL/min |
| Dynamic 5000 | 3 µL/min | 5000 | 5000 | 800 mL/min | 800 mL/min |
Table 2:
Relevant shear forces at the investigated Reynolds numbers
| Reynolds Number | Shear Rate (s−1) | Wall Shear Stress |
|---|---|---|
| 500 | 900 | 9 |
| 5000 | 9000 | 90 |
Flow treatments
The custom-made sample holder and flow loop (Figure S4) were designed to test the behavior of the hydrogel-coated nitinol and the attachment of CON-conjugate nanoparticles in response to different flow conditions. The open flow loop was closed to permit circulation (Figure 3B). The sample holder (Figure 3A) was made from 3D printed components using clear resin on a Form 2 printer (Formlabs, Somerville MA). The printed sample holder formed a closed testing chamber with a cross-sectional profile of 1.5×4 mm. The length of the chamber was 80 mm, coupled to Tygon tubing by a resin-printed luer connecter. The nitinol strip was positioned inside the channel within a printed groove that prevented any slipping or tilting. Rubber gaskets placed in printed slots prevented leakage or damage by clamping. The upper channel component was placed onto the flat channel bottom and clamped to maintain the channel profile and prevent leakage. A printed assembly component held the channel components and permitted the placement of mirrors for side view visualization of hydrogel thickness. Between uses, the loop was cleaned with 70% ethanol and washed with deionized water. The flow loop circuit consisted of the peristaltic pump, a printed compliance chamber, and a reservoir. PBS was circulated within the flow loop.
Once the sample was placed in the channel, the flow rate was gradually increased (to prevent air intake from reservoir) until the desired Reynolds number was achieved. After each trial, the working fluid was replaced to remove all detached nanoparticles. Volumetric flow rate was determined from the channel hydraulic diameter and the velocity derived from the Reynolds number equation (1), and the pump was calibrated to achieve the nominal flow rates. Here, ρ is defined as fluid density, V is defined as mean velocity, D is defined as characteristic length or hydraulic diameter of the channel, and µ is defined as dynamic viscosity of the fluid. The flow experiments were performed at these laminar and turbulent flow conditions. Specifically, the desired flow rate resulted in Reynolds numbers of 500 and 5000 based on the channel cross-section and fluid properties. The kinematic viscosity of water at 22°C (9.55 cSt) was used with a hydraulic diameter (D) of 2.18 mm. The analytically determined velocities within the testing chamber were validated with a PIV system; velocity fields were acquired and processed in Insight 4G (TSI, Minneapolis MN). Shear rates were calculated according to Eqn. (2) and wall shear stress from Eqn. (3). Here, γ is mean wall shear rate on the surface of the hydrogel, Q denotes volumetric flow rate, w denotes the horizontal width of the flow channel, and h denotes the height of the flow channel. Shear rates and wall shear stress (WSS) are listed in Table 2. Nanoparticle treated samples were subjected to the different flow conditions for 1 hour before removal and imaging. Fluorescent images of the biotinylated nanoparticles were taken before and after the flow treatment to examine the stability of the nanoparticle attachment to the hydrogel coated nitinol surface. The notched line on the nitinol was used to spatially locate the area of interest on the nitinol.
| (1) |
| (2) |
| (3) |
Imaging
Fluorescent imaging was performed on an Olympus IX71 inverted fluorescent microscope (10x, 0.3 N.A.) using a CMOS DP74 camera with 20.7 MP resolution. CellSens imaging software was used to acquire images from live feed. Raw images were processed in ImageJ (NIH). Nanoparticle attachment was calculated using ImageJ software and plotted in Matlab (Mathworks, CA) and GraphPad (San Diego, CA).
Quantification of Nanoparticle Attachment
Two attachment conditions were used prior to flow experiments. First, the previously discussed static incubation was used to allow nanoparticles to bind to the functionalized hydrogel. The second method made use of an upstream injection port (Figure 4A) in which a nanoparticle solution was dynamically injected into a single-pass system. The main channel where PBS was introduced operated at Re = 500 or 5000 depending on the flow regime investigated. All samples (for either nanoparticle attachment method) were removed from the testing chamber and imaged before flow experiments were conducted (Figure 5). Samples then underwent one hour of flow treatment under laminar or turbulent conditions. Nanoparticle retention was determined by calculating the percentage of nanoparticles lost after flow treatment. Eqn. 2 quantifies the percentage of nanoparticles lost. Here, NP1 denotes the number of nanoparticles adhered before any flow treatment. NP2 denotes the number of nanoparticles adhered after flow treatment.
Figure 5.

Attachment efficacy of nanoparticle adhesion prior to flow experiment. A. Adhered nanoparticles for all flow conditions. B. Adhered nanoparticles for both attachment methods Data is presented as mean ± standard error of the mean.
| (4) |
Statistical Analysis
Significance was calculated using a one-way ANOVA test, and pairwise comparisons were calculated via student t-test on GraphPad. A minimum sample size (N = 12 separate flow experiments) was analyzed for each group. Results were considered statistically significant if p < 0.05. Quantitative data were reported as mean ± standard deviation.
Results
Synthesis and characterization of ON-functionalized hydrogel coating on nitinol surface
The hydrogel was coated on nitinol surface via photo-initiated polymerization. As the ON sequence was modified with acrydite group at the 5’ end it can participate in free radical polymerization (Table S1). This allows for fast and easy incorporation of ONs into the hydrogel. The nitinol was successfully coated with polyacrylamide hydrogel (Figure 1A). The hydrogel surface was visibly smooth with a consistent thickness of 100±28 µm when observed under brightfield microscopy (Figure 1B). To verify the incorporation of ON into the hydrogel coating and assess its distribution, the hydrogel was treated with Cy-5 labeled CON. As shown in Figure 1C, the hydrogel surface with ON showed high fluorescence while no signal was observed on a control coating without ON.
Nanoparticle characterization and adsorption on nitinol surface
CON-functionalized nanoparticles were incubated with ON-functionalized hydrogel coated nitinol samples to assess specific nanoparticle adsorption mediated via DNA hybridization. Before the hydrogel coating, the nanoparticle (NP) absorption capability of the bare nitinol strip was examined as a control. Non-conjugated NPs were not adsorbed by the bare nitinol (Figure 2A-1) whereas CON-conjugated nanoparticles (CON-NP) were non-specifically adsorbed (Figure 2A-2). However, hydrogel coating of the nitinol, with and without functionalization by ONs, prevented the non-specific attachment of non-conjugated NPs (Figure 2A-3,4). ON functionalization of the hydrogel or CON-conjugation of the nanoparticle separately did not induce any nanoparticle adsorption onto the nitinol surface (Figure 2A-3-5). ON functionalization of the hydrogel in conjunction with CON-nanoparticle conjugation produced a robust specific binding response (Figure 2A-6). An increase in the concentration of ON from 5 µM to 10 µM increased the degree of nanoparticle attachment from ~1500 to ~4500 NPs/mm2 (Figure 2B, C).
Figure 2.

Nanoparticle (NP) adsorption on the surface of ON-functionalized hydrogel coated nitinol. A. Hydrogel coated nitinol strips are treated with nanoparticles (Scale bar - 100 µm. (1) Prevention of NP binding to bare nitinol (2) Binding of CON-NP to bare nitinol. (3–4) Prevention of NP binding to hydrogel and ON-functionalized hydrogel. (5). Lack of binding of CON-NP to nonfunctionalized hydrogel. (6) Binding of CON-NP on the ON-functionalized hydrogel surface. B. ON concentration dependent NP adsorption on functionalized hydrogel coated nitinol surface. (Scale bar - 100 µm) C. Quantitative analysis of CON-functionalized nanoparticle attachment to the functionalized surface. Data is presented as mean ± standard deviation.
Hydrogel Durability under flow
After the initial hydrogel layer thickness characterization, hydrogel-coated nitinol strips were inserted under testing chamber (Figure 3A) and exposed to one hour of closed loop flow (Figure 3B). The strips were then removed to determine if there was any measurable loss in hydrogel thickness. Images of fluorescently labelled hydrogel before and after flow treatment revealed that there was insignificant loss in hydrogel thickness (Figure 3C), indicating resilience to flow-induced stripping of the coating. The average loss in hydrogel thickness was 3.1 µm (3.9% of initial thickness), which was not statistically significant (p = 0.7258). This result was consistent for both flow regimes.
Nanoparticle attachment under variable conditions
To determine the efficacy of different methods of nanoparticle attachment and surface functionalization under potential clinical flow conditions, static, laminar, and turbulent flow attachment conditions were investigated. The dynamic injection process and the depository rates of NPs were assessed under both laminar and turbulent conditions in an open loop system (Figure 4A). Moreover, the retention of statically adhered NPs was examined under laminar and turbulent flow. There was no observable difference in NP loss for statically adhered samples when treated under the two flow conditions (Figure 4B). The attachment of CON-functionalized NPs on the ON-functionalized hydrogel coated nitinol samples were similar under both laminar and turbulent condition (Figure 4C). NPs were shown to deposit at altered levels dependent on the methodology of attachment. NPs that were allowed to passivate on the hydrogel-coated nitinol under stagnant flow adhered at a higher rate than the NPs that adhered under dynamic injection (Figure 5A). When comparing static vs dynamic attachment (Figure 5A–B), this distinction was statistically significant (p < 0.01). There was no discernable difference between statically-adhered NPs that were later exposed to either laminar or turbulent flow (Figure 5A). The clearest difference in efficacy of initial nanoparticle attachment was between the statically attached NPs, and the NPs dynamically injected under laminar flow. A pairwise comparison between dynamic injection in laminar flow and static attachment indicated statistical significance (p < 0.01). A one-way ANOVA analysis indicated that Reynolds number was a significant factor in NP attachment (p < 0.01), assuming a Reynolds number of 0 for the static attachment condition.
Nanoparticle retention under flow
Nanoparticle retention was quantified using Eqn. (4). For nanoparticles that were statically conjugated to the functionalized nitinol, there was a mean nanoparticle loss of 38.2% for Re 500 and 36.1% for Re 5000 (Figure 4B). Nanoparticles that were adhered under dynamic injection conditions experienced a mean loss of 50.4% for Re 500 and 53.8% for Re 5000 (Figure 4C). A two-way ANOVA was performed (Figure 6A), indicating significance of attachment method (p < 0.05), while Reynolds number was determined to be not significant, as was the interaction between the two factors. This aligned with the pairwise comparison between retention when binned between only two factors, in which attachment method was significant (Figure 6B). The most pronounced difference was between the nanoparticle retention of the two groups treated with turbulent flow, in which significantly more nanoparticles were lost in the dynamically injected group (Figure 6A). Based on comparison of the retention rate between the two attachment methods, the dynamically attached nanoparticles seemed to have a weaker adhesion strength on average than the nanoparticles that were allowed to statically passivate (Figure 6A–B).
Figure 6.

Depletion of adhered nanoparticles due to flow treatment. A. Loss of adhered nanoparticles of all groups. No difference in static attachment of nanoparticles used for laminar or turbulent experiments. B. Loss of nanoparticles related to attachment method Data is presented as mean ± standard error of the mean.
Discussion
Coating and specificity of ON-functionalized hydrogel
The hydrogel surface with ON demonstrated high fluorescence while no fluorescence was detected on a control coating without ON (Figure 1C). These results clearly demonstrate that the acrydite-labeled ON was chemically conjugated to the hydrogel network and that attachment was specific to the reactive site rather than nonspecific localization. Following experiments investigating the specificity of the ON-CON reaction revealed that the bare nitinol surface permitted attachment of CON-functionalized NPs in the absence of any ON binding sites. The hydrogel coating without any ON functionalization blocked this attachment, as did the lack of CON-NP functionalization when the hydrogel was functionalized (Figure 2A-4,5). The only reactive attachment involving the hydrogel-coated nitinol occurred when both the hydrogel and the NP were functionalized (2A-6). Thus, the data suggest that the hydrogel coating has the dual benefit of blocking nonspecific attachment of molecules or particles to the surface of metal device and mediating hydrogel-NP reactivity based on the presence of conjugated ONs and CONs. The blocking of attachment to the bare nitinol could potentially be reproduced in the context of blocking protein absorption. Further study of nitinol-serum interactions under similar flow conditions would be useful in quantifying the degree of biofouling prevention in the turbulent conditions of mechanical circulatory support devices.
Nanoparticle characterization and adsorption on functionalized hydrogel
NPs and hydrogels were necessarily functionalized with complementary single-stranded DNA sequences for the NPs to attach to the surface of ON-functionalized hydrogel coated nitinol surface. Moreover, the adsorption is dependent on the concentration of ONs used to functionalize the hydrogel (Figure 2B). The NP-hydrogel interaction displayed a dose-dependent response to ON concentration within the hydrogel (Figure S1). The size of the NPs limited the level of CON functionalization. The fairly predictable level of hydrogel functionalization from initial ON concentration is attractive to elicit a desired rate of NP attachment. Based on these results, nitinol remains a viable biomaterial candidate for programmable drug delivery [1–2, 7, 11].
Hydrogel durability
The hydrogel demonstrated excellent durability in response to both laminar and turbulent flow. Loss in hydrogel thickness as measured by brightfield microscopy was negligible (Figure 3C), and there was no observed reduction ON-functionalization. Hydrogel thickness was maintained despite the application of high shear forces by both the laminar and turbulent flow regimes [4, 7, 33]. Previously published work has demonstrated that erosion of hydrogel happens rather rapidly due to repeated mechanical loading [24], suggested that the time scale of flow stripping of the hydrogel would fall within the examined one hour time frame. Studies exposing hydrogel to flow in an aneurysmal model also demonstrated robust resistance to fatigue over a month-long period [37], supporting the assertion that hydrogel durability is resistant to chronic flow exposure. The swelling of the hydrogel reached equilibrium within seconds due to the low thickness of the coating. The lack of any difference in changes in hydrogel thickness suggests that the change in thickness reaches a plateau within the one-hour time frame of flow treatment.
Nanoparticle attachment under flow
The reduction of nanoparticle adhesion for dynamic injection was nonintuitively less pronounced for nanoparticles that were injected under turbulent conditions. The overnight incubation of statically adhered particles permitted robust adhesion and resistance to flow stripping, likely due to the time permitted increase in bonding events with hydrogel ON’s. Despite the higher residence time within the testing chamber, only the nanoparticles injected in laminar flow were significantly different from the statically adhered nanoparticles. This could be a result of greater mixing and the altered flow profile of turbulent flow [7]. Based on knowledge of the mixing properties of turbulence [7, 33], it seems likely that a greater number of nanoparticles are introduced to the fluid layer contacting the functionalized hydrogel. An unexpected result is the increased attachment of dynamically injected nanoparticles in the turbulent case relative to the laminar case. While the difference was not statistically significant, only the dynamic laminar case exhibited significantly reduced adhesion when compared to the static case. Further investigation at pulsatile conditions and other Reynolds numbers, such as a transitional flow, could shed more light on the difference in adhesion [4, 7, 25] and provide opportunities for in vitro modeling of thrombosis [5, 6, 8, 11]. One possible explanation for the lesser reduction in adhesion for the turbulent case could be the high incidence of vorticity and secondary flows associated with turbulence [8, 36]. Results from studies of turbulence in rectangular channel flow [38, 39] support the assertion that flow within the specified channel at a Reynold’s number of 5,000 demonstrates significant mixing and fluctuations characteristic of turbulence. The altered flow patterns might increase mixing rates and the number of nanoparticles that come into contact with complementary oligonucleotides [7, 9]. Additionally, nanoparticles that are removed by flow may repenetrate the hydrogel in turbulent conditions.
Nanoparticle retention under flow
While nanoparticle retention was unaffected by the difference in Reynolds number for either statically or dynamically adhered samples, method of attachment was a significant factor in predicting retention rates. A t-test comparison confirmed that there was no difference in the statically attached groups that would be exposed to either laminar and turbulent flow (Figure 4B). The only significant pairwise comparison between the four groups was between statically adhered turbulent samples and dynamically adhered turbulent samples. This could indicate that the dynamic injection method permits the formation of some less stable conjugate bonds than the static passivation method. Functionalized hydrogel samples performed well in conditions characteristic of blood-contacting devices, coating the commonly used biomaterial nitinol and retaining performance in the turbulent conditions associated with mechanical circulatory support devices [4, 12–13].
The ON-functionalized hydrogel was chemically conjugated onto the surface of Nitinol. The bare nitinol exhibited nonspecific binding to CON-NPs, but hydrogel-coated nitinol dramatically prevented nonspecific binding. Binding between the functionalized hydrogel coating and nanoparticles was evident only when both ONs and CONs were present. The dose-dependent response of nanoparticle attachment to ON concentration during the functionalization process exhibited a strong and specific interaction between nanoparticles and hydrogel-functionalized nitinol.
The functionalized hydrogel demonstrated excellent durability in resisting mechanical deformation by flow. The nanoparticles that were allowed to attach under static conditions adhered at ~150% of the rate of dynamically attached nanoparticles. This result is unsurprising due to the increased time of passivation compared to the transient contact during the dynamic injection. The functionalized hydrogel demonstrated comparable retention of bound nanoparticles in both flow regimes. The main indicator of nanoparticle loss during flow treatment was the attachment method used. Differentiating between static and dynamic attachment is important in designing a clinical protocol for hydrogel functionalization. Static attachment of nanoparticles would be useful for biomaterial functionalization prior to implantation, while dynamic attachment is relevant to nanoparticle replenishment of an implanted device. The flow regimes tested are related to potential physiological flow conditions [40, 41], showing evidence that a hydrogel-coated biomaterial treated in the manner shown could be re-functionalized in follow up procedures without requiring device retrieval and replacement.
This study had some limitations. First, hydrogel durability should be quantified at chronic time scales, to establish that the demonstrated resilience to stripping would also be present in a clinical setting for long-term use. Second, the influence of local shear rates within the flow regime would be an informative investigation, as this examination could be a better predictor of nanoparticle loss than the Reynolds number. Additionally, the study of a transitional and/or a pulsatile flow would be both interesting and physiologically relevant. Increasing the duration of an experiment relevant to clinical studies would be of interest, to match estimated time scales of a realistic surgical procedure. While outside the scope of this study, extending the length of flow treatment to investigate both hydrogel degradation and nanoparticle retention would be useful for informing the design of more rigorous in vivo studies. This experiment would shed more light on nanoparticle long term performance under device relevant conditions. Nitinol is the current gold standard of implanted device materials, but other biomaterials such as Ti6Al4V might be investigated. The results of hydrogel coating of the device would be dependent on the ability of the material to be functionalized by oligonucleotides. Due to the similar structure of the two titanium alloys, we believe the results would be similar, and possibly hold for many metallic biomaterials with similar surface properties. Finally, the simplified biochemical scenario of non-reactive PBS is not a good indicator of most biofluids. Further studies making use of blood are necessary when introducing antithrombogenic drugs.
Conclusions
The overall study clearly supports the hypothesis that ON-functionalized nitinol remains an attractive biomaterial in turbulent flow. Elements of turbulence are characteristic of blood-contacting devices, and little has been published regarding biomaterial performance under turbulent flow. To remain clinically relevant, hydrogel coatings must be structurally robust and retain their specific binding properties even under the high shear stresses of turbulent flow. Nitinol had a suitable response to hydrogel coating, and coated samples displayed a robust resistance to structural hydrogel degradation due to flow. Once functionalized, the hydrogel exhibited the attractive qualities of a high affinity for complementary oligonucleotides and specificity for the ON-CON binding reaction. Nonspecific binding was prohibited by the hydrogel coating in the absence of either ON or CON. The functionalized nitinol samples also displayed suitable retention of adhered nanoparticles under both laminar and turbulent flow conditions. Static adhesion of nanoparticles was demonstrated to be superior to dynamic injection in both initial adhesion and retention, however, dynamic injection still permitted comparable adhesion efficacy and retention. Combined with the ON dose-dependent response of CON-binding, an implanted biomaterial could be functionalized initially with a statically attached complementary sequence and replenished over longer periods of time with upstream injections. The hydrogel’s ability to allow ON-mediated binding of dynamically injected nanoparticles within even rapid turbulent flows would be extremely useful for clinical replenishment of functionalized hydrogels without interrupting device operation. While a pre-functionalized implanted device could remain antithrombogenically active for a period of time, it would likely become necessary to replenish drug loss. This suggests that refunctionalization of an implanted device could be possible with an upstream injection of nanoparticles, without the need to replace the device.
The success of functionalized nitinol in a generalized context has a number of applications for drug delivery under dynamic flow, but the most obvious is use for antithrombogenic modification. The performance of the functionalized nitinol within the described flow experiments is a positive indicator for future use in mechanical circulatory support devices. These encouraging results warrant the further investigation of ON conjugation with antithrombogenic drugs and of the interaction between functionalized hydrogel surfaces and blood.
Supplementary Material
Acknowledgments
This research was supported by American Heart Association Grant 19IPLOI34770159.
Footnotes
Statements and Declarations:
The authors have no conflicting or financial interests in regards to the subject of this manuscript
Conflicts of Interest
No benefits in any form have been received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- [1].Abune L, Davis B, and Wang Y. Aptamer-functionalized hydrogels: an emerging class of biomaterials for protein delivery, cell capture, regenerative medicine, and molecular biosensing. WIRES. Nanomed. Nanobio e1731, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abune L, Zhao N, Lai J, Peterson B, Szczesny S, and Wang Y. Macroporous hydrogels for stable sequestration and sustained release of vascular endothelial growth factor and basic fibroblast growth factor using nucleic acid aptamers. ACS. Biomater. Sci. Eng 5(5):2382–2390, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Battig MR, Soontornworajit B, and Wang Y. Programmable release of multiple protein drugs from aptamer-functionalized hydrogels via nucleic acid hybridization. J. Am. Chem. Soc 134(30):12410–12413, 2012. [DOI] [PubMed] [Google Scholar]
- [4].Bortot M, Sharifi A, Ashworth K, Walker F, Cox A, Ruegg K, Clendenen N, Neeves KB, Bark D, and Di Paola J. Pathologic shear and elongation rates do not cause cleavage of von Willebrand factor by ADAMTS13 in a purified system. Cell. Mol. Bioeng 13(4):379–90, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen S, Li L, Zhao C, and Zheng J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer. 51(23):5283–93, 2010. [Google Scholar]
- [6].R. W., Marder VJ, Clowes AW, George JN, and Goldhaber SZ. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Philadelphia: Lippincott Williams & Wilkins, 2006, 1827 pp. [Google Scholar]
- [7].Dimotakis PE (2000). The mixing transition in turbulent flows. J. Fluid. Mech 409:69–98, 2000. [Google Scholar]
- [8].Furie B, and Furie BC. Mechanisms of thrombus formation. New. Engl. J. Med 359(9):938–949, 2008. [DOI] [PubMed] [Google Scholar]
- [9].Gaddes ER, Gydush G, Li S, Chen N, Dong C, and Wang Y. Aptamer-based polyvalent ligands for regulated cell attachment on the hydrogel surface. Biomacromolecules. 16(4):1382–1389, 2015. [DOI] [PubMed] [Google Scholar]
- [10].Good BC, Deutsch S, and Manning KB. Hemodynamics in a pediatric ascending aorta using a viscoelastic pediatric blood model. Ann. Biomed. Eng 44(4):1019–1035, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Irwin NJ, McCoy CP, and Trotter JL. Hydrogel coatings for medical device applications. In Hydrogels. CRC Press:89–101, 2018. [Google Scholar]
- [12].Jaffer IH, Fredenburgh JC, Hirsh J, and Weitz JI. Medical device-induced thrombosis: what causes it and how can we prevent it?. J. Thromb. Haemost 13:72–81, 2015. [DOI] [PubMed] [Google Scholar]
- [13].Jhun CS, Siedlecki C, Xu L, Lukic B, Newswanger R, Yeager E, Reibson J, Cysyk J, Weiss W, and Rosenberg G. Stress and exposure time on von Willebrand factor degradation. Artif. Organs 43(2):199–206, 2019. [DOI] [PubMed] [Google Scholar]
- [14].Jiang P, Li S, Lai J, Zheng H, Lin C, Shi P, and Wang Y. Nanoparticle-programmed surface for drug release and cell regulation via reversible hybridization reaction. ACS. Appl. Mater. Inter 9(5):4467–4474, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kingshott P and Griesser HJ. Surfaces that resist bioadhesion. Curr. Opin. Solid. St. M 4(4):403–412, 1999. [Google Scholar]
- [16].Li S, Chen N, Zhang Z, and Wang Y, Y. Endonuclease-responsive aptamer-functionalized hydrogel coating for sequential catch and release of cancer cells. Biomaterials, 34(2):460–469,2013. [DOI] [PubMed] [Google Scholar]
- [17].Li S, Gaddes ER, Chen N, and Wang Y. Molecular Encryption and Reconfiguration for Remodeling of Dynamic Hydrogels. Angew. Chem., Int. Ed,54:5957–5961, 2015. [DOI] [PubMed] [Google Scholar]
- [18].Liang Y and Kiick KL. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta. Biomater 10(4):1588–1600, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee JH, Lee HB, and Andrade JD. Blood compatibility of polyethylene oxide surfaces. Prog. Polym. Sci 20(6):1043–1079, 1995. [Google Scholar]
- [20].Lowe S, O’Brien-Simpson NM, and Connal LA. Antibiofouling polymer interfaces: poly (ethylene glycol) and other promising candidates. Polym. Chem 6(2):198–212, 2015. [Google Scholar]
- [21].Ma H, Li D, Sheng X, Zhao B, and Chilkoti A. Protein-resistant polymer coatings on silicon oxide by surface-initiated atom transfer radical polymerization. Langmuir 22(8):3751–3756, 2006. [DOI] [PubMed] [Google Scholar]
- [22].Manning KB, Nicoud F, and Shea SM. Mathematical and computational modeling of device-induced thrombosis. Curr. Opin. Biomed. Eng 20:100349, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Navitsky MA, Taylor JO, Smith AB, Slattery MJ, Deutsch S, Siedlecki CA, and Manning KB. Platelet adhesion to polyurethane urea under pulsatile flow conditions. Artif. Organs 38(12):1046–1053, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nicodemus GD, Shiplet KA, Kaltz SR, and Bryant SJ. Dynamic compressive loading influences degradation behavior of PEG-PLA hydrogels. Biotechnol. Bioeng 102(3):948–959, 2009. [DOI] [PubMed] [Google Scholar]
- [25].Obiweluozor FO, Tiwari AP, Lee JH, Batgerel T, Kim JY, Lee D, Park CH, and Kim CS. Thromboresistant semi-IPN hydrogel coating: towards improvement of the hemocompatibility/biocompatibility of metallic stent implants. Mater. Sci. Eng: C 99:1274–1288, 2019. [DOI] [PubMed] [Google Scholar]
- [26].Palacio ML and Bhushan B. Bioadhesion: a review of concepts and applications. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 370(1967):2321–2347, 2012. [DOI] [PubMed] [Google Scholar]
- [27].Rabenstein DL Heparin and heparan sulfate: structure and function. Nat. Prod. Rep 19(3):312–331, 2002. [DOI] [PubMed] [Google Scholar]
- [28].Sakiyama-Elbert SE 2014. Incorporation of heparin into biomaterials. Acta Biomater 10(4):1581–1587, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Soontornworajit B, Zhou J, and Wang Y. A hybrid particle–hydrogel composite for oligonucleotide-mediated pulsatile protein release. Soft. Matter 6(17):4255–4261, 2010. [Google Scholar]
- [30].Taylor JO, Yang L, Deutsch S, and Manning KB. Development of a platelet adhesion transport equation for a computational thrombosis model. J. Biomech 50:114–120, 2017. [DOI] [PubMed] [Google Scholar]
- [31].Taylor JO, Meyer RS, Deutsch S, and Manning KB. Development of a computational model for macroscopic predictions of device-induced thrombosis. Biomech. Model. Mechan 15(6):1713–1731, 2016. [DOI] [PubMed] [Google Scholar]
- [32].Topper SR, Navitsky MA, Medvitz RB, Paterson EG, Siedlecki CA, Slattery MJ, Deutsch S, Rosenberg G, and Manning KB. The use of fluid mechanics to predict regions of microscopic thrombus formation in pulsatile VADs. Cardiovasc. Eng. Techn 5(1):54–69, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Uhl V Mixing V1: Theory and Practice. Elsevier, 2012, 350 pp. [Google Scholar]
- [34].Yang N, Deutsch S, Paterson EG, and Manning KB. Comparative study of continuous and pulsatile left ventricular assist devices on hemodynamics of a pediatric end-to-side anastomotic graft. Cardiovasc. Eng. Techn 1(1):88–103, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yang N, Deutsch S, S., Paterson EG, and Manning KB. Hemodynamics of an end-to-side anastomotic graft for a pulsatile pediatric ventricular assist device. J. Biomech. Eng 132:031009, 2010. [DOI] [PubMed] [Google Scholar]
- [36].Zhang Z, Chen N, Li S, Battig MR, and Wang Y. Programmable hydrogels for controlled cell catch and release using hybridized aptamers and complementary sequences. J. ACS 134(38):15716–15719, 2012. [DOI] [PubMed] [Google Scholar]
- [37].Poupart O, Conti R, Schmocker A, Pancaldi L, Moser C, Nuss KM, Sakar MS, Dobrocky T, Grützmacher H, Mosimann PJ and Pioletti DP. Pulsatile Flow-Induced Fatigue-Resistant Photopolymerizable Hydrogels for the Treatment of Intracranial Aneurysms. Frontiers in Bioengineering and Biotechnology, 8, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sano M, Tamai K: A universal transition to turbulence in channel flow. 15 (2016). 10.1038/NPHYS3659 [DOI] [Google Scholar]
- [39].Wang J, Wang G, Rongsheng Z, Wang Z, Zhang R, Yu T, Liu Z, Huang Y, Wang J, Cong T: Article 67 J and Cong T (2020) Numerical Study on Laminar-Turbulent Transition Flow in Rectangular Channels of a Nuclear Reactor. Front. Energy Res 8, 67 (2020). 10.3389/fenrg.2020.00067 [DOI] [Google Scholar]
- [40].Mcgurk KA, Owen B, Watson WD, Nethononda RM, Cordell HJ, Farrall M, Rider OJ, Watkins H, Revell A, Keavney BD: Heritability of haemodynamics in the ascending aorta. 10, 14356 (2020). 10.1038/s41598-020-71354-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wan Ab Naim WN, Ganesan PB, Sun Z, Osman K, Lim E: The Impact Of The Number Of Tears In Patient-Specific Stanford Type B Aortic Dissecting Aneurysm: Cfd Simulation. J. Mech. Med. Biol 14, 1450017 (2014). 10.1142/S0219519414500171 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


