Abstract
Background and Objectives
The inherited backlog of 16,000 medicines applications of the South African Health Products Regulatory Authority (SAHPRA) was cleared through facilitated review pathways that included reliance on prior work by trusted regulators. This research aimed at determining the economic impact of reliance on national regulatory authorities (NRAs) in terms of lower assessors’ costs, especially to offset the financial efforts required to attain a higher World Health Organization (WHO) maturity level and understanding the way fees can sustain NRA activities.
Methods
To this end, the assessor costs associated with reliance and full review applications were calculated and compared. A high-level review of African NRA fee structures was also carried out and pharmaceutical industry input was solicited regarding the feasibility of alternative tariff modalities for low- and middle-income (LMIC) NRAs.
Results
The investigation showed a marked reduction in time spent in reliance assessments compared to full reviews, with an associated decrease in reviewers’ costs; SAHPRA conserved US$277,413 across the 188 applications applying reliance principles. The NRA fee structure review revealed outdated fees with little differentiation between full and reliance assessment. NRAs lack the financial resources to strengthen regulatory systems; WHO Global Benchmarking Tool activities are not directly covered by levied fees. Overall, the pharmaceutical industry was supportive of advancing the maturity of African NRAs and was willing to pay increased fees for reliance reviews when authorities adhere to published timelines. More expensive fast-track services were cited, making an argument for higher fees for reliance assessment when this enables medicines to reach markets quicker.
Conclusions
Reliance is a tool to safeguard NRA resources and support regulatory and information systems strengthening. The study illustrates the return on investment of reliance for NRAs and, if optimally implemented, the benefits for patients.
Key Points
| This research was carried out to determine the economic impact of reliance on national regulatory authorities (NRAs) especially relative to costs to attain a higher World Health Organization maturity level. |
| Pharmaceutical industry input was solicited regarding alternative tariff modalities for low- and middle-income NRAs. |
| Results showed a marked reduction in time and decrease in costs for reliance assessments; the pharmaceutical industry was willing to pay increased fees for reliance reviews with regulatory timeline adherence. |
Introduction
Prior to the establishment of the South African Health Products Regulatory Authority (SAHPRA) in 2018, its predecessor, the Medicines Control Council (MCC), operated as a division under the National Department of Health (NDoH), which provided oversight and guidance to the MCC. In 2008, in view of a significant medicine application backlog that had formed in the MCC, a Ministerial Task Team (MTT) was appointed to review the structure of the MCC and provide recommendations [1]. The South African medicines regulator faced several challenges, especially in the areas of clinical trial evaluation and marketing authorization of products, with these directly linked to overarching issues, such as limited assessor capacity, inhibited financial capabilities and the lack of an electronic application tracking system [1].
The MCC relied on a combination of government allocations through the NDoH budget and application fees for product evaluation and registration; the latter not commensurate with the activities performed and not in line with fees charged by other regulators at the time [1]. Furthermore, although fees were collected, these did not accrue to the MCC but rather to the National Treasury [1]. This dependency on the NDoH severely challenged the MCC in fulfilling its legislative mandate and, as such, the MTT recommended a replacement of the MCC with an entity that would safeguard the autonomy of the South African medicines regulator, both regarding independent decision-making and oversight of financial sustainability [1].
Through promulgation of an amended Medicines and Related Substances Act 101 of 1965 on 24 February 2017 [2], and the enactment thereof in May 2017, SAHPRA was classified as a National Schedule 3A Public Entity through the Public Finance Management Act (PFMA) (Act 1 of 1999) [3], thereby enabling the establishment of a separate entity from the NDoH. Through the model of an independent agency of government, SAHPRA is enabled to recover costs from fees charged to applicants, thereby reducing the burden on the fiscus [1]. The MTT also recommended a partial cost recovery (establishing and collecting fees) with SAHPRA able to generate revenue to supplement its budget and the remaining expenses supported by the fiscus [1]. As a Schedule 3A Entity, SAHPRA has a 5-year strategic plan [4] and an annual performance plan [5] linked to a yearly budget, which is approved by the Minister of Health annually. Moreover, through the PFMA, SAHPRA is required to ensure transparent, accountable and sound administration of its revenue, expenditure, assets and liabilities [3] and to provide financial and performance reports to the NDoH and National Treasury on a quarterly basis.
One of the current medium-term strategic priorities for SAHPRA is “financial sustainability achieved through revenue-generated and enhanced operational efficiencies” [6]. The latter addresses another of the MTT recommendations, which was the increased efficiency in the evaluation of dossiers through reliance on prior product reviews by recognized regulatory authorities (RRAs) in other jurisdictions, enabled via memoranda of understanding between SAHPRA and the RRAs [1]. The clearance of the backlog of approximately 16,000 marketing authorizations and variation applications was a high priority for the new regulator and was given “immediate attention” [1, 7]. An ambitious 2-year timeframe was set by SAHPRA to clear this backlog, which required an increased level of agility and flexibility, with the organization willing to transform culturally by adopting best practices as advocated by the World Health Organization (WHO) [7]. As a consequence of the revision of the SAHPRA legislative framework, facilitated review pathways (FRPs) were now permissible [2]. Given this context, regulatory reliance and risk-based assessment were piloted within its Backlog Clearance Project (BCP) [7].
The same year SAHPRA was established, the WHO first field-tested its Global Benchmarking Tool (GBT) [8, 9]. The aim of the tool is to enable health product regulators to assess their regulatory capabilities within the global context through a gap analysis, thereby resulting in corrective and preventative action plans (CAPAs) to address disparities, so-called “Institutional Development Plans” (IDPs). By the end of 2019, “26 countries had undergone formal benchmarking, and a further 54 countries had used the GBT to conduct self-benchmarking exercises assisted by WHO” [8]. In November 2022, SAHPRA was the second national regulatory authority (NRA) on the African continent, after the Egyptian Drug Authority, to achieve WHO maturity level 3 (ML3) for vaccine regulation and testing, with ML4 for its lot release function [6].
Although the GBT is a sound framework with the impetus on increasing the maturity levels of NRAs, it may, at times, compound resource constraints within low- and middle-income country (LMIC) medicines regulators. The GBT necessitates an information and communication technology (ICT) infrastructure for metrics collation and reporting, as well as an extension of an NRA’s regulatory scope relating to country-specific activities, such as vigilance, post-marketing and port-of-entry surveillance, amongst others [9]. While the GBT identifies the requirements in terms of a mature medicines regulatory system, some LMIC NRAs struggle to meet these due to compromised resources, infrastructure deficiencies and a lack of internal expertise, compelling them to rely on external development partners to strengthen their regulatory systems to achieve the goal of WHO ML3. The WHO Coalition of Interested Parties (CIP) initiative is an example of the manner in which stakeholders collaborate to support NRAs in this regard [10].
The WHO is a known proponent of reliance practice implementation at national regulatory authority level, as these practices have demonstrated to not only alleviate the pressure on an NRA specifically related to human endeavour, with less duplication of efforts [11], but also to provide timely access to safe and efficacious medicines of good quality to LMIC populations, when optimally implemented [7]. However, there is yet to be a formalised approach, as well as accessible information containing metrics, in understanding the benefits (or limitations) of reliance practice implementation by an NRA, as well as to acknowledge the return-on-investment (ROI) this may have for an authority [12]. In the search of such a methodology, the concept “relianomics” has been developed, with it being “a structured framework for the assessment of the impact of regulatory reliance pathways on regulatory, economic, societal, and other systems” [12].
In view of the above, the purpose of this study was to investigate the economic impact on an African medicines regulatory agency of investing in reliance practices, as well as to reflect the pharmaceutical industry perspectives regarding this approach and its economic benefits.
The objectives of this study were to:
Determine the major direct and indirect costs influencing the operational capacity of an African NRA, in this case SAHPRA, especially when aiming for WHO ML3 attainment and maintenance.
Analyse the assessment duration and costs associated with abridged and full review in SAHPRA’s BCP.
Reflect on NRA funding models and the level of differentiation within fee structures for full and facilitated review pathways across a subsection of African medicines regulators.
Assess the views and perception of the pharmaceutical private sector on the economic benefits for a LMIC NRA stemming from reliance review implementation.
Illuminate and assist with the further development of the concept of relianomics.
Provide recommendations regarding potential financial sustainability mechanisms for NRAs.
Study Hypotheses
The alternative or research hypothesis is that the adoption of facilitated regulatory pathways, such as reliance on unredacted assessment reports obtained from a reference agency that initially granted the product marketing authorisation, would result in economic efficiency and save resources. The null hypothesis entails that there would be no difference between the assessor costs for full versus reliance product applications.
Methods
South African Health Products Regulatory Authority (SAHPRA) Operating and Capital Expenditure
The initial aim of the study was to review the direct and indirect operational costs (OPEX) and capital expenditure (CAPEX) of SAHPRA to demonstrate the costs associated with a LMIC NRA. This aspect of the research examined the day-to-day costs, such as the building rental, other facilities and human resource (HR) costs, required to enable SAHPRA to accomplish its mandate.
SAHPRA Costs for WHO Maturity Level 3 (ML3) Activities
Additional analyses were conducted to ascertain the financial and direct/indirect resource consequences for SAHPRA in attaining and sustaining its current maturity status for vaccine regulation oversight. The costs for specific GBT requirements, such as national regulatory system (RS) facets like human resource management, sound financial governance and the implementation of a robust quality management system (QMS), were investigated in this regard. Also in scope was the high-cost ML3 activities, for example post-marketing surveillance and control (MC) and laboratory testing (LT), which, although part of the GBT criteria, do not directly attract fees from the pharmaceutical industry.
SAHPRA Backlog Clearance Project Assessment Time-and-Cost Metrics
This study further endeavoured to establish the difference between the assessment duration (number of hours) and costs (in United States dollars (USD)) associated with abridged and full review of the chemistry, manufacturing and controls (CMC) and/or bioequivalence (BE) data of new chemical entities (NCEs) and generic new registration applications in SAHPRA’s BCP. These applications were submitted electronically to SAHPRA’s dossier hosting platform, where they were tracked and assessed from 2019 to 2022 [7]. The abridged review was conducted in line with the SAHPRA reliance guideline [13] and contingent on the availability of unredacted assessment reports from the references agencies with whom SAHPRA aligns itself, namely the European Medicines Agency (EMA), the Australia Therapeutic Goods Administration (TGA), Swissmedic, the United Kingdom Medicines and Healthcare products Regulatory Agency (MHRA), the United States Food and Drug Administration (US FDA), and Health Canada, amongst others. Leveraging the findings from a prior study in terms of reliance-assessment outcomes in relation to the SAHPRA BCP [7], the complete sampling frame in terms of CMC and/or BE reviews was considered.
It is important to note that within SAHPRA, both the CMC and BE assessments for generic molecules were, at that time, carried out by the same evaluation unit [7]. As such, the review of both the CMC and BE data contained in generic product dossiers were in scope for this research. Furthermore, no analyses of the different clinical review pathways for generic products were conducted, as these applications seldom contain additional clinical data to be reviewed. SAHPRA, moreover, requires that generic product labelling be aligned to the latest SAHPRA-approved innovator’s Professional Information (PI) [7]. In terms of assessors’ costs, this type of verified review against the innovator’s labelling is consistent across generic applications and does not impact the final costs in terms of abridged or full reviews. Any clinical review costs (other than BE studies) were thus disregarded for this research.
Assessor Demographics
It is noteworthy that the SAHPRA BCP utilised four subsets of assessors, each remunerated via a different policy, and who were employed based on their availability and relevant expertise and the type of assessment required:
International assessors, comprising mostly of reviewers from the Southern African Development Community (SADC) NRAs, with assessors from the Medicines Control Authority of Zimbabwe (MCAZ), the Zambia Medicines Regulatory Authority (ZAMRA), the Namibia Medicines Regulatory Council (NMRC), the Botswana Medicines Regulatory Authority (BoMRA), the Tanzania Medicines and Medical Devices Authority (TMDA) and the Ghana Food and Drugs Authority (FDA), as well as a limited number of assessors previously or currently employed by RRAs, such as the TGA, the EMA and the US FDA. This group of assessors were remunerated per individual hourly rate in USD and made up the largest group within the BCP.
External South African assessors from universities or retired assessors who had previously performed reviews for the then MCC, who were paid according to a fixed South African Rand (ZAR) rate per type of assessment, based on their experience level, that is level 1 (entry-level) to level 3 (expert-level) (SAHPRA, Committee Members and External Evaluator Policy, Internal document, 2020; SAHPRA, External Evaluator Policy, Internal document, 2022). For subsequent response or additional information evaluations, these assessors were remunerated in ZAR per the number of hours spent assessing the responses.
SAHPRA-employed assessors, reviewing BCP applications on an overtime basis, that is, after working hours and over weekends. The SAHPRA overtime policy allowed for 10 hours per week with a capped number of overtime assessment hours of 40 per month per employee (SAHPRA, Overtime Policy, Internal document, 2020). This remuneration was calculated based on the individual assessor’s fixed SAHPRA hourly rate, which was multiplied by 1.5 and 2 for hours spent reviewing after hours during the week or on Saturdays, and on Sundays, respectively. At any given time during the BCP, this group consisted of approximately 10–15 reviewers based in different units of the South African medicines regulator.
The Backlog Clearance Project staff performed assessments during their normal working day, as well as did three (3) CMC and/or BE senior SAHPRA assessors, who were seconded to the project for its duration. The former cohort was used especially for initial abridged reviews and the average time spent by these in-house reviewers was set at 12 h for an initial review, with 4 h for any response reviews. The cost of the reviews was calculated based on their individual monthly salary, which was divided by 195 working hours (9 h/day × 21.67 days/month) [14], to arrive at an hourly rate for each staff member. For the seconded assessors, their time assessing was calculated in a similar manner as the project staff.
For the purpose of this study, all the claims submitted in ZAR were converted to USD based on the average exchange rate for the month and year in which the assessment had taken place [15].
Individual assessors’ remuneration claims for each application in the sample cohort were collected and the costs for initial primary and secondary reviews, as well as for assessment of applicant responses to SAHPRA requests for additional information and any quality assurance (QA) steps, if and when required, were determined. The time-and-cost metrics were collated for both the NCE and generic applications detailed in the previous study [7].
Exclusion Criteria
Since at the initial stages of the BCP, claims were still being processed manually and the COVID-19 pandemic disrupted some of these processes, not all assessor remuneration information could be located. This has led to exclusions, as no claim information (duration and/or cost) was available for these applications.
Selection of New Chemical Entity New Registration Applications
The complete sampling frame from the initial BCP research, spanning 72 NCE applications (full = 29; abridged = 43), was considered [7]. However, 21 applications were excluded due to unavailability of claim information and the final cohort consisted of 51 (71%) applications. For these, the assessment duration and the aggregated individual assessors’ cost for full review of the CMC data (n = 18: 35%) and the same metrics for CMC assessment conducted via an abridged review pathway (n = 33: 65%) were collected.
Selection of Generic Molecule New Registration Applications
Similarly, the complete sampling frame included in the prior research was taken into account, that is, all 153 applications (full = 72; abridged = 81) [7]. In this instance, the exclusions amounted to 16 generic product applications and the final cohort was 137 (90%). As with the NCE applications, both the assessment duration and aggregate costs for assessment of the CMC and BE data (the latter, if applicable) were reviewed in full (n = 67: 49%) or in an abridged manner (n = 70: 51%).
Based on the above data, analyses were performed in terms of the duration and NRA costs associated with abridged and full reviews of CMC data (NCE applications) and of CMC and/or BE data (generic applications).
Statistical Analysis
Data between groups were analysed using both parametric and non-parametric methods. In the parametric assessment, it was first determined if the data were normally distributed using the Shapiro–Wilks test assessing the null hypothesis to ensure there was no significant departure from normality, followed by a two-sample t-test. In terms of non-parametric tests, the Kruskall–Wallis test was applied by testing the null hypothesis that there was no significant difference between medians. Based on the normality testing outcome, box-plots were compared using the non-parametric method. All the statistical analyses were conducted assuming probability of type I error at 5% level using SAS Enterprise Guide (version 7.15) software.
Data Validation
In order to validate the data extraction, there was a random double extraction of 30% of the data points by the same researcher. As such, the accuracy of the data collected was assured.
Review of Funding Models and Fee Structures of African National Regulatory Authorities (NRAs)
Moreover, an overview of the different fee structures employed by certain African NRAs was conducted, with a specific review of the differences in fees levied for full and reliance review of new registration applications. Further investigation was also carried out to determine the level of financial support for African NRAs, by examining the difference between governmental grants and income from application fees received, as this ratio may influence the financial sustainability of an African regulatory authority.
Digital Pharmaceutical Industry Opinion Surveys
A digital questionnaire, the Reliance Economic Impact Questionnaire (REIQ), was generated, in collaboration with the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), wherein international Heads of Medical and Regulatory Affairs of innovative pharmaceutical companies were asked to provide their views regarding the possible economic benefits of reliance practices, specifically related to African NRAs. The survey also sought to elicit responses from the participants as to whether the pharmaceutical sector, in view of the perceived benefits of reliance, believes a differentiated fee structure for different review pathways within African NRAs should be considered. It further investigated how the industry regards the cost-savings arising from reliance implementation by NRAs in terms of the ROI this may have for African regulators. Lastly, the questionnaire tested innovative medicine manufacturers’ appetite for contributing to an annual developmental fee to enable NRAs to attain ML3 or 4, given the benefits of a mature NRA, or whether their organisation would rather consider a cost-based fee structure within African NRAs. In addition, in collaboration with the Global Medicines Development Professionals Academy (GMDP Academy), certified by King’s College, London, their alumni members and global fellows in medicines development were recruited to complete the REIQ.
Figure 1 provides a flow diagram of the research conducted.
Fig. 1.
Flow diagram of the study. BE bioequivalence, CAPEX capital expenditure, CMC chemistry, manufacturing and controls, ML3 maturity level 3, NRA national regulatory authority, OPEX operational costs, SAHPRA South African Health Products Regulatory Authority
Results
SAHPRA Operating and Capital Expenditure
For the 2022/2023 financial year, SAHPRA’s annual report statistics reflected 58% revenue from application fees and 42% from a grant through the South African National Treasury, excluding other revenue [6]. This split, however, fluctuates depending on the availability of funds from the government and revenue generation from fees. In its Annual Performance Plan for 2023/2024, SAHPRA indicated that the weak economic growth in South Africa will have an impact on the public health sector in terms of fewer resources than required; in its SWOT (strengths, weaknesses, opportunities, threats) analysis this is also listed as a threat [5]. It is estimated that the NDoH grant contribution to the total funding will be reduced from 45 to 34% between 2022 and 2027 due to the fiscal contribution decreasing and the projection of increased own revenue generation. This funding model requires a greater dependence on fees paid by the pharmaceutical industry for services rendered by SAHPRA [5].
In a measure to remain financially self-sustaining, SAHPRA aims to recover 100% of all its direct evaluation costs through application fees. It currently pools product retention fees received from applicants and the operational grant received from the NDoH to support any indirect activities. SAHPRA assessor expenditure for the 2023/2024 financial year amounted to R32.5 million (~US$1.8 million) per annum and this only included remuneration of externally contracted evaluators and expert committee members and is expected to increase due to the lack of funding for full-time evaluators. For its 2023/2024 financial year, the main SAHPRA expenditure was projected to be staff remuneration (68% of its total budget of approximately US$21,340,304), followed by its operating costs at 20%, and the contracted National Control Laboratory costs, office rental and capital expenditure at 6, 5 and ~ 1%, respectively [5].
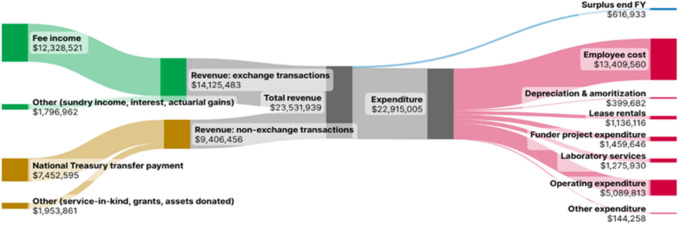
The Sankey diagram in Fig. 2 details SAHPRA’s actuals in terms of revenue and expenditure for the 2023/2024 financial year [16]. The revenue from application fees for the year had a year-on-year increase of 10.7% and exceeded the budgeted expectations by 7.2%. The year-on-year revenue growth was mainly due to a higher number of applications submitted by the industry than anticipated, while grants and service-in-kind realised during the financial year were unbudgeted for, resulting in an increase in other revenue and expenditure. The majority of the external funding received related to employment of internal and external evaluators and digitisation of manual processes. It represented 8.3% of the NRA’s total revenue for the 2023/2024 financial year.
Fig. 2.
South African Health Products Regulatory Authority (SAHPRA)’s actual revenue versus expenditure for financial year (FY) 2023–2024 (United States dollars) (exchange rate of 18.5) [16]
The accounting surplus for the financial year, indicated in Fig. 2, amounts to ~ 3% of the South African medicines agency’s total income, and it is from this surplus that SAHPRA draws to fund some of the activities required for a WHO ML3 NRA, as described in Sect. 3.2.
SAHPRA Costs for WHO ML3 Activities
In its efforts to attain and further sustain the status of WHO ML3 as a regulator able to provide oversight of in-country vaccine production, as well as its ongoing endeavour to attain ML3 for medicines regulation, SAHPRA has incurred additional costs. Table 1 provides examples of SAHPRA budgeted costs associated with activities to be conducted as required by the WHO GBT as an ML3 regulator [SAHPRA, 2023/2024 Annual Budget, Internal document, 2024]. It is important to note that under the GBT sub-indicator RS07.01, an NRA and its affiliated institutions should have adequate funding available to perform all regulatory functions within its mandate, while RS07.04 requires that an ML4 agency has the authority to manage the governmental grants and/or the revenue it generates through fees [9].
Table 1.
South African Health Products Regulatory Authority (SAHPRA) expenditure (US dollars) in relation to activities performed in line with its maturity level 3 status
| GBT function [9] | GBT sub-indicator [9] | Activity [9] | SAHPRA budgeted cost in 2023/2024 (SAHPRA, Annual budget, 2023/2024, internal document) |
|---|---|---|---|
| 01: National regulatory systems (RS) | RS01.07 |
NRA should develop regulations to be implemented and enforced Proper enforcement of laws, regulations and guidelines by an NRA is required |
Review of Act 101 of 1965 [2]: ~ $600,000 Legal and administrative costs pertaining to court cases: ~ $216,000 |
| RS02.03 | NRA should constitute scientific and advisory committees to advise it on topics of scientific and regulatory interest and on future strategies | Provision of recommendations and expert review of evaluations: ~ $430,000 | |
| RS05 |
A quality and risk management system to be implemented and maintained, with the NRA demonstrating the financial, human, infrastructure and equipment allocated for the QMS Internal and external audits of the QMS to be conducted at regular intervals |
QMS personnel costs: ~ $335,000 Auditing fees: ~ $13,000 |
|
| RS06 | Adequate HR capacity to perform regulatory functions within the NRA with ongoing training and development of regulatory personnel | Staff training: ~ $76,000 | |
| RS08.03 | The equipment provided for performing the regulatory functions is adequate, that is digitalisation and information and communication infrastructure to allow for optimum NRA functioning |
Licenses: ~ $700,000 Communication: ~ $140,500 Hardware: ~ $65,000 Regulatory information management system development (once-off): ~ $2,270,000 |
|
| RS09.01 | NRA is required to collaborate in regional and/or global networks to promote regulatory convergence and harmonisation efforts | International collaboration and networking costs (travel, participation in workshops and meetings, coordination costs): ~ $211,000 | |
| 03: Vigilance (VL) | VL04.01 | NRA has implemented vigilance procedures and tools, to be used for collection and assessment of adverse drug reactions and adverse events | Vigiflow annual license fee: $5000 |
| 04: Market surveillance and control (MC) | MC01.01 | Permanent border medicines control technicians stationed at ports of entry and exits to monitor the movement of medicines |
Staff cost: ~ $100,000 Port office rentals: ~ $31,000 |
| MC01.02 | Post-market surveillance: Laboratory testing of quality of risk-based sampled products from the market | Contracted small molecule laboratory to test sampled products: ~ $135,000 (SFMP destruction included in above budget) | |
| MC04.08 | Storage and destruction of detected substandard and falsified medical products | ||
| 06: Regulatory inspection (RI) | RI03 | Human resources to perform regulatory inspection activities; these resources are required to be experienced inspectors with all the necessary tools of the trade (independent modes of transport, laptops and internet connectivity) |
Inspector salaries: ~ $590,000 Travel and accommodation:a Domestic inspections: ~ $122,000 International inspections: ~ $100,000 |
| 07: Laboratory testing (LT) | LT01.01 | An NRA requires access to an NCL, whether its own or an external, sub-contracted laboratory, to supply the required testing services (costs inclusive of method transfers, validation and reference standards) | Contracted NCL for vaccine testing: ~ $1,300,000 |
GBT global benchmarking tool, HR human resources, NCL national control laboratory, NRA national regulatory authority, QMS quality management system, SFMP substandard and falsified medical products
aPartially recoverable through good manufacturing practice (GMP) inspection fees
SAHPRA Backlog Clearance Project Assessment Time-and-Cost Metrics
New Chemical Entity (NCE) Chemistry, Manufacturing and Controls (CMC) Data Assessment
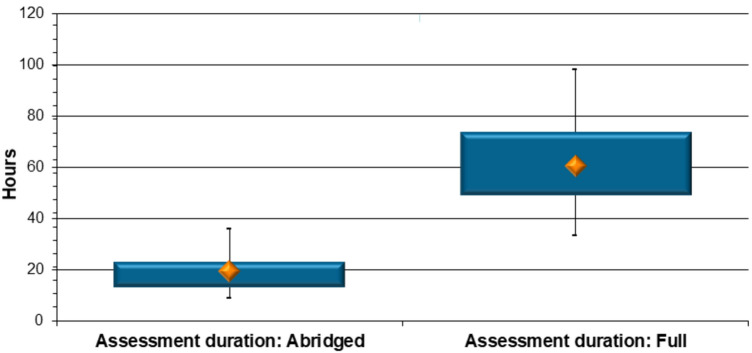
Assessment duration The time it took SAHPRA assessors to evaluate the CMC aspects of an NCE product was determined through the review of all the relevant claim information for each of the 51 NCE applications, with 33 applications going through an abridged review and 18 applications being fully assessed. The results revealed that it took assessors a median of 19.4 h to complete the abridged evaluation of the CMC aspects of an NCE product application, a third of the time, compared to a median of 60.5 h for assessment and approval of CMC data undergoing a full review (p < 0.0001) (Fig. 3).
Fig. 3.
New chemical entity product comparison: duration of abridged versus full review of chemistry, manufacturing and controls data (hours)
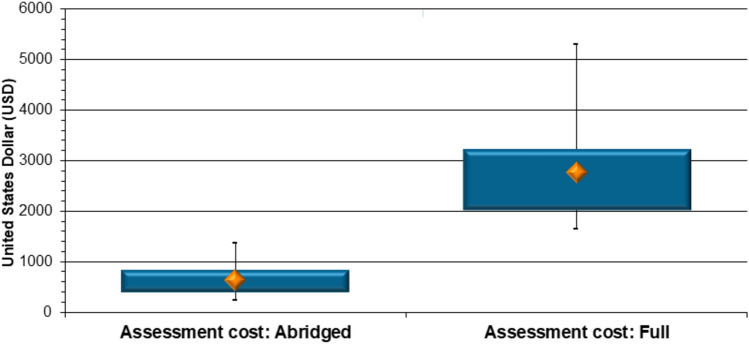
Assessment cost The decrease in review time (Fig. 3) brought with it a commensurate reduction in remuneration claimed by the assessors. A median of US$636.00 was claimed by evaluators for assessing the CMC aspects of an NCE product application through the abridged pathway, while a median cost of US$2773.00 was attached to a full review of these data (Fig. 4). Reliance on prior work conducted by RRAs resulted in a 77% decrease (p < 0.0001) in the payments SAHPRA had to make to the NCE quality assessors.
Fig. 4.
New chemical entity product comparison (2019–2022): assessors’ costs for abridged versus full review of chemistry, manufacturing and controls data (United States dollars)
Generic CMC and BE Data Assessment
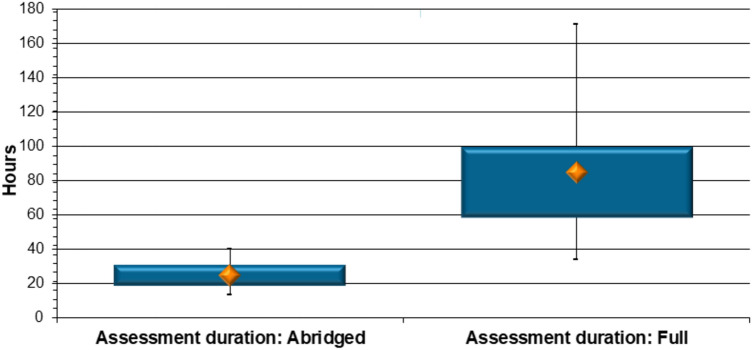
Assessment duration The assessment duration was also investigated for the 137 generic applications in the selected cohort. Seventy of these were assessed via an abridged review pathway as unredacted RRA assessment reports were submitted by the applicants. The investigation revealed a median of 24.6 h were required for an abridged review of the CMC and/or BE data of a generic product. This contrasted with the results for the 67 generic product applications that were fully reviewed. A significantly higher median of 84.7 h was collectively spent on the review of the same type of data when these underwent full assessment (p < 0.0001) (Fig. 5).
Fig. 5.
Generic product comparison: duration of abridged versus full review of chemistry, manufacturing and controls and/or bioequivalence data (hours)
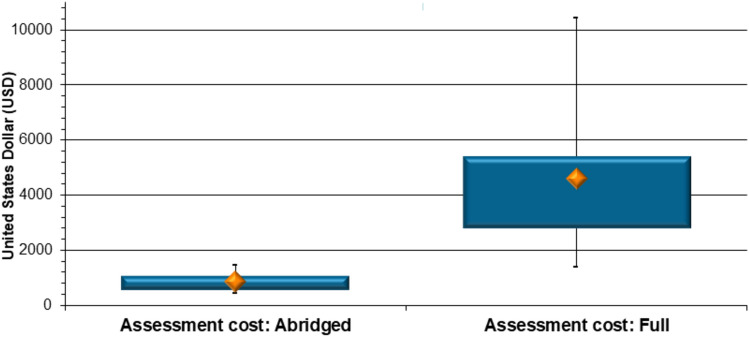
Assessment costs The respective assessors’ costs in USD for the same 137 generic applications, assessed via the two types of review, were furthermore collected and collated for each individual application. The costs to SAHPRA for applications assessed via the abridged and full review correlated with the duration of assessment, even though many different assessor cohorts, with different remuneration schedules, were utilised. It was found that SAHPRA paid a median of US$855.00 for an abridged review of CMC and/or BE data contained in a generic application, and a significantly higher value of US$4602.00 (median) for a full review of these data (p < 0.0001) (Fig. 6). The abridged review pathway enabled an 81% decrease in costs to SAHPRA, when the generic CMC and/or BE data were reviewed via a reliance mechanism.
Fig. 6.
Generic product comparison (2019–2022): assessors’ costs for abridged versus full review of chemistry, manufacturing and controls and/or bioequivalence data (United States dollars)
Overall Cost Reduction
When comparing the total cost for the abridged assessments of both the NCE and generic product applications (US$80,857.00) with the total cost incurred for full reviews of the selected NCE and generic applications (US$358,270.00), there was an overall cost-saving of US$277,413.00 (77% decrease in cost) when the data were reviewed via the reliance pathway.
Review of Funding Models and Fee Structures of African NRAs
A number of NRAs on the African continent, such as SAHPRA and the Rwanda Food and Drugs Authority (RFDA), have recently been established as autonomous entities, and with this comes the need for functional operating systems and a fully staffed organisational structure. These requirements contribute to the operational costs of running a medicines regulatory authority, especially when aspiring to WHO ML3, as detailed in Table 1 [9, SAHPRA, Annual budget 2023/2024, Internal document, 2023]. Although the majority of NRAs function on grants from their respective governments, many have to remit any surplus to the government at the end of each financial year, which inhibits expansion possibilities. Table 2 provides an overview of the funding models in selected African NRAs, together with information on differentiated fee structures within these [6, 17–28].
Table 2.
Funding model and fee structure overview for selected low- and middle income African national regulatory authorities (NRAs)
| National regulatory authority | Year established | Current funding model [17, 18] | Differentiated fee structure | ||
|---|---|---|---|---|---|
| Government grant | Application fees | For new active substances vs. generics | For reliance vs. full review | ||
| SAHPRA | 2018 | Yes (~ 43%) [6] | Yes (~ 57%) [6] | Yes [19] | Nob [19] |
| Rwanda FDA | 2018 | Yes (22%) | Yes (76%) | No [20] | No [20] |
| NAFDAC | 1993 | Yesa | Yesa | No [21] | No [21] |
| Ethiopia FDA | 2010 | Yesa | Yesa | Yes [22] | No [22] |
| Uganda NDA | 1993 | No | Yes (98.25%) | No [23] | Nob [23] |
| Namibia NMRC | 2003 | Yes (100%) | No | No [24] | No [24] |
| Kenya PPB | 1959 | No | Yes (100%) | No [25] | No [25] |
| Tanzania TMDA | 2003 | Yes (11.7%) + 11.4% balance from previous budget | Yes (76.3%) | Yes (biologicals) [26] | Nob [26] |
| Zambia ZAMRA | 2013 | No (5% from other sources) | Yes (95%) | Yes [27] | Yes [27] |
|
Zimbabwe MCAZ |
1998 | Yes (100%) | No | Yes [28] | Nob [28] |
aNo percentages in terms of funding were available for these NRAs
bAgencies with fast-tracked or expedited review pathways for which they charge higher application fees
Any funding gap not covered by the NRA’s current funding models is derived from other sources, such as development partners and funders.
Digital Pharmaceutical Industry Opinion Survey
A total of 33 IFPMA and GMPD Academy members completed the REIQ with two follow-up communications. It was not possible to calculate the response rate as the authors were not able to obtain an accurate account of the membership of the two organisations. The collated results are detailed in Table 3.
Table 3.
Reliance Economic Impact Questionnaire responses on the economic impact of reliance implementation and financial sustainability of national regulatory authorities
| Survey questions | Yes | No | Maybe | No response |
|---|---|---|---|---|
| In instances where reliance review pathways work effectively, would your organisation consider paying higher application fees to ensure quicker market access for your products? | 25 (76%) | 5 (15%) | 3 (9%) | 0 (0%) |
| Should an NRA not adhere to its service charter timelines for reliance pathways, would your organisation recommend a credit on future applications? | 22 (67%) | 6 (18%) | 1 (3%) | 4 (12%) |
| Would your organisation consider paying an annual developmental fee to enable NRAs to attain ML3/4, given the benefits of a mature NRA within the country/region both for patients and the pharmaceutical industry? | 20 (61%) | 9 (27%) | 2 (6%) | 2 (6%) |
| Would your organisation be supportive of a cost-based fee model implemented within NRAs? | 25 (76%) | 3 (9%) | 3 (9%) | 2 (6%) |
NRA national regulatory authority
When questioned whether their company would consider paying higher application fees to ensure quicker market access for their products through effective reliance review pathways, 76% of respondents indicated in the affirmative and 15% in the negative. Another 67% felt their organisation would be in favour of a credit from NRAs for future applications if authorities do not adhere to their published service charter timelines, while 18% responded that this was not an option they would support. The question as to whether companies would be open to paying an annual development fee to enable NRAs to advance their WHO maturity was raised and 61% of respondents were in favour of this, contrasting to the 27% who were not. Lastly, 76% of survey participants were supportive of a cost-based fee model implemented within NRAs, with 9% not in agreement.
Discussion
For many LMICs, various factors have recently affected the economic climate, most importantly the COVID-19 pandemic and the resultant lock-down periods. This has led to the sharpest economic contraction since the Great Depression in the 1930s and has exacerbated the dire economic situation in developing countries already burdened by fiscal deficits and excessive public debt [29]. Four years on from the start of the pandemic, many governments across Africa are implementing austerity measures to curb spending [30, 31]. That said, the majority of the NRAs in developing countries are dependent on government grants to operate, the notable exceptions being the Uganda National Drug Authority (NDA), the Kenya Pharmacy and Poisons Board (PPB) and the Zambia Medicines Regulatory Authority (ZAMRA), with these three agencies relying heavily on fees collected from the pharmaceutical industry [17, 18, 32]. As Ndomondo-Sigonda and colleagues further highlight, the fact that the Uganda NDA relies almost solely on application fees is a direct result of its government’s deliberate transition away from its former 100% reliance on donor funding. In contrast, the medicines regulator in Burundi depends exclusively on fiscal contributions to enable its operations [32]. To remain financially afloat, NRAs are increasingly reaching out to donor organisations to support their operations, but this funding varies significantly from country to country [32].
The weak economic growth in South Africa has impacted the flow of fiscal support to SAHPRA, with the government grant anticipated to decline by an estimated 11% over the next four years. Consequently, the authority has become more dependent on fees paid by the pharmaceutical sector. Until the end of 2024, SAHPRA only charged fees for medicines registration and lifecycle management applications (including retention fees), clinical trial applications, licence fees (for manufacturers, distributors, importers, exporters), good practice (GxP) inspections, vaccine lot release and ancillary fees for a few administrative services [19]. However, some of these tariffs are not aligned with SAHPRA expenditure for the individual activity, with the contracted NCL for vaccine testing making up 6% of SAHPRA’s annual budget, but with only a 21% cost-recovery in the 2023/2024 financial year. Given that many of the activities falling within the SAHPRA mandate do not generate fees, the current fee income stream cannot resolve its budgeting shortfalls.
The main SAHPRA expenditure relates to assessor and other authority personnel costs, followed by its operational expenditure. It is noteworthy that the majority of assessors contracted to the BCP to clear the SAHPRA application backlog were supported by external funders, as the in-agency capacity was not sufficient. The cost for these external assessors was approximately US$2,671,000 over the duration of the project, this excluding the BCP staff and SAHPRA overtime assessors’ costs. During the period 2019–2022, in parallel to these clearance efforts, SAHPRA was investing heavily in optimising its operational capacity and systems with a view of reaching WHO ML3. Many of the South African regulator’s current undertakings to maintain this status, such as defending its regulations, as well as the much-needed revision of its Medicines and Related Substances Act, maintenance of the QMS with regular audit cadences, capacitating its regulatory personnel, setting up a national system to curb substandard and falsified medical products (SFMPs), inclusive of contract laboratory testing, as well as vigilance endeavours, are not at present being compensated for by the private sector, although the latter is reaping the benefits these initiatives bring about in terms of a more securely regulated environment in which to operate and serve patients. In view of this, SAHPRA has revised its fees to ensure future financial viability and to “enhance the ability of the regulator to respond to the needs of the regulated environment”, as per the MTT recommendation [1].
With SAHPRA budget deficits in mind, research into the financial benefits of reliance implementation by SAHPRA was undertaken, with the outcome revealing a significant impact in terms of assessment duration and associated costs. The results pointed to a marked reduction in assessors’ costs for both NCE and generic new registration applications, when the CMC and/or BE data were assessed by relying on prior assessments and authorisation by medicines regulators SAHPRA trusts, with the authority conserving more than US$270,000 through this approach for the 188 product applications assessed. Investing in reliance practices not only enabled expedited medicine access, but also allowed SAHPRA to utilise its financial resources more effectively.
On further review of the fee schedules of ten selected African NRAs, it is apparent that half of these differentiate between the fees they charge for NAS and generic product assessment, with higher fees for innovator or biological product applications (the South African, Ethiopian, Tanzanian, Zambian and Zimbabwean NRAs). However, upon examining whether any of the authorities distinguished between full and reliance review when setting fees, it became apparent that only ZAMRA charges different tariffs for these types of assessments, in that it charges a reduced fee for an abridged review. Out of the reviewed NRAs, only 40% (SAHPRA, Uganda NDA, TMDA and MCAZ) have a fast-track review pathway through which applicants can theoretically, at a higher cost, receive an expedited assessment of their products. Across various industries, fast-track services are generally more expensive to ensure speedier outcomes. Two examples of WHO-Listed Authorities (WLAs) who have subscribed to this approach are the Singapore HSA in charging higher fees for a verified versus an abridged review of health product applications [33] and Swissmedic, where a procedure with prior-announcement (3–6 pre-submission notification) secures an applicant, under certain conditions, a 20% faster process but with doubled fees, as the alert allows Swissmedic to plan appropriately for the assessment of the incoming NAS application and ensure the “efficient processing of applications” [34].
Upon evaluating the industry perspectives on reliance practices and how successful implementation of these may benefit not only NRAs but also the private sector, the majority of respondents were in favour of financially incentivising NRAs to adopt reliance practices, through which NRAs could be supported on their journey to WHO ML3. They also acceded to a cost-based fee model for NRAs and/or an annual fee contributing to the expansion and maturity of individual African NRAs. Some respondents indicated that, in return, there should be a stricter observance of published timelines by NRAs. Increased trust amongst African medicines regulators and applicants, whether local or international, was also cited as a key ingredient to successful reliance implementation and the industry’s willingness to aid with the proliferation of mature NRAs on the African continent.
An ML3 NRA brings great benefit to a country, as well as to a region, in terms of safer, more efficacious medicines of good quality available to its people, but the financial and resource requisites for attaining and sustaining this status are significant. Many African NRAs are heavily dependent on their governments’ contributions to carry out agency operations, and given the current economic climate, this revenue stream has become reduced, with resources thinly spread within the NRAs. The paucity of skilled staff, directly equating to poor NRA performance outcomes, and the industry’s expectations are two sides of the same coin. However, the study results have shown reliance to be a lever for not only expediting medicine authorisation, but to, in addition, conserve the financial resources of the implementing authority. It illustrated the ROI of an NRA investing in reliance practices and, if optimally implemented, the benefits for the pharmaceutical industry and its patients.
If less financial (and human) resources are required for marketing authorisation by employing reliance mechanisms, more funds would be available to NRAs for regulatory and information system-strengthening initiatives. The redirected funds would enable advancement of country-specific activities required by the WHO, such as border control of medicines, vigilance, post-marketing surveillance and legislative overhauls. Any cost-saving would be re-invested into the regulator to not only sustain or improve the maturity level of that NRA, but also to aid improved service delivery through an increased number of expert evaluators, especially for specialised products, such as cutting-edge biological therapies, digital health technologies (DHTs) and artificial intelligence (AI)-operated medical devices, optimised and integrated data management systems, with record linkages, and accurate tracking of all the NRAs key performance indicators (KPIs).
This proposal aligns well with current global thinking on the topic. In its position paper on EMA fees, the European Federation of Pharmaceutical Industries and Associations (EFPIA) suggests tenets for a proportionate fee structure for EMA, as well as for national competent authorities (NCAs) [35], and by extension, one could argue, applicable to African NRAs. Among these principles are transparency, fairness and proportionality, and sustainability. EFPIA argues for an expanded maintenance fee for all marketing authorisations, which would cover minor variations, renewals and PV activities, and which would taper off as a product nears the end of its lifecycle [35]. Through this income, it is anticipated that a regulatory authority may be adequately funded, considering the primary costs associated with the services an NRA provides, fully cognisant of the SWOT analysis of an agency, and with a future-forward perspective. It is anticipated that a product-specific MA maintenance fee (and this does not refer to the low-cost annual retention fee currently paid by the pharmaceutical private sector) would provide an NRA with suitably skilled experts in a variety of regulatory disciplines, which would enable dynamic regulatory assessments, with continued dialogue between stakeholders throughout the assessment process [35]. However, EFPIA concludes that fees should be fair and proportionate to all stakeholders. Aligned with this approach, the IFPMA published its own position paper [36] on regional joint assessment procedures in Africa, propounding that “fees should be determined on actual costings and linked to agreed regulatory performance indicators”, with periodic agency performance reviews to justify any fee amendments. In this regard it would be important to holistically consider the overall costs of the NRA authorising the product, as disregard of this could risk the agency being financially disadvantaged in terms of reliance reviews.
As a progressive example of fee modelling, the United States Prescription Drug User Fee Act (PDUFA), which was first enacted in 1992 and now in its sixth reauthorisation, guarantees that the US FDA, in addition to the annual funding supplement from Congress, “will continue to receive a source of stable and consistent funding during fiscal years 2023–2027 that will allow the agency to fulfil its mission to protect and promote public health by helping to bring to market critical new medicines for patients” [37]. The 2024 PDUFA fee for a human medicine application requiring clinical data review was US$4,048,695 [38] and, should the product qualify for a priority review, an additional fee of US$1,314,206 was levied [39]. The PDUFA fees are not only used for medicine application evaluations, but also for facility inspections, maintenance of US FDA information databases, and other programmatic support such as research and policy development [40]. Furthermore, the US FDA does not only collect fees from industry, but also from certain accreditation and certification entities. Importantly, the tariffs are not a “fee-for-service payment” as the payments contribute to all the above activities and assist in funding the US FDA payroll and achieving its programmatic goals [40]. The US FDA fee model is an example of a holistic approach to safeguarding agency sustainability and development, but with assured deliverables, as expected by the private sector.
Limitations
As a result of the manual processing of the assessors’ claims at the beginning of the project, with the COVID-19 pandemic then disrupting their processing, some of the claim information was not available. In addition, no assessment of NCE clinical evaluation was performed, as the previous study [7] concluded that clinical reliance had been less successfully implemented during the SAHPRA BCP, due to several reasons; these included a heavy reliance on external advisory committee members, with the experts exhibiting a reduced risk appetite, leading to a reticence to adopting reliance review practices. Hence, analysing these data, which showed less reliance impact on timelines, would not have contributed to the outcomes of this research. Another limitation of this study could potentially be the fact the analysis is based on data from one ML3 regulatory authority in Africa or not having included any of the ML2 authorities to carry out comparison of resource consumption use across two different maturity levels. However, since WHO GBT requirements for obtaining and maintaining ML3 is the same across the board, it is highly unlikely that having included another ML3 authority would have made any difference in the outcome of the study.
Conclusions
In view of the above fee model illustrations and taking cognisance of the African market of 55 countries, NRAs on the African continent should consider different options to preserve financial stability. The crux of the matter is that without adequate revenue, either to maintain operations or to achieve a higher level of maturity, NRAs will not be able to deliver on their mandates or the expectations of the private sector. As such, medicines regulators should consider differentiated fees for different review pathways, but also other expedients such as development funds or cost-based fee models. Ultimately, it is a case where capacitation of African NRAs is required to guarantee improved performance outcomes to ensure access of medicines to the Africans whom both regulators and industry serve.
Recommendations
To ensure financial sustainability, NRAs should:
Consider a differentiated fee structure for different review pathways, with possibly higher fees for quicker market authorisation through reliance review.
Encourage governments to perform a cost analysis of the NRA needs, thereby ensuring the relevant funding model is applied.
Develop and present a strategic plan to governments to ensure surplus funds are retained and re-invested into strengthening the regulatory system.
Institute a development fund for additional income, thereby improving sustainability independent of outside funders’ contributions.
Introduce an annual fee for pharmaceutical companies to cover the wide range of activities not explicitly funded through application fees, with many of these required for maintaining ML3 status.
Acknowledgements
The authors would like to acknowledge the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) for its collaboration on this research and its valuable input from a private sector perspective. The authors further wish to thank Sharon Naidoo and Naazneen Babamia from the SAHPRA Finance Department for providing the raw data required for this research, as well as their willingness to respond to questions from the authors. The authors appreciate the collaboration of the Global Medicines Development Professionals Academy (GMDP Academy) for their invaluable contribution to the success of this study.
Declarations
Funding
No external funding was utilized for this research.
Conflict of interest
Lorraine Danks, Boitumelo Semete-Makokotlela, Regardt Gouws and Kennedy Otwombe have no competing interests to declare that are relevant to the content of this article. Sam Salek and Stuart Walker are Editorial Board members of Pharmaceutical Medicine. Professors Salek and Walker were not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethics approval
The study was approved by Health, Science, Engineering and Technology ECDA, University of Hertfordshire, United Kingdom [Reference Protocol number: LMS/PGR/UH/05160].
Consent to participate
All participants were contacted and provided written consent to participate in the study.
Consent for publication
Not applicable.
Availability of data and material
The data are available from the corresponding author upon reasonable request.
Author contributions
Lorraine Danks: Conceptualization, data curation, formal analysis, writing – original draft, writing – review and editing. Boitumelo Semete-Makokotlela: Conceptualization, writing – review and editing. Regardt Gouws: Conceptualization, writing – review and editing. Kennedy Otwombe: Formal analysis, writing – review and editing. Stuart Walker: Conceptualization, writing – original draft, writing – review and editing. Sam Salek: Conceptualization, writing – original draft, Writing – review and editing. All the authors have read and approved the final submitted manuscript, and have agreed to be accountable for the work.
References
- 1.South African Government. Report of the Ministerial Task Team on the restructuring of the Medicines Regulatory Affairs and Medicines Control Council and recommendation for the new regulatory authority for health products for South Africa. 2008. https://www.gov.za/documents/other/report-ministerial-task-team-restructuring-medicines-regulatory-affairs-and. Accessed 06 Jan 2024.
- 2.South African Health Products Regulatory Authority (SAHPRA). The South African Medicines and Related Substances Act No. 101 of 1965 (as amended by Act 72 of 2008 and Act 14 of 2015). 2015. https://www.sahpra.org.za/wp-content/uploads/2019/09/Medicines-and-Related-Substances-Act_101-of-1965_Act_GG-40869_2017-05-26.pdf. Accessed 24 Jan 2024.
- 3.South African National Treasury. The South African Public Finance Management Act No 1 of 1999 (as amended by Act 12 of 2013). 2013. https://www.treasury.gov.za/legislation/pfma/act.pdf. Accessed 24 Jan 2024.
- 4.South African Health Products Regulatory Authority (SAHPRA) 2020/21—2024/25 strategic plan. January 2022. 2022. https://www.sahpra.org.za/sahpra-planning-documents/. Accessed 24 Jan 2024.
- 5.South African Health Products Regulatory Authority (SAHPRA). 2023/24 annual performance plan. 2023. https://www.sahpra.org.za/document/sahpra-annual-performance-plan-2023-2024/. Accessed 24 Jan 2024.
- 6.South African Health Products Regulatory Authority (SAHPRA). 2022/23 annual report. 2023. https://www.sahpra.org.za/document/annual-report-2022-2023/. Accessed 24 Jan 2024.
- 7.Danks L, Semete-Makokotlela B, Otwombe K, Parag Y, Walker S, Salek S. Evaluation of the impact of reliance on the regulatory performance in the South African Health Products Regulatory Authority: implications for African regulatory authorities. Front Med. 2023. 10.3389/fmed.2023.1265058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broojerdi AK, Sillo HB, Dehaghi ROA, Ward M, Refaat M, Parry J. The World Health Organization Global Benchmarking Tool an instrument to strengthen medical products regulation and promote universal health coverage. Front Med. 2020. 10.3389/fmed.2020.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. The World Health Organization (WHO) global benchmarking tool (GBT) for evaluation of national regulatory systems of medicines and vaccine. 2021. https://www.who.int/tools/global-benchmarking-tools/VI. Accessed 29 Dec 2023.
- 10.World Health Organization. Coalition of Interested Parties (CIP) Network. Global Steering Group: strategic plan (January 2023–December 2027). 2023. https://cdn.who.int/media/docs/default-source/medicines/regulatory-systems/cip/cip_gsg_strategic-plan_oct2023.pdf. Accessed 29 Dec 2023.
- 11.World Health Organization. WHO Technical Report Series 1033: 55th report of the WHO Expert Committee on Specifications for Pharmaceutical Preparations (ECSPP). Annex 10 Good reliance practices in the regulation of medical products: high level principles and considerations. 2021. https://www.who.int/publications/i/item/55th-report-of-the-who-expert-committee-on-specifications-for-pharmaceutical-preparations. Accessed 30 Dec 2023.
- 12.Liberti L, Garza MA, Van Der Zee IT, Vreman RA, Bujar M. Relianomics: A proposed framework for the assessment of the societal, economic and efficiency impacts of regulatory pathways. FRPanorama Adv Regul Sci. 2023. https://img1.wsimg.com/blobby/go/24af6e56-5955-4473-832e-abea104f734e/downloads/FARS%20Issue%2020230410%20final.pdf?ver=1715955692298.
- 13.South African Health Products Regulatory Authority (SAHPRA) SAHPGL-BAU-01_v4 Reliance Guideline (version 4). 2024. https://www.sahpra.org.za/document/reliance-guideline/. Accessed 24 July 2024.
- 14.Singh A. The South African Payroll Society. Labour laws affecting the payroll. 2011. https://www.sapayroll.co.za/Portals/2/Documents/events/2011/SAPA%20conference%202011/Labour%20Laws%20Affecting%20the%20Payroll%20-%20Ashmini%20Singh.pdf?ver=2013-03-27-105447-000. Accessed 21 Mar 2024.
- 15.Exchange Rates.org.uk. US Dollar (USD) to South African Rand (ZAR) exchange rate history. 2024. https://www.exchangerates.org.uk/ZAR-USD-exchange-rate-history.html. Accessed 21 Mar 2024.
- 16.South African Health Products Regulatory Authority (SAHPRA). 2023/24 annual report. 2024. https://www.sahpra.org.za/document/annual-report-2023-2024/. Accessed 02 Jan 2025.
- 17.Ngum N, Ndomondo-Sigonda M, Habonimana R, Mbwiiri P, Irasabwa C, Ojukwa J, Apolinary F, Okello A, Ahmada S, Walker S, Salek S. Evaluation of good review practices in member agencies of the East African Medicines Regulatory Harmonisation Initiative: strategies for alignment with African Medicines Agency. Front Med. 2024;11:1437970. 10.3389/fmed.2024.1437970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sithole T, Mahlangu G, Capote V, Sitoie T, Shifotoka S, Gaeseb J, Padayachee S, Sehloho T, Khea A, Fimbo A, Munkombwe Z, Mwale B, Salek S, Walker S. Evaluation of the good review practices of countries participating in the Southern African Development Community: alignment and strategies for moving forward. Front Med (Lausanne). 2021;27(8): 742181. 10.3389/fmed.2021.742181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.South African Health Products Regulatory Authority (SAHPRA). The Republic of South Africa. Regulation Gazette. 2020;666:11214. https://www.sahpra.org.za/document/regulations-regarding-fees-payable-in-terms-of-the-provisions-of-the-medicines-and-related-substances-act-1965-act-no-101-of-1965/. Accessed 24 Mar 2024.
- 20.Rwanda Food and Drugs Authority (RFDA). Regulations related to regulatory service: tariff/fees and fines. Rwanda FDA Law No. 003/2018 of 09/02/2019, Article 9. 2019. https://rwandafda.gov.rw/wp-content/uploads/2022/11/Regulation_Related_to_Regulatory_service_tariff_fees_and_fines-1.pdf. Accessed 24 Mar 2024.
- 21.Nigeria National Agency for Food and Drug Administration and Control (NAFDAC) tariff. 2020. https://nafdac.gov.ng/regulatory-resources/nafdac-tariff/. Accessed 24 Mar 2024.
- 22.Ethiopian Food and Drug Authority (EFDA). Federal Negazit Gazette of the Federal Democratic Republic of Ethiopia. Regulation No. 370/2015. 2016. http://www.efda.gov.et/publication/rate-of-service-fees-regulation-no-370-2015/. Accessed 24 Mar 2024.
- 23.Uganda National Drug Authority (NDA). The National Drug Policy and Authority (Fees) Regulations. The Uganda Gazette. 2022;Volume CXV, no. 5, Statutory Instruments Supplement No. 3. https://www.nda.or.ug/wp-content/uploads/2022/03/NDA-FEES-REGULATIONS-2022-1.pdf. Accessed 24 Mar 2024.
- 24.Namibia Medicines Regulatory Council (NMRC) Government Notice No 316. Amendment to schedules: Medicines and Related Substances Control Act, 2003. 31 Government Gazette of the Republic of Namibia. 2015. https://nmrc.gov.na/documents/81630/81765/NMRC+Fees.pdf/309a8c02-7dbd-3fb1-f916-246bfc25c107. Accessed 24 Mar 2024.
- 25.Republic of Kenya Pharmacy and Poisons Board. (PPB) Schedule of fees for regulatory activities. 2023. https://web.pharmacyboardkenya.org/download/schedule-of-fees-for-regulatory-activities/. Accessed 24 Mar 2024.
- 26.Tanzania Medicines & Medical Devices Authority (TMDA). The Tanzania Medicines and Medical Devices Act (CAP 219). Fees and Charges, Regulations. 2021. https://www.tmda.go.tz/uploads/publications/en1621327639-FEES%20AND%20CHARGES%20REGULATIONS%202021%20-%20ENGLIS%20VERSION%2025%20APRIL.pdf. Accessed 24 Mar 2024.
- 27.Zambia Medicines Regulatory Authority (ZAMRA). Fees for marketing authorisation. 2023. https://www.zamra.co.zm/wp-content/uploads/2023/05/fees. Accessed 24 Mar 2024.
- 28.Medicines Control Authority of Zimbabwe (MCAZ). Fee schedule. 2023. https://www.mcaz.co.zw/documents/approved-fees/fee-schedule. Accessed 24 Mar 2024.
- 29.United Nations. Department of Economic and Social Affairs. COVID-19 to slash global economic output by $8.5 trillion over next two years. 2020. https://www.un.org/en/desa/covid-19-slash-global-economic-output-85-trillion-over-next-two-years. Accessed 15 Apr 2024.
- 30.Godongwana E. Republic of South African National Treasury Medium Term Budget Policy Statement (MTBPS). 2023. https://www.treasury.gov.za/documents/mtbps/2023/speech/speech.pdf. Accessed 15 Apr 2024.
- 31.Tegegn YT Ethiopia’s silent turn to austerity while attempting economic liberalisation. Addis Fortune News. 2023;03. https://addisfortune.news/ethiopias-silent-turn-to-austerity-while-attempting-economic-liberalisation. Accessed 15 Apr 2024.
- 32.Ndomondo-Sigonda M, Miot J, Naidoo S, Ng’andu B, Ngum N, Masota NE, Kaale E. National medicines regulatory authorities financial sustainability in the East African Community. PLoS ONE. 2023;15(7): e0236332. 10.1371/journal.pone.0236332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singapore Health Sciences Authority (HSA) Fees and turnaround time for therapeutic products: New Drug Application (NDA) for product registration. 2024. https://www.hsa.gov.sg/therapeutic-products/fees#. Accessed 13 July 2024.
- 34.Swiss Agency for Therapeutic Products (Swissmedic). Voluntary prior notification of new applications with new active substance for human medicinal products. 2024. https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/authorisations/information/freiwillige-vorankuendigung-na-mit-neuem-ws.html. Accessed 15 Apr 2024.
- 35.European Federation of Pharmaceutical Industries and Associations (EFPIA). EFPIA Position Paper on EMA Fees. 2020. https://www.efpia.eu/media/676986/efpia-position-paper-on-ema-fees_aug-2020.pdf. Accessed 15 Apr 2024.
- 36.International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) and Innovation Pharmaceutical Association of South Africa (IPASA). IFPMA & IPASA Position Paper on fee system for regional joint assessment procedures in Africa. 2023. https://www.ifpma.org/wp-content/uploads/2023/03/20230301_Position-Paper-on-fee-system-for-regional-joint-assessment-procedures-in-Africa.pdf. Accessed 15 Apr 2024.
- 37.United States Food and Drug Administration (USFDA) fee rate for using a priority review voucher in fiscal year 2024. 2023. https://www.federalregister.gov/documents/2023/09/29/2023-21513/fee-rate-for-using-a-priority-review-voucher-in-fiscal-year-2024. Accessed 15 Apr 2024.
- 38.United States Food and Drug Administration (USFDA). Prescription drug user fee amendments. FY 2023 and FY 2024 user fee rates. 2024. https://www.fda.gov/industry/fda-user-fee-programs/prescription-drug-user-fee-amendments. Accessed 15 Apr 2024.
- 39.United States Food and Drug Administration (USFDA). Prescription drug user fee amendments. PDUFAVII: fiscal years 2023–2027. 2023. https://www.fda.gov/industry/prescription-drug-user-fee-amendments/pdufa-vii-fiscal-years-2023-2027. Accessed 15 Apr 2024.
- 40.United States Food and Drug Administration (USFDA). FDA: user fees explained. 2024. https://www.fda.gov/industry/fda-user-fee-programs/fda-user-fees-explained. Accessed 15 Apr 2024.