In March 2014, The Lancet reported the successful results of the efficacy and safety trial of 116E, the first Indian-manufactured rotavirus vaccine to complete phase 3 clinical testing.[1] In a multicenter study conducted in three geographically and culturally diverse cities of India – Delhi, Pune, and Vellore – despite intensive health care provision which may have modified the severity of disease, the 116E vaccine showed efficacy comparable to that of two internationally licensed rotavirus vaccines manufactured by Merck and Co. and GlaxoSmithKline. Several other indigenously manufactured rotavirus vaccines are in development in India, some of which are in late stages of clinical testing. With an effective, indigenously produced rotavirus vaccine on the near-term horizon, India, which singularly accounts for almost one fifth of the world’s burden of rotavirus deaths in children[2], is poised to have a new tool in the arsenal of interventions to reduced morbidity and mortality from childhood diarrhea. To help assess the public health value of the vaccine, understanding the current rotavirus disease burden and epidemiology, circulating strains, and economic burden of rotavirus in India is important. This supplement contains papers summarizing the most up-to-date data on these issues. In addition, the supplement addresses areas relevant for post-introduction monitoring of rotavirus vaccine, including potential safety concerns associated with other rotavirus vaccines such as intussusception, a condition in which one portion of the bowel telescopes into another causing a blockage. Finally, this supplement contains papers looking at the performance of rotavirus vaccines, both the indigenous and internationally available vaccines, in India and explores strategies to improve vaccine performance. This collection of papers will help provide a complete picture of rotavirus disease in India and the potential for a rotavirus vaccination program, and also set the platform to assess the impact of vaccines post-introduction.
Rotavirus disease burden in India
Rotavirus persists as a major cause of severe acute diarrhea in Indian children. By 5 years of age, an estimated 1 out of every 344 Indian children will die from rotavirus diarrhea, 1 in every 23 to 46 children will be hospitalized for rotavirus diarrhea, and 1 in every 6 to 12 children will have an outpatient visit due to rotavirus diarrhea.[3] This translates into 78,500 deaths, 872,000 hospitalizations, over 3.2 million outpatient visits and 11.37 million diarrhea episodes due to rotavirus in children <5 years of age each year in India.[3] Most previous disease burden estimates have provided figures for mortality and hospitalizations alone, and hence the availability of these updated estimates, which include outpatient visits and diarrheal episodes managed at home, will provide a tool to better assess the health and economic burden of disease that might be alleviated by rotavirus vaccination.
Rotavirus causes a significant proportion of the severe health burden due to diarrhea. Sentinel hospital-based surveillance, often conducted as part of the Indian Rotavirus Surveillance Network, found the proportion of diarrheal hospitalizations among children <5 years of age associated with rotavirus ranging from 26% in Vellore, 35% in Pune, 38%−40% in Delhi, 50% Trichy, and 53% in Kolkata.[4–8] (Figure 1). Rotavirus was detected year round in southern India but peaks in rotavirus disease were seen during the cool months in northern India.[4, 7, 9] Rotavirus hospitalization tended to occur in young children; of all rotavirus hospitalizations in children under five, 43%−73% occurred in children <1 year of age and 70%−89% occurred by 2 years of age.[4, 5, 9] (Figure 2) Rotavirus was often found to cause more severe disease than non-rotavirus causes of diarrhea, with children with rotavirus more likely to have higher Vesikari severity scores and more likely to have vomiting associated with their illnesses than children not infected with rotavirus.[5] Younger children (0 to 5 months of age) with rotavirus were also found to have more severe disease than older children (6 to 23 months of age), including an increased risk of complications of severe dehydration, severe acidosis, severe acidemia, and have a hospital stay of 7 days or longer.[6]
Figure 1.

Rotavirus detection among children <5 years of age hospitalized with acute gastroenteritis at facilities throughout India.
Figure 2.
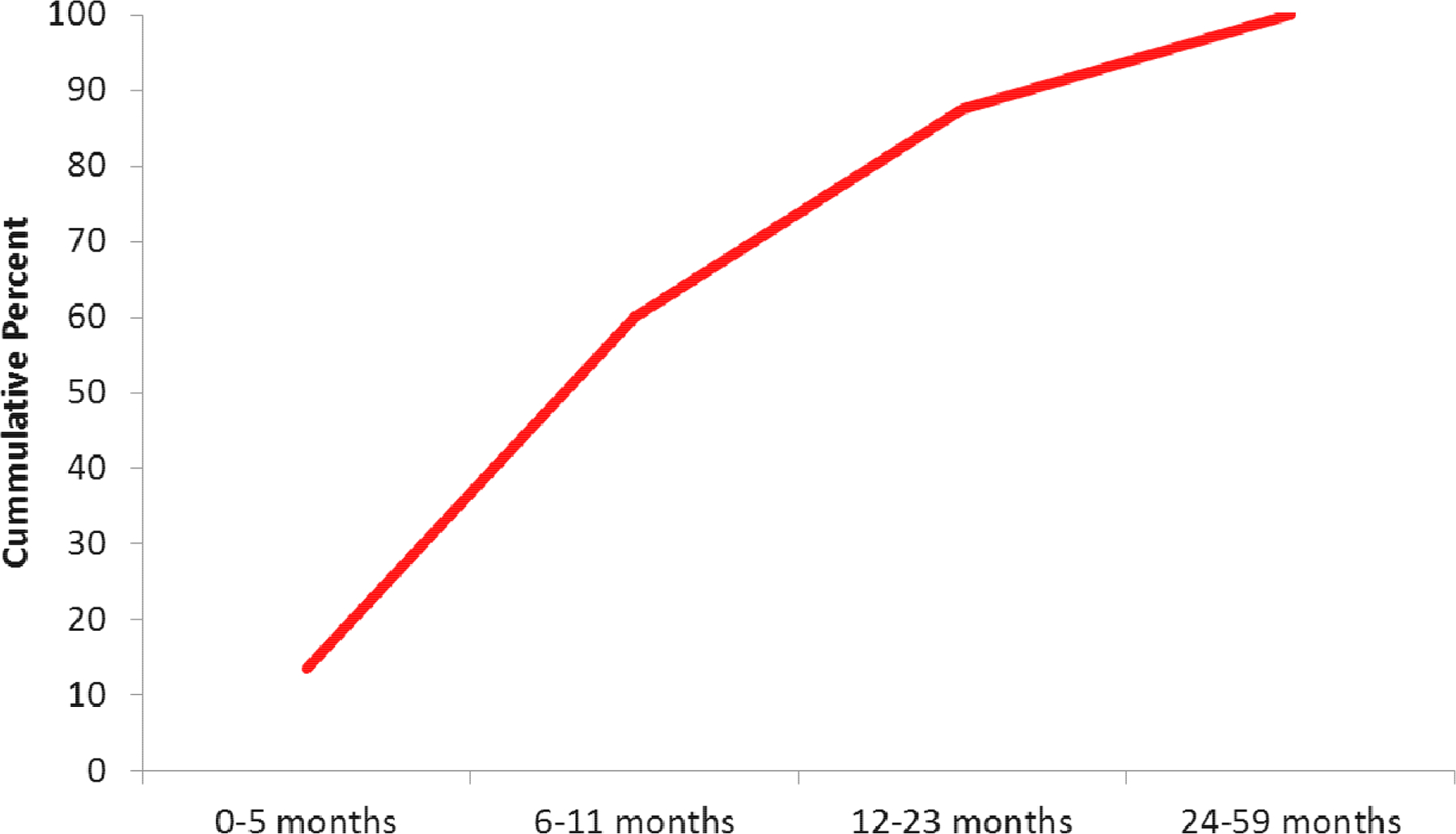
Cumulative age distribution of children hospitalized with rotavirus gastroenteritis in India
Rotavirus was also found to cause significant disease burden in among children<5 years of age treated in the outpatient setting. One multicenter study detected rotavirus in 23% of enrolled outpatients during the 11 month surveillance period.[10] In another study in Kolkata, 48% of outpatients tested positive for rotavirus over a 36 month surveillance period.[8] As with hospitalized children, the majority of children (86%) that tested positive for rotavirus in the outpatient setting were <2 years of age and had more severe disease including high proportions of children with vomiting, fever, and abnormal behavior than children with non-rotavirus diarrhea.[10]
While the brunt of severe rotavirus disease is borne by young children, rotavirus is also a cause of morbidity in older age groups in India. In a 6-month pilot study among children >12 years of age and adults seeking care for diarrhea in Vellore during 2012–2013, rotavirus was detected in approximately 4% of enrolled specimens.[11] Rotavirus was also detected among adolescents (>10 years of age) and adults in Pune, with 9.4% of those enrolled testing positive for rotavirus.[12] However, the proportion rotavirus positive in this study declined during the surveillance period from 18.0% in 2008 to 3.9% in 2012.
Two studies of a birth cohort in Vellore shed light on the natural history of rotavirus disease.[13, 14] Approximately 95% of children in the birth cohort were infected with rotavirus by 3 years of age including 18% of children who were infected as neonates.[13] Based on stool testing, the incidence of rotavirus infection was 1.04 per child-year including 0.75 asymptomatic infections per child-year and 0.29 symptomatic infections per child-year.[13] As was seen in the sentinel site based surveillance, vomiting and fever were more common among children with rotavirus diarrhea than with other causes of diarrhea.[13] Many children in the cohort had multiple infections over the first three years of life but the interval between infections became increasingly longer between subsequent infections.[13] The risk of rotavirus infection and diarrhea decreased with increasing age, corresponding with an increase in IgG and IgA antibody titers increased with increasing age.[14] However, no threshold level of protection was observed for either IgG or IgA.[14]
Rotavirus Detection, Strain Diversity, and Molecular Epidemiology
The globally common G1P[8], G2P[4], and G9P[8] rotavirus strains were also the most frequently detected strains in numerous studies in India in both inpatients and outpatients<5 years of age.[4, 5, 7–10] G12 and G9P[4] were also detected in many studies.[4, 5, 7–10] In the birth cohort study in Vellore, G10P[11] was frequently detected in infections in neonates.[13] Another study compared circulating rotavirus strains in children <5 years of age and in animals collected in the same area in south India during similar time periods.[15] The common G types in children were similar to those detected in other hospital based surveillance studies (G1, G2, and G9). Of the animals tested for rotavirus, 35 (5.5%) of 627 were positive for rotavirus with G6, G2, and G10 as the most common G types and P[6] and P[4] as the most common P-types. G2 infections, which are predominately detected in humans, are rare in animals suggesting anthroponotic transmission occurs in southern India. One unusual P-type, P[15], was detected in combination with G10.
Several studies noted a high false positivity rate using ELISA ranging from 13% of results as false positives in children to over 50% in adolescents and adults.[11, 16] These false positive detections complicated interpretation of the ELISA results and often required additional testing to determine true positives. For example, samples that are untypeable using standard PCR-based methods may be due to false positive results on ELISA. To help characterize untypeable strains, Babji and colleagues propose a typing strategy based on available primers but using alternate extraction methods and showed that this strategy, combined with sequencing, is able to resolve the majority of untypeable strains.[16]
In sequencing studies of circulating strains, naturally circulating G1P[8] strains differ from subgenotypic linages of the G1P[8] strains in both of the currently available international vaccines, Rotarix and RotaTeq, but the relationship of these sublineages to vaccine effectiveness is unknown.[17] Circulation of intergenogroup reassortants was detected among adolescents and adults.[12]
Economic Burden of Rotavirus and Cost-Effectiveness of a National Rotavirus Vaccination Program
Rotavirus diarrhea results in a significant economic burden to India.[3] Rotavirus hospitalizations among children <5 years of age are estimated to cost INR 4.9 billion (USD ~81.6 million) each year in India and rotavirus outpatient visits an additional INR 5.38 billion (USD ~89.5 million) per year. A national rotavirus vaccination program if implemented by the Government of India would cost Rs 60 (USD 1) per dose with a total cost of INR 4.47 billion per year which is less than the annual cost of rotavirus hospitalizations. In a study focusing on outpatients, rotavirus diarrhea was found to have higher direct costs (INR 3,177 (USD 52.88)) per visit compared to non-rotavirus outpatient visits (INR 1,787 (USD 29.74)).[10]
A national rotavirus vaccination program would be cost-effective in India although given the heterogeneity of rotavirus disease burden across geographic and socioeconomic subgroups, its impact and cost-effectiveness will not be uniform. One study found that a rotavirus vaccination program would prevent 35,000 deaths nationally at an average cost of USD 118 (INR 7,081) per DALY averted.[18] Reductions geographic and socioeconomic disparities could prevent an additional 9,400 deaths. In poorer states with high mortality, the primary justification for vaccine introduction would be the potential reduction in diarrhea mortality whereas in wealthier states with lower mortality, the primary benefit would be averted costs.[18] A second cost effectiveness study using the IndiaSim model also examined the cost-effectiveness of a national rotavirus vaccination taking into account the geographic variability of health and wealth. In this study, three scenarios were examined including one where rotavirus vaccine was introduced at the routine coverage levels of the other routine EIP vaccines, a second where coverage was increased to 90% randomly across the population, and a third where targeted rural and urban regions with coverage below 90% at baseline were targeted.[19] In all three scenarios, rotavirus vaccines were cost saving but the impact of vaccination was greatest under scenario 3. Rotavirus vaccine introduction averted 21.2 deaths and $248,203 (INR 14.9 million) in out-of-pocket costs per 100,000 children <5 years of age under scenario 1 and deaths and cost averted increased under the other two scenarios. The reduced burden was highest for the poor and in rural areas.
Intussusception
Following its introduction into the US, a first generation rotavirus vaccine was found to have an increased risk of intussusception of ~1 excess case of intussusception for every 10,000 children vaccinated and was subsequently withdrawn from the market less than one year after its introduction.[20] For the two second generation vaccines that are currently available internationally, large safety studies were conducted as part of the clinical trials and found no increased risk of intussusception within 31 or 42 days of vaccination.[21, 22] However, continued post-marketing surveillance has detected a small increased risk 1–5 cases of intussusception per 100,000 children vaccinated mainly within the first week following the first dose.[23–29] While there was no association with intussusception was observed in the clinical trial of 116E vaccine[1], post-marketing monitoring of intussusception with this and other Indian-manufactured rotavirus vaccines is important, especially within specified risk windows. Understanding the epidemiology of intussusception in India and how monitoring efforts could influence presentation and severity will guide such efforts.
Several studies contribute to the understanding of the epidemiology of intussusception in India. In a 6-year retrospective review from 2007–2012 of intussusception cases among children <5 years of age presenting to two facilities, one in Manipal in southern India and one in north-central India in Lucknow,175 cases of intussusception were identified with 75% of the cases occurring in males.[30] The median age was 8 months with 56% of cases in children <5 years of age occurring by the first birthday. The classic triad of symptoms, vomiting, passage of blood through the rectum, and abdominal pain, were present in only 19% of cases. All cases were diagnosed by either ultrasound or abdominal radiology. The median length of stay was 10 days with 72% of cases managed surgically, 26% managed by radiological reduction, and 3% of cases spontaneously reduced. No fatalities were observed.
In a study in Vellore, data from retrospective surveillance of intussusception cases among children <2 years of age who presented to a large tertiary referral center during January 2010 through August 2013 were compared to data on cases of intussusception identified through active surveillance as part of a clinical trial conducted in the region during the same time period.[31] The findings from the retrospective review were similar to those from the two center retrospective study in Manipal and Lucknow. Intussusception peaked in children 4–6 months of age with 85% occurring in the first year of life. Two thirds of intussusception cases occurred in males. Almost all cases, 97%, met the Brighton Collaboration Intussusception Working Group level 1 criteria for diagnostic certainty with a median of 48 hours between symptom onset and arrival at the hospital. Approximately half of the cases required surgery and of those requiring surgery, half had resection performed. There were no deaths identified through retrospective surveillance. In sharp contrast, the active surveillance conducted as part of the phase 3 clinical trial identified 16 cases in the trial population, all of which were outside the known risk window associated with rotavirus vaccination, and only 7 (44%) met the Brighton Collaboration Intussusception Working Group level 1 criteria for diagnostic certainty with a median interval between symptom onset and follow-up of 10 hours. None of these cases require surgery, half were <1 year of age, and none of the children died.
Another study further examines the intussusception data from the phase 3 clinical trial and included data from all three clinical trial sites, Vellore, Pune, and Delhi.[32] Of the 1432 suspected intussusception events that were screened, only 23 cases of intussusception were identified by ultrasound, of which a total of 11 (48%) met the Brighton Collaboration Intussusception Working Group level 1 criteria for diagnostic certainty. In the placebo group the rate of ultrasound detected intussusception was 141 per 100,000 child-years and the rate of Brighton level 1 confirmed intussusception was 71 per 100,000 child-years. Incidence varied greatly by geography with the highest rate ultrasound-detected intussusception of 581 per 100,000 child-years detected in the south (Vellore) and the lowest rate of 28 per 100,000 child-years detected in the north (Delhi). Approximately half (52%) of the intussusceptions were transient and none required surgery. No cases occurred within 28 days of vaccination and no children died.
Rotavirus vaccines
The initial clinical trial results for the indigenously produced rotavirus vaccine, Rotavac, showed that the vaccine was 56% effective against severe rotavirus gastroenteritis during the first year of life which is comparable to the efficacy of the other internationally available vaccines in developing country settings.[1, 33–35]In a follow-up analysis, the vaccine efficacy was shown to be sustained through the second year of life with an efficacy of 49% in the second year of life unlike the other available vaccines which showed a substantially reduced efficacy during the second year of life in some developing country settings.[36] The vaccine provided comparable protection against a wide variety of strains. Forty infants would need to be vaccinated to prevent a severe episode of rotavirus gastroenteritis and 21 infants would need to be vaccinated to prevent an episode of rotavirus gastroenteritis of any severity.
There are additional oral rotavirus vaccines in the pipeline in India.(Table 1) One such vaccine is an oral bovine rotavirus pentavalent vaccine (BRV-PV) containing bovine-human reassortant strains of serotype G1, G2, G3, G4, and G9 that has been developed by the Serum Institute of India, Ltd. in collaboration National Institutes of Health (NIH) in the United States.[37] This vaccine has completed animal toxicity studies and Phase I and II clinical trials in adults, toddlers, and infants and was found to be safe and immunogenic. Seroconversion rates were similar to those reported for Rotarix in India. Phase III trials to assess its efficacy against severe rotavirus gastroenteritis are planned. Another bovine human reassortant vaccine under development by Shantha Biotechnics Limited and based on the National Institutes of Health’s bovine-human reassortant strains.[38] This oral bovine human reassortant tetravalent vaccine (BRV-TV) expresses serotypes G1, G2, G3, and G4. In Phase I/II clinical trials, all three concentrations of antigen tested were immunogenic and resulted in an increase in anti-rotavirus IgA antibodies. The vaccine arm with the highest concentration of antigen had the highest sero-response rate and also exceeded that of the RotaTeq arm.
Table 1.
Indian manufactured rotavirus vaccines
| Name | Composition | Organization/Company | Stage of development |
|---|---|---|---|
| ROTAVAC | Live attenuated neonatal rotavirus strain, G9P[11] (aka 116E) | Bharat Biotech | Recently licensed for use in India; pursuing WHO pre-qualification |
| BRV-PV | Bovine-human reassortant strains of serotype G1, G2, G3, G4, and G9 | Serum Institute of India | Phase I and II completed; phase III trials planned |
| BRV-TV | Bovine-human reassortant strains of serotype G1, G2, G3, and G4 | Shantha Biotech | Phase I and II completed |
As live, oral vaccines including rotavirus vaccines have been shown to have lower efficacy in developing country settings, several studies have been conducted to examine strategies to improve the immune response to such vaccines. Reasons for the lower efficacy are not well understood but several hypotheses include higher levels of maternal antibody, neutralization of the vaccine by breast milk, high level of other infections in the intestines, and malnutrition. To address the question of interference by neutralizing factors in breast milk, a randomized control trial was conducted in which mother-infant pairs were randomized into two groups, where mothers were either encouraged to breastfeed or withhold breastfeeding during the 30 minutes before and after each dose of Rotarix vaccine.[39] There was no difference in the proportion of infants who seroconverted in the two groups which is consistent with other recently published studies.[40] Another study examined the effect of an increasing the number of doses on the infants’ immune response to the vaccine. In this study, children were randomized to receive either 3 or 5 doses of Rotarix vaccine.[41] Seroconversion rates in both groups were low and there was no difference in the proportion of infants seroconverting in the 3 and 5 dose arms.
Interpreting and synthesizing data
Finally, several papers provide insight into the debate surrounding rotavirus vaccine introduction and offer insights into interpreting results from the clinical trials and applying lessons learned from the international experience with rotavirus vaccine introduction. In a synthesis of the debate and of the available evidence for rotavirus vaccines, Panda et al examine disease burden data, host and environmental factors, vaccine efficacy, immunization program issues, and economic considerations surrounding rotavirus vaccine in India.[42] The authors note that the overall immunization system performance in India needs to be strengthened but scientific, economic, and societal factors suggest that rotavirus vaccine introduction would be a good investment for India.
As various point estimates of rotavirus vaccine efficacy for different rotavirus vaccines are now available, Neuzil et al propose a framework for evaluating new rotavirus vaccines with a special focus on design characteristics of the clinical trials.[43] This framework identifies co-administration with oral polio vaccines, age at vaccine administration, measure of severe disease and specificity of outcome, and length of follow-up period as some of the key design effects to review when comparing point estimates from clinical trials. Comparing the Rotavac vaccine to the currently available international vaccine, Neuzil et al conclude that the point estimate for efficacy of Rotavac compares quite favorably to the point estimate for efficacy from clinical trials of RotaTeq and Rotarix performed in low-income settings.
Finally, Rao et al review global data on licensed rotavirus vaccine performance in terms of impact on disease, strain diversity, safety, and cost-effectiveness to provide a framework for decision-making regarding rotavirus vaccine introduction in India.[44] Countries that have introduced rotavirus vaccines have seen dramatic declines in deaths, hospitalizations, and outpatient visits due to rotavirus gastroenteritis among children <5 years of age and in their associated economic costs. Several countries have also seen such declines in disease in older children and adults but such data from developing country settings in more limited. Many countries have shown substantial diversity in circulating strains as has been seen in India and available vaccines have been shown to provide heterotypic protection against a wide range of genotypes. Risk benefit analyses have shown that rotavirus vaccine benefits greatly outweigh risk especially in high disease burden settings like India.
Conclusion
With the potential availability of multiple indigenously manufactured rotavirus vaccines in the next few years, Indian policy-makers will need to weigh available local data on disease and economic burden with cost-effectiveness, safety, and efficacy of the vaccines in their decision to introduce rotavirus vaccines into the national immunization program. This supplement contains up-to-date data on these issues, highlighting the tremendous health and economic burden of rotavirus in Indian children, the lack of any safety signals in clinical testing so far and underscoring the potential value of vaccination. While a wide diversity of circulating rotavirus strains in Indian children was noted, it is reassuring from both global data and from clinical trial data for 116E that rotavirus vaccines provide good protection against a range of circulating strains, including those that are not included in the vaccines. Nevertheless, on-going surveillance for rotavirus gastroenteritis through the Indian Rotavirus Surveillance System will continue to provide valuable information about rotavirus disease burden and strain diversity in India, and should provide a valuable platform to assess the large anticipated health benefits of vaccination.
Footnotes
Conflict of interest: None.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- [1].Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. The Lancet infectious diseases. 2012;12:136–41. [DOI] [PubMed] [Google Scholar]
- [3].John J, Sarkar R, Muliyil J, Bhandari N, Bhan MK, Kang G. Rotavirus gastroenteritis in India, 2011–2013: Revised estimates of disease burden and potential impact of vaccines. Vaccine. 2014:DOI: 10.1016/j.vaccine.2014.03.004. [DOI] [PubMed] [Google Scholar]
- [4].Babji S, Arumugam R, Sarvanabhavan A, Moses PD, Simon A, Aggarwal I, et al. Multi-center surveillance of rotavirus diarrhea in hospitalized children <5yrs of age in India, 2009–2012. Vaccine. 2014:DOI: 10.1016/j.vaccine.2014.03.001. [DOI] [PubMed] [Google Scholar]
- [5].Chitambar SD, Ranshing SS, Pradhan GN, Kalrao VR, Dhongde RK, Bavdekar AR. Changing trends in circulating rotavirus strains in Pune, western India in 2009–2012: emergence of a rare G9P[4] rotavirus strain. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.027. [DOI] [PubMed] [Google Scholar]
- [6].Mathew A, Rao PSS, Sowmyanarayanan TV, Kang G. Severity of rotavirus gastroenteritis in an Indian population: Report from a 3 year surveillance study. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.038. [DOI] [PubMed] [Google Scholar]
- [7].Tiku VR, Sharma S, Verma A, Kumar P, Raghavendhar S, Aneja S, et al. Rotavirus diversity among diarrheal children in Delhi, India during 2007–2012. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.005. [DOI] [PubMed] [Google Scholar]
- [8].Mullick S, Mandal P, Nayak MK, Ghosh S, De P, Rajendran K, et al. Hospital based Surveillance and Genetic Characterization of Rotavirus strains in Children (<5years) with acute gastroenteritis in Kolkata, India, revealed Resurgence of G9 and G2 Genotypes during 2011–2013. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.018. [DOI] [PubMed] [Google Scholar]
- [9].Saluja T, Sharma SD, Gupta M, Kundu R, Kar S, Dutta A, et al. A multicenter prospective hospital based surveillance to estimate the burden of rotavirus gastroenteritis in children less than five years of age in India. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.030. [DOI] [PubMed] [Google Scholar]
- [10].Namjoshi GS, Mitra M, Lalwani SK, Sachdeva A, Balasubramanian S, Babji S, et al. Rotavirus gastroenteritis among children less than 5 years of age in private outpatient setting in urban India. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.070. [DOI] [PubMed] [Google Scholar]
- [11].Anandan S, Peter R, Aramugam R, Ismail N, Veeraraghavan B, Kang G. Group A rotavirus gastroenteritis in older children and adults at a hospital in southern India. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.008. [DOI] [PubMed] [Google Scholar]
- [12].Tatte VS, Chothe NS, Chitambar SD. Characterization of rotavirus strains identified in adolescents and adults with acute gastroenteritis highlights circulation of nontypeable strains: 2008–2012. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.009. [DOI] [PubMed] [Google Scholar]
- [13].Paul A, Gladstone BP, Mukhopadhya I, Kang G. Rotavirus infections in a community based cohort in Vellore, India. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.039. [DOI] [PubMed] [Google Scholar]
- [14].Premkumar P, Lopman B, Ramani S, Paul A, Gladstone B, Muliyel J, et al. Association of serum antibodies with protection against rotavirus infection and disease in South Indian children. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rajendran P, Kang G. Molecular epidemiology of rotavirus in children and animals and characterization of an unusual G10P[15] strain associated with bovine diarrhea in south India. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.026. [DOI] [PubMed] [Google Scholar]
- [16].Babji S, Arumugam R, Sarvanabhavan A, Gentsch JR, Kang G. Approach to molecular characterization of partially and completely untyped samples in an Indian rotavirus surveillance program. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kulkarni R, Arora R, Chitambar SD. Sequence analysis of VP7 and VP4 genes of G1P[8] rotaviruses circulating among diarrhoeic children in Pune, India : A comparison with Rotarix and RotaTeq vaccine strains. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.080. [DOI] [PubMed] [Google Scholar]
- [18].Rheingans R, Anderson IV JD, Anderson B, Chakraborty P, Atherly D, Pindolia D. Estimated impact and cost-effectiveness of rotavirus vaccination in India: Effects of geographic and economic disparities. Vaccine. 2014. [DOI] [PubMed] [Google Scholar]
- [19].Megiddo I, Colson AR, Nandi A, Chatterjee S, Prinja S, Khera A, et al. Analysis of the Universal Immunization Programme and Introduction of a Rotavirus Vaccine in India with IndiaSim. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.04.080. [DOI] [PubMed] [Google Scholar]
- [20].Peter G, Myers MG, National Vaccine Advisory C, National Vaccine Program O. Intussusception, rotavirus, and oral vaccines: summary of a workshop. Pediatrics. 2002;110:e67. [DOI] [PubMed] [Google Scholar]
- [21].Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. The New England journal of medicine. 2006;354:23–33. [DOI] [PubMed] [Google Scholar]
- [22].Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. The New England journal of medicine. 2006;354:11–22. [DOI] [PubMed] [Google Scholar]
- [23].Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, et al. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011;29:3061–6. [DOI] [PubMed] [Google Scholar]
- [24].Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s National Immunization Program. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57:1427–34. [DOI] [PubMed] [Google Scholar]
- [25].Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliveira LH, Bautista Marquez A, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. The New England journal of medicine. 2011;364:2283–92. [DOI] [PubMed] [Google Scholar]
- [26].Velazquez FR, Colindres RE, Grajales C, Hernandez MT, Mercadillo MG, Torres FJ, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. The Pediatric infectious disease journal. 2012;31:736–44. [DOI] [PubMed] [Google Scholar]
- [27].Haber P, Patel M, Pan Y, Baggs J, Haber M, Museru O, et al. Intussusception after rotavirus vaccines reported to US VAERS, 2006–2012. Pediatrics. 2013;131:1042–9. [DOI] [PubMed] [Google Scholar]
- [28].Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, et al. Intussusception risk after rotavirus vaccination in U.S. infants. The New England journal of medicine. 2014;370:503–12. [DOI] [PubMed] [Google Scholar]
- [29].Weintraub ES, Baggs J, Duffy J, Vellozzi C, Belongia EA, Irving S, et al. Risk of intussusception after monovalent rotavirus vaccination. The New England journal of medicine. 2014;370:513–9. [DOI] [PubMed] [Google Scholar]
- [30].Singh JV, Kamath V, Shetty R, Kumar V, Prasad R, Saluja T, et al. Retrospective Surveillance for Intussusception in Children Aged Less than Five Years at two tertiary care centers in India. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.007. [DOI] [PubMed] [Google Scholar]
- [31].Jehangir S, John J, Mani B, Srinivasan R, Kang G. Intussusception in southern India: Comparison of retrospective analysis and active surveillance. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.028. [DOI] [PubMed] [Google Scholar]
- [32].John J, Kawade A, Rongsen-Chandola T, Bavdekar A, Bhandari N, Taneja S, et al. Active surveillance for intussusception in a phase III efficacy trial of an oral monovalent rotavirus vaccine in India. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.036. [DOI] [PubMed] [Google Scholar]
- [33].Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–23. [DOI] [PubMed] [Google Scholar]
- [34].Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–14. [DOI] [PubMed] [Google Scholar]
- [35].Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. The New England journal of medicine. 2010;362:289–98. [DOI] [PubMed] [Google Scholar]
- [36].Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, et al. Efficacy of a Monovalent Human-Bovine (116E) Rotavirus Vaccine in Indian Children in the Second Year of Life. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.04.079. [DOI] [PubMed] [Google Scholar]
- [37].Zade JK, Kulkarni PS, Desai SA, Sabale RN, Naik SP, Dhere RM. Bovine Rotavirus Pentavalent Vaccine Development in India. Vaccine. 2014;doi: 10.1016/j.vaccine.2014.03.003. [DOI] [PubMed] [Google Scholar]
- [38].Dhingra MS, Kundu R, Ganguly N, Gupta M, Singh M, Kanungo S, et al. Evaluation of Safety and Immunogenicity of a Live Attenuated Tetravalent (G1-G4) Bovine-Human Reassortant Rotavirus vaccine (BRV-TV) in Healthy Indian Adults and Infants. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.069. [DOI] [PubMed] [Google Scholar]
- [39].Rongsen-Chandola T, Strand TA, Goyal N, Flem E, Rathore SS, Arya A, et al. Effect of Withholding Breastfeeding on the Immune Response to a Live Oral Rotavirus Vaccine in North Indian Infants. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.04.078. [DOI] [PubMed] [Google Scholar]
- [40].Groome MJ, Moon SS, Velasquez D, Jones S, Koen A, van Niekerk N, et al. Effect of breastfeeding on immunogenicity of oral live-attenuated human rotavirus vaccine: a randomized trial in HIV-uninfected infants in Soweto, South Africa. Bulletin of the World Health Organization. 2014;92:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kompithra RZ, Paul A, Manoharan D, Babji S, Sarkar R, Mathew LG, et al. Immunogenicity of a Three Dose and Five Dose Oral Human Rotavirus Vaccine (RIX4414) Schedule in South Indian Infants. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.002. [DOI] [PubMed] [Google Scholar]
- [42].Panda S, Das D, Samanta S. Synthesizing evidences for policy translation: a public health discourse on rotavirus vaccine in India. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.037. [DOI] [PubMed] [Google Scholar]
- [43].Neuzil KM, Zaman K, Victor JC. A Proposed Framework for Evaluating and Comparing Efficacy Estimates in Clinical Trials of New Rotavirus Vaccines. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.04.074. [DOI] [PubMed] [Google Scholar]
- [44].Rao TS, Arora R, Khera A, Tate JE, Parashar U, Kang G. Insights from Global Data for Use of Rotavirus Vaccines in India. Vaccine. 2014:doi: 10.1016/j.vaccine.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


