Abstract
Efficient long-term cultivation of chicken primordial germ cells (cPGCs) is essential for various avian research and biotechnology applications. Our study aimed to address the challenge of inconsistent culture success by investigating strain-specific variations and optimizing culture conditions using two distinct media: Ovotransferrin-enriched medium (OTM) and chicken serum-supplemented medium (CSM). We demonstrated that each chicken strain has unique nutritional requirements, with Hubbard cPGCs thriving in OTM and Bovans cPGCs favoring CSM. This strain-specific variation was effective in derivation and proliferation rates and the expression of stem cell-specific markers such as POU5F3/OCT4 and NANOG. Furthermore, our study confirmed the sustained germ cell identity of long-term cultured cPGCs through the expression of DAZL, DDX4, and EMA1 germ cell markers. We also showed that cultured cPGCs retained their migratory abilities and transfectability, successfully generating G0 germline chimeras and G1 transgenic Bovans chickens. These findings highlight the importance of optimized culture conditions depending on the genotype to enhance the viability and genetic stability of cPGCs, paving the way for more effective genetic modifications and conservation strategies in avian species.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-93777-w.
Keywords: Chicken primordial germ cells, Strain-specific culture, Genetically-engineered birds, Transgenesis
Subject terms: Biotechnology, Animal biotechnology, Stem-cell biotechnology
Introduction
cPGCs are unique cells found in developing embryos and able to colonize gonads1. They can transmit genetic and epigenetic information to the next generation through their differentiation into sperm and eggs. cPGCs possess distinct developmental features, such as their formation mechanism and ability to migrate to the gonads through the bloodstream2. Moreover, cPGCs exhibit a specialized mechanism for sex determination, involving both autonomous factors within the cPGCs and external factors from the testes and ovaries3–5.
The efficient culture of cPGCs is crucial for many downstream applications, including maintaining long-term poultry genetic biodiversity in cryobanks and tools for efficient genetic engineering6. Notably, the robust proliferation of cPGCs diminishes over time in culture, which limits the use of biotechnological methods in these cells. Also, the ability of cPGCs to pass down their genome to future generations decreases with long-term cultures including the risk of accumulated undetected genomic mutations7–9. Therefore, achieving the optimal conditions for the long-term cultivation of cPGCs is necessary. In this regard, choosing an appropriate medium becomes a bottleneck for successfully cultivating cPGCs. As reported in various studies, persistent variations in cPGC growth and development emphasize improving the nutrition medium required for cPGC culture10.
Two common types of media used for the culture of cPGCs are defined and undefined. A defined medium is a precisely controlled culture medium with chemically defined known components and concentrations, offering a well-defined environment for cell growth, reproducibility, and the study of specific cellular responses. In contrast, an undefined medium includes complex components, often of animal origin, such as animal sera, conditioned medium, and feeder cells, whose precise compositions and concentrations are not entirely determined. While undefined media may supply essential nutrients and growth factors for cell growth, their variable composition across different batches can introduce inconsistencies in cell culture experiments, potentially affecting reproducibility and the interpretation of results.
Despite numerous studies investigating the nutritional requirements and environmental factors influencing cPGC culture, a given culture medium may not guarantee optimal results when culturing cPGCs derived from different chicken strains or maintained under various laboratory conditions. This study was conducted in response to developing an appropriate medium for the culture of cPGCs from various strains. To address this issue, we conducted a comparative study on the growth and expansion of cPGCs derived from two distinct chicken strains: the Bovans White layer (hereafter called Bovans) and the Hubbard JA57 broiler (hereafter called Hubbard). We employed different culture media formulations to investigate optimal growth conditions. We observed variations in the two strain culture behaviors and proliferation/stemness features of the two strains, emphasizing the need for customized culture media specific to each strain. These findings hold significant promise for a more efficient culture of cPGCs according to their genetic background.
The generation of genetically engineered chickens requires the primary culture of cPGCs, their long-term and optimized maintenance in vitro, and the introduction of desired genetic modifications in their genome. Hence, the development of an appropriate culture medium for the proper growth and proliferation of these cells is of great importance.
The use of genetically engineered chickens capable of producing recombinant proteins can be beneficial in the development of various vaccines, monoclonal antibodies, and orphan drugs due to reduced production costs and increased production scale. Additionally, genetically engineered chickens with resistance to diseases can be important to increase food security. Moreover, genetically engineered chickens can serve as models for various diseases, development of drugs, and diagnostic kits.
Materials and methods
Animal experiments
This study was performed in two centers; INSERM, INRAE, Stem Cell and Brain Research Institute, Claude Bernard University Lyon 1, Lyon, France and Research Institute of Biotechnology, Ferdowsi University of Mashhad, Mashhad, Iran. Fertilized eggs and recipient embryos of Hubbard and Bovans were obtained from Elevage Grand Buisson, France and from Seamorgh Company, Iran, respectively. cPGCs were collected from 2.5-day-old embryos, Hamburger–Hamilton (HH) stages 14–16, of both strains. Using chicken embryos before one-third of the incubation period did not require ethical approval. The study was conducted following the ARRIVE guidelines.
Culture media for cPGCs
To cultivate and maintain cPGCs, we utilized two media formulations: CSM and OTM. The base cPGC medium was prepared as described in our previous study7,11. CSM was created by supplementing the base medium with 0.5% (v/v) chicken serum. For OTM, we substituted chicken serum with 10 µg/mL ovotransferrin (Sigma). All media components were sourced from Thermo Fisher Scientific, USA unless otherwise specified (Table 1).
Table 1.
Media components used in this study.
| Component | Ovotransferrin-enriched Medium (OTM) |
Chicken serum-supplemented Medium (CSM) |
|---|---|---|
| DMEM- high Glucose (Calcium free) | 250 mOsm* | 250 mOsm * |
| GlutaMAX (100X) | 1X | 1X |
| Sodium Pyruvate (100X) | 1X | 1X |
| Non-Essential Amino Acid (100X) | 1X | 1X |
| Nucleoside (100X) | 1X | 1X |
| Penicillin/Streptomycin (100X) | 1X | 1X |
| Sodium heparin | 0.1 mg/mL | 0.1 mg/mL |
| Calcium chloride | 0.15 mM | 0.15 mM |
| 2-mercaptoethanol | 0.1 mM | 0.1 mM |
| B27 supplement (50X) | 1X | 1X |
| h-FGF | 5 ng/ml | 5 ng/ml |
| Activin | 30 ng/ml | 30 ng/ml |
| Ovalbumin | 0.2% | 0.2% |
| Ovotransferrin | 10 µg/ml | – |
| Chicken serum | – | 0.5% |
* DMEM medium (high glucose, no glutamine, no calcium; Cat#: 21068028) is diluted to a final osmolarity of 250 mOsm by adding distilled water.
Isolation and long-term culture of PGCs
Primary cultures of cPGCs were established from both Bovans and Hubbard embryos. The details of the isolation and cultivation methods of cPGCs were as described in our previous study7,11. Briefly, Embryonic blood (1 µL) was isolated from the dorsal aorta of stage HH 14–16 embryos and cultured in 100 µL of CSM or OTM media in 96-well plates, as previously explained7,11. The sex of embryos was determined from tissue samples. After 14 days, proliferated cPGCs were transferred to 48-well plates for expansion and long-term culture. The culture medium was refreshed every two days throughout the experiment. Established cell lines were defined as those exceeding 100 days in culture (100-day-old cPGCs). Male cPGC lines from both strains, cultured in either CSM or OTM for 100 days, were used for subsequent experiments.
Proliferation assay
A proliferation assay was performed on three male cPGC lines from each strain cultured in CSM or OTM media. Cells were seeded into a 96-well plate at a density of 2 × 103 cells per well (2000 cells/well on Day 0). After that, cell numbers were counted on days 2, 4, 6, 8, and 10. At each time point, 10 µL of cPGCs were collected from each well, stained with Trypan Blue (Thermo Fischer, USA), and counted using a hemocytometer.
Transfection of PGCs
For lipofection, each Bovans cPGC culture, whether in CSM or OTM, was treated with 1 µL of Lipofectamine LTX reagent diluted in 50 µL of Opti-MEM medium (referred to as ‘diluted Lipofectamine’). Concurrently, two µg of DDX4-tdTomato or DAZL-tdTomato plasmids11 were diluted in 50 µL of Opti-MEM medium (referred to as ‘diluted DNA’). The diluted DNA was combined with the diluted Lipofectamine to form a Lipoplex, which was incubated for 30 min. Subsequently, 0.1 × 106 of cPGC lines were counted, pelleted, and resuspended in 100 µL of Opti-MEM medium. The cell suspension was transferred to a 96-well plate, and then 100 µL of Lipoplex was added. Six hours post-transfection, the medium was replaced with fresh CSM or OTM.
For electroporation, Hubbard-derived cPGCs maintained in either CSM or OTM were processed using a NEON electroporation instrument (Neon NxT, Thermo Fischer Scientific, USA), following the protocol outlined in previous work7. Briefly, two µg of either DDX4-tdTomato or DAZL-GFP transposon plasmids, along with two µg of piggyBac transposase plasmid, were electroporated by applying 850 V for 50 milliseconds with a single pulse. The expression of tdTomato or GFP in the transfected cPGCs was assessed using fluorescence microscopy 72 h post-transfection for each strain cultured in the respective media.
Immunocytochemistry
Hubbard cPGCs cultured for over 100 days were characterized for the presence of germ cell markers (EMA1, DAZL, DDX4) and a stem cell marker (SSEA1) using immunocytochemistry (detailed methods in7). Primary antibodies against EMA1 (mouse IgG; DSHB, USA), DAZL (mouse IgG; Abcam ab34139, UK), DDX4 (mouse IgG; custom made by Biotem, France), and SSEA1 (mouse IgG; DSHB, USA) were employed to detect their corresponding protein markers. FITC-labeled anti-mouse IgM (JIR, USA) and FITC-labeled anti-rabbit IgG (JIR, USA) were used to detect primary antibodies. DNA was counterstained using TO-PRO-3.
Flow cytometry
Expression of EMA1 and SSEA1 was further assessed using specific antibodies and flow cytometry. cPGCs were labeled with primary antibodies EMA1 (1:100; DSHB, USA) and SSEA1- Alexa Fluor 647 (1:125; Santa Cruz, USA). Anti-mouse IgM AF647 (1:500; JIR, USA) was used as a secondary antibody for EMA1-labeled cells. The labeling process consisted of resuspending cPGCs in PBS with 5% FBS, incubating with primary antibodies for 1 h, washing, and incubating with the secondary antibody for 30 min. Control cells were labeled with only the secondary antibody. All centrifugation steps were performed at 1200 rpm for 3 min. The labeled cells were analyzed using a BD flow cytometer (BD Biosciences, USA).
Promoter assay
The activity of germ cell promoters in Bovans cPGCs cultured for extended periods (> 100 days) was assessed using a promoter assay technique. Cells were transfected with plasmids containing the tdTomato reporter gene under the control of DDX4 or DAZL promoters, and the expression of the red fluorescent protein was evaluated under a fluorescence microscope.
RT-PCR gene expression assay
The expression of stem cell-related genes, including core pluripotency genes (NANOG, SOX2, and POU5F3) and pluripotency-associated genes (KLF4, c-MYC, and LIN28A), was evaluated by RT-PCR. Total RNA was isolated from 1 × 105 cPGCs and reverse-transcribed into cDNA with random hexamer using a Thermostable RT kit, following the manufacturer’s instructions (DENAzist Asia, Iran). The transcription of the ACTB gene was used as a control for the reverse transcription step. PCR reactions included 10 µL from 2X Master Mix RED (Ampliqon, Denmark), 1µL of each forward and reverse primers for each gene (Table 2), 2 µL cDNA template (This was equivalent to 2000 ng of input RNA), and 7 µl sterile-deionized water. The PCR steps consisted of an initial denaturation at 94 °C for 3 min, followed by 45 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for specific genes and 55 °C for the control gene for 30 s, extension at 72 °C for 15 s, and a final extension at 72 °C for 5 min.
Table 2.
PCR primers used in this study.
| Gene | Accession number | Sequence (5’ to 3’) | Product (bp) |
|---|---|---|---|
| NANOG | NM_001146142.2 | F: GGGATTTATCTACCACAGAATGG | 183 bp |
| XM_046906232.1 | R: CACAGCCATGAACGGATA | ||
| SOX2 | NM_205188.3 | F: GGTGGTCAAGACGGAATC | 132 bp |
| R: GGTTCTGGTACTTCAGCA | |||
| POU5F3 | NM_001309372 | F: ACGCTCTATGGGAAGATGTTC | 87 bp |
| R: CTTCAGCTTGCACATGTTCTTA | |||
| KLF4 | XM_004949369.5 | F: AGTACCAAGAGCTGATGCCG | 107 bp |
| XM_046905543.1 | |||
| XM_046936072.1 | R: GTCACAGGTGTGAGTGGCTG | ||
| XM_046936073.1 | |||
| c-MYC | NM_001030952.2 | F: TTACATTAGCTGAAGCGAACG | 193 bp |
| R: CTGTCCAACTTTAGCCTCTTG | |||
| LIN28A | NM_001031774.2 | F: TCACCCGTCGATGTCTTC | 102 bp |
| R: GGAGGATTTCTTGAAGGTGAATTC | |||
| ACTB | NM_205518.2 | F: GAGAAGATGACACAGATC | 118 bp |
| R: CAGAGTCCATCACAATAC | |||
| tdTomato | – | F: GTCATCAAAGAGTTCATGCGCTTCAAGG | 737 bp |
| R: GCGGATAACAATTTCACACAGG |
qPCR gene expression assay
We evaluated the expression levels of NANOG and POU5F3 by RT-qPCR in six established Bovans and Hubbard cPGC lines cultured in two different media. Total RNA was isolated from these cPGC lines using the RNA Isolation Kit (Column RNA Isolation Kit, DENAzist Asia, IRAN for Bovans cPGCs and RNeasy Mini Kit, Qiagen, Germany for Hubbard cPGCs). From 500 ng of input RNA, cDNA was synthesized using the cDNA Synthesis Kit with random hexamer (Thermo Fisher Scientific, USA). qPCR was conducted with 50 ng cDNA in a 20 µL reaction of RealQ Plus 2×Master Mix Green (Ampliqon, Denmark) containing specific primers (Table 2) and an Applied Biosystems Real-Time PCR instrument (Thermo Fisher Scientific, USA). The qPCR temperature steps were the initial denaturation step at 94 °C for 15 min, followed by 45 cycles of denaturation at 94 °C for 30 s, annealing (at 50 °C for NANOG and POU5F3 and 55 °C for ACTB) for 30 s, and extension at 72 °C for 20 s, with a final 10 s extension at 72 °C for signal detection. A melting curve analysis was conducted between 60 °C and 95 °C. Gene expression levels of NANOG and POU5F3 were calculated using the Pfaffl method and ACTB as a calibrator.
PKH26-Red staining of PGCs
The Bovans cPGC line was stained with the PKH26-Red fluorescent cell linker kit (Sigma, USA) for general cell membrane labeling. Briefly, 1 × 106 cPGCs were counted and pelleted. The cells were washed once with a serum-free medium. After centrifugation, the supernatant was cautiously removed without disturbing the cell pellet. Then, 0.5 mL of diluent C reagent was added to the cell pellet and gently pipetted to ensure complete resuspension (2x cell suspension). 2 µL of the PKH26-Red dye solution was added to the 0.5 mL of diluent C and mixed well (2x dye solution). The 2x cell suspension was added to the 2x dye solution, immediately mixed by gentle pipetting, and incubated for 2 min with periodic mixing. The staining procedure was stopped by adding an equal volume (1 mL) of chicken serum, followed by a 1-minute incubation at room temperature. Then, the cells were pelleted at 1200 rpm for 3 min and the supernatant was cautiously discarded. The cell pellet was resuspended in 2 mL of complete medium and transferred to a new sterile conical tube. The cells were washed by pelleting at 1200 rpm for 3 min. To remove the unbound dye, the washing step was repeated two more times with 2 mL of the complete medium. After the final wash, the cell pellet was resuspended in a complete medium for injection into HH 14–16 stage embryos.
Generation and analysis of transgenic Bovans chickens
Male and female Bovans cPGCs cultured in CSM (each 0.1 × 106 cells) were transfected with DDX4-tdTomato transposon and transposase plasmids using Lipofectamine LTX, as described above. Transfection was confirmed by the detection of red fluorescence. Pooled male and female cPGCs were injected into 30 embryonated eggs at the stage of HH 14–16 (around 0.5 × 104 cPGCs per egg).
Only male G0 chickens were raised to puberty. DNA extraction from sperm was conducted using the Animal Tissue DNA Isolation Kit (DENAzist Asia, Iran). Extracted sperm DNA was evaluated for transgene integration using PCR with specific primers (tdTomato) designed for the tdTomato sequence (Table 2). The PCR protocol consisted in an initial denaturation step at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 15 s, with a final extension at 72 °C for 10 min. The PCR amplicon was 737 bp. To obtain G1 heterozygous chicken, the G0 germline tdTomato-positive roosters were mated with wild-type hens. The same PCR protocol was performed on DNA extracted from the root of the feathers of G1 chickens.
The 737 bp amplicon amplified from DDX4-tdTomato plasmid (as a positive control) and all positive G0 or G1 chickens were recovered from agarose gel using the Gel Extraction Kit (DENAzist Asia, IRAN) and then was digested with the BstXI enzyme (Thermo Fisher, USA) at 55 °C for 3 h. Then digested amplicons were run on a 2% agarose gel.
Results
Evaluation of strain-specific differences in morphology, derivation rate, and proliferation capacity
The time required for culturing cPGCs and achieving an optimal cell population for subsequent applications can be influenced by several factors, including the choice of culture method, proper formulation of culture media, and the inherent growth characteristics of cPGCs. In this study, we investigated the culture conditions, including the derivation rate and culture behavior of two breeds in two different culture media. cPGCs were obtained from Hubbard and Bovans chicken embryos at HH stage 14–16. We employed two distinct culture media: OTM, which is classified as defined, and CSM, which is categorized as an undefined medium. The specific components of each medium are outlined in Table 1.
We assessed the derivation rate of isolated cPGCs in both media types for each group, consisting of 15 blood samples collected from embryos of two different strains. Our experiment focused on male embryos to exclude potential biases introduced by sex-specific cell behavior and developmental differences. The derivation success rate of Hubbard’s cPGCs was more successful in OTM, while the derivation rate for Bovans was better in CSM. The derivation success rate of cPGCs from Hubbard was 73.3% (11 out of 15 isolated blood samples) in OTM and 53.3% (8 out of 15 isolated blood samples) in CSM (Fig. 1A). For Bovans, the derivation rate of cPGCs was 73.3% (11 out of 15 isolated blood samples) in CSM and 40% (6 out of 15 isolated blood samples) in OTM (Fig. 1B).
Fig. 1.
Evaluation of strain-specific differences in morphology, derivation rate, and proliferation capacity. (A) Derivation and long-term culture of Hubbard cPGCs in OTM and CSM. 100-day-old H-OTM (a and b) and H-CSM cPGCs (c and d) are shown. The stacked bars (e) show the number of derived and long-term cultured cPGCs in OTM and CSM. H-OTM: Hubbard cPGCs cultured in OTM; H-CSM: Hubbard cPGCs cultured in CSM. (B) Derivation and long-term culture of Bovans cPGCs in OTM and CSM. 100-day-old B-OTM (a and b) and B-CSM cPGCs (c and d) are shown. The stacked bars (e) show the number of derived and long-term cultured cPGCs in OTM and CSM. B-OTM: Bovans cPGCs cultured in OTM; B-CSM: Bovans cPGCs cultured in CSM. (C) Morphological Characterization of cPGCs from Hubbard and Bovans Strains. Hubbard cPGCs (a-c) distinctive protruded spikes (a) are indicated by an arrow. PAS-positive staining of freshly isolated H-OTM-1 cells is shown by arrows (b and c). Bovans cPGCs (d-h) morphological features of long-term cultured B-CSM cPGCs are shown (d-h). The presence of eccentric refractive granules and a large colony of cPGCs are indicated by an arrowhead and dashed square, respectively (d). G2 stage (e), doublet form (f), freshly divided (g), and a small colony (h) of cPGCs are shown. (D) Proliferation Assay of 100-Day-Old cPGCs. Proliferation was assessed in H-OTM (a), H-CSM (b), B-OTM (c), and B-CSM (d) cPGCs.
The derived cPGCs of both strains were cultured for more than 100 days (Fig. 1A-a–d for Hubbard and Fig. 1B-a–d for Bovans). Some cultures stopped proliferating without any apparent reasons as previously reported in our publication7. For Hubbard cPGCs, successful long-term culture was 62.5% (5 out of 8 derived cPGCs) in CSM and 81.8% (9 out of 11 derived cPGCs) in OTM (Fig. 1A). For Bovans, the successful long-term culture rate was 50% (3 out of 6 derived cPGCs) in OTM and 72.7% (8 out of 11 derived cPGCs) in CSM (Fig. 1B).
Cultured cPGCs were carefully examined for their size and morphological features throughout their long-term culture (Fig. 1A–C). We did not detect any significant differences in the appearance or morphology between the two strains when cultured in two distinct media types (Fig. 1A, B, C). The round shape of long-term cultured cPGCs closely resembled freshly isolated ones (Fig. 1A-a–d, B-a–d), while their size varied with the cell cycle stage (Fig. 1C-e–g). cPGCs exhibited a size of approximately 15–20 μm in the G2 stage (Fig. 1C-e) and a size of 7.5–15 μm in doublets (freshly divided) (Fig. 1C-f, g). The presence of cPGC clusters was observed in the long-term culture (Fig. 1C-h). Additionally, the presence of eccentric refractive granules in cPGCs was evident (Fig. 1C-d), and the presence of glycogen was confirmed by Periodic Acid-Schiff (PAS) staining (Fig. 1C-b, c). Distinctive protruded spikes were noted on the cell membrane surface of cPGCs (Fig. 1C-a; arrow in the inset), particularly during the initial two weeks of culture.
Proliferation rates and doubling time of two distinct strains of cPGCs (Hubbard and Bovans) maintained in OTM and CSM were assessed. In each experimental group, three highly-proliferating lines of cPGCs were used. The results revealed that Hubbard cPGCs in OTM (with doubling times ranging from 29 to 30 h) displayed a shorter doubling time and higher proliferation rate than in the CSM medium with doubling times of 32 to 34 h (Fig. 1D-a, b). In contrast, Bovans cPGCs exhibited a shorter doubling time and higher proliferation rate in CSM than in OTM (Fig. 1D-c, d).
Verification of germ cell identity in long-term cultured cPGCs from different strains cultured in two distinct media.
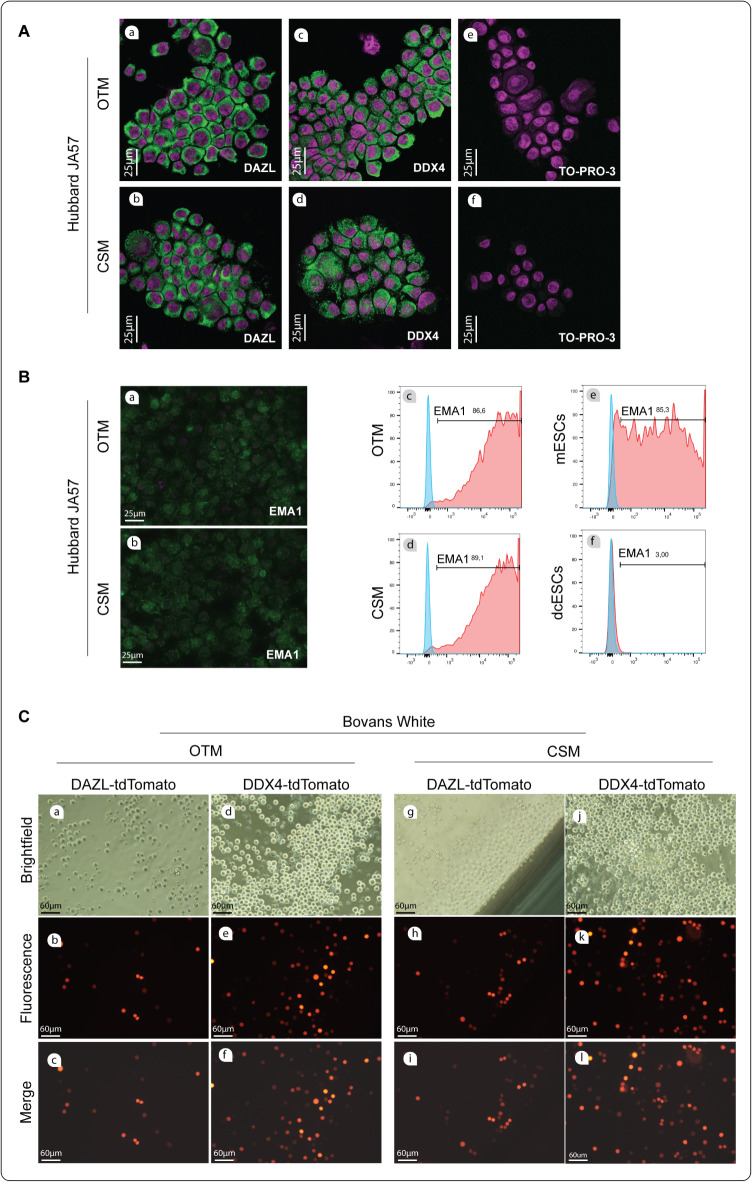
To analyze the germ cell characteristics of long-term cultured cPGCs, we evaluated the expression of well-defined germ cell-specific cytoplasmic markers in cPGCs, including DAZL12 and DDX413. The expression of DAZL (Fig. 2A-a, b) and DDX4 (Fig. 2A-c, d) was immunocytochemically confirmed in Hubbard cPGCs cultured in OTM and CSM. Negative controls were counterstained using TO-PRO-3 (Fig. 2A-e, f). Additionally, the presence of the germ-cell specific membrane marker EMA1 was also confirmed in these cells by immunocytochemistry (Fig. 2B-a, b) and flow cytometry (Fig. 2B-c, d). EMA1 was positive for mouse embryonic stem cells (mESCs) and negative for differentiated chicken stem cells (dcESCs), as determined by flowcytometry (Fig. 2B-e, f). In Bovans cPGCs, a promoter assay was conducted, and the activity of endogenous DAZL and DDX4 promoters was confirmed in CSM and OTM culture conditions (Fig. 2C).
Fig. 2.
Verification of germ cell identity in long-term cultured cPGCs from different strains cultured in two distinct media. (A) DAZL-positive (a; H-OTM, b; H-CSM) and DDX4-positive cPGCs (c; H-OTM, d; H-CSM), as well as unstained cPGCs (e, f; negative control: stained only with secondary antibody), are shown using confocal microscopy. (B) Evaluation of EMA1 presence in Hubbard cPGCs. H-OTM and H-CSM cPGCs were evaluated for EMA1 presence using fluorescence microscopy (a, b) and flow cytometry (c, d). Mouse embryonic stem cells (mESCs) and differentiated chicken stem cells (dcESCs) were used as flow cytometry positive and negative controls, respectively (e, f). (C) DAZL-tdTomato expressing cPGCs (a-c; B-OTM, g-i; B-CSM) and DDX4-tdTomato expressing cPGCs (d-f; B-OTM, j-l; B-CSM) are shown using fluorescence microscopy with the promoter assay technique. (only the cells that were successfully transfected can express tdTomato)
Evaluating stem cell characteristics in long-term cultured cPGCs of different strains and media
SSEA1 presence was observed by flow cytometry (Fig. 3A) and immunofluorescence (Fig. 3B-a, b) in both OTM and CSM long-term cultured cPGCs derived from Hubbard and Bovans strains. Fluorescence microscopy analysis revealed that the the SSEA1 marker was present in both lines in both conditions, suggesting similar stemness status. To validate the expression of stem cell-specific pluripotency and transcription factors, we evaluated the expression of core transcriptional network genes (NANOG, SOX2, and POU5F3) and pluripotency-associated genes (KLF4, c-MYC, and LIN28A) in Hubbard and Bovans cPGCs cultured in OTM and CSM, respectively, on days 20 and 100. Our findings confirmed that NANOG, LIN28A, KLF4, and c-MYC were not expressed in Hubbard cPGCs cultured in OTM on day 20. This pattern was also observed in Bovans cPGCs culture in CSM, except for c-MYC which was expressed on day 20 (Fig. 3C and Supplementary Fig. S1). Moreover, we found that NANOG, SOX2, POU5F3, c-MYC, and LIN28A and not KLF4 were expressed in Bovans cPGCs cultured in CSM on day 100. This pattern was slightly similar to what observed in Hubbard cPGCs cultured in OTM, except for LIN28A and KLF4 which were not expressed on day 100 (Fig. 3C and Supplementary Fig. S1).
Fig. 3.
Evaluating stem cell characteristics in long-term cultured cPGCs of different strains and media. (A) Flow cytometry analysis of SSEA1 expression in Hubbard (a-d) and Bovans cPGCs (e–h). Mouse embryonic stem cells (mESCs) (i) and differentiated chicken stem cells (dcESCs) (j) were used as positive and negative controls for SSEA1 expression, respectively. (B) Immunofluorescence staining of SSEA1 in Hubbard cPGCs. (C) RT-PCR expression analysis of stem cell-specific pluripotency and transcription factors in Hubbard and Bovans cPGCs cultured in OTM and CSM, respectively, on days 20 and 100. bp; base pairs. SM; size marker. (D) Expression levels of NANOG (a) and POU5F3 (b) transcripts in Hubbard cPGCs cultured in OTM and CSM. (E) Expression levels of NANOG (a) and POU5F3 (b) transcripts in Bovans cPGCs cultured in OTM and CSM.
To quantify the expression levels of NANOG and POU5F3, we performed RT-qPCR analysis on cPGCs derived from both strains cultured in OTM and CSM media (Fig. 3D and E). The results revealed no significant difference in NANOG expression between Hubbard cPGCs cultured in OTM and CSM (Fig. 3D-a). However, POU5F3 expression was significantly higher in Hubbard cPGCs cultured in OTM (Fig. 3D- b; p < 0.05). In contrast, Bovans cPGCs cultured in CSM exhibited significantly higher expression levels of both NANOG and POU5F3 compared to cells cultured in OTM (Fig. 3E-a, and b; p < 0.05).
Gonad colonization capacity of cPGCs isolated from two different chicken strains
To explore the migration capability of cPGCs, two different methods were applied. Hubbard cPGC lines cultured in OTM were transfected with DAZL promoter-GFP and DDX4 promoter-tdTomato transposons (Fig. 4A-a, b). After antibiotic selection, they were mixed and injected into the blastoderm of recipient embryos at stage X (Fig. 4A-c). On the other hand, Bovans cPGCs cultured in CSM were stained with PKH26-Red dye (Fig. 4B-a, b) and injected into embryos at HH stage 14–16 (Fig. 4-c). The injected embryos from both strains were dissected on embryonic day 8 (HH stage 26–28), and their gonads were observed under a fluorescence microscope. We found that cPGCs from both strains could successfully migrate to the gonads of the recipient embryos (Fig. 4A-d, e, f, g, B-d, e, f and g).
Fig. 4.
Gonad colonization capacity of cPGCs isolated from two different chicken strains. (A) Hubbard cPGCs containing DAZL promoter-GFP (a) and DDX4 promoter-tdTomato (b) were mixed and injected into stage HH X chicken embryos (c). Chimeric gonads were imaged using brightfield microscopy (d), green (e, showing GFP-positive cPGCs), and red (f, showing tdTomato positive cPGCs) channels of a fluorescence microscope. (g) A merged image. R and L denote right and left gonads, respectively. (B) Bovans cPGCs cultured in CSM were stained with the PKH-26 fluorescence dye and injected into stage HH 14–16 chicken embryos (a, brightfield; b, red channel). No red cPGCs were visible in the negative control gonads from chickens injected with un-stained cPGCs. R and L denote right and left gonads, respectively.
Generation of G0 germline chimera and G1 transgenic Bovans chickens
Since Bovans cPGCs cultured in CSM showed higher proliferation rate and stemness features than cells in other experimental groups, these cells were used for generating transgenic chickens. To generate G0 germline chimeras, we injected 50 Bovans chicken embryos at HH stage 14–16 with a mixture of male and female Bovans cPGCs cultured in CSM and expressing tdTomato under the control of the DDX4 promoter (Fig. 5A-a–d). The hatching success rate was 30% (15 out of 50 embryos). We raised 9 of the resulting male chicks to sexual maturity at approximately 5 to 6 months of age (Fig. 5A-e). PCR analysis of the sperm from these roosters identified the transgene in two individuals (Fig. 5B-a and Supplementary Fig. S2A). These G0 germline chimera roosters were then mated with wild-type hens to produce the G1 generation (Fig. 5B-b). Feather PCR analysis of the nine G1 offspring revealed that 44.44% (4 out of 9; two males and two females) carried the tdTomato transgene (Fig. 5B-c and Supplementary Fig. S2A-a). The 737 bp amplicon amplified from DDX4-tdTomato plasmid (as a positive control) and from all positive G0 or G1 chickens was digested with the BstXI enzyme. The resulting 525 bp and 212 bp bands confirmed the correct amplicon (Fig. 5B-d, e and Supplementary Fig. S2A-b).
Fig. 5.
Generation of G0 germline chimera and G1 transgenic Bovans chickens. (A) Generation of G0 Germline Chimera. Bovans cPGCs cultured in CSM and expressing tdTomato, driven by the DDX4 promoter (a, brightfield; b, red channel) were injected into the dorsal aorta of 2.5-day-old chicken embryos at stage HH 14–16 (c). The tdTomato-expressing cPGCs migrated and colonized the gonads of 6-day-old embryos (d). Nine Potential chimeric male chickens were raised (e). (B) Generation of G1 Transgenic Chickens. Sperm was collected from nine G0 roosters. Sperm DNA was analyzed by PCR to detect the presence of the tdTomato gene (sperms indicated by asterisk harbor tdTomato transgene in their genome). 737 bp amplicons were digested by BstXI restriction enzyme (d). Two G0 roosters (named Ghand and Nabat) positive for the tdTomato gene were mated with wild-type hens (b). G1 chicken progeny were screened for the presence of the tdTomato gene in their feathers. 737 bp PCR products were digested by BstXI restriction (e). M: Male, F: Female, bp: Base pairs, NTC: Non-template control, CTRL+: Positive control (DDX4-tdTomato plasmid), SM50: Size marker 50 bp.
Discussion
Efficient long-term cultivation of cPGCs is fundamental for various applications in avian research and biotechnology. Our study addressed the persistent challenge of highly variable culture success by investigating strain-specific differences and optimizing culture conditions using two distinct culture media: OTM and CSM. OTM favored Hubbard cPGCs survival, while CSM supported a better long-term culture for Bovans cPGCs (Fig. 1).
We found that both OTM and CSM effectively sustain the viability and proliferation of cPGCs from both strains. However, distinct strain-specific capabilities for optimal growth were evident. OTM notably boosted the proliferation and culture success of Hubbard cPGCs, whereas CSM supported superior outcomes for Bovans cPGCs (Fig. 1). Our results indicate that the choice of culture media impacts the derivation and proliferation phases of cPGC culture and long-term success rate for cPGCs from different strains. In cultures that did not achieve long-term viability, the density of dead cells increased over time. This highlights the delicate balance needed to maintain cPGC cultures beyond early developmental stages14,15. While regular media changes can mitigate some of these issues, they may not be sufficient to prevent all the challenges associated with long-term cell culture. Continuous optimization of culture conditions, including media composition and culture protocols, is essential for improving the viability and stability of cPGC cultures.
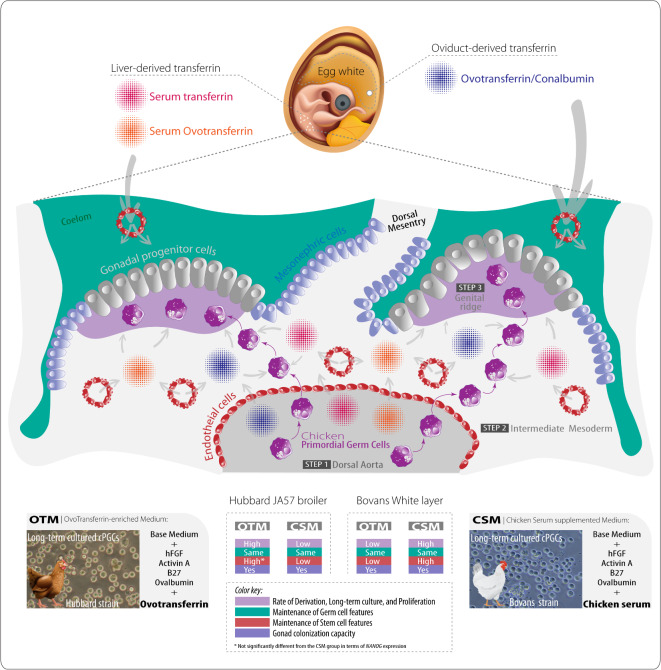
Here we hypothesized that the type of transferrin influences the culture of cPGCs (Fig. 6). To our knowledge, there are no existing studies on the effect of transferrin on the growth of cPGCs from different breeds. We found that cPGCs from broiler breeds thrive in the presence of ovotransferrin (found in OTM), while cPGCs from laying breeds grow well in the presence of liver-derived transferrins (available in CSM), such as serum ovotransferrin and serum transferrin. Interestingly, although cPGCs from broiler breeds grew well in the presence of ovotransferrin, the expression level of the NANOG gene did not significantly increase compared to the medium containing serum transferrin. In birds, there are generally two types of transferrin: liver-derived and oviductal-derived transferrin. Serum transferrin and serum ovotransferrin are of liver origin and circulate in the blood, while conalbumin/ovotransferrin originates from the oviduct and is stored in the egg white16. Developing germ cells with high mitotic activity require rapid and abundant iron availability due to the demands of high DNA synthesis and mitochondriogenesis17. Transferrin has a high capacity to transport and rapidly release iron, whereas ovotransferrin, despite its high iron storage capacity, releases iron more slowly18. Therefore, it would be reasonable to use transferrin or a combination of transferrin and ovotransferrin in the culture of cPGCs. It appears that the early stages of cPGCs development prior to the development of liver cells, may depend on the presence of oviduct-derived transferrin. However, after liver cell development, the growth of these cells may be influenced by liver-derived transferrins.
Fig. 6.
A schematic hypothesis on the effect of transferrin on the embryonic development of cPGCs. At the onset of Hamburger-Hamilton (HH) stage 11 (after 40 h of incubation), most chicken primordial germ cells (cPGCs) are found in a region anterior to the head. At this stage, cPGCs also start appearing in blood vessels. In the second half of stage 11 (after 44 h of incubation), the number of cPGCs in the anterior head region decreases, while their presence within the vascular system is increased. This indicates that cPGCs enter the blood vessels (especially into dorsal aorta) from the anterior region (STEP 1). The number of cPGCs in the bloodstream peaks at HH stage 14 (after 52 h of incubation). Up to HH stage 14, no cPGCs are observed in the intermediate mesoderm, which is the area destined to become the gonads. cPGCs are first detected in the intermediate mesoderm at HH stage 15 (after 54 h of incubation) and become more abundant by HH stage 17 (after 60 h of incubation) (STEP 2). Between HH stages 15 and 17, cPGCs settle in the genital ridges (future gonads) (STEP 3). Before liver cell development (before HH stage 30), early cPGCs development seems to rely on oviduct-derived transferrin (Conalbumin/Ovotransferrin) stored in the egg white. However, after liver cell development (after HH stage 30), the growth and survivability of these cells may be influenced greatly by liver-derived transferrins (Serum transferrin and serum ovotransferrin) and slightly by oviduct-derived transferrin. In this paper, we found that the rates of derivation, long-term culture, and proliferation are higher in OTM compared to CSM for Hubbard-derived cPGCs, while they are higher in CSM compared to OTM for Bovans-derived cPGCs. All the media maintain the germ cell features of both strains. Stem cell features for Hubbard-derived cPGCs are highly maintained by OTM compared to CSM, while these features for Bovans-derived cPGCs are highly maintained by CSM. The capacity for gonad colonization is not affected by the type of culture media. In conclusion, this study demonstrated that the nutritional requirements of cPGCs can be strain-specific, with Hubbard-derived cPGCs thriving in OTM, while Bovans-derived cPGCs favor CSM.
It has been observed that primary cells of the chick embryo, such as mesoderm cells, exhibit distinct nutritional needs at various developmental stages. For instance, the growth of mesodermal cells at HH stage 5 is influenced by transferrin, whereas at HH stage 12, the addition of transferrin to the culture medium does not affect their growth compared to the control group. Furthermore, the type of transferrin is crucial; human transferrin has a lesser effect on the growth of these cells compared to chicken transferrin. Thus, the nutritional requirements of cells can vary during different stages of development19. It has been reported that defined FA-ITS (FGF-activin containing insulin/human transferrin/selenium) could not completely support the growth of cPGCs compared to FA-ovotransferrin or FA-chicken serum11,20.
Recently, it has been reported that male and female cPGCs are transcriptionally distinct21. Additionally, reports highlight challenges for the long-term culture of female cPGCs9,10,22–25. Therefore, by culturing only male cPGCs in this study, we tried to minimize the potential sex-based confounding effects. This approach allowed us to more precisely investigate how strain and media components influence derivation rates, proliferation, and other culture outcomes.
The strain-specific differences observed in the derivation and proliferation rates of two different cPGCs highlight the complex interplay between genetic factors and culture conditions. The higher derivation rate of Hubbard cPGCs in the OTM medium and Bovans cPGCs in the CSM medium suggests that each strain has evolved to thrive in distinct biochemical environments. This is further supported by the differential proliferation rates and doubling times of cPGCs when cultured in these media. Yuxiao et al. showed that there is a subtle difference in the expression of specific meiotic genes between layer and broiler breeds, indicating that the genetic background of these strains can impact the molecular mechanisms governing germ cell development26. Our previous study showed that culturing cPGCs in an enriched medium containing Knock Out Serum Replacement (KOSR) compared to a defined medium not only maintains the expression of germ cell markers but also significantly enhances their self-renewal, pluripotency and proliferation7. Additionally, we demonstrated that GlutaMax, a stabilized form of glutamine, in the culture medium significantly enhances the proliferation, viability, and long-term culture performance of cPGCs, making it a preferable choice for their cultivation and genetic manipulation27.
A main concern in cPGC culture is preserving their characteristics, including morphological, germline, stem cell, and migratory features. In this study, normal morphological features including the presence of eccentric refractive granules and glycogen content was confirmed in all experimental groups (Fig. 1). These morphological features are consistent with those described in earlier reports on cPGC culture25. Additionally, distinctive cell-membrane-protruded spikes were observed in some cultured cPGCs. The presence of these spikes during the initial weeks of culture may be related to a transient developmental stage of cPGCs or a morphological adaptation of cPGCs to the in vitro culture conditions, possibly aiding in cell-cell communication, nutrient uptake, or differentiation status. As the cells adapt to the culture conditions and progress through in vitro cultivation, the spikes disappear, suggesting that these protrusions may be a temporary response to the initial stress of the artificial environment.
This study successfully employed established germ cell markers to evaluate germ cell identity in long-term cultured PGCs. We investigated the expression of DAZL, DDX4, EMA1, and SSEA1.
Also, we investigated the expression of core transcriptional network genes (NANOG, SOX2, and POU5F3/OCT4) along with pluripotency-associated genes (KLF4, c-MYC, and LIN28A) in Hubbard and Bovans cPGCs cultured in OTM and CSM, respectively, on days 20 and 100. Expression of some of these genes was evaluated in several studies20,25,28. However, the exact age of cPGCs under study has not been indicated. We found that on day 20, NANOG, LIN28A, and KLF4 were not expressed in Bovans cPGCs cultured in CSM. Similarly, we did not observe the expression of NANOG, LIN28A, KLF4, as well as c-MYC in Hubbard cPGCs cultured in OTM. This finding aligns with previous studies that demonstrated the downregulation of certain pluripotency regulators in cPGCs, including KLF4 and c-MYC29. Conversely, some studies reported the expression of NANOG, KLF4 and c-MYC20,25. There could be two reasons for this discrepancy. The first reason is that previous studies did not specify the exact day of culture on which gene expression analysis was performed. The second reason is that analyses on the early-stage cultures might yield unreliable PCR results due to low cell numbers or low gene expression levels. In our preliminary study, we performed the PCR with 35 cycles using 500 nanograms of input RNA and no expression of c-MYC and KLF4 on day 100 (data not shown) was detected. By increasing the number of cycles and the amount of input RNA (2000ng), c-MYC became detectable, while KLF4 remained undetectable in both strains and media. Not being able to detect the expression of c-MYC with 35 cycles of PCR could indicate a lower expression of this gene in long-term cultured cPGCs, consistent with previous reports30,31. However, the downregulated or potentially undetectable levels of gene expression for some genes may be attributed to different strains and intrinsic properties of cPGCs on different days of culture.
We focused on analyzing NANOG and POU5F3 expression due to their well-established roles in orchestrating the complex network of pathways that regulate pluripotency, self-renewal, and cell fate determination32. Interestingly, we observed a strain-specific influence of media composition on the expression of the pluripotency marker POU5F. Hubbard cPGCs displayed significantly higher POU5F3 expression in OTM than CSM (Fig. 3D). Conversely, Bovans cPGCs exhibited higher levels of both NANOG and POU5F3 when cultured in CSM (Fig. 3E).
Given that NANOG plays a crucial role in maintaining pluripotency and cell proliferation during early embryonic development of cPGCs33–36, the upregulation of NANOG in Bovans-derived cPGCs cultured in CSM medium may contribute to their improved condition in this environment. Thus, media composition influences the expression of specific stem cell markers in a strain-dependent manner.
The successful migration of GFP- and tdTomato-expressing cPGCs from Hubbard and PKH26-Red-stained cPGCs from Bovans strains to the gonads of recipient embryos confirms the preservation of their migration abilities (Fig. 4). In addition, the expression of GFP and tdTomato reporter genes under the control of cPGC-specific promoters (DAZL and DDX4) proves that maintaining cPGCs in culture does not affect their transfectability. Thus, these cells are amenable to genetic modification, and genetically manipulated cPGCs retain their germline features during migration. The generation of G0 germline chimeras and G1 transgenic Bovans chickens demonstrated the feasibility of using long-term cultured cPGCs in chicken transgenesis (Fig. 5). Transgenic cells could be engineered by CRISPR37–40 or CRISPR/transposase23,41,42 technologies.
Conclusion
In conclusion, our findings underscore the importance of strain-specific media formulations to address the unique nutritional requirements of cPGCs derived from different genetic backgrounds and strain-specific preferences for culture media for the successful long-term cultivation of cPGCs from distinct chicken strains.
Our research has revealed that for Hubbard-derived cPGCs, the rates of derivation, long-term culture, and proliferation are higher in OTM compared to CSM. Conversely, Bovans-derived cPGCs exhibit higher rates in CSM compared to OTM. Both media are effective in maintaining the germ cell features of both strains. Specifically, OTM highly maintains the stem cell features of Hubbard-derived cPGCs compared to CSM, whereas for Bovans-derived cPGCs, CSM highly maintains these features. Importantly, the capacity for gonad colonization remains unaffected by the type of culture media used. Collectively, while both OTM and CSM effectively supported the viability and proliferation of cPGCs from both strains, distinct strain-specific preferences for optimal growth were evident.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We want to thank Seamorgh Company, Mashhad, Iran, especially Dr. Reza Hoseini-Shahidi, for providing the Bovans embryonated eggs.
Abbreviations
- cPGCs
Chicken Primordial Germ Cells
- OTM
Ovotransferrin-enriched medium
- CSM
Chicken serum-supplemented medium
- HH
Hamburger–Hamilton
- PAS
Periodic Acid-Schiff
- H-OTM
Hubbard PGCs cultured in OTM
- H-CSM
Hubbard PGCs cultured in CSM
- B-OTM
Bovans PGCs cultured in OTM
- B-CSM
Bovans PGCs cultured in CSM
Author contributions
S.Y.: Conceptualization, Investigation, Methodology, Formal analysis, Visualization, Writing—original draft. N.D.: Conceptualization, Investigation, Methodology, Formal analysis, Visualization, Writing—original draft. L.G.: Investigation, Methodology. C.K.: Investigation, Methodology. S.R.G.: Investigation, Methodology. G.M.: Investigation, Methodology. R.E.V.: Investigation, Methodology. B.P.: Conceptualization, Supervision, Visualization, Resources, Writing—original draft, Writing—review & editing, Project administration, Funding acquisition. H.D.: Conceptualization, Supervision, Visualization, Resources, Writing—original draft, Writing—review & editing, Project administration, Funding acquisition.
Funding
We extend our gratitude to DENAzist Asia Co. and ANR, project CRB-ANIM-ANR-11-INBS-0003 for financially supporting this study.
Data availability
Correspondence and requests for materials should be addressed to H.D. or B.P.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
No ethics approval was needed. The experiments were conducted in accordance with relevant guidelines.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sara Yousefi Taemeh and Nima Dehdilani.
Contributor Information
Bertrand Pain, Email: bertrand.pain@inserm.fr.
Hesam Dehghani, Email: dehghani@um.ac.ir.
References
- 1.Dehdilani, N., Yousefi Taemeh, S., Goshayeshi, L. & Dehghani, H. Genetically engineered birds; pre-CRISPR and CRISPR era†. Biol. Reprod.106(1), 24–46 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Kim, Y. M. & Han, J. Y. The early development of germ cells in chicken. Int. J. Dev. Biol.62(1-2-3), 145–152 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Ichikawa, K. et al. Prediction of sex-determination mechanisms in avian primordial germ cells using RNA-seq analysis. Sci. Rep.12(1), 13528 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ichikawa, K., Ezaki, R., Furusawa, S. & Horiuchi, H. Comparison of sex determination mechanism of germ cells between birds and fish: cloning and expression analyses of chicken forkhead box L3-like gene. Dev. Dyn. Off Publ Am. Assoc. Anat.248(9), 826–836 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yousefi Taemeh, S., Mahdavi Shahri, N., Lari, R., Bahrami, A. R. & Dehghani, H. Meiotic initiation in chicken germ cells is regulated by Cyp26b1 and mesonephros. J. Exp. Zool. B Mol. Dev. Evol.332(7), 269–278 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Fallahshahroudi, A. et al. A male-essential MicroRNA is key for avian sex chromosome dosage compensation. BioRxiv (2024).
- 7.Dehdilani, N. et al. Enhanced cultivation of chicken primordial germ cells. Sci. Rep.13(1), 12323. 10.1038/s41598-023-39536-1 (2023). [DOI] [PMC free article] [PubMed]
- 8.Kong, L. et al. Long-term in vitro culture and preliminary establishment of chicken primordial germ cell lines. PLoS One13(4), e0196459 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van De Lavoir, M. C. et al. Germline transmission of genetically modified primordial germ cells. Nature441(7094), 766–769 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Mathan, Z. G. et al. Formation, application, and significance of chicken primordial germ cells: a review. Anim. Open. Access. J. MDPI13, 6 (2023). [DOI] [PMC free article] [PubMed]
- 11.Chen, Y. C. et al. Three-dimensional culture of chicken primordial germ cells (cPGCs) in defined media containing the functional polymer FP003. PLoS One13(9), e0200515 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maegawa, S., Yasuda, K. & Inoue, K. Maternal mRNA localization of zebrafish DAZ-like gene. Mech. Dev.81(1–2), 223–226 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Aduma, N., Izumi, H., Mizushima, S. & Kuroiwa, A. Knockdown of DEAD-box helicase 4 (DDX4) decreases the number of germ cells in male and female chicken embryonic gonads. Reprod. Fertil. Dev.31(5), 847–854 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Krampe, B. & Al-Rubeai, M. Cell death in mammalian cell culture: molecular mechanisms and cell line engineering strategies. Cytotechnology62(3), 175–188 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, Y. et al. Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell. Death Dis.4(12), e950 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horrocks, N. P. C., Irene Tieleman, B. & Matson, K. D. A simple assay for measurement of ovotransferrin—a marker of inflammation and infection in birds. Methods Ecol. Evol.2(5), 518–526. 10.1111/j.2041-210X.2011.00096.x (2011).
- 17.Leichtmann-Bardoogo, Y. et al. Compartmentalization and regulation of iron metabolism proteins protect male germ cells from iron overload. Am. J. Physiol. Endocrinol. Metab.302(12), E1519–E1530 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Abdallah, F. B. & Chahine, J. M. Transferrins, the mechanism of iron release by ovotransferrin. Eur. J. Biochem.263(3), 912–920 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Sanders, E. J. Changes in the transferrin requirement of cultured chick embryo mesoderm cells during early differentiation. J. Embryol. Exp. Morphol.95, 81–93 (1986). [PubMed] [Google Scholar]
- 20.Whyte, J. et al. FGF, insulin, and SMAD signaling cooperate for avian primordial germ cell Self-Renewal. Stem Cell. Rep.5(6), 1171–1182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doddamani, D. et al. The transcriptome of chicken migratory primordial germ cells reveals intrinsic sex differences and expression of hallmark germ cell genes. Cells12, 8 (2023). [DOI] [PMC free article] [PubMed]
- 22.Naito, M., Harumi, T. & Kuwana, T. Long-term culture of chicken primordial germ cells isolated from embryonic blood and production of germline chimaeric chickens. Anim. Reprod. Sci.153, 50–61. 10.1016/j.anireprosci.2014.12.003 (2015). [DOI] [PubMed]
- 23.Park, T. S. & Han, J. Y. piggyBac transposition into primordial germ cells is an efficient tool for transgenesis in chickens. Proc. Natl. Acad. Sci. U. S. A.109(24), 9337–9341 (2012). [DOI] [PMC free article] [PubMed]
- 24.Miyahara, D. et al. Culture conditions for maintain propagation, long-term survival and germline transmission of chicken primordial germ cell-like cells. J. Poult. Sci.51(1), 87–95 (2014). [Google Scholar]
- 25.Macdonald, J., Glover, J. D., Taylor, L., Sang, H. M. & McGrew, M. J. Characterisation and germline transmission of cultured avian primordial germ cells. PLoS One5, 11 (2010). [DOI] [PMC free article] [PubMed]
- 26.Ma, Y. et al. The synchronized progression from mitosis to meiosis in female primordial germ cells between layers and broilers. Genes (Basel)14, 4 (2023). [DOI] [PMC free article] [PubMed]
- 27.Yousefi Taemeh, S., Mehrzad, J. & Dehghani, H. Effect of glutamine stability on the long-term culture and line establishment of chicken primordial germ cells. J. Cell Mol. Res.13(1), 44–53 (2021).
- 28.Naeemipour, M., Dehghani, H., Bassami, M. & Bahrami, A. Expression dynamics of pluripotency genes in chicken primordial germ cells before and after colonization of the genital ridges. Mol. Reprod. Dev.80(10), 849–861 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Rengaraj, D. et al. Chicken blastoderms and primordial germ cells possess a higher expression of DNA repair genes and lower expression of apoptosis genes to preserve their genome stability. Sci. Rep.12(1), 49 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tonus, C. et al. Long term-cultured and cryopreserved primordial germ cells from various chicken breeds retain high proliferative potential and gonadal colonisation competency. Reprod. Fertil. Dev.28(5), 628–639 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Ibrahim, M. et al. The effect of short- and long-term cryopreservation on chicken primordial germ cells. Genes (Basel) ;15, 5 (2024). [DOI] [PMC free article] [PubMed]
- 32.Loh, Y. H. et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet.38(4), 431–440 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Choi, H. J. et al. Chicken NANOG self-associates via a novel folding-upon-binding mechanism. FASEB J. Off Publ Fed. Am. Soc. Exp. Biol.32(5), 2563–2573 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Lavial, F. et al. The Oct4 homologue PouV and Nanog regulate pluripotency in chicken embryonic stem cells. Development134(19), 3549–3563 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Han, J. Y. et al. Acquisition of pluripotency in the chick embryo occurs during intrauterine embryonic development via a unique transcriptional network. J. Anim. Sci. Biotechnol.9, 31 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi, H. J. et al. Differential transcriptional regulation of the NANOG gene in chicken primordial germ cells and embryonic stem cells. J. Anim. Sci. Biotechnol.12(1), 40 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dehdilani, N. et al. Integrating omics and CRISPR technology for identification and verification of genomic safe harbor loci in the chicken genome. Biol Proced Online25(1), 18. 10.1186/s12575-023-00210-5 (2023). [DOI] [PMC free article] [PubMed]
- 38.Dehdilani, N., Fathi Najafi, M. & Dehghani, H. A multi-faceted approach for prediction of genome safe harbor loci in the chicken genome. J. Cell Mol. Res.13(1), 1–9 (2021).
- 39.Yousefi Taemeh, S. et al. Study of the regulatory elements of the ovalbumin gene promoter using CRISPR technology in chicken cells. J. Biol. Eng.17(1), 46 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yousefi Taemeh, S., Dehdilani, N., Goshayeshi, L. & Dehghani, H. Exploring the function of gene promoter regulatory elements using CRISPR tools. Methods Mol. Biol.2844, 145–156 (2024). [DOI] [PubMed] [Google Scholar]
- 41.Goshayeshi, L. et al. CRISPR/dCas9-mediated transposition with specificity and efficiency of site-directed genomic insertions. FASEB J.35, 2 (2021). [DOI] [PubMed]
- 42.Rezazade Bazaz, M., Ghahramani Seno, M. M. & Dehghani, H. Transposase-CRISPR mediated targeted integration (TransCRISTI) in the human genome. Sci. Rep.12(1), 3390 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Correspondence and requests for materials should be addressed to H.D. or B.P.