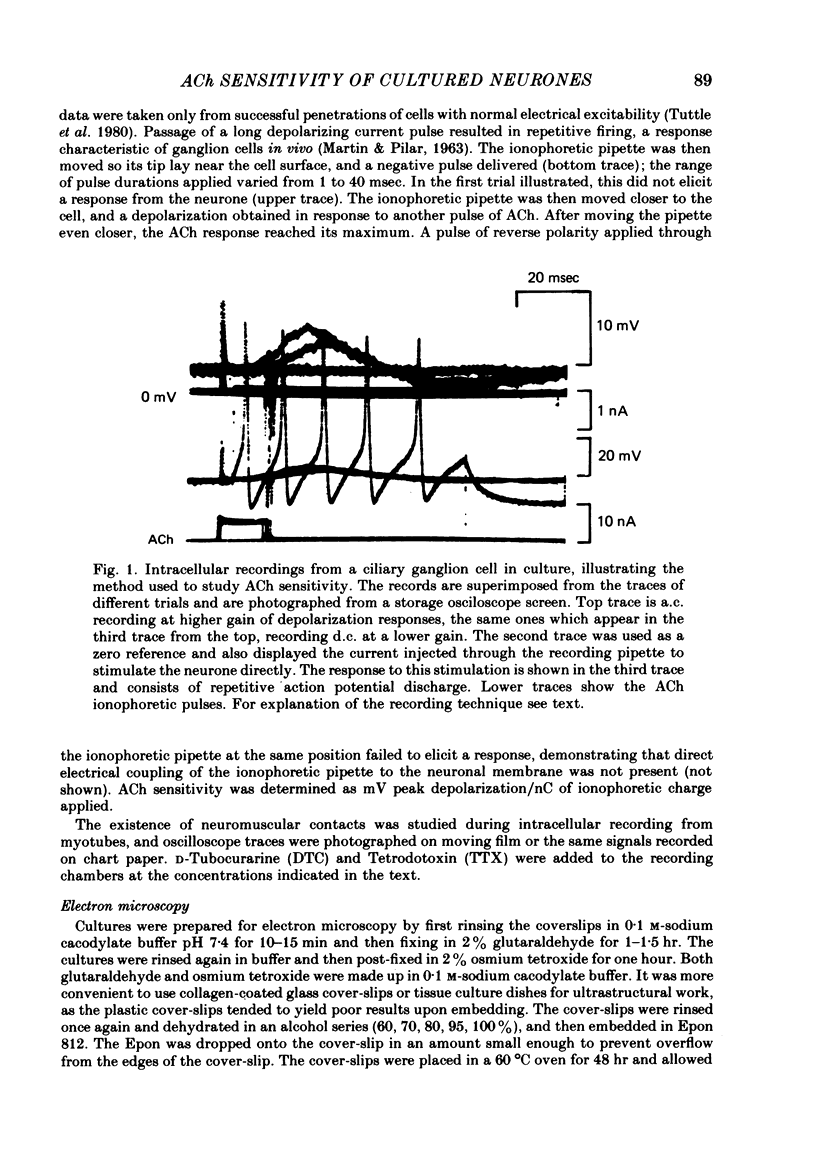
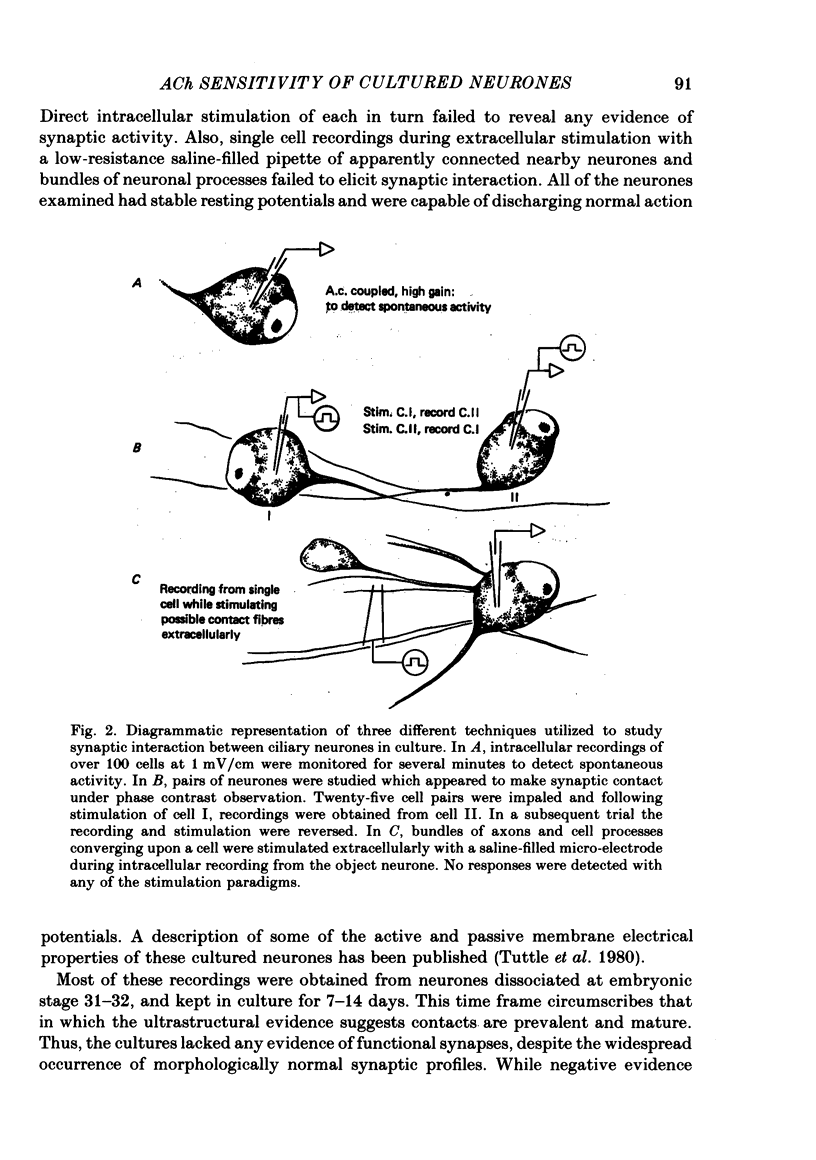
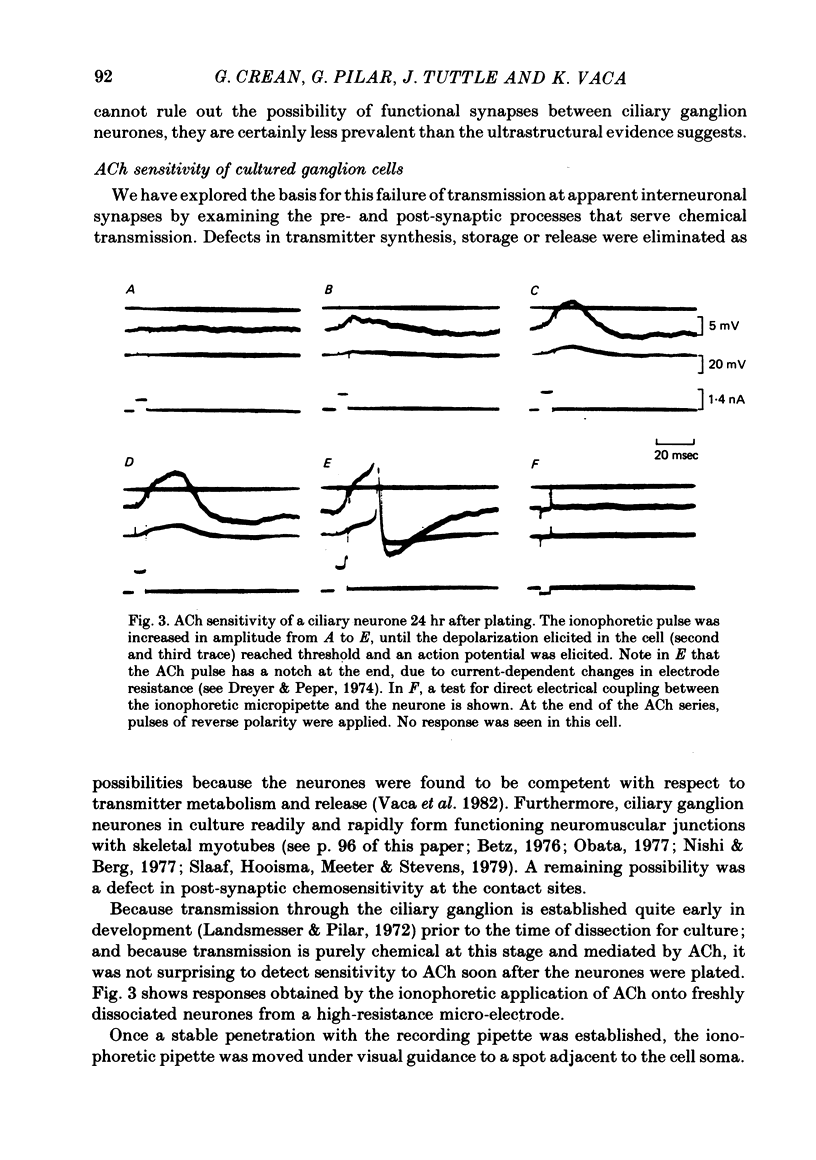
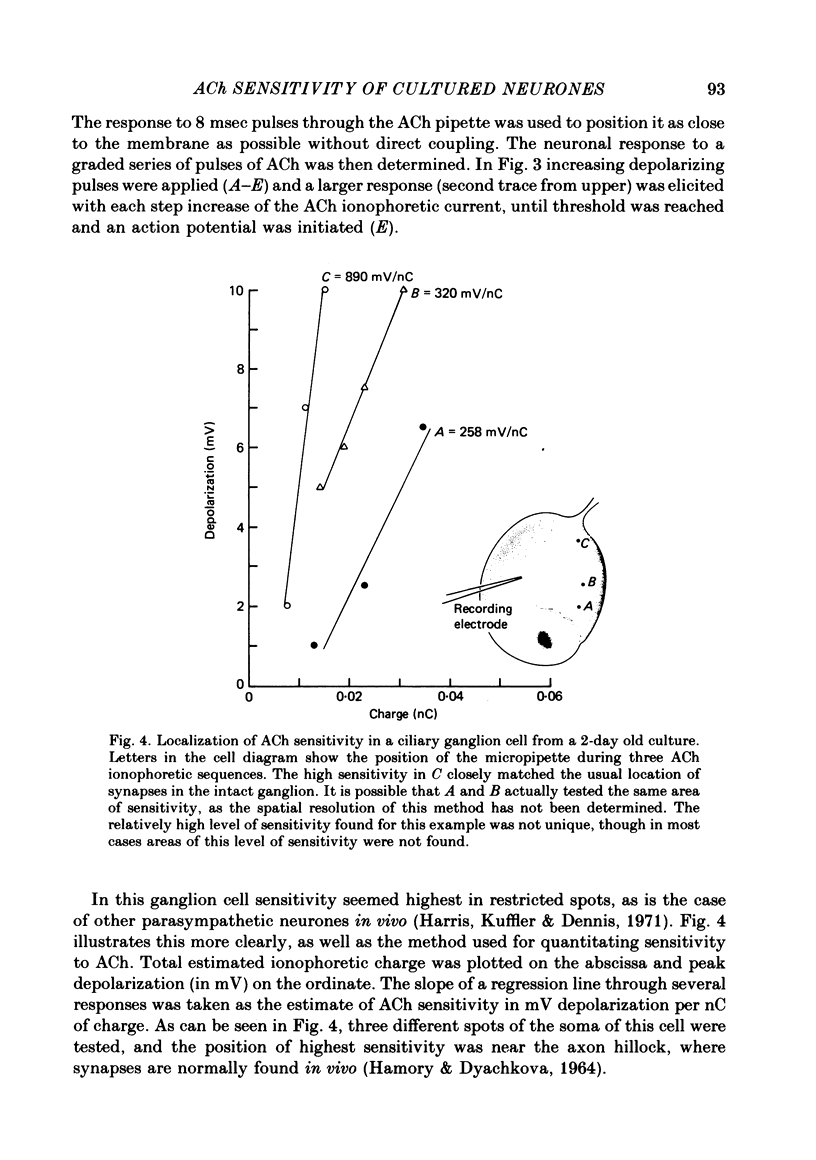
Abstract
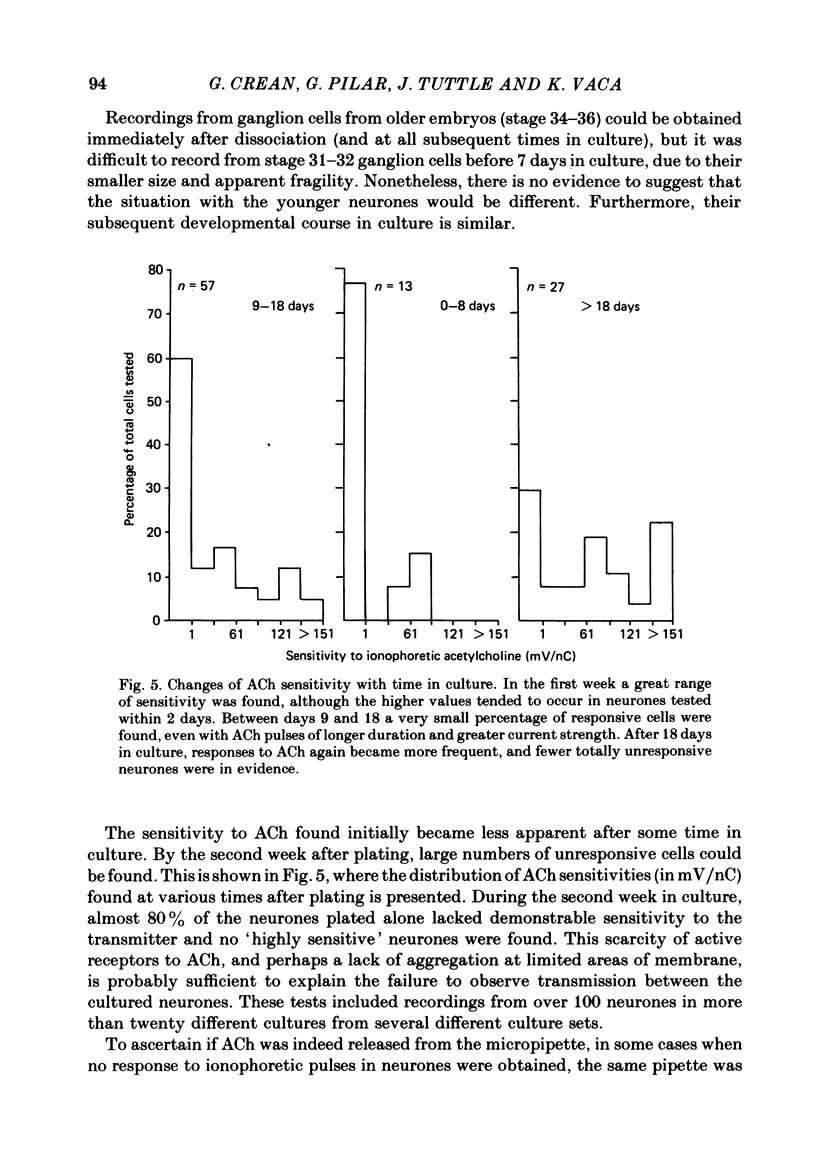
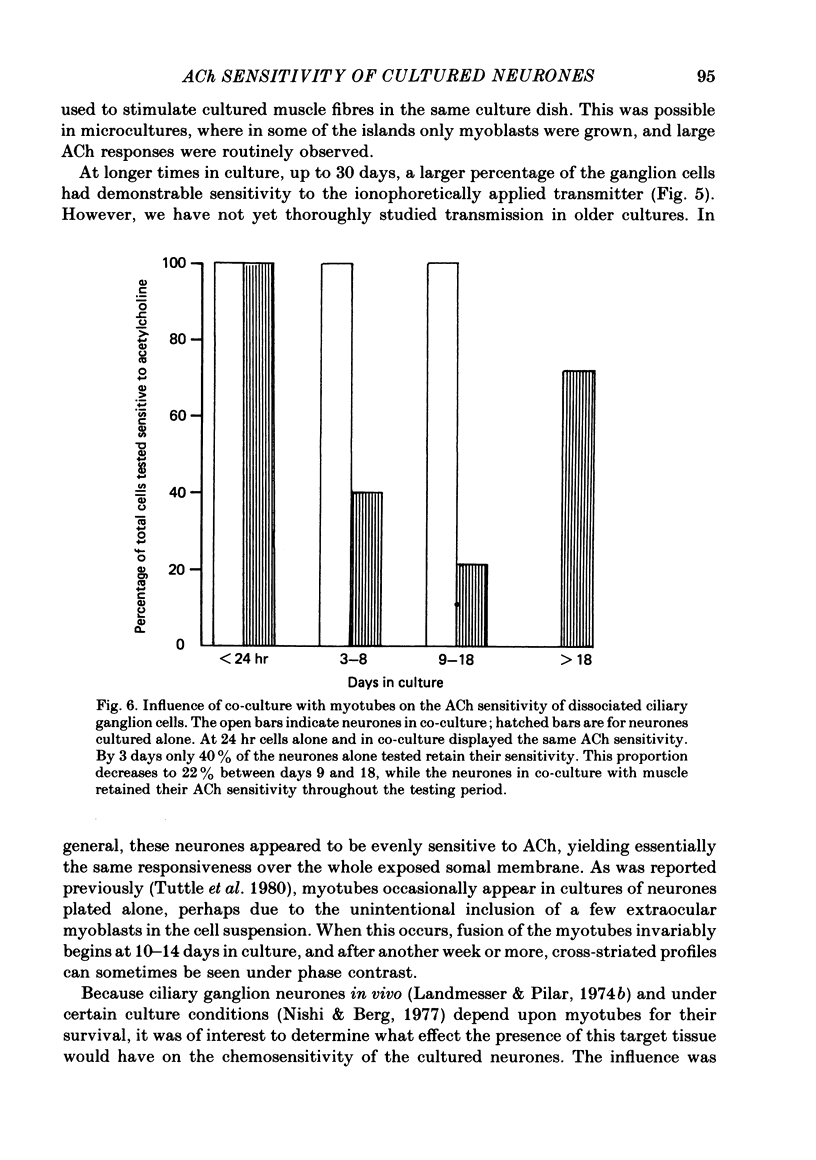
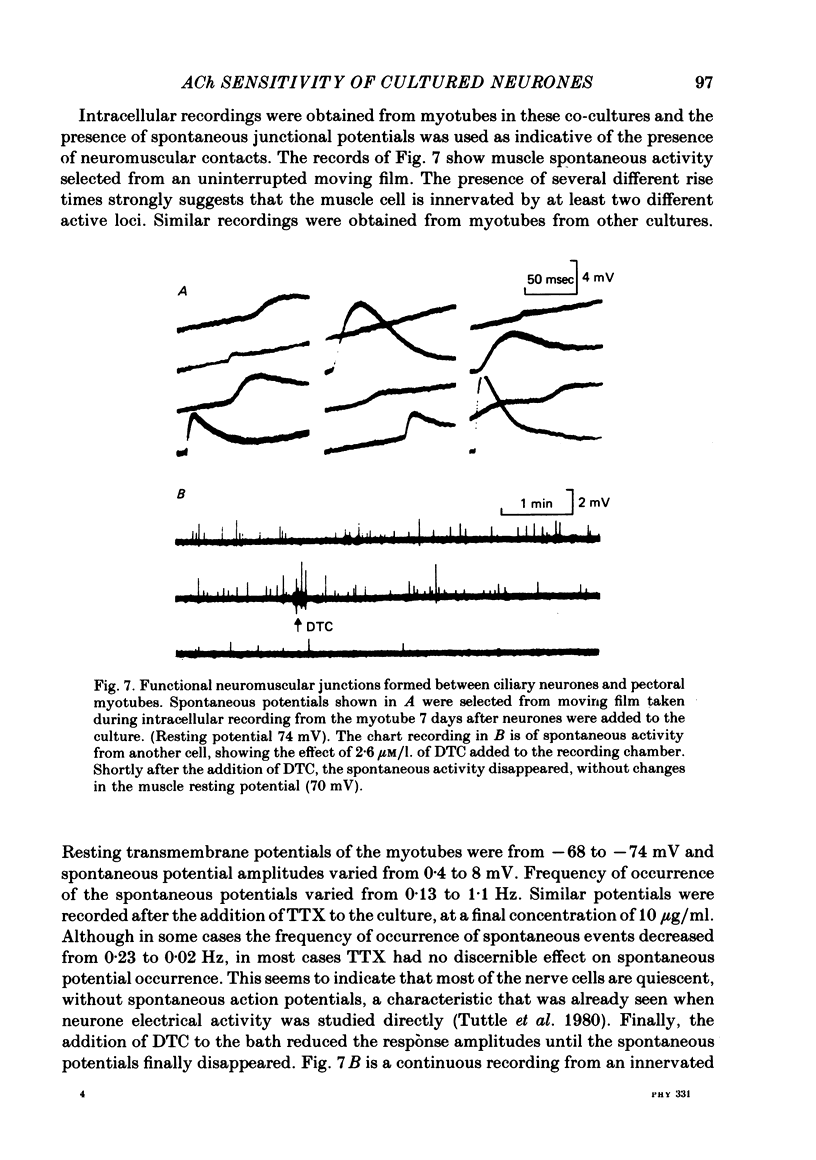
1. The influence of target interaction upon the electrophysiological properties of dissociated ciliary ganglion cells was investigated by testing the sensitivity of the neuronal somal membrane to ionophoretically applied acetylcholine (ACh). Variations in the percentage of cells responsive to the transmitter were measured with time in culture. 2. Twenty-four hours after plating, all cells respond to an ionophoretic pulse of ACh with a depolarization. However, 1 week after plating (between 7 and 14 days) most of the neurones are unresponsive, and highly responsive cells (greater than 100 mV peak depolarization/nC) are extremely rare. At even later times in culture, neurones sensitive to the transmitter are again more frequent. 3. When neurones are plated onto pre-formed pectoral myotubes, however, ACh sensitivity is maintained throughout a 3 week culture period. Neuromuscular junctions are formed by the neurones, and when sufficient neurones are present, all the muscle fibres tested show evidence of functional synaptic transmission. Chemosensitivity to ACh is not maintained by neurones in muscle-free microcultures are present on the same cover-slip. 4. Interneuronal synaptic contacts, defined by ultrastructural criteria, are formed in cultures of neurones alone, but evidence of widespread functional synaptic interaction between cells was not found at 7-14 days in culture. 5. It is concluded that the maintenance of ACh sensitivity of cultured ciliary ganglion cells is enhanced by the presence of muscle in co-culture. The interneuronal synaptic contacts observed are apparently not as potent a stimulus as co-culture with muscle for the full expression of the cholinergic phenotype under these culture conditions.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz W. The formation of synapses between chick embryo skeletal muscle and ciliary ganglia grown in vitro. J Physiol. 1976 Jan;254(1):63–73. doi: 10.1113/jphysiol.1976.sp011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B. Regulation of autonomic development. Annu Rev Neurosci. 1978;1:183–214. doi: 10.1146/annurev.ne.01.030178.001151. [DOI] [PubMed] [Google Scholar]
- Bonyhady R. E., Hendry I. A., Hill C. E., McLennan I. S. Characterization of a cardiac muscle factor required for the survival of cultured parasympathetic neurones. Neurosci Lett. 1980 Jun;18(2):197–201. doi: 10.1016/0304-3940(80)90326-2. [DOI] [PubMed] [Google Scholar]
- Brenner H. R., Martin A. R. Reduction in acetylcholine sensitivity of axotomized ciliary ganglion cells. J Physiol. 1976 Aug;260(1):159–175. doi: 10.1113/jphysiol.1976.sp011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetto S. T., Fambrough D. M., Muller K. J. Nonequivalence of alpha-bungarotoxin receptors and acetylcholine receptors in chick sympathetic neurons. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1016–1020. doi: 10.1073/pnas.75.2.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetto S., Fambrough D. M. Synthesis, insertion into the plasma membrane, and turnover of alpha-bungarotoxin receptors in chick sympathetic neurons. J Cell Biol. 1979 Jun;81(3):555–569. doi: 10.1083/jcb.81.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappinelli V. A., Giacobini E. Time course of appearance of alpha-bungarotoxin binding sites during development of chick ciliary ganglion and iris. Neurochem Res. 1978 Aug;3(4):465–478. doi: 10.1007/BF00966328. [DOI] [PubMed] [Google Scholar]
- Chiappinelli V., Giacobini E., Pilar G., Uchimura H. Induction of cholinergic enzymes in chick ciliary ganglion and iris muscle cells during synapse formation. J Physiol. 1976 Jun;257(3):749–766. doi: 10.1113/jphysiol.1976.sp011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J., MILEDI R. A study of foetal and new-born rat muscle fibres. J Physiol. 1962 Aug;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J. Development of the neuromuscular junction: inductive interactions between cells. Annu Rev Neurosci. 1981;4:43–68. doi: 10.1146/annurev.ne.04.030181.000355. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Iontophoretic application of acetylcholine: advantages of high resistance micropipettes in connection with an electronic current pump. Pflugers Arch. 1974 Apr 22;348(3):263–272. doi: 10.1007/BF00587417. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Berg D. K., Cohen S. A., Frank E. Enrichment of nerve--muscle synapses in spinal cord--muscle cultures and identification of relative peaks of ACh sensitivity at sites of transmitter release. Cold Spring Harb Symp Quant Biol. 1976;40:347–357. doi: 10.1101/sqb.1976.040.01.034. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Frank E., Jessell T. M., Rubin L. L., Schuetze S. M. Accumulation of acetylcholine receptors and acetylcholinesterase at newly formed nerve-muscle synapses. Pharmacol Rev. 1978 Dec;30(4):411–428. [PubMed] [Google Scholar]
- Furshpan E. J., MacLeish P. R., O'Lague P. H., Potter D. D. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E. Neurotrophic relations. Annu Rev Physiol. 1976;38:177–216. doi: 10.1146/annurev.ph.38.030176.001141. [DOI] [PubMed] [Google Scholar]
- HAMORI J., DYACHKOVA L. N. ELECTRON MICROSCOPE STUDIES ON DEVELOPMENTAL DIFFERENTIATION ON CILIARY GANGLION SYNAPSES IN THE CHICK. Acta Biol Acad Sci Hung. 1964;15:213–230. [PubMed] [Google Scholar]
- Harris A. J., Kuffler S. W., Dennis M. J. Differential chemosensitivity of synaptic and extrasynaptic areas on the neuronal surface membrane in parasympathetic neurons of the frog, tested by microapplication of acetylcholine. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):541–553. doi: 10.1098/rspb.1971.0046. [DOI] [PubMed] [Google Scholar]
- Harris W. A. Neural activity and development. Annu Rev Physiol. 1981;43:689–710. doi: 10.1146/annurev.ph.43.030181.003353. [DOI] [PubMed] [Google Scholar]
- Helfand S. L., Smith G. A., Wessells N. K. Survival and development in culture of dissociated parasympathetic neurons from ciliary ganglia. Dev Biol. 1976 Jun;50(2):541–547. doi: 10.1016/0012-1606(76)90174-3. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Dennis M. J., Harris A. J. The development of chemosensitivity in extrasynaptic areas of the neuronal surface after denervation of parasympathetic ganglion cells in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):555–563. doi: 10.1098/rspb.1971.0047. [DOI] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Fate of ganglionic synapses and ganglion cell axons during normal and induced cell death. J Cell Biol. 1976 Feb;68(2):357–374. doi: 10.1083/jcb.68.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Synapse formation during embryogenesis on ganglion cells lacking a periphery. J Physiol. 1974 Sep;241(3):715–736. doi: 10.1113/jphysiol.1974.sp010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Synaptic transmission and cell death during normal ganglionic development. J Physiol. 1974 Sep;241(3):737–749. doi: 10.1113/jphysiol.1974.sp010681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. The onset and development of transmission in the chick ciliary ganglion. J Physiol. 1972 May;222(3):691–713. doi: 10.1113/jphysiol.1972.sp009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. DUAL MODE OF SYNAPTIC TRANSMISSION IN THE AVIAN CILIARY GANGLION. J Physiol. 1963 Sep;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing A., Kim S. U. Developments of alpha-bungarotoxin receptors in cultured chick ciliary ganglion neurons. Brain Res. 1981 Mar 16;208(2):479–486. doi: 10.1016/0006-8993(81)90581-3. [DOI] [PubMed] [Google Scholar]
- Nishi R., Berg D. K. Dissociated ciliary ganglion neurons in vitro: survival and synapse formation. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5171–5175. doi: 10.1073/pnas.74.11.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Lague P. H., Potter D. D., Furshpan E. J. Studies on rat sympathetic neurons developing in cell culture. III. Cholinergic transmission. Dev Biol. 1978 Dec;67(2):424–443. doi: 10.1016/0012-1606(78)90210-5. [DOI] [PubMed] [Google Scholar]
- Obata K. Development of neuromuscular transmission in culture with a variety of neurons and in the presence of cholinergic substances and tetrodotoxin. Brain Res. 1977 Jan 1;119(1):141–153. doi: 10.1016/0006-8993(77)90096-8. [DOI] [PubMed] [Google Scholar]
- Patterson P. H. Environmental determination of autonomic neurotransmitter functions. Annu Rev Neurosci. 1978;1:1–17. doi: 10.1146/annurev.ne.01.030178.000245. [DOI] [PubMed] [Google Scholar]
- Pilar G., Landmesser L., Burstein L. Competition for survival among developing ciliary ganglion cells. J Neurophysiol. 1980 Jan;43(1):233–254. doi: 10.1152/jn.1980.43.1.233. [DOI] [PubMed] [Google Scholar]
- Pilar G., Landmesser L. Ultrastructural differences during embryonic cell death in normal and peripherally deprived ciliary ganglia. J Cell Biol. 1976 Feb;68(2):339–356. doi: 10.1083/jcb.68.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilar G., Tuttle J., Vaca K. Functional maturation of motor nerve terminals in the avian iris: ultrastructure, transmitter metabolism and synaptic reliability. J Physiol. 1981 Dec;321:175–193. doi: 10.1113/jphysiol.1981.sp013978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin P. M., Berg D. K. Inhibition of neuronal acetylcholine sensitivity by alpha-toxins from Bungarus multicinctus venom. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2072–2076. doi: 10.1073/pnas.76.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B. S., Engelbert V. E., Fisher K. C. Morphological and electrophysiological characteristics of dissociated chick embryonic spinal ganglion cells in culture. Exp Neurol. 1969 Feb;23(2):230–248. doi: 10.1016/0014-4886(69)90060-0. [DOI] [PubMed] [Google Scholar]
- Slaaf D. W., Hooisma J., Meeter E., Stevens W. F. Effects of innervation by ciliary ganglia on developing muscle in vitro. Brain Res. 1979 Oct 12;175(1):87–107. doi: 10.1016/0006-8993(79)90516-x. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Tuttle J. B., Suszkiw J. B., Ard M. Long-term survival and development of dissociated parasympathetic neurons in culture. Brain Res. 1980 Feb 3;183(1):161–180. doi: 10.1016/0006-8993(80)90127-4. [DOI] [PubMed] [Google Scholar]
- Van der Loos H., Glaser E. M. Autapses in neocortex cerebri: synapses between a pyramidal cell's axon and its own dendrites. Brain Res. 1972 Dec 24;48:355–360. doi: 10.1016/0006-8993(72)90189-8. [DOI] [PubMed] [Google Scholar]
- Varon S., Manthorpe M., Adler R. Cholinergic neuronotrophic factors: I. Survival, neurite outgrowth and choline acetyltransferase activity in monolayer cultures from chick embryo ciliary ganglia. Brain Res. 1979 Sep 7;173(1):29–45. doi: 10.1016/0006-8993(79)91093-x. [DOI] [PubMed] [Google Scholar]
- Wakshull E., Johnson M. I., Burton H. Postnatal rat sympathetic neurons in culture. I. A comparison with embryonic neurons. J Neurophysiol. 1979 Sep;42(5):1410–1425. doi: 10.1152/jn.1979.42.5.1410. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]





