Abstract
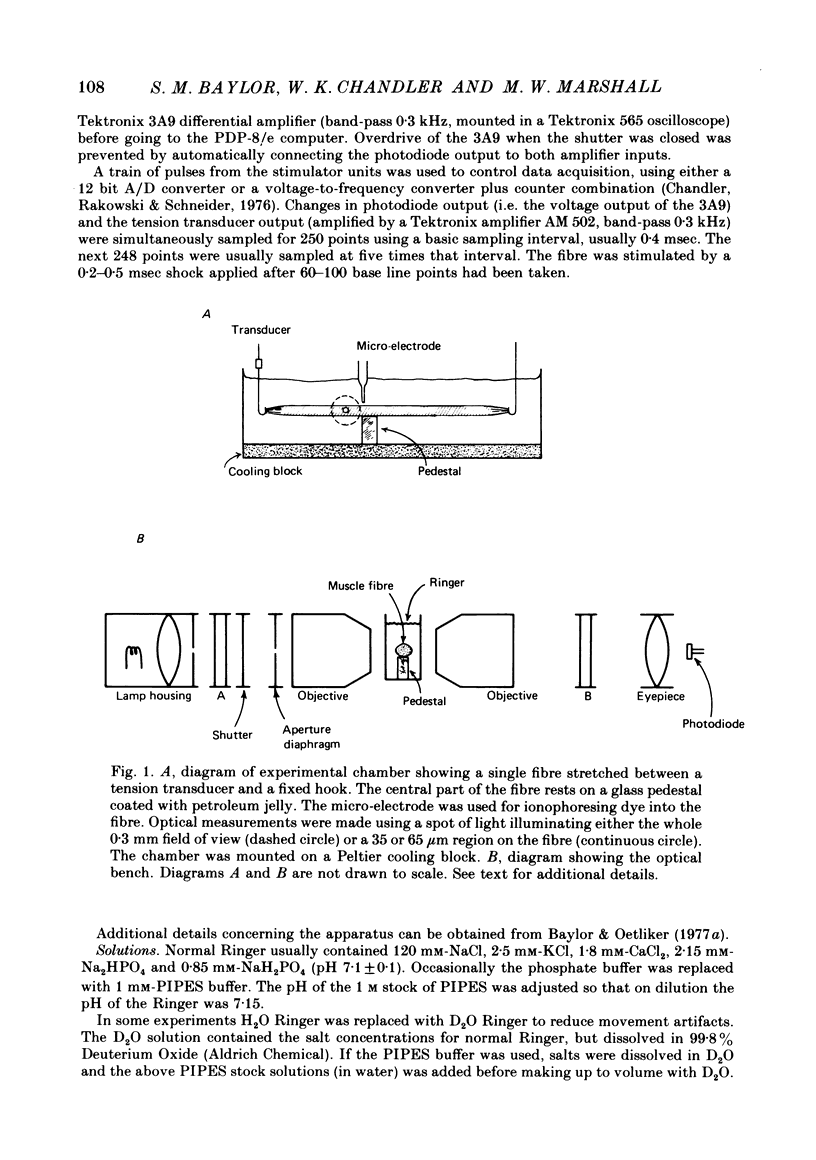
1. Single twitch fibres were isolated from frog muscle, then mounted in a chamber which was positioned on an optical bench. The fibres were immobilized by high stretch (sarcomere spacing 3·9-4·3 μm) and by placement on a pedestal. Their optical properties were determined by illuminating a 35-65 μm diameter spot with quasimonochromatic light of intensity I0 and measuring the intensity I of the transmitted light. Since the main purpose of the experiments was to draw inferences from the absorbance spectra of different indicator dyes injected into the fibres, all results were expressed in terms of absorbance A calculated from the equation [Formula: see text]. Changes in absorbance ΔA were calculated from the differential form of the equation [Formula: see text].
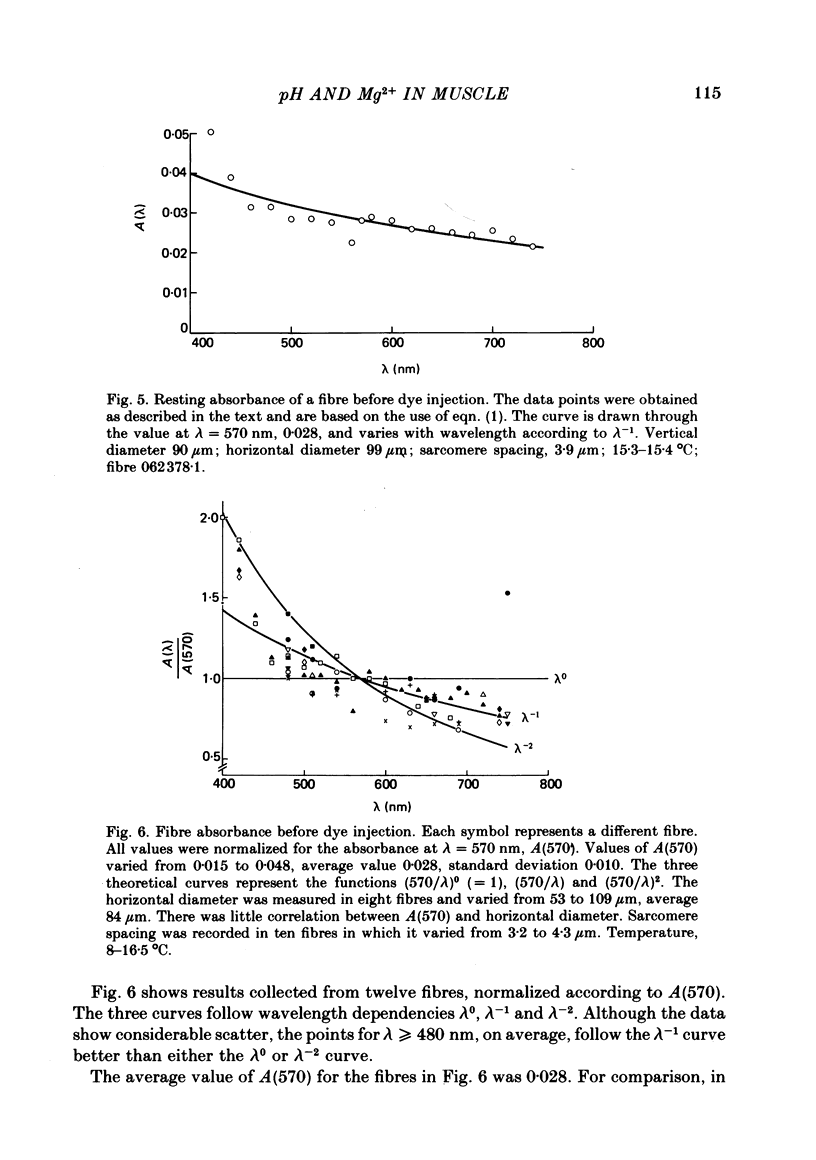
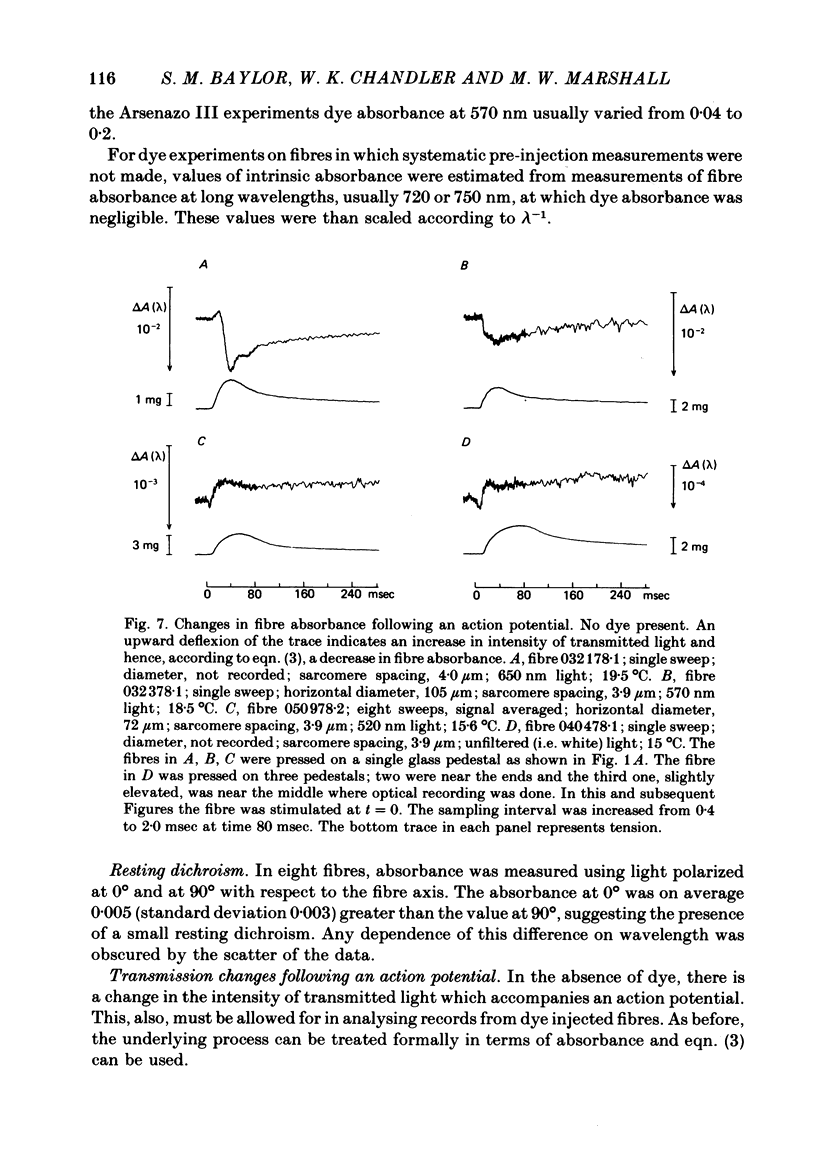
2. The absorbance of a normal, non-injected fibre was, on average, equal to 0·03 at 570 nm and varied approximately inversely with wavelength between 450 and 750 nm.
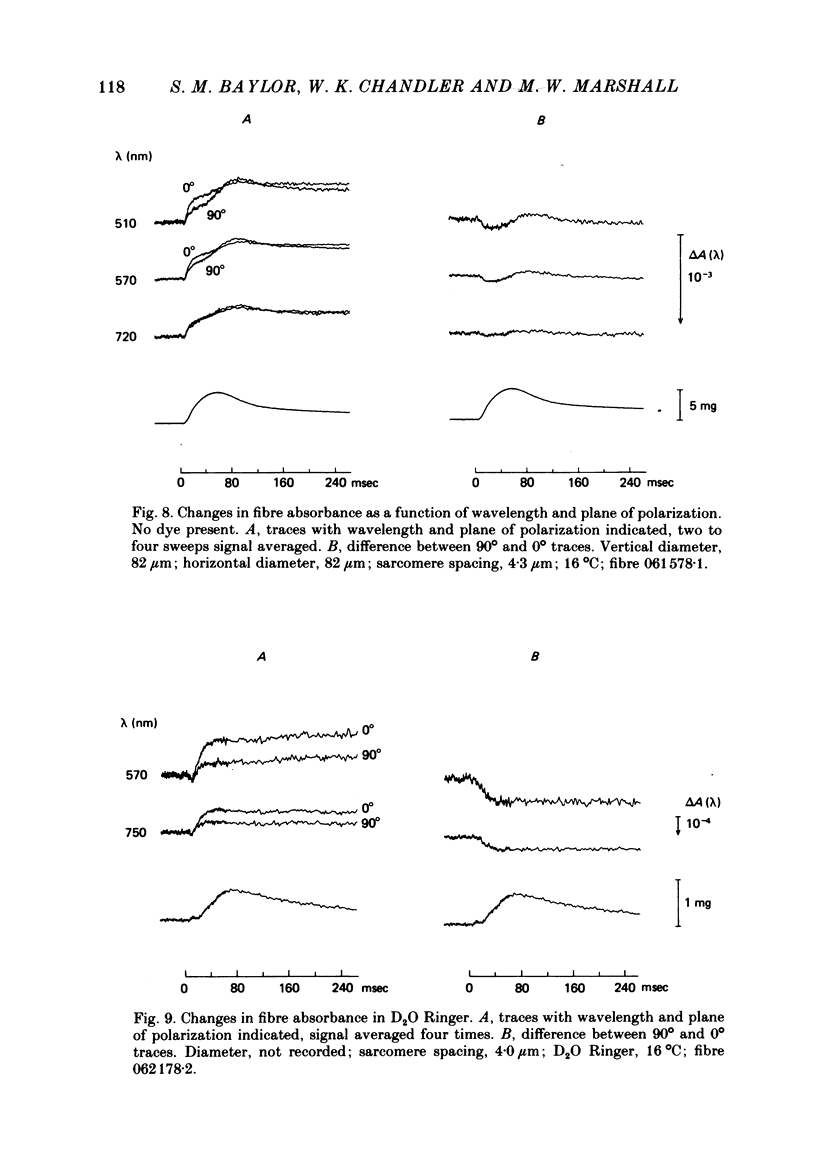
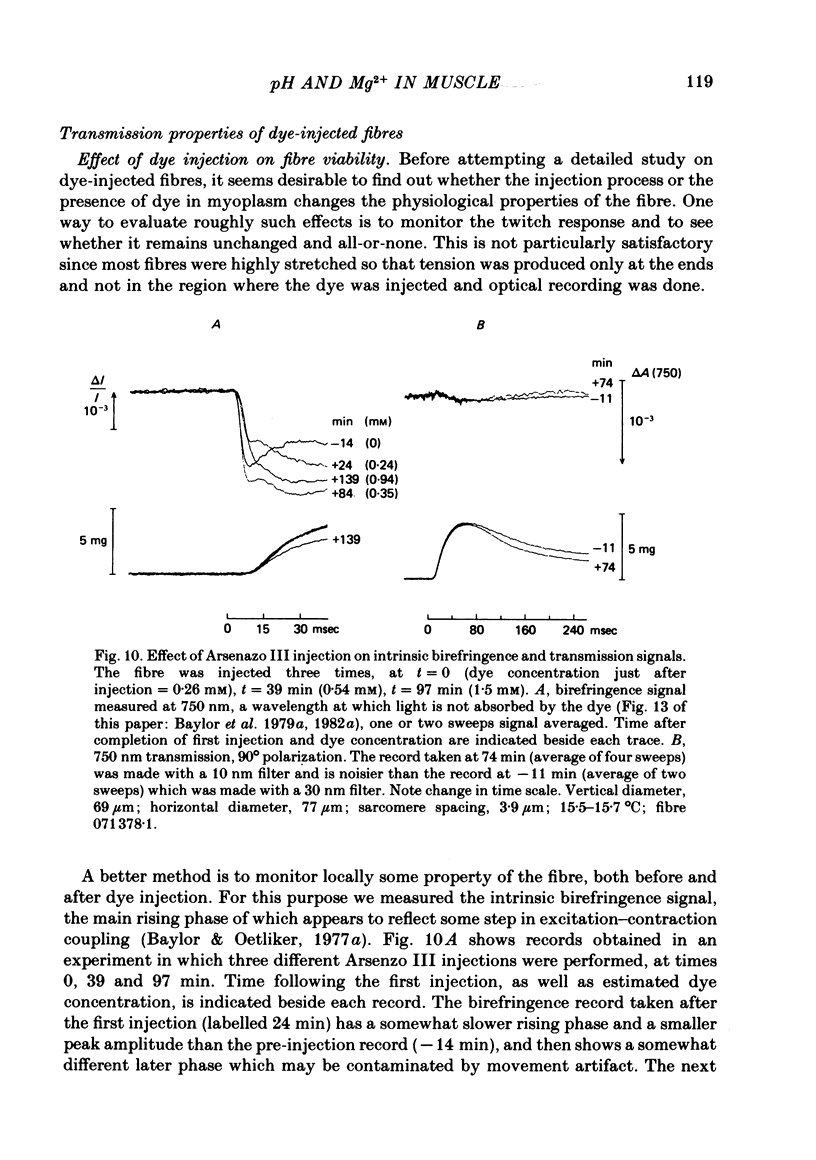
3. The earliest change in absorbance following an action potential was a small, transient increase which was followed by a larger decrease. The decrease in fibre absorbance varied from 0·5 × 10-4 to 3 × 10-4 units.
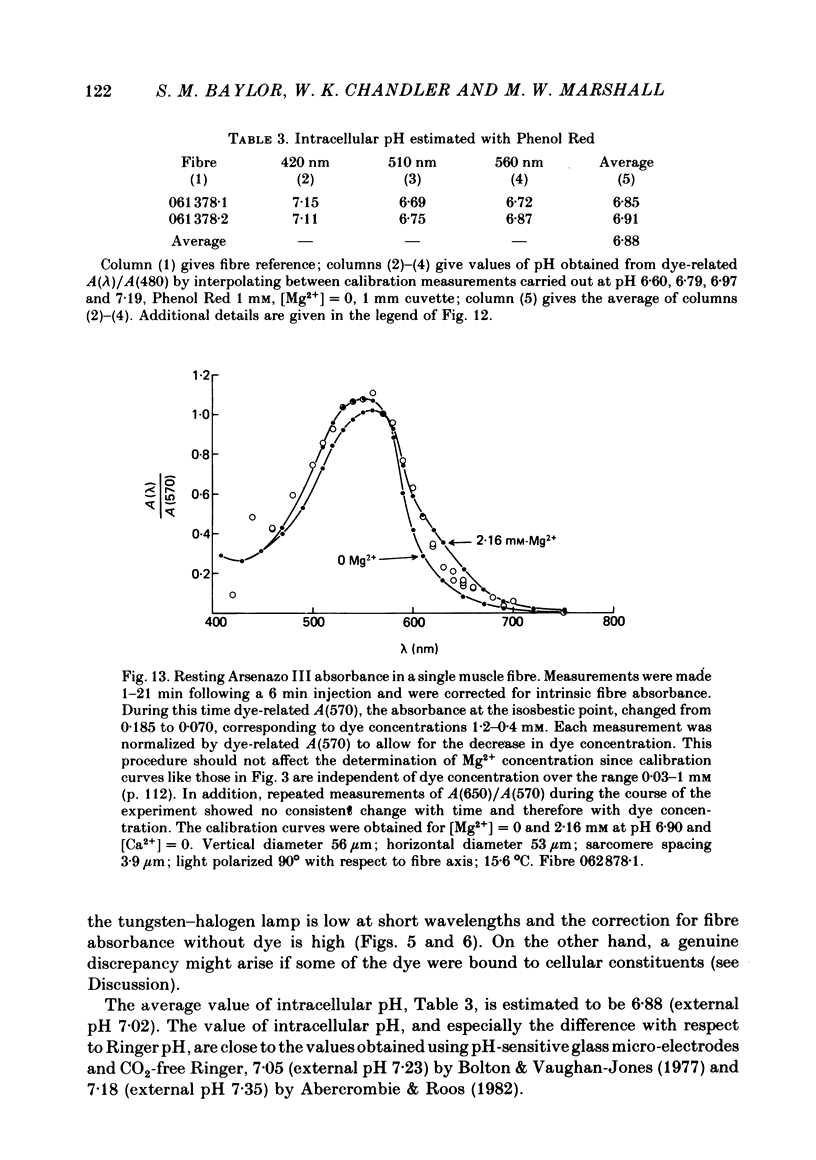
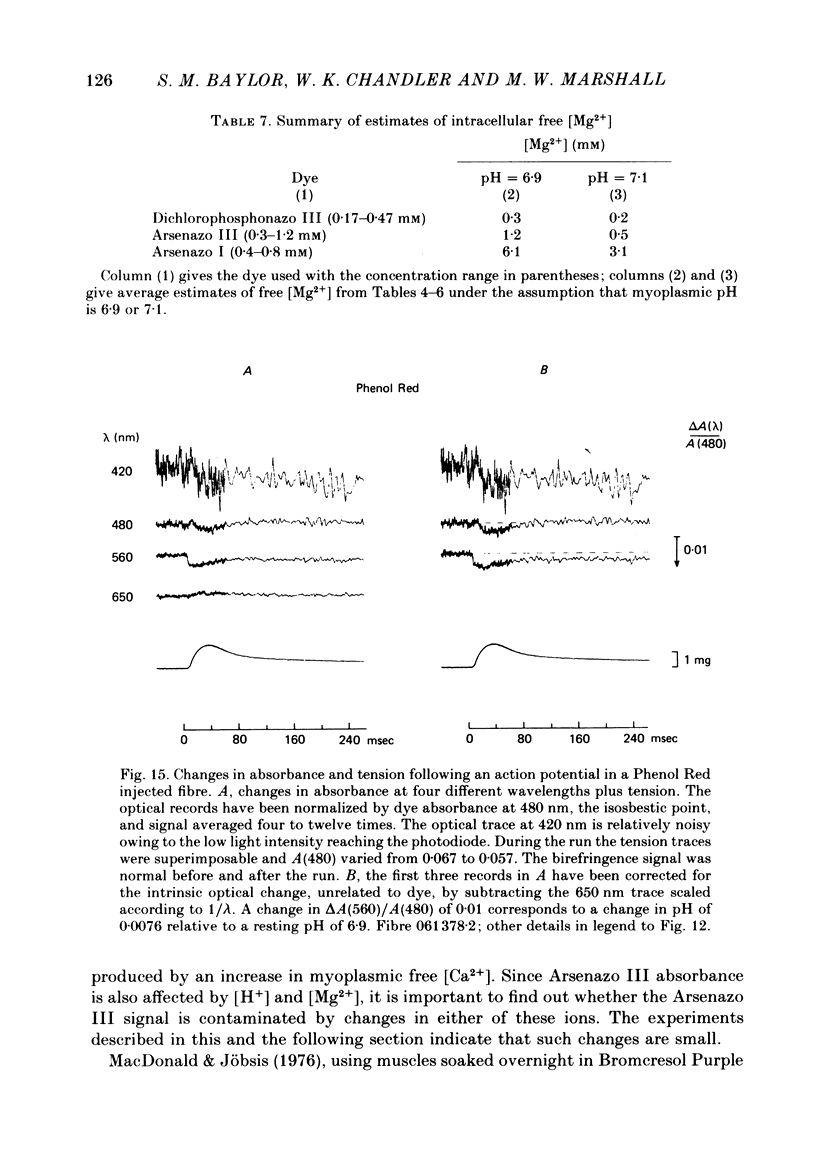
4. Resting myoplasmic pH was determined by comparing the absorbance spectrum from fibres injected with Phenol Red with that obtained from calibrating solutions in cuvettes. The muscle measurements were corrected for the intrinsic absorbance of fibre without dye. The average value of pH in two fibres was 6·9. The change in absorbance following an action potential in these highly stretched fibres was small. In one experiment the change, if due to pH alone, corresponded to an increase in pH of 0·004 peak and 0·002 maintained (relative to a resting level of 6·9). The maintained signal can be satisfactorily explained by the known amount of phosphocreatine hydrolysis.
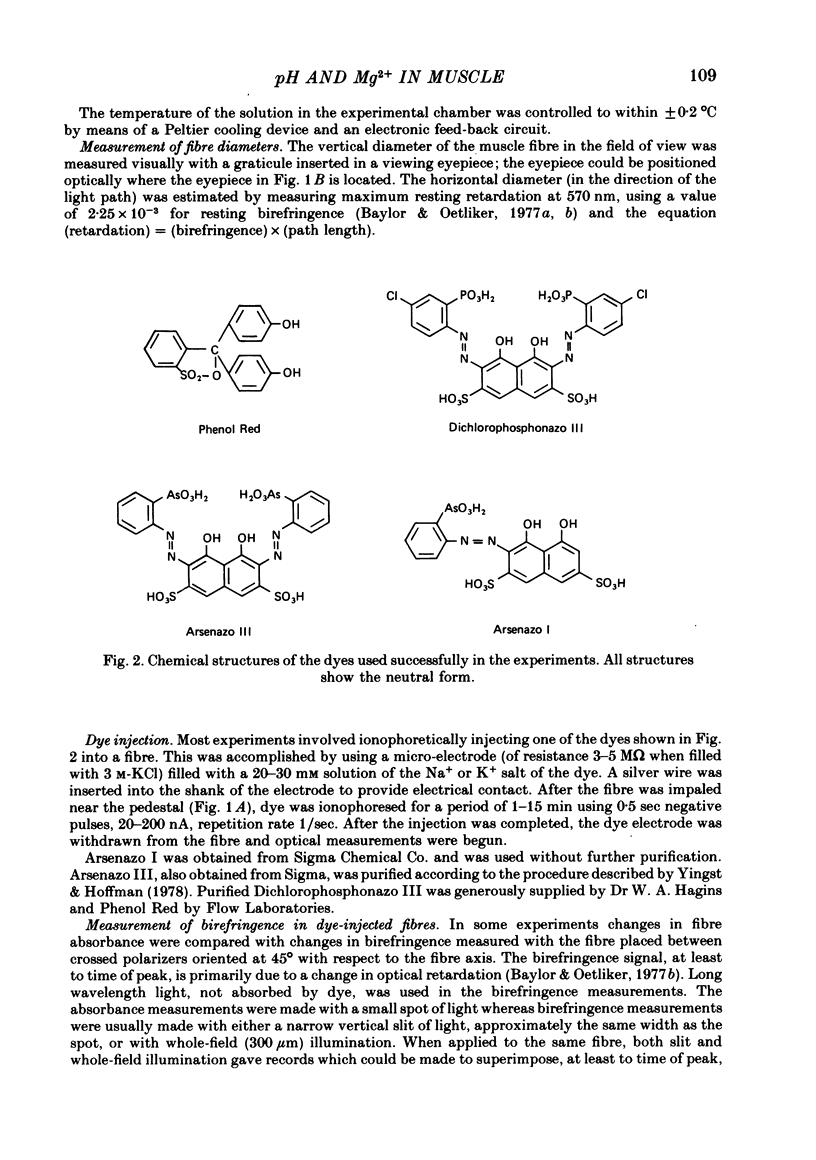
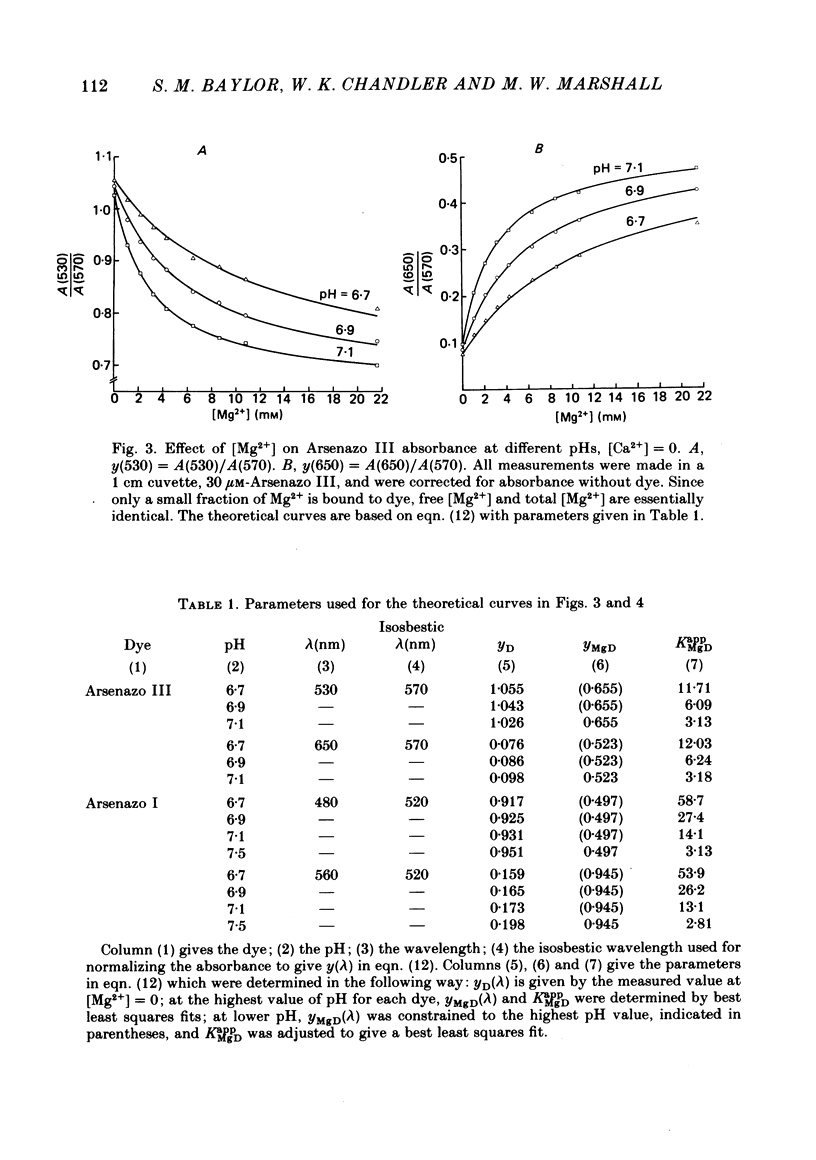
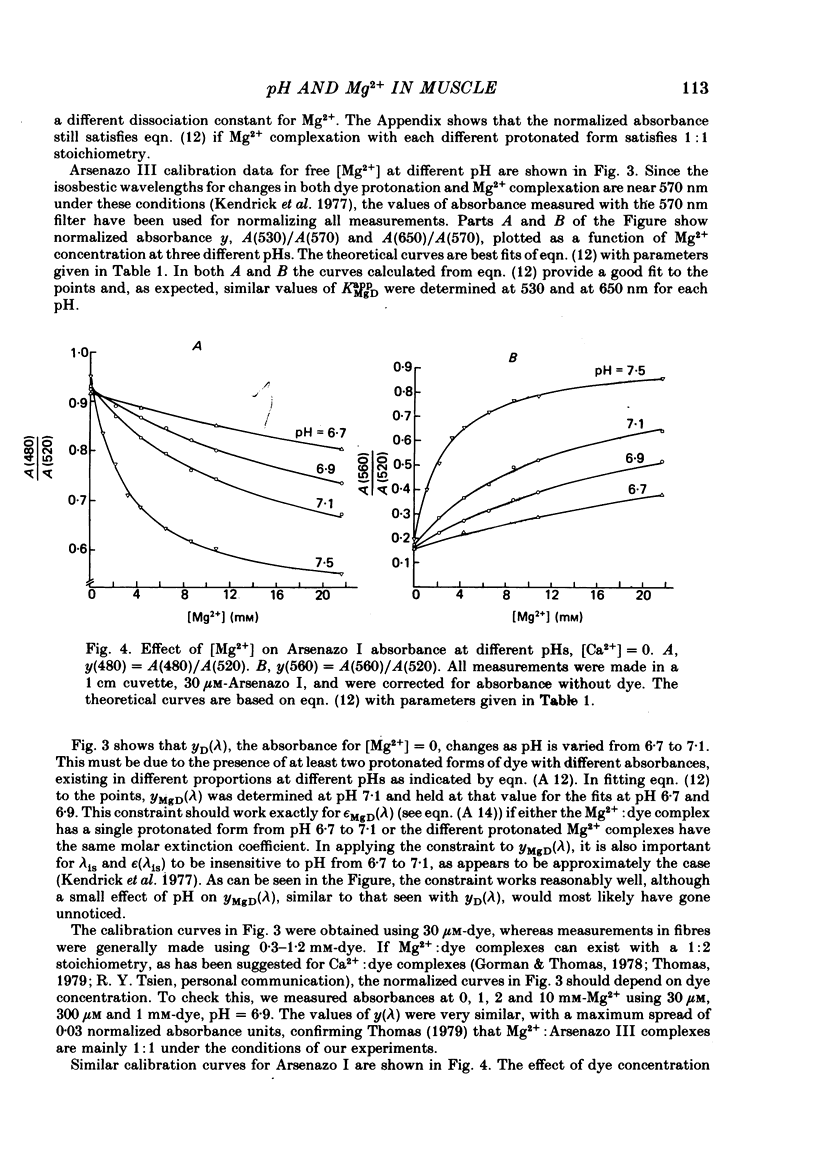
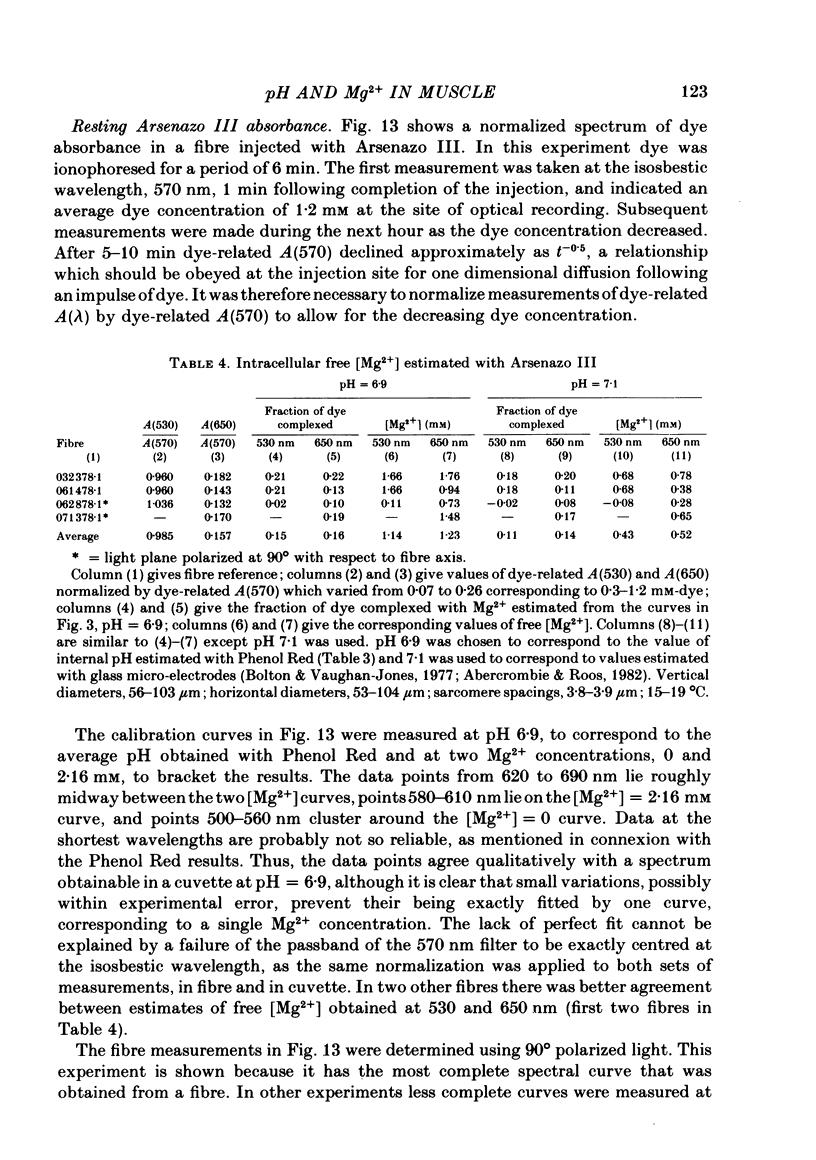
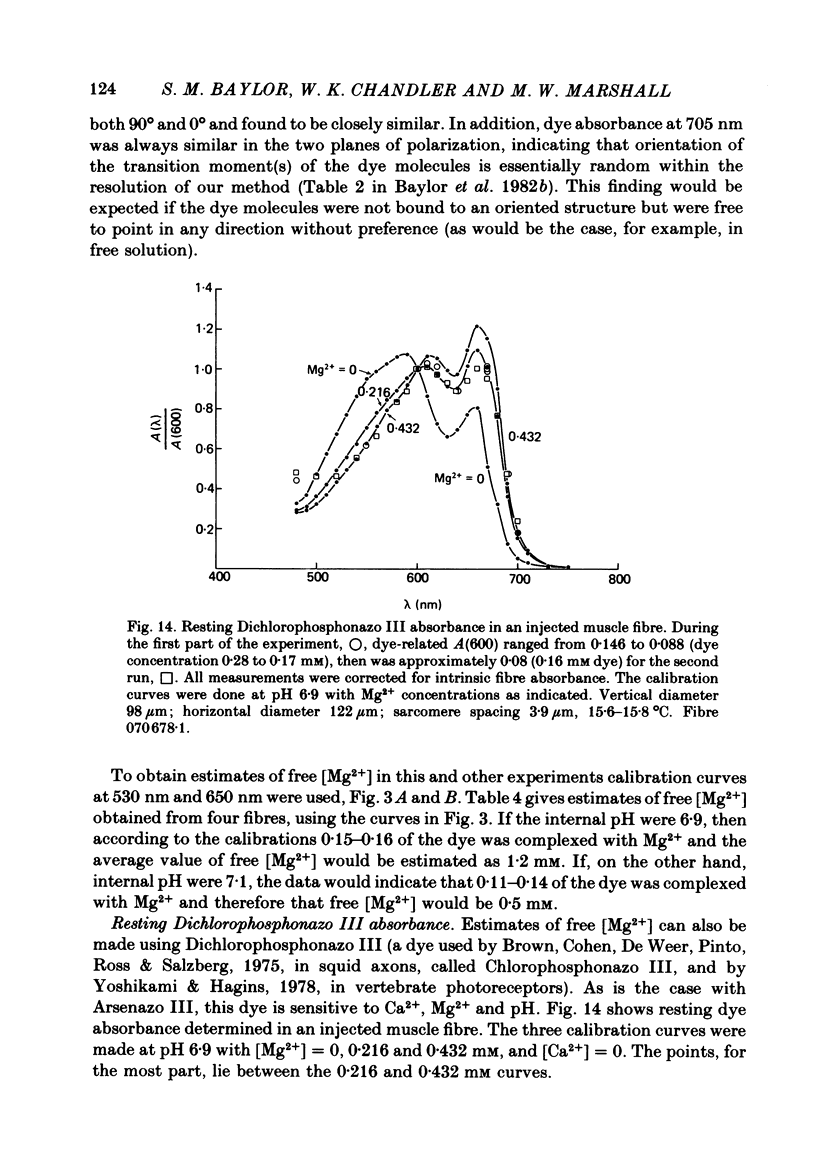
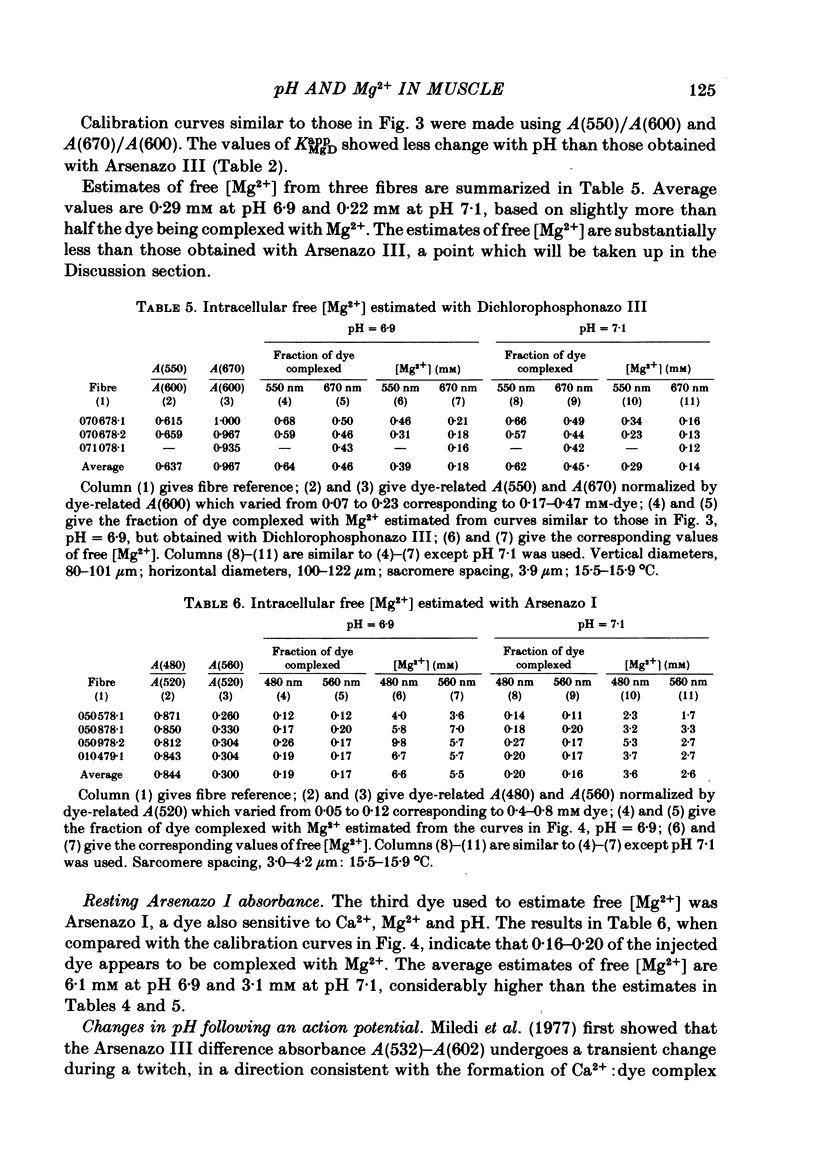
5. Estimates of myoplasmic free [Mg2+] were made using three metallochromic indicator dyes. A different estimate was obtained with each dye as indicated below. Since these dyes are sensitive to pH, as well as [Mg2+], the estimate depends on the assumed value of intracellular pH. [List: see text] This variability probably means that at least two, and possibly all three dyes behave differently inside muscle fibres than they do in calibrating solutions. The most likely explanation is that dye, once injected, can bind to cellular contents and that this alters its properties.
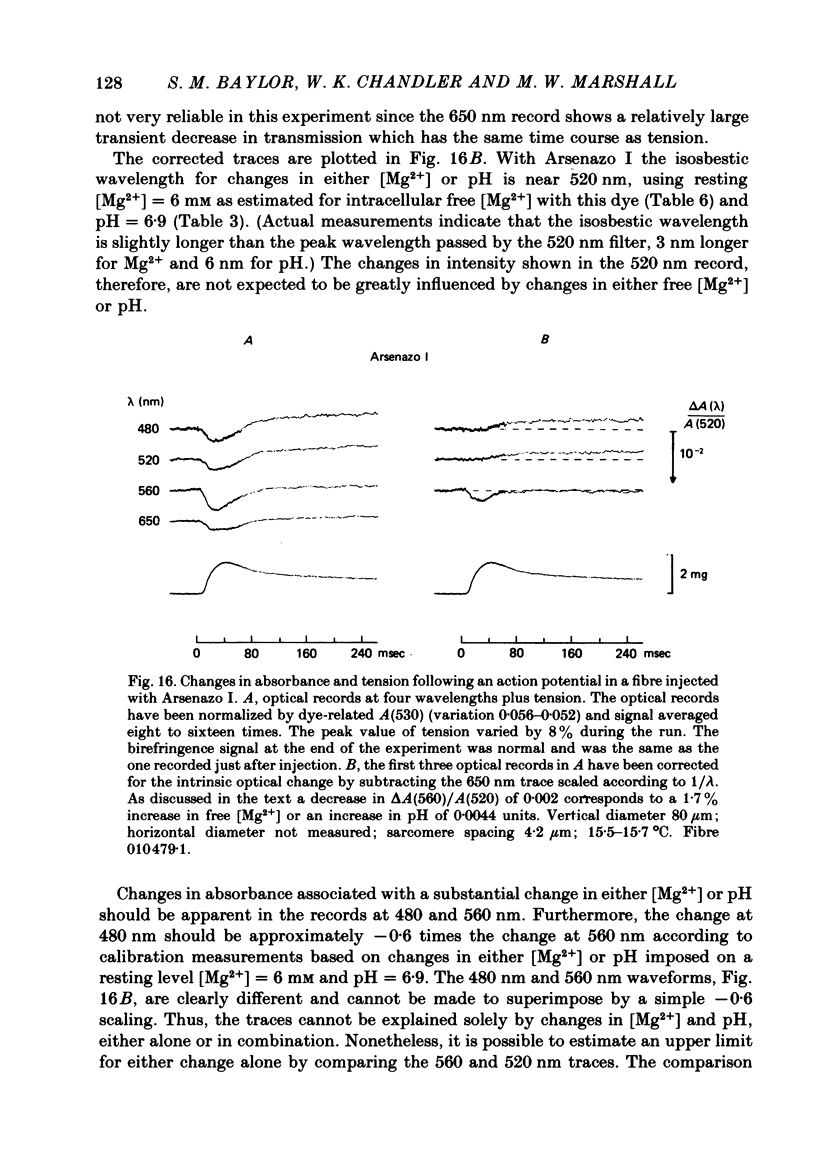
6. Changes in absorbance of fibres injected with Arsenazo I, a dye three times more sensitive to Mg2+ than to Ca2+, were used to determine whether changes in free [Mg2+] occur following an action potential. The observed changes were small and could be due to a small increase in pH, of the magnitude measured with Phenol Red, and/or free [Mg2+]. In terms of a change in free [Mg2+], the results set an upper limit of 2%.
7. The conclusion from the action potential experiments is that neither intracellular pH nor free [Mg2+] changes appreciably in highly stretched fibres. Changes in these two quantities can therefore be neglected in analysing the relatively large 650-660 nm Ca2+ signal in fibres injected with the Ca2+ (but also pH and Mg2+) sensitive indicator dye Arsenazo III.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Arsenazo III signals in singly dissected frog twitch fibres [proceedings]. J Physiol. 1979 Feb;287:23P–24P. [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Dichroic components of Arsenazo III and dichlorophosphonazo III signals in skeletal muscle fibres. J Physiol. 1982 Oct;331:179–210. doi: 10.1113/jphysiol.1982.sp014369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Use of metallochromic dyes to measure changes in myoplasmic calcium during activity in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:139–177. doi: 10.1113/jphysiol.1982.sp014368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. A large birefringence signal preceding contraction in single twitch fibres of the frog. J Physiol. 1977 Jan;264(1):141–162. doi: 10.1113/jphysiol.1977.sp011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. The optical properties of birefringence signals from single muscle fibres. J Physiol. 1977 Jan;264(1):163–198. doi: 10.1113/jphysiol.1977.sp011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler T. J., Schibeci A., Martonosi A. The binding of arsenazo III to cell components. Biochim Biophys Acta. 1980 May 7;629(2):317–327. doi: 10.1016/0304-4165(80)90104-x. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Vaughan-Jones R. D. Continuous direct measurement of intracellular chloride and pH in frog skeletal muscle. J Physiol. 1977 Sep;270(3):801–833. doi: 10.1113/jphysiol.1977.sp011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Cohen L. B., De Weer P., Pinto L. H., Ross W. N., Salzberg B. M. Rapid changes in intracellular free calcium concentration. Detection by metallochromic indicator dyes in squid giant axon. Biophys J. 1975 Nov;15(11):1155–1160. doi: 10.1016/S0006-3495(75)85891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M., Davila H. V., Ross W. N., Landowne D., Waggoner A. S., Wang C. H. Changes in axon fluorescence during activity: molecular probes of membrane potential. J Membr Biol. 1974;19(1):1–36. doi: 10.1007/BF01869968. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Burt C. T. 31P nuclear magnetic relaxation studies of phosphocreatine in intact muscle: determination of intracellular free magnesium. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4271–4275. doi: 10.1073/pnas.74.10.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Energy changes and muscular contraction. Physiol Rev. 1978 Jul;58(3):690–761. doi: 10.1152/physrev.1978.58.3.690. [DOI] [PubMed] [Google Scholar]
- DESMEDT J. E. Electrical activity and intracellular sodium concentration in frog muscle. J Physiol. 1953 Jul;121(1):191–205. doi: 10.1113/jphysiol.1953.sp004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Changes in the intracellular concentration of free calcium ions in a pace-maker neurone, measured with the metallochromic indicator dye arsenazo III. J Physiol. 1978 Feb;275:357–376. doi: 10.1113/jphysiol.1978.sp012194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. K., Moore R. D. 31P NMR studies of intracellular free Mg2+ in intact frog skeletal muscle. J Biol Chem. 1980 May 10;255(9):3987–3993. [PubMed] [Google Scholar]
- HUXLEY A. F., PEACHEY L. D. The maximum length for contraction in vertebrate straiated muscle. J Physiol. 1961 Apr;156:150–165. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. Analysis of the membrane capacity in frog muscle. J Physiol. 1972 Feb;221(1):121–136. doi: 10.1113/jphysiol.1972.sp009743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöbsis F. F., O'Connor M. J. Calcium release and reabsorption in the sartorius muscle of the toad. Biochem Biophys Res Commun. 1966 Oct 20;25(2):246–252. doi: 10.1016/0006-291x(66)90588-2. [DOI] [PubMed] [Google Scholar]
- Kendrick N. C., Ratzlaff R. W., Blaustein M. P. Arsenazo III as an indicator for ionized calcium in physiological salt solutions: its use for determination of the CaATP dissociation constant. Anal Biochem. 1977 Dec;83(2):433–450. doi: 10.1016/0003-2697(77)90052-5. [DOI] [PubMed] [Google Scholar]
- Kovács L., Ríos E., Schneider M. F. Calcium transients and intramembrane charge movement in skeletal muscle fibres. Nature. 1979 May 31;279(5712):391–396. doi: 10.1038/279391a0. [DOI] [PubMed] [Google Scholar]
- Lisman J. E., Strong J. A. The initiation of excitation and light adaptation in Limulus ventral photoreceptors. J Gen Physiol. 1979 Feb;73(2):219–243. doi: 10.1085/jgp.73.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. The effect of change in length on conduction velocity in muscle. J Physiol. 1954 Jul 28;125(1):215–220. doi: 10.1113/jphysiol.1954.sp005151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald V. W., Jöbsis F. F. Spectrophotometric studies on the pH of frog skeletal muscle. PH change during and after contractile activity. J Gen Physiol. 1976 Aug;68(2):179–195. doi: 10.1085/jgp.68.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Measurement of calcium transients in frog muscle by the use of arsenazo III. Proc R Soc Lond B Biol Sci. 1977 Aug 22;198(1131):201–210. doi: 10.1098/rspb.1977.0094. [DOI] [PubMed] [Google Scholar]
- Mobley B. A., Eisenberg B. R. Sizes of components in frog skeletal muscle measured by methods of stereology. J Gen Physiol. 1975 Jul;66(1):31–45. doi: 10.1085/jgp.66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Gilai A., Dingeman D. Dye absorption changes in single muscle fibers: an application of an automatic balancing circuit. Pflugers Arch. 1976 Apr 6;362(3):285–287. doi: 10.1007/BF00581183. [DOI] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Rall J. A. Effects of temperature on tension, tension-dependent heat, and activation heat in twitches of frog skeletal muscle. J Physiol. 1979 Jun;291:265–275. doi: 10.1113/jphysiol.1979.sp012811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdel R., Taylor S. R. Aequorin luminescence during contraction of amphibian skeletal muscle. J Physiol. 1973 Aug;233(1):5P–6P. [PubMed] [Google Scholar]
- Thomas M. V. Arsenazo III forms 2:1 complexes with Ca and 1:1 complexes with Mg under physiological conditions. Estimates of the apparent dissociation constants. Biophys J. 1979 Mar;25(3):541–548. doi: 10.1016/S0006-3495(79)85322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R., Eccleston J. F., Bagshaw C. R. Kinetic analysis of ATPase mechanisms. Q Rev Biophys. 1976 May;9(2):217–281. doi: 10.1017/s0033583500002419. [DOI] [PubMed] [Google Scholar]
- Yingst D. R., Hoffman J. F. Changes of intracellular Ca++ as measured by arsenazo III in relation to the K permeability of human erythrocyte ghosts. Biophys J. 1978 Sep;23(3):463–471. doi: 10.1016/S0006-3495(78)85462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikami S., Hagins W. A. Calcium in excitation of vertebrate rods and cones: retinal efflux of calcium studied with dichlorophosphonazo III. Ann N Y Acad Sci. 1978 Apr 28;307:545–561. doi: 10.1111/j.1749-6632.1978.tb41981.x. [DOI] [PubMed] [Google Scholar]


