Abstract
1. Changes in transmission of quasi-monochromatic light were measured in singly dissected, dye-injected twitch fibres following a single propagated action potential. The records, after correction for the intrinsic transmission signal, indicate changes in dye-related absorbance, ΔA. This paper describes the different components of dye-related signals in fibres injected with either Arsenazo III, Antipyrylazo III or Dichlorophosphonazo III.
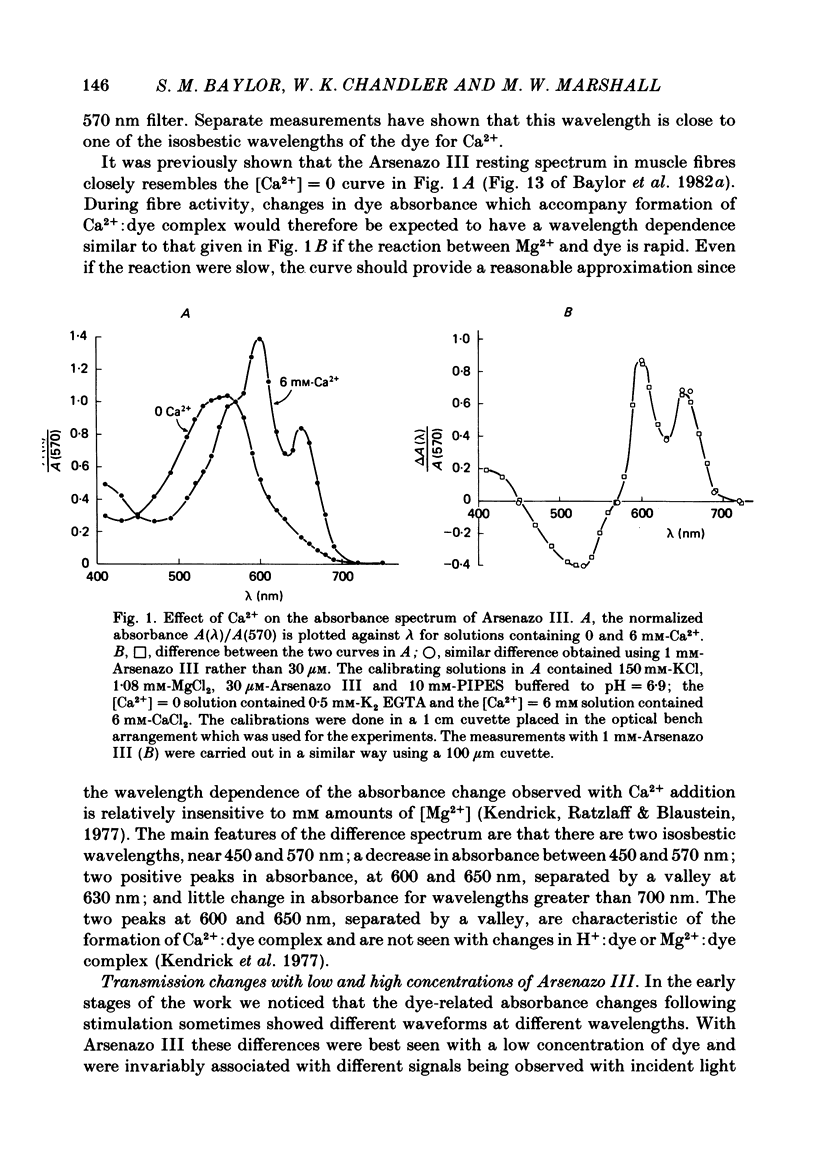
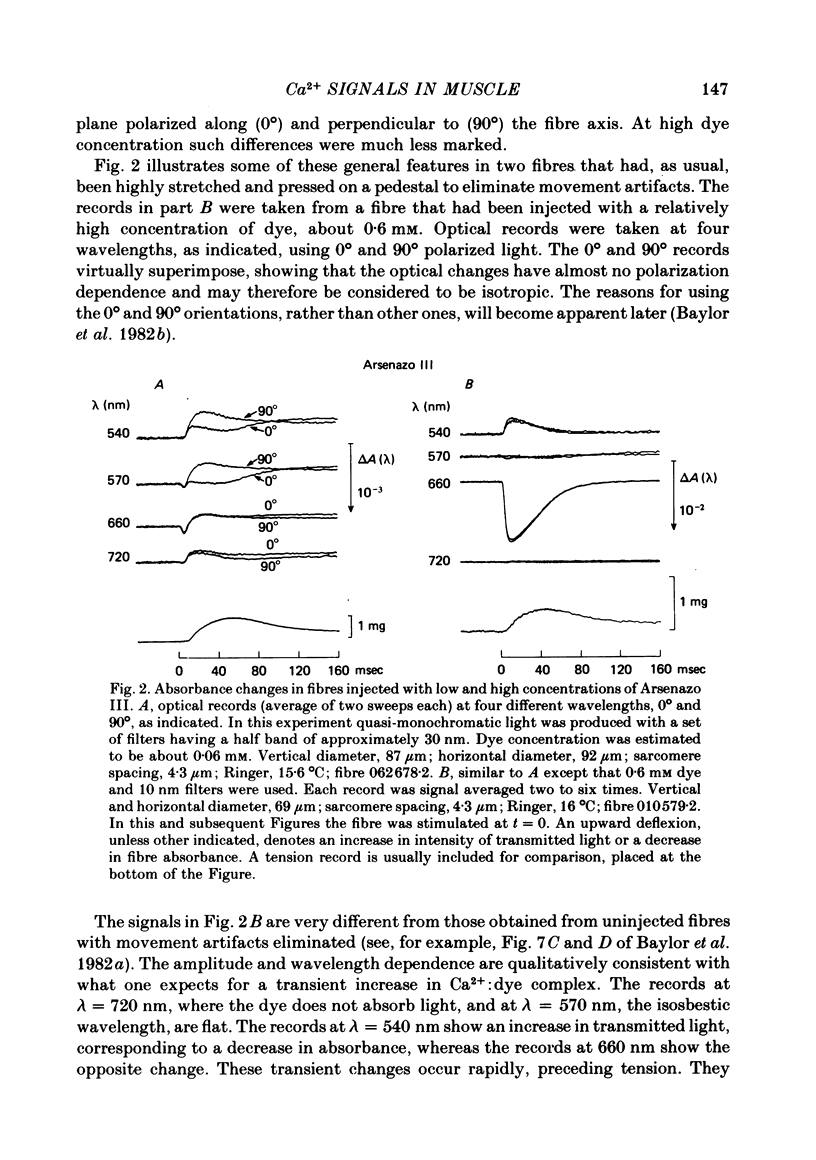
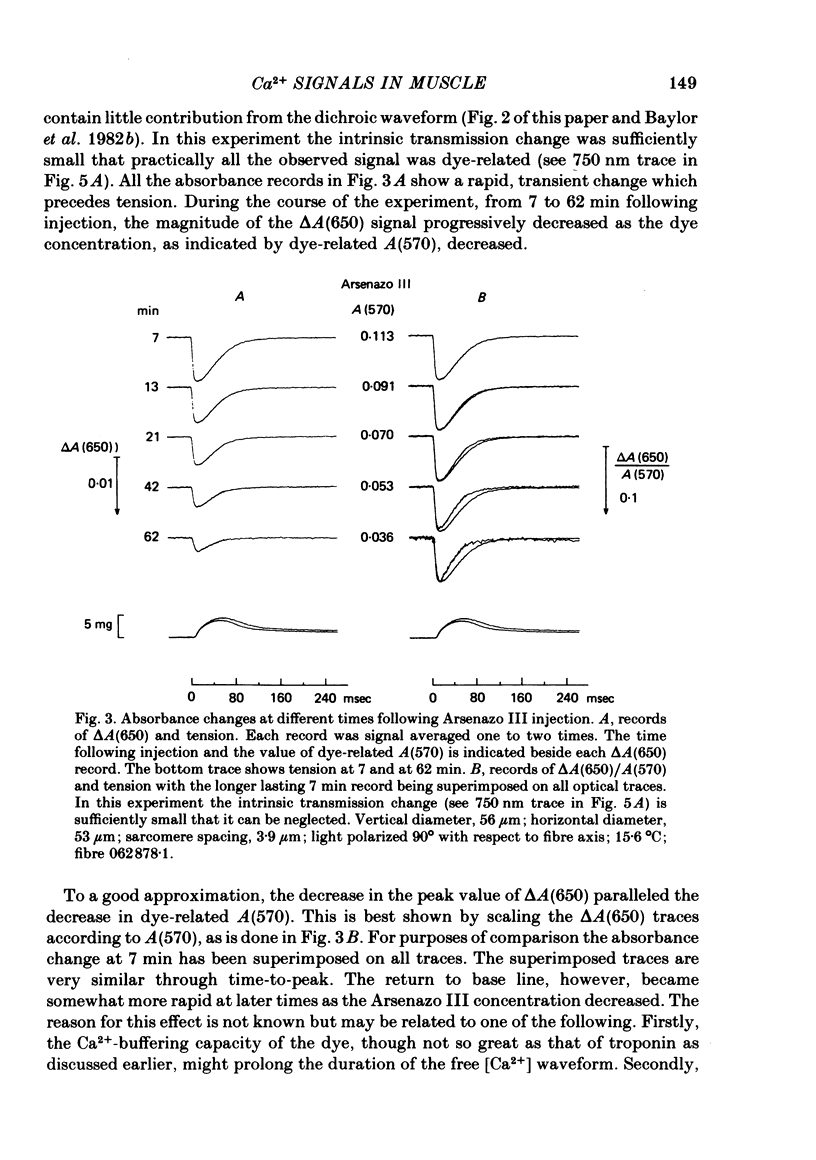
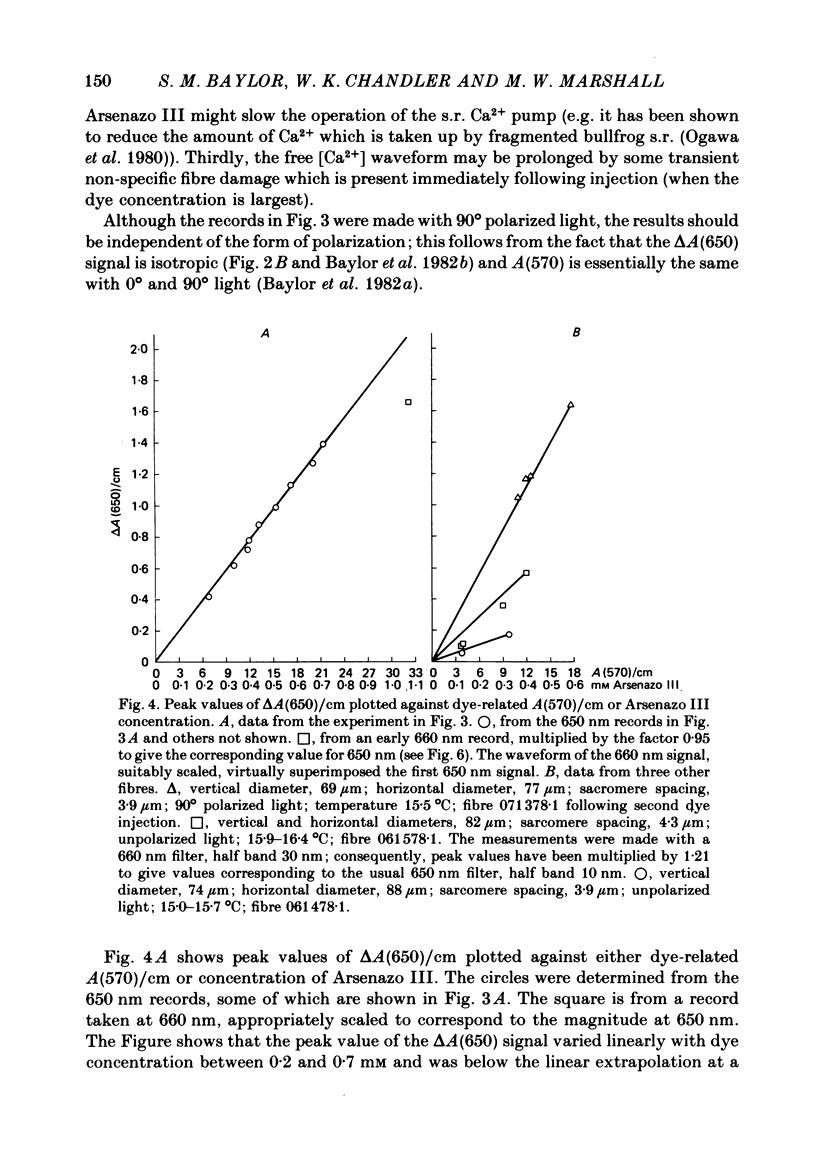
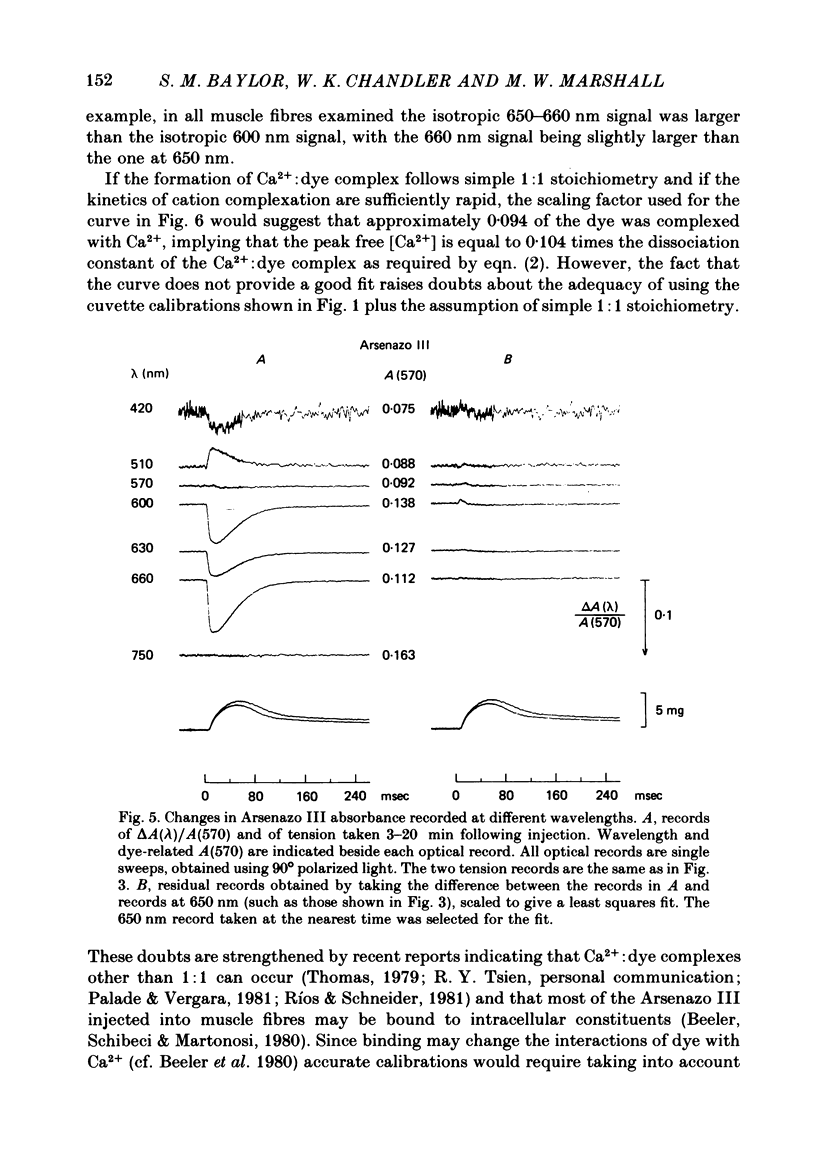
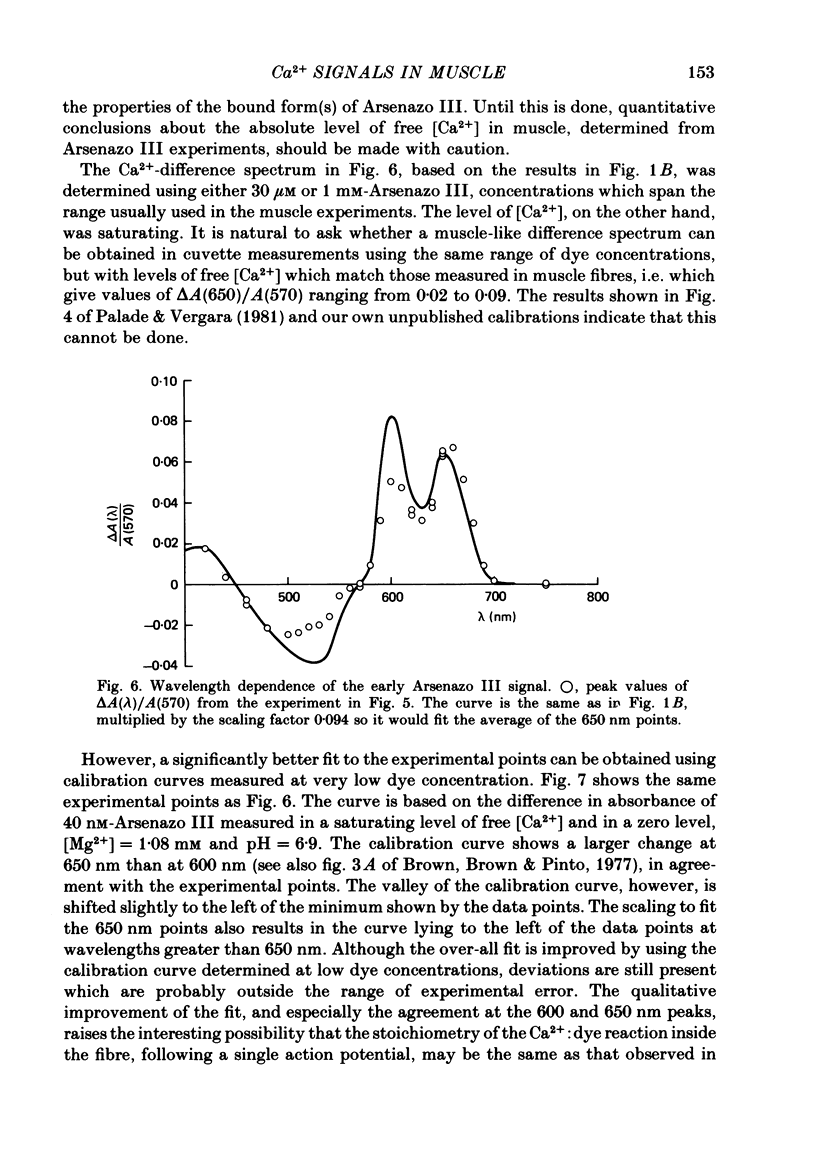
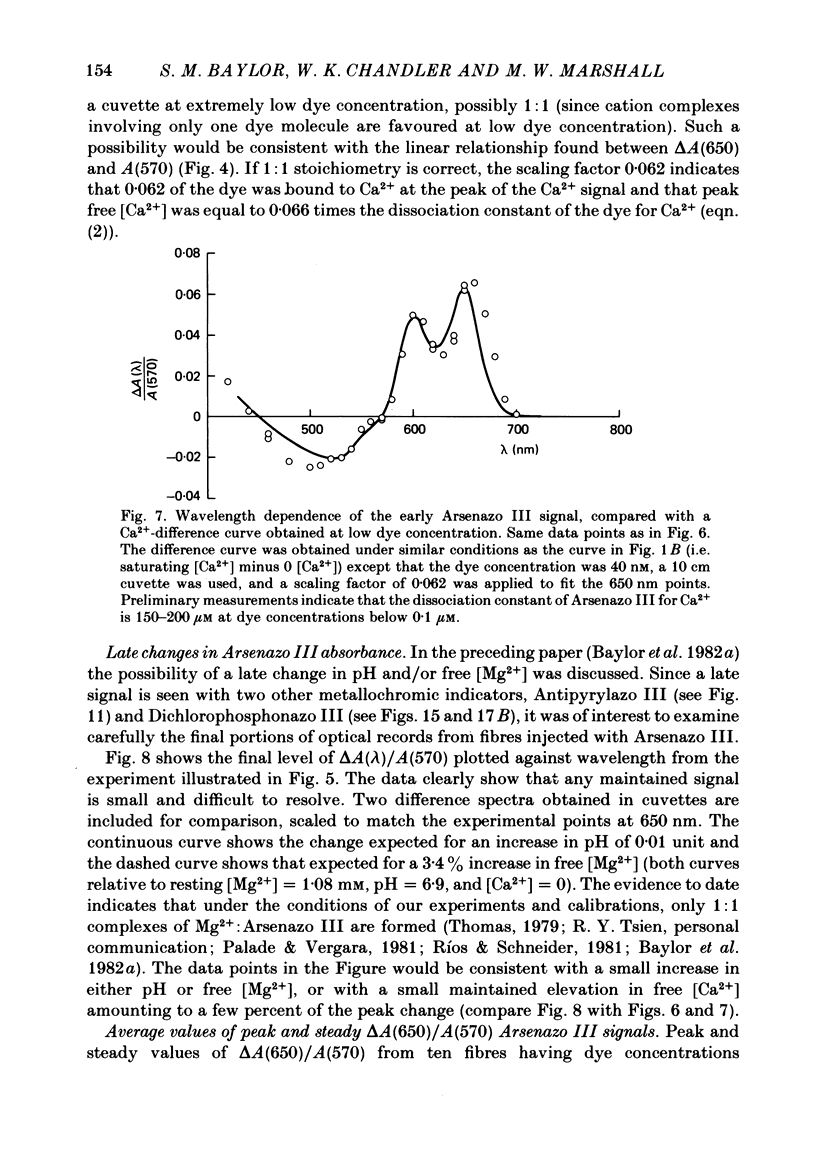
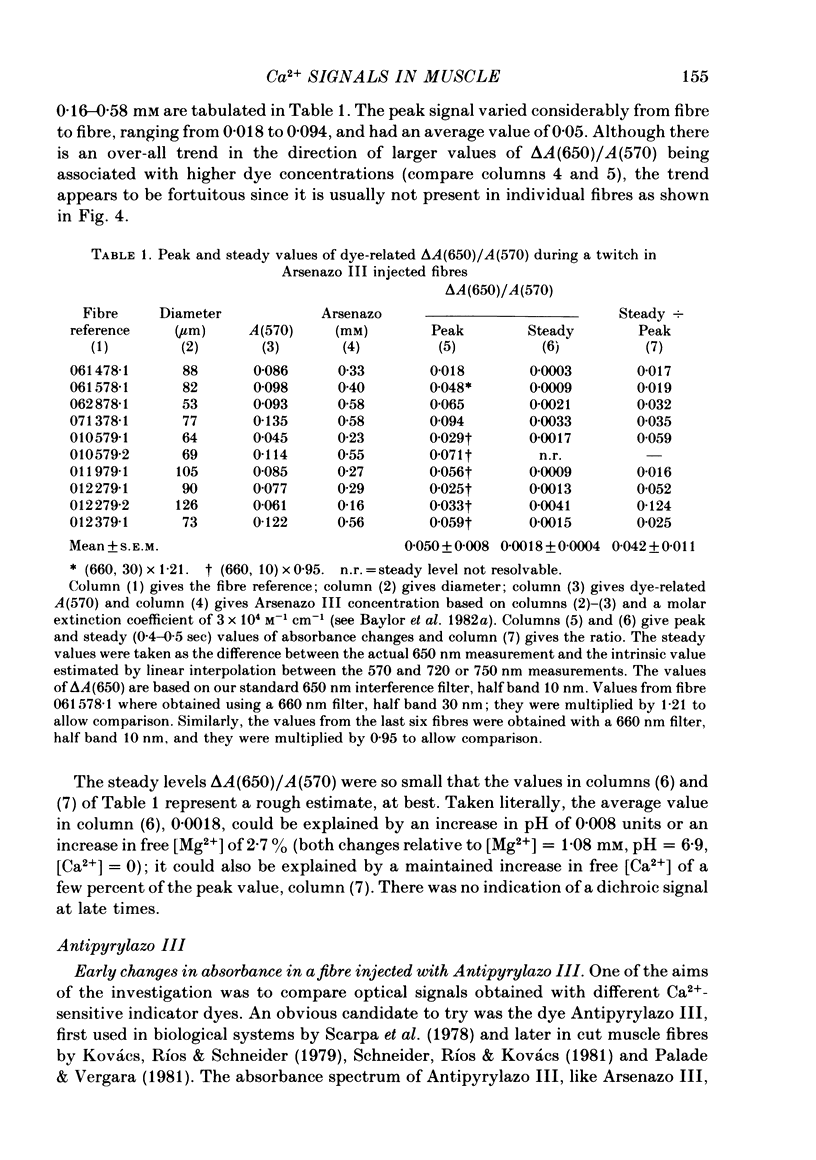
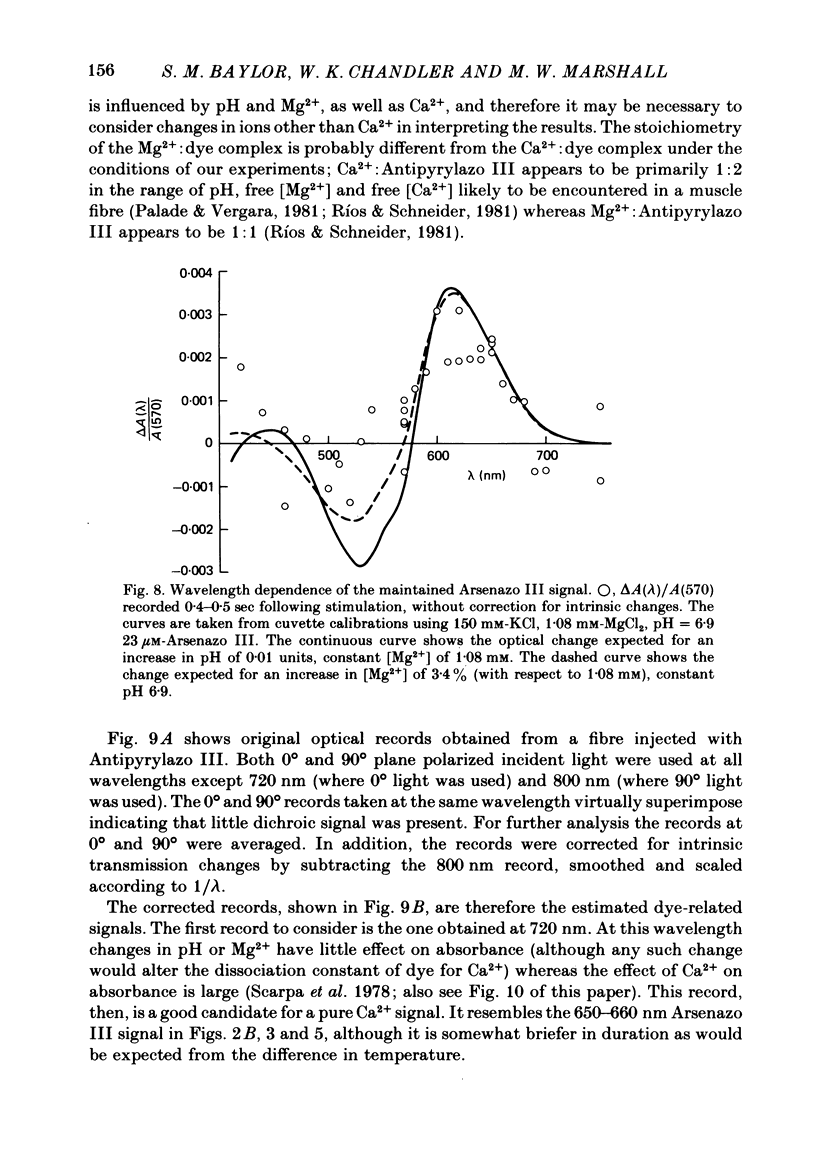
2. Fibres injected with Arsenazo III can show two kinds of changes in dye-related absorbance, an early isotropic change and a later dichroic change. The isotropic signal, which is the main subject of this paper, is transient in nature; it starts to develop before tension, reaches a peak in about 10 msec and is nearly over by 0·1 sec (16 °C). This signal is largest at 650-660 nm and measurements in this range indicate that the peak ΔA varies approximately linearly with dye concentration between 0·2 and 0·7 mM. The wavelength dependence of the peak amplitude can be qualitatively fitted by the Ca2+-difference spectrum determined from cuvette calibration measurements. There may be a small maintained (0·4-0·5 sec) absorbance change of a few percent of the peak value at 650-660 nm, possibly reflecting a maintained increase in myoplasmic pH or free [Mg2+].
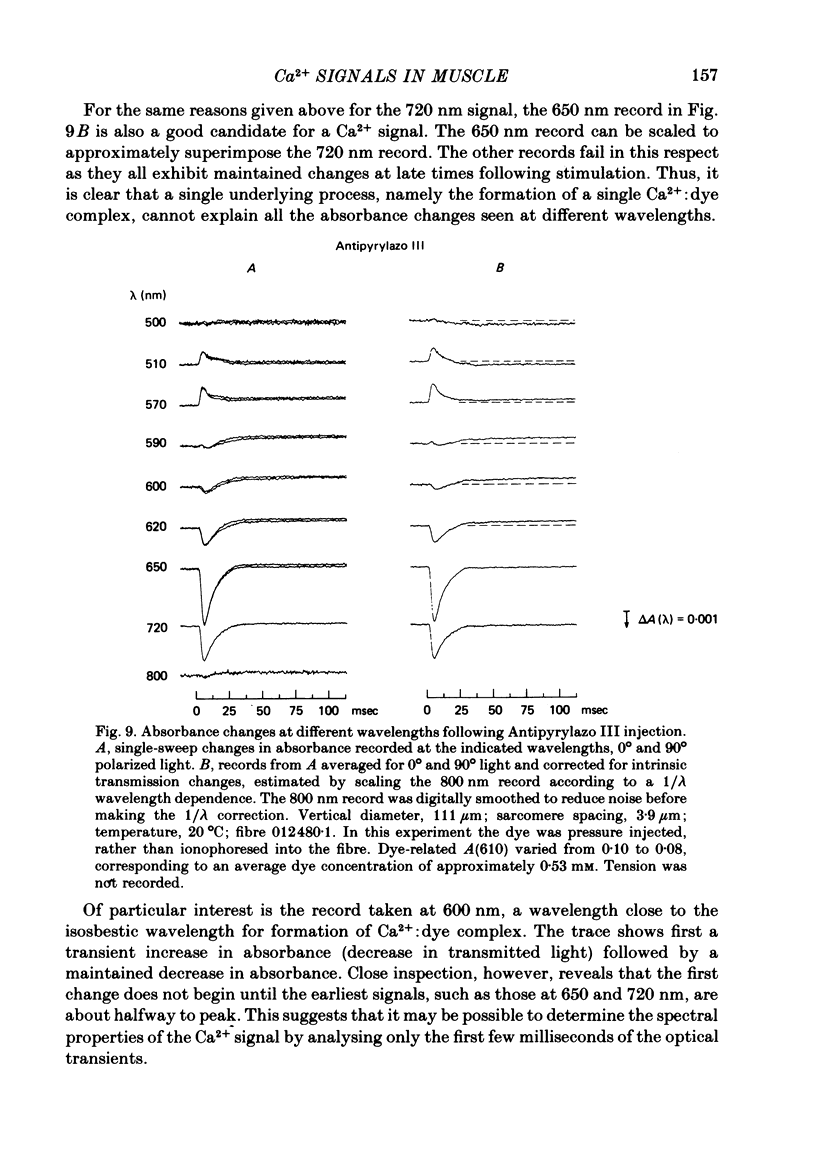
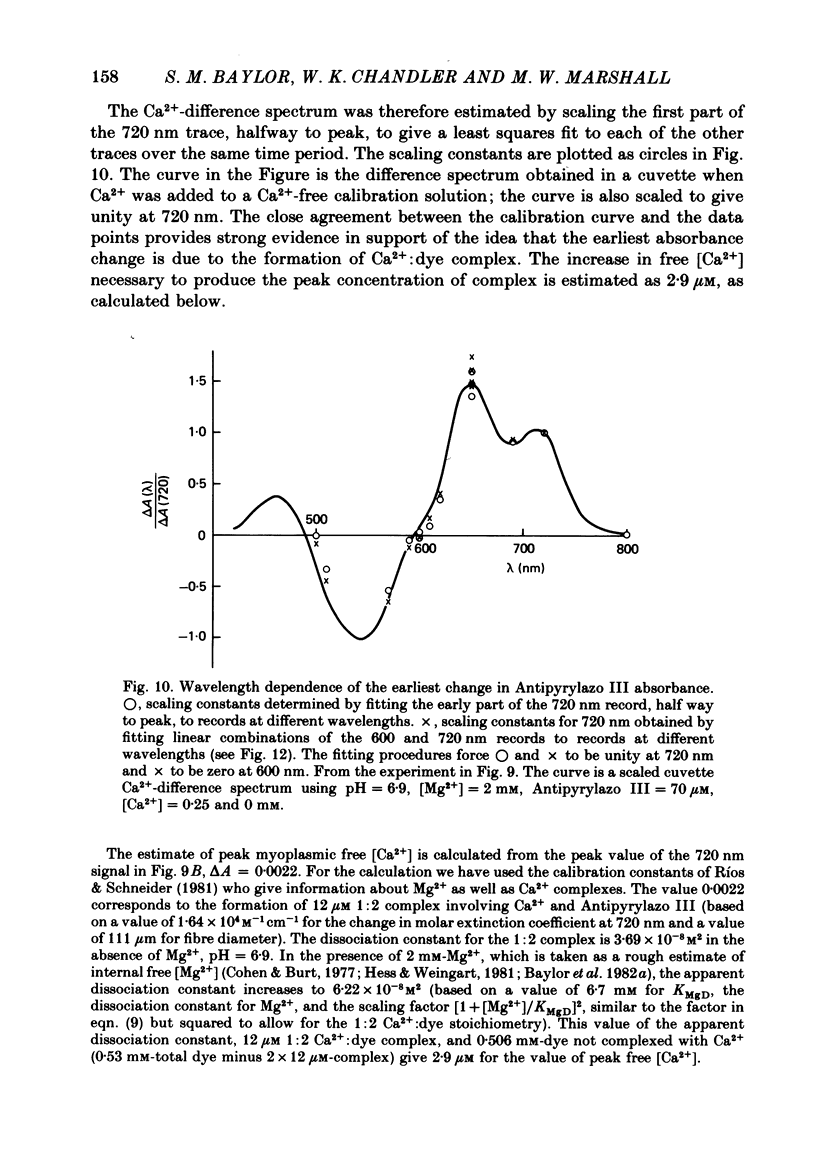
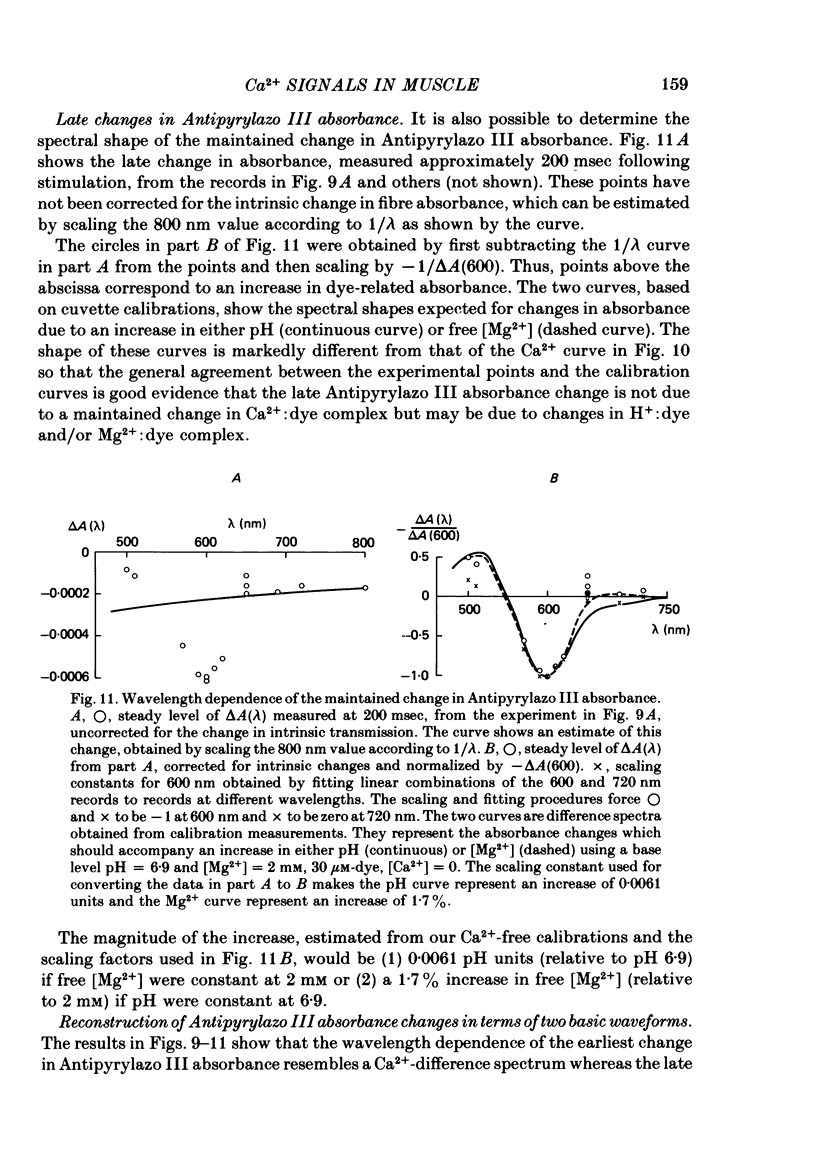
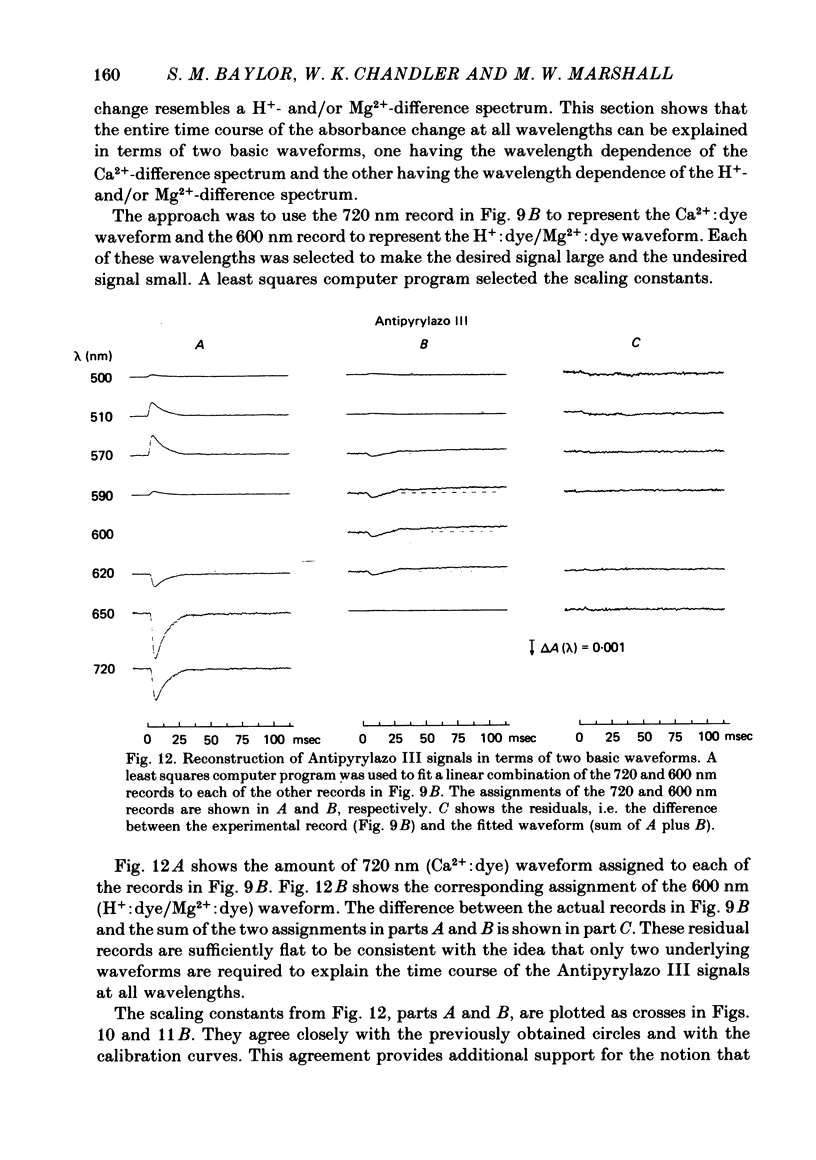
3. In a fibre injected with approximately 0·5 mM-Antipyrylazo III, there were two kinds of dye-related absorbance signals, both of which were isotropic. There was no signal that was obviously dichroic. The earlier signal was similar in time course to the early isotropic Ca2+ signal which was measured with Arsenazo III, and its magnitude followed the wavelength dependence of the Ca2+-difference spectrum determined from cuvette calibration measurements. By contrast, the wavelength dependence of the later absorbance change was similar to either the H+ or Mg2+-difference spectrum. The direction of this late signal (0·2 sec after stimulus) would correspond to an increase in either myoplasmic pH or free [Mg2+]. Records of the absorbance change at all wavelengths can be fitted by a linear combination of the Ca2+ waveform and the H+/Mg2+ waveform.
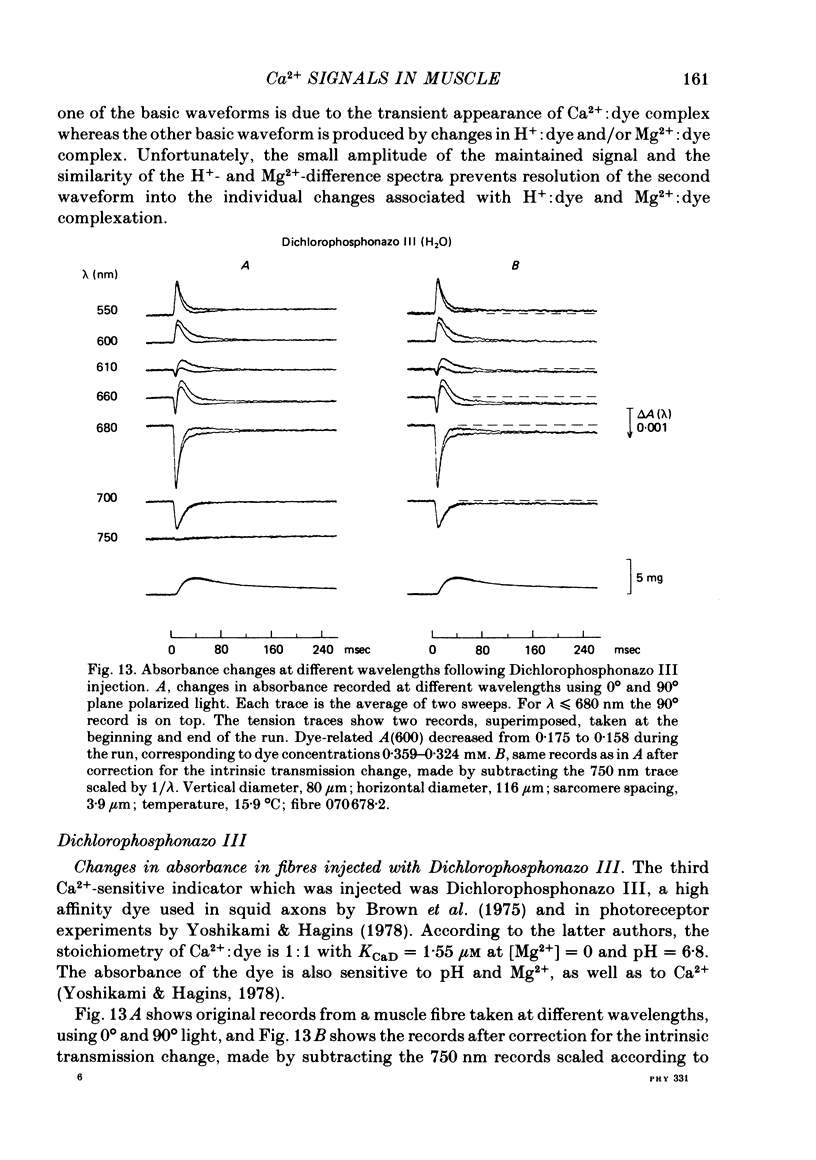
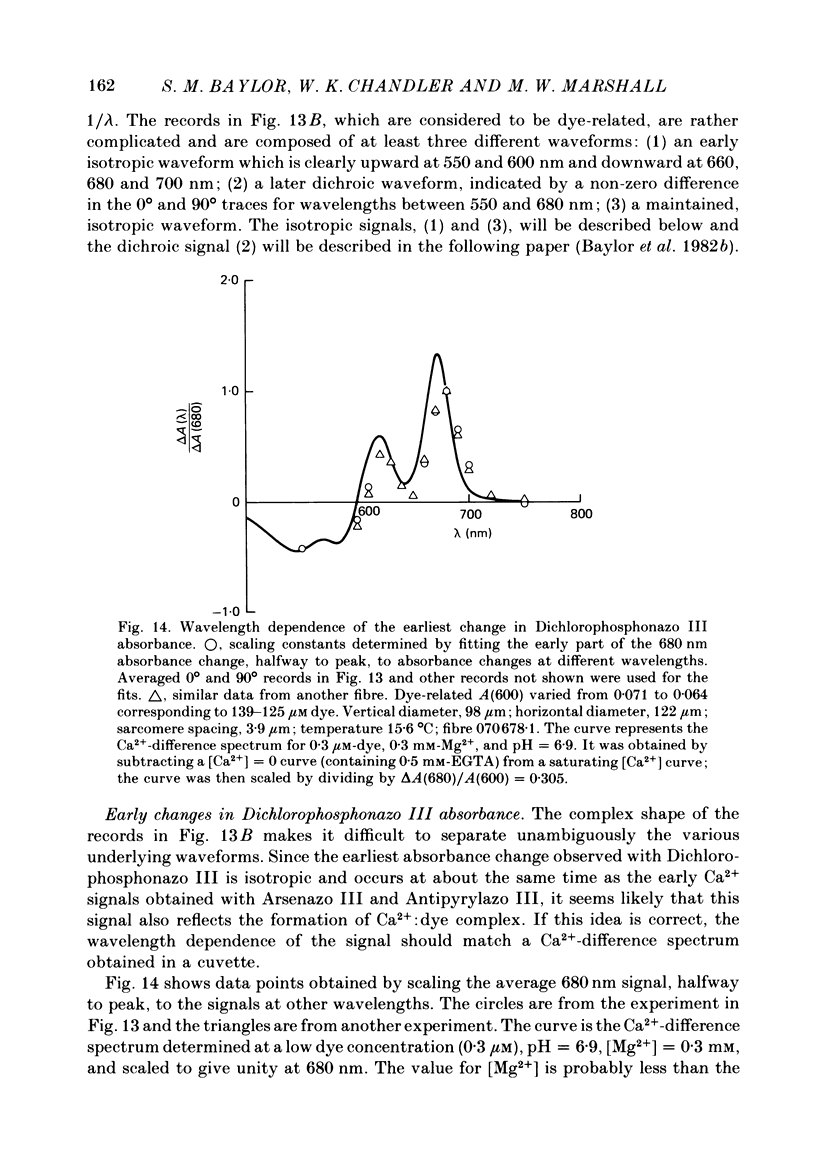
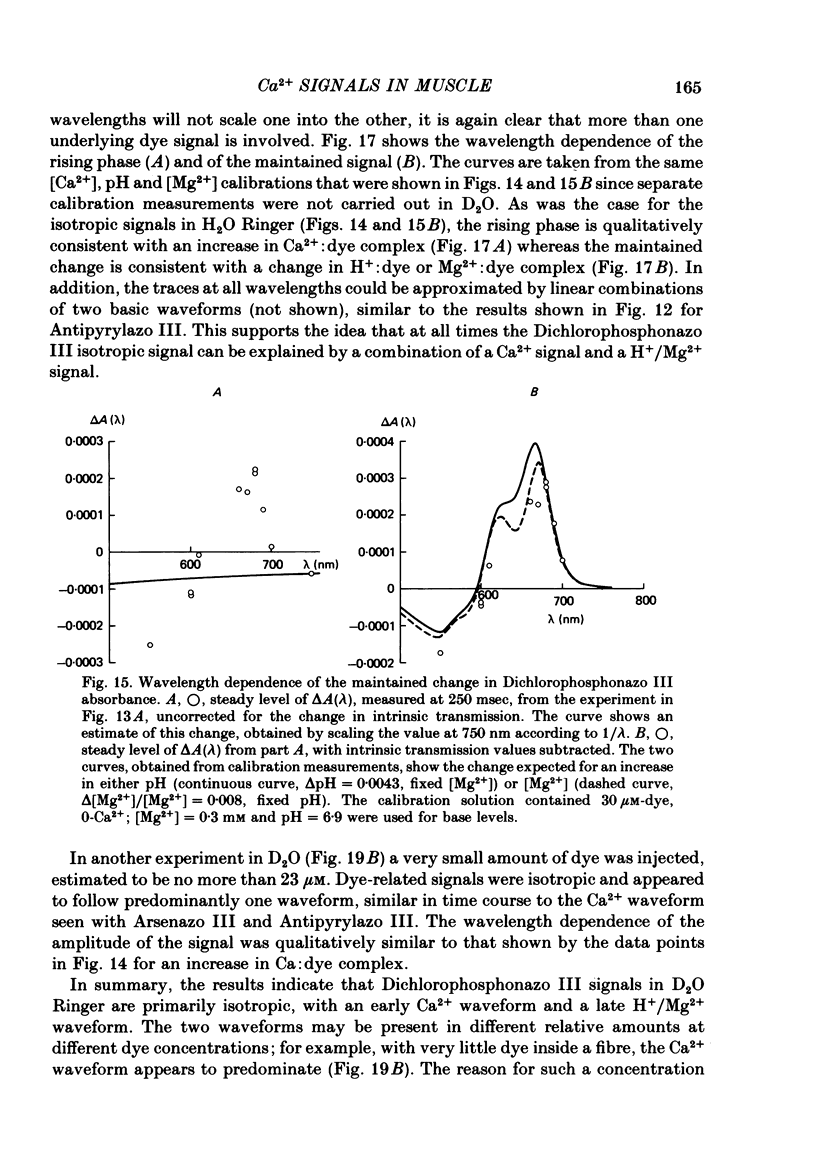
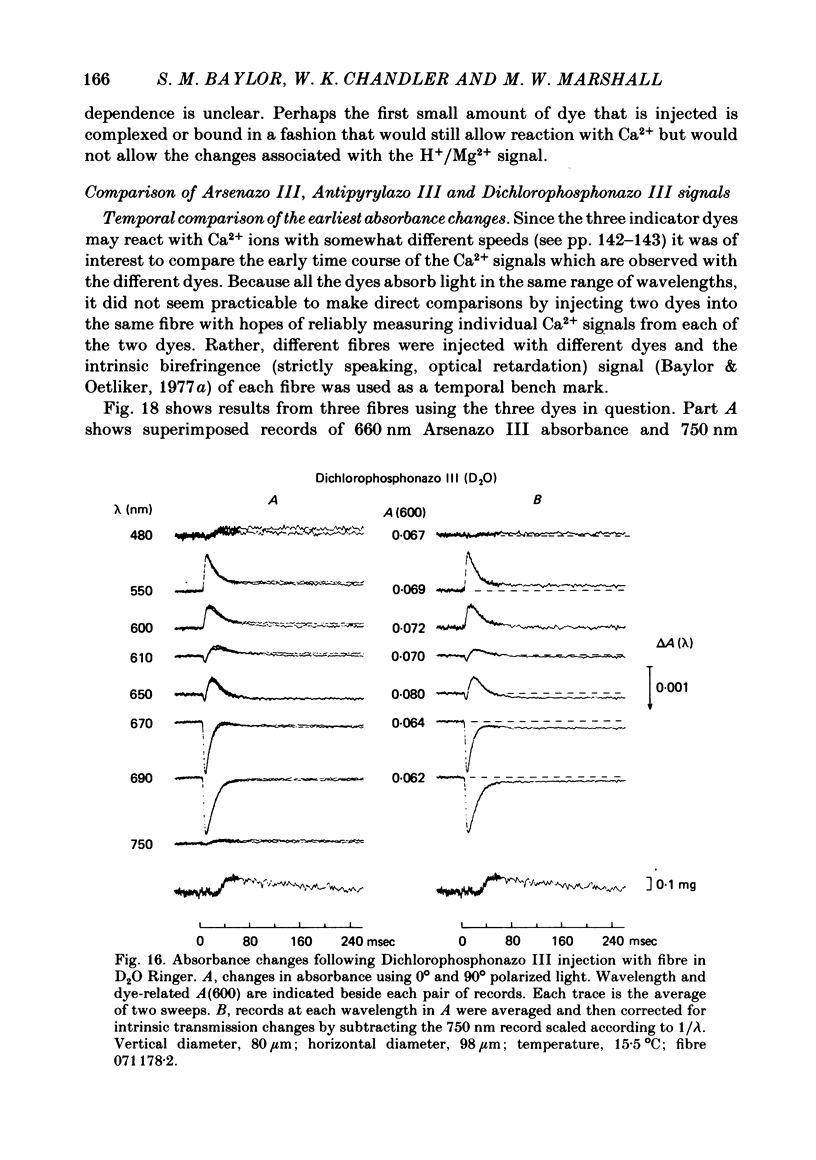
4. Fibres injected with Dichlorophosphonazo III showed three dye-related absorbance changes. There was an early isotropic signal, a later dichroic signal and a second isotropic signal. The wavelength dependence of the first part of the early signal is similar to the Ca2+-difference spectrum whereas the wavelength dependence of the second isotropic signal is similar to the H+- or Mg2+-difference spectrum. As was the case with Arsenazo III and Antipyrylazo III, the direction of the second signal at late times would correspond to an increase in either pH or free [Mg2+]. Replacing H2O with D2O resulted in a marked diminution of the dichroic signal. In D2O, linear combinations of two basic isotropic waveforms were sufficient to account for the absorbance changes measured at all wavelengths.
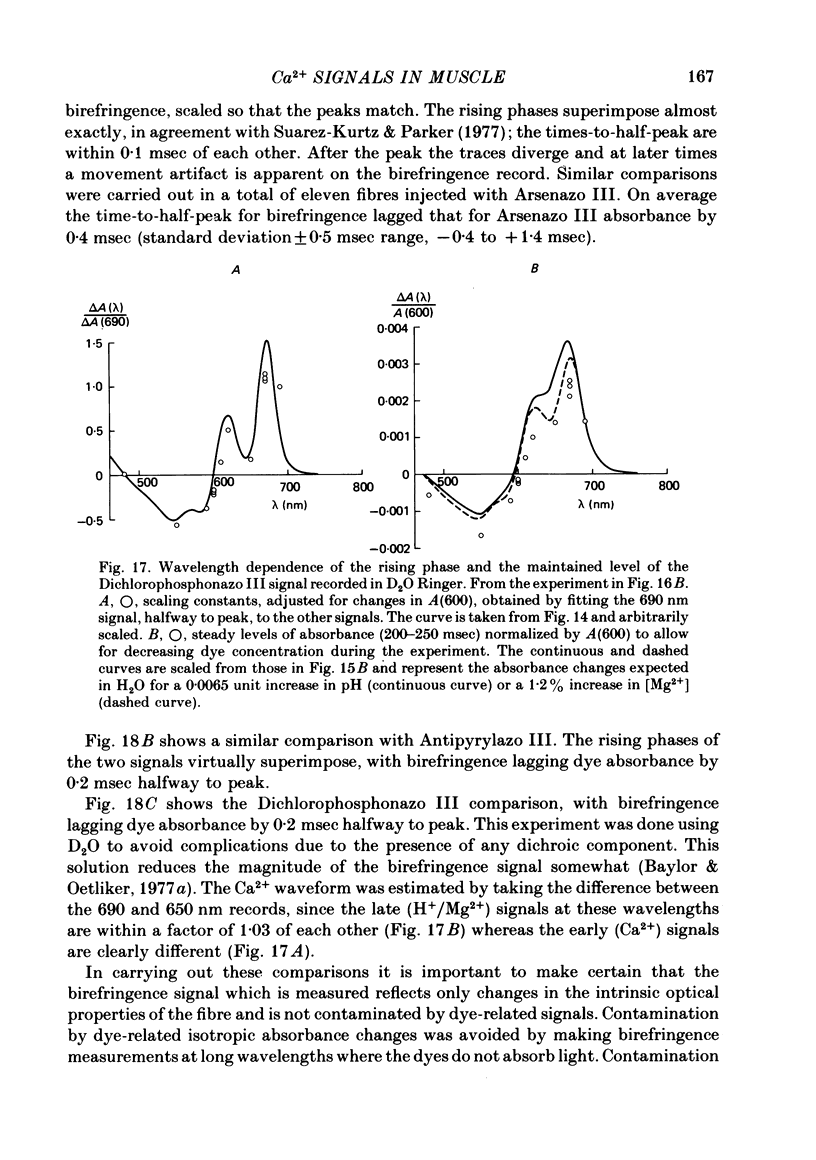
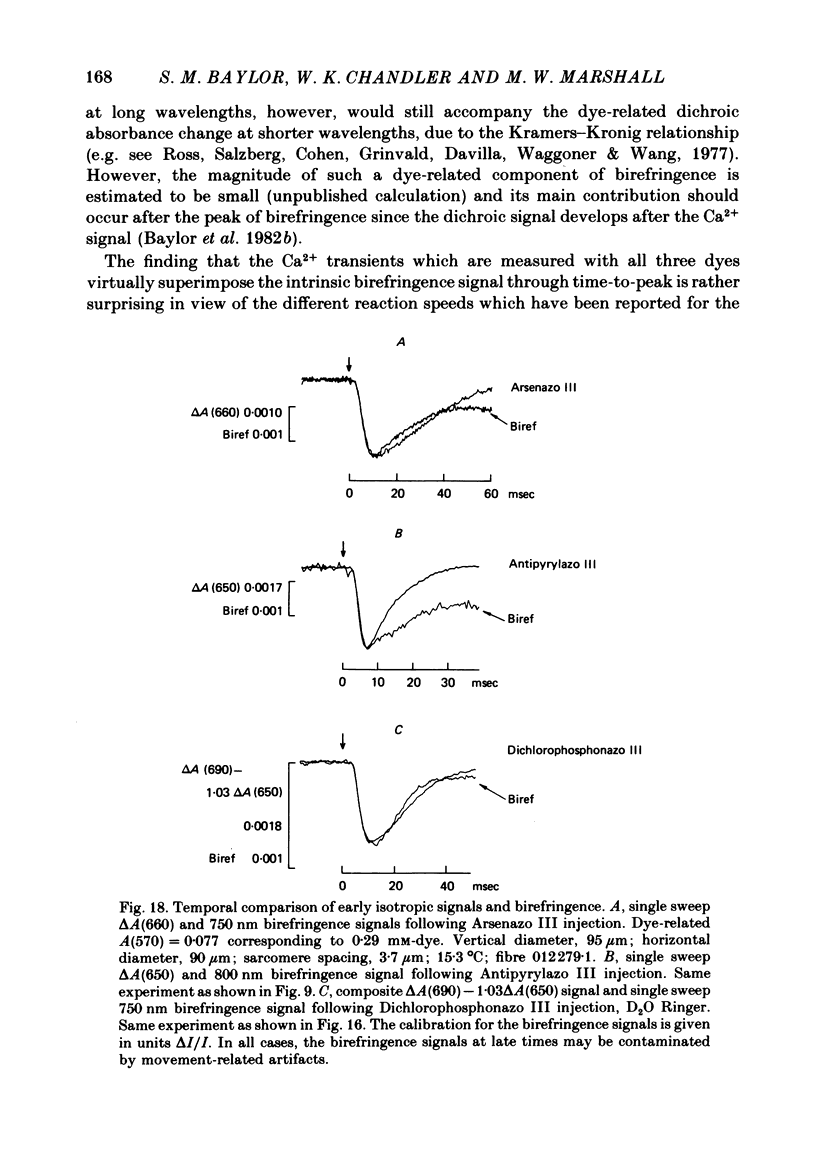
5. With all three metallochromic dyes, the time course of the early isotropic signal is similar to that of the second component of the intrinsic birefringence signal, at least to time of peak. On the assumption that this birefringence signal bears a unique temporal relationship to the myoplasmic free [Ca2+] waveform, at least to time of peak, the similarity suggests that all three dyes track free [Ca2+] with similar speed.
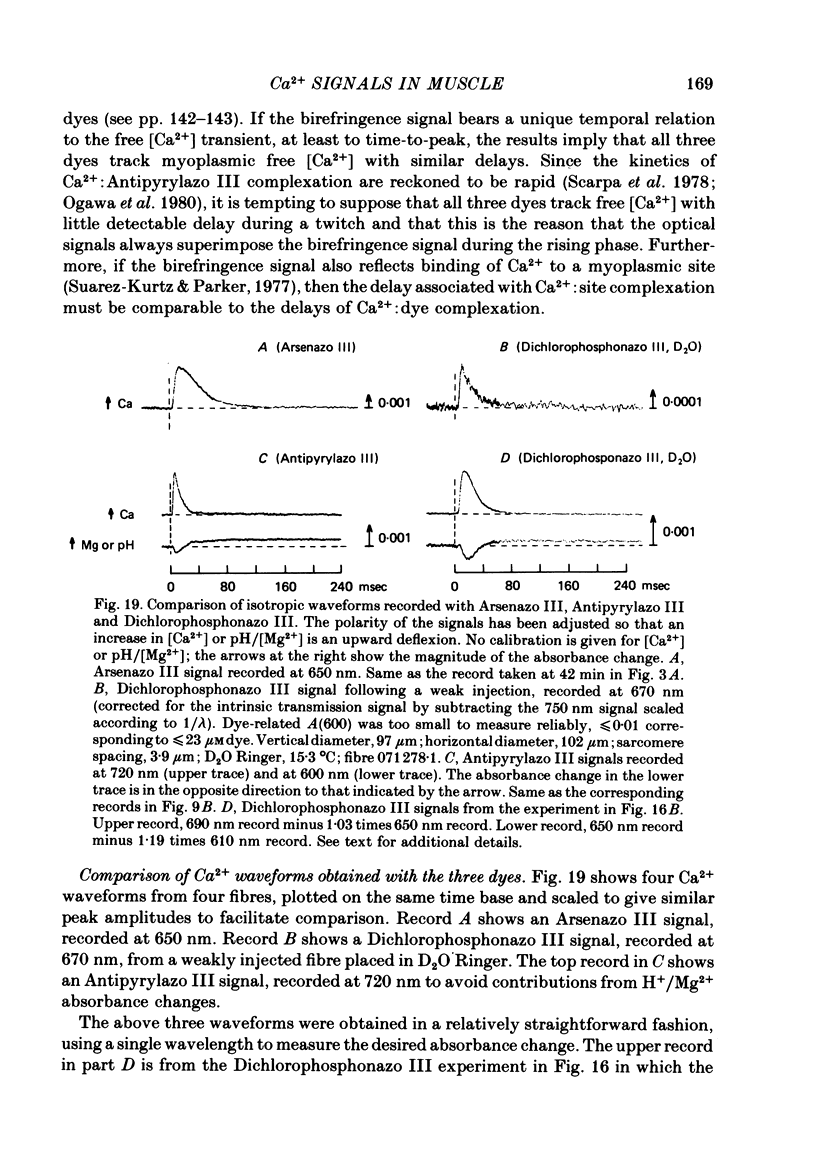
6. The conclusion from the experiments is that there are, in general, two dye-related isotropic absorbance signals seen with Arsenazo III, Antipyrylazo III and Dichlorophosphonazo III. One has an early, transient time course and appears to be due to the formation of Ca2+: dye complex in response to a transient increase in myoplasmic free [Ca2+]. The other signal persists after the free [Ca2+] transient has decayed. This appears to be due to a change in H+: dye or Mg2+: dye complex, such as would occur if there were a small maintained increase in myoplasmic pH or free [Mg2+].
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor S. M., Chandler W. K., Marshall M. W. Arsenazo III signals in singly dissected frog twitch fibres [proceedings]. J Physiol. 1979 Feb;287:23P–24P. [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Dichroic components of Arsenazo III and dichlorophosphonazo III signals in skeletal muscle fibres. J Physiol. 1982 Oct;331:179–210. doi: 10.1113/jphysiol.1982.sp014369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Optical measurements of intracellular pH and magnesium in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:105–137. doi: 10.1113/jphysiol.1982.sp014367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. A large birefringence signal preceding contraction in single twitch fibres of the frog. J Physiol. 1977 Jan;264(1):141–162. doi: 10.1113/jphysiol.1977.sp011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. The optical properties of birefringence signals from single muscle fibres. J Physiol. 1977 Jan;264(1):163–198. doi: 10.1113/jphysiol.1977.sp011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler T. J., Schibeci A., Martonosi A. The binding of arsenazo III to cell components. Biochim Biophys Acta. 1980 May 7;629(2):317–327. doi: 10.1016/0304-4165(80)90104-x. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Brown P. K., Pinto L. H. Detection of light-induced changes of intracellular ionized calcium concentration in Limulus ventral photoreceptors using arsenazo III. J Physiol. 1977 May;267(2):299–320. doi: 10.1113/jphysiol.1977.sp011814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Cohen L. B., De Weer P., Pinto L. H., Ross W. N., Salzberg B. M. Rapid changes in intracellular free calcium concentration. Detection by metallochromic indicator dyes in squid giant axon. Biophys J. 1975 Nov;15(11):1155–1160. doi: 10.1016/S0006-3495(75)85891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M., Burt C. T. 31P nuclear magnetic relaxation studies of phosphocreatine in intact muscle: determination of intracellular free magnesium. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4271–4275. doi: 10.1073/pnas.74.10.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Hastings J. W., Mitchell G., Mattingly P. H., Blinks J. R., Van Leeuwen M. Response of aequorin bioluminescence to rapid changes in calcium concentration. Nature. 1969 Jun 14;222(5198):1047–1050. doi: 10.1038/2221047a0. [DOI] [PubMed] [Google Scholar]
- Kendrick N. C., Ratzlaff R. W., Blaustein M. P. Arsenazo III as an indicator for ionized calcium in physiological salt solutions: its use for determination of the CaATP dissociation constant. Anal Biochem. 1977 Dec;83(2):433–450. doi: 10.1016/0003-2697(77)90052-5. [DOI] [PubMed] [Google Scholar]
- Kovács L., Ríos E., Schneider M. F. Calcium transients and intramembrane charge movement in skeletal muscle fibres. Nature. 1979 May 31;279(5712):391–396. doi: 10.1038/279391a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Measurement of calcium transients in frog muscle by the use of arsenazo III. Proc R Soc Lond B Biol Sci. 1977 Aug 22;198(1131):201–210. doi: 10.1098/rspb.1977.0094. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Harafuji H., Kurebayashi N. Comparison of the characteristics of four metallochromic dyes as potential calcium indicators for biological experiments. J Biochem. 1980 May;87(5):1293–1303. doi: 10.1093/oxfordjournals.jbchem.a132867. [DOI] [PubMed] [Google Scholar]
- Potter J. D., Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1975 Jun 25;250(12):4628–4633. [PubMed] [Google Scholar]
- Robertson S. P., Johnson J. D., Potter J. D. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+. Biophys J. 1981 Jun;34(3):559–569. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Salzberg B. M., Cohen L. B., Grinvald A., Davila H. V., Waggoner A. S., Wang C. H. Changes in absorption, fluorescence, dichroism, and Birefringence in stained giant axons: : optical measurement of membrane potential. J Membr Biol. 1977 May 6;33(1-2):141–183. doi: 10.1007/BF01869514. [DOI] [PubMed] [Google Scholar]
- Ríos E., Schneider M. F. Stoichiometry of the reactions of calcium with the metallochromic indicator dyes antipyrylazo III and arsenazo III. Biophys J. 1981 Dec;36(3):607–621. doi: 10.1016/S0006-3495(81)84755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A., Brinley F. J., Jr, Dubyak G. Antipyrylazo III, a "middle range" Ca2+ metallochromic indicator. Biochemistry. 1978 Apr 18;17(8):1378–1386. doi: 10.1021/bi00601a004. [DOI] [PubMed] [Google Scholar]
- Suarez-Kurtz G., Parker I. Birefringence signals and calcium transients in skeletal muscle. Nature. 1977 Dec 22;270(5639):746–748. doi: 10.1038/270746a0. [DOI] [PubMed] [Google Scholar]
- Thomas M. V. Arsenazo III forms 2:1 complexes with Ca and 1:1 complexes with Mg under physiological conditions. Estimates of the apparent dissociation constants. Biophys J. 1979 Mar;25(3):541–548. doi: 10.1016/S0006-3495(79)85322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikami S., Hagins W. A. Calcium in excitation of vertebrate rods and cones: retinal efflux of calcium studied with dichlorophosphonazo III. Ann N Y Acad Sci. 1978 Apr 28;307:545–561. doi: 10.1111/j.1749-6632.1978.tb41981.x. [DOI] [PubMed] [Google Scholar]


