Abstract
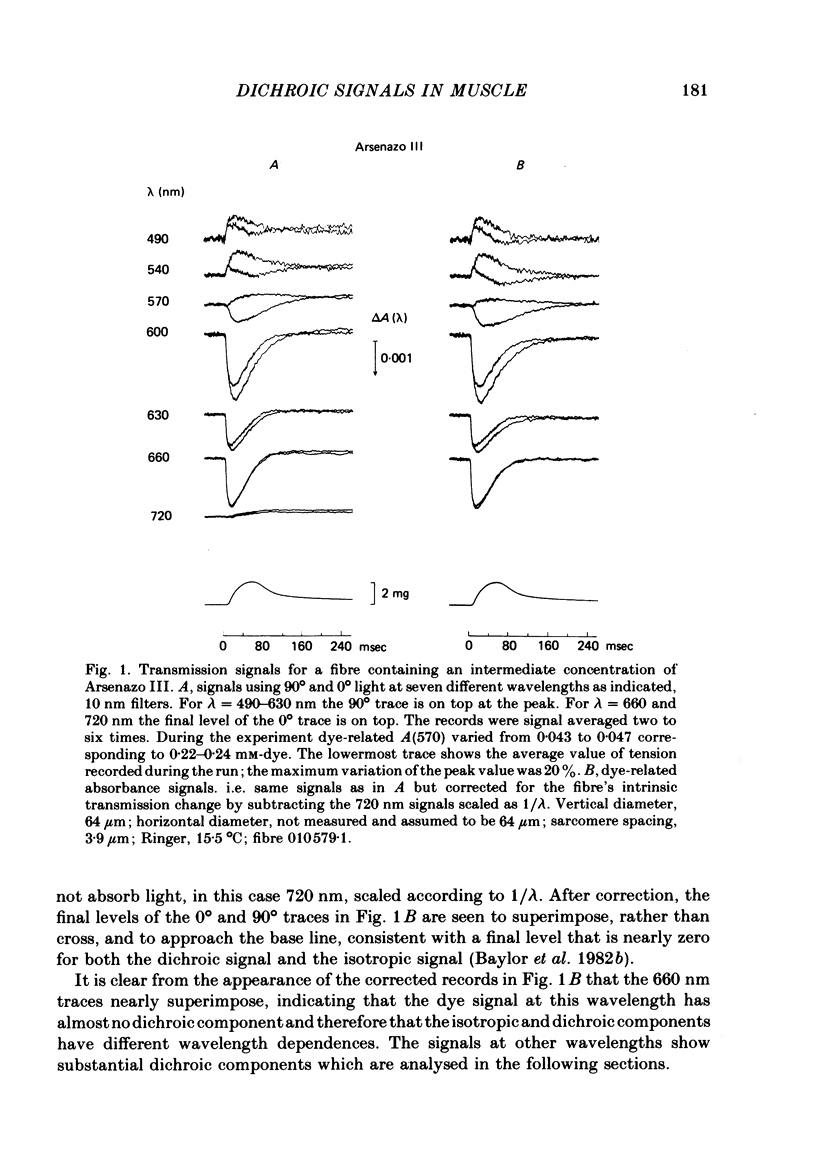
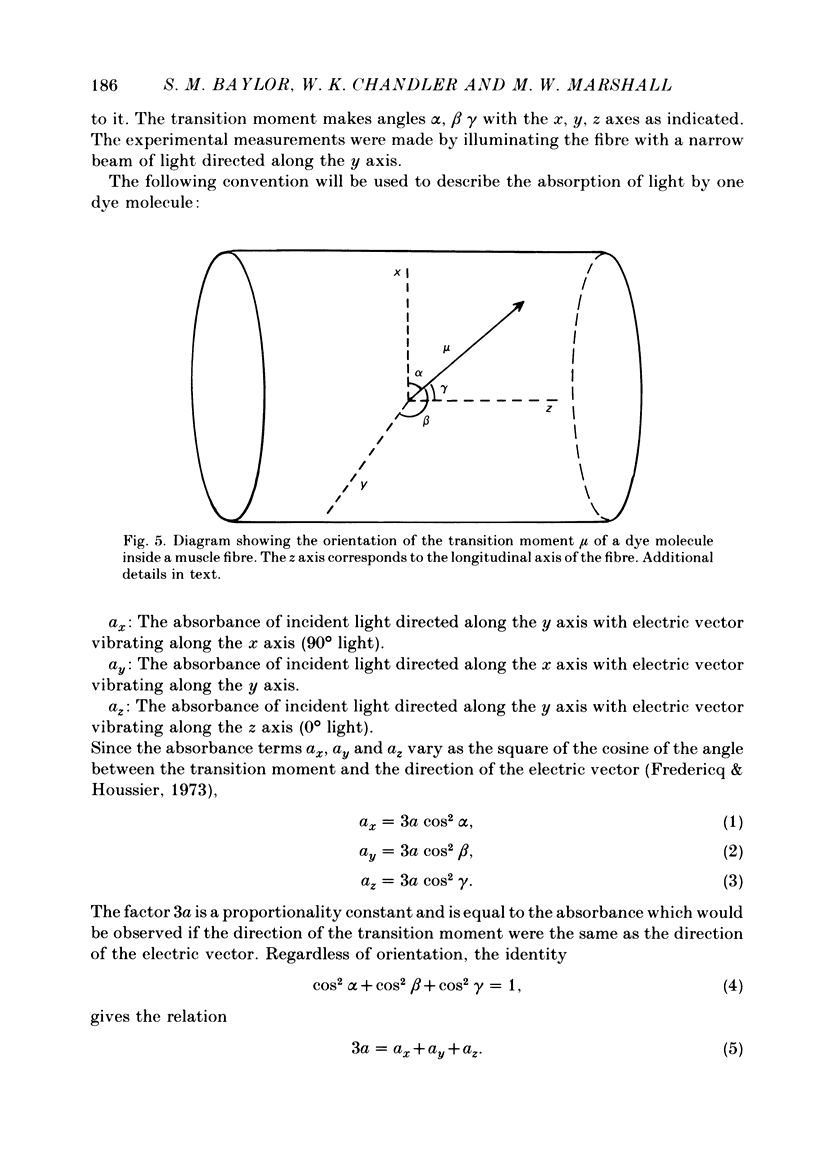
1. Absorbance changes were measured following stimulation of single muscle fibres injected with the metallochromic indicator dye Arsenazo III. Two dye-related signals can be clearly resolved: (1) an early, transient isotropic signal that appears to be due to the formation of Ca2+:dye complex and (2) a slower, transient signal that is `dichroic' in nature. The dichroic signal is obtained by taking the difference between absorbance changes measured with light plane polarized along the fibre axis (0° light) and at right angles to the axis (90° light).
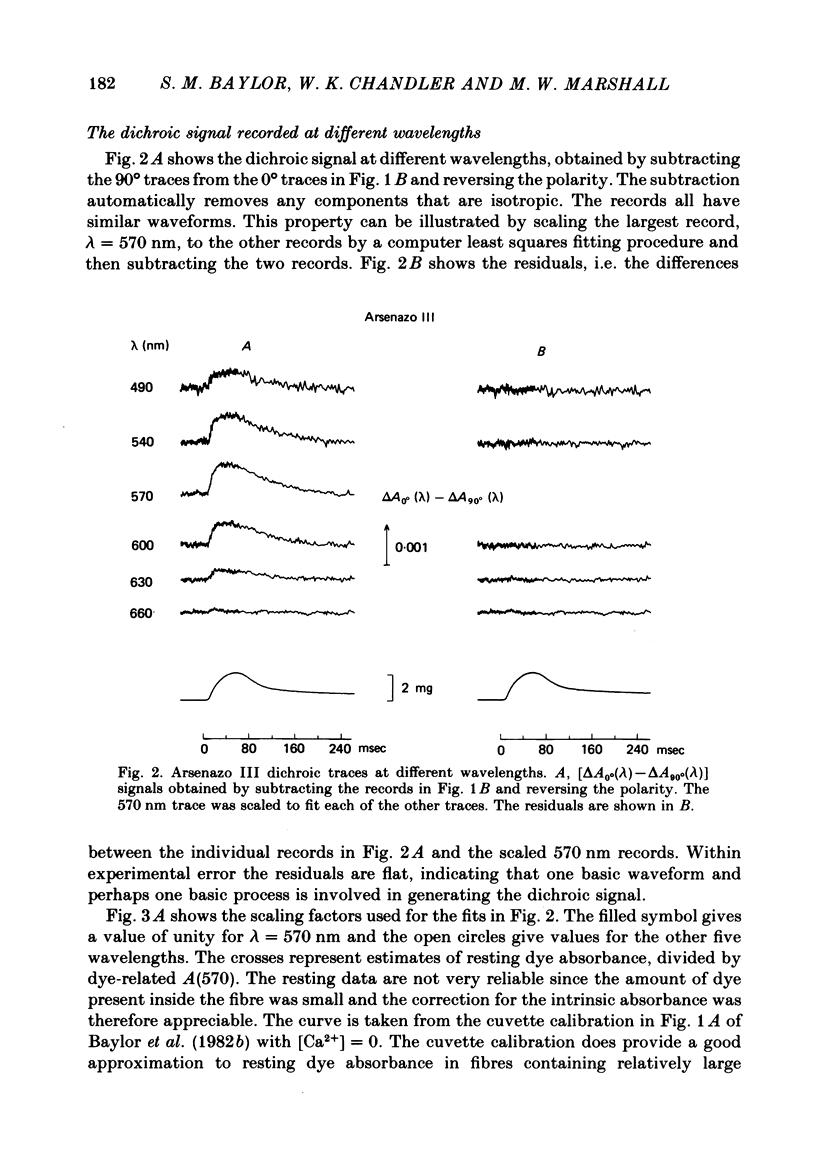
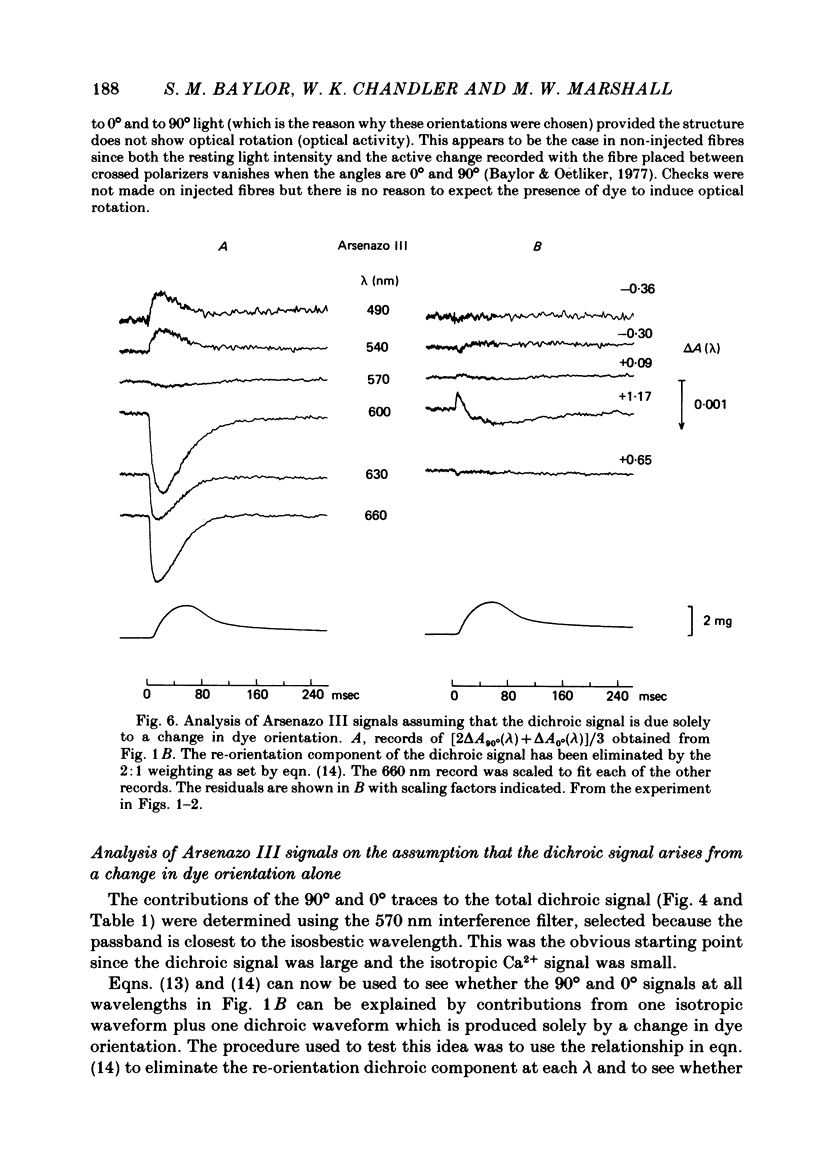
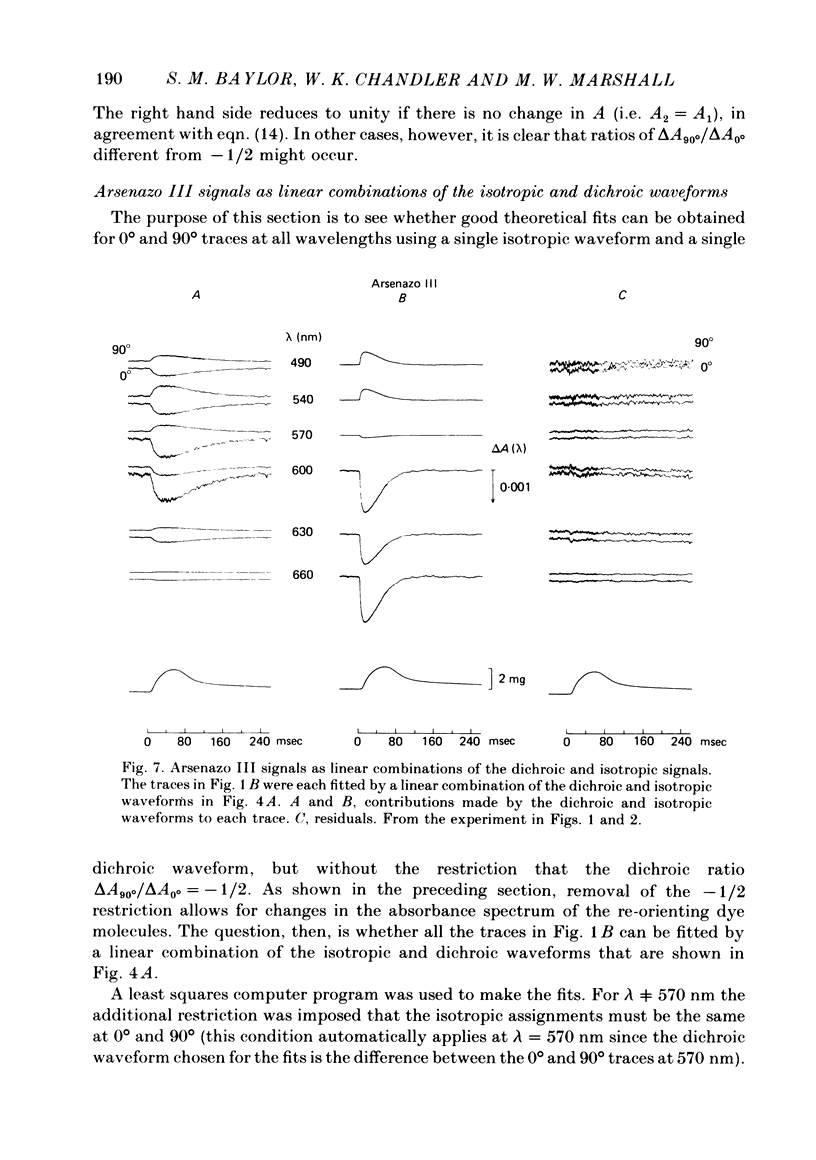
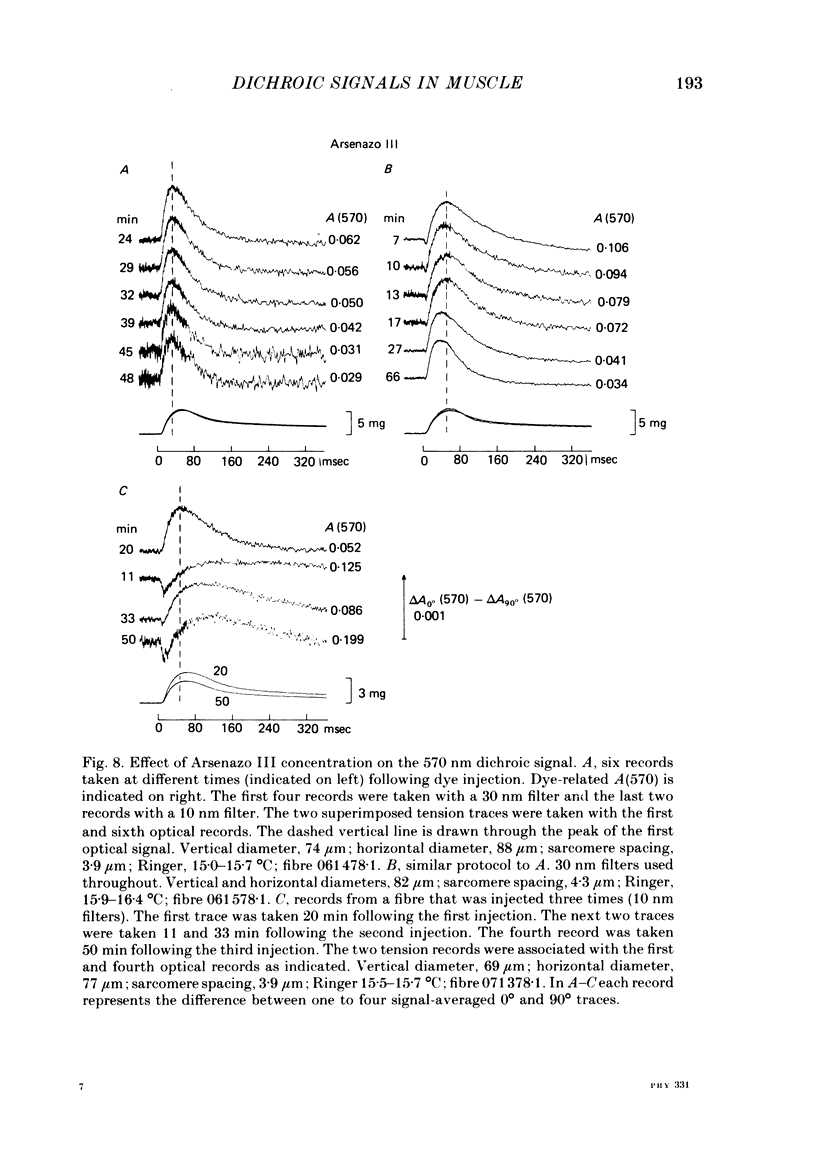
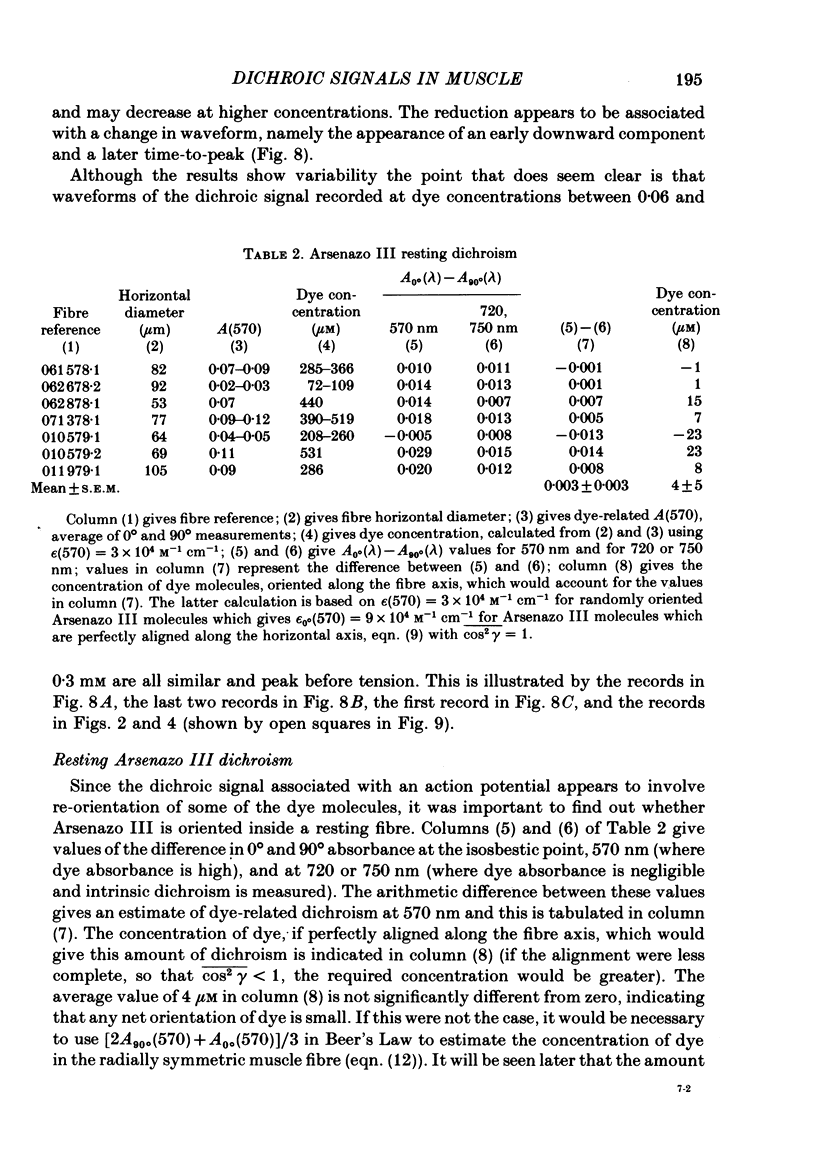
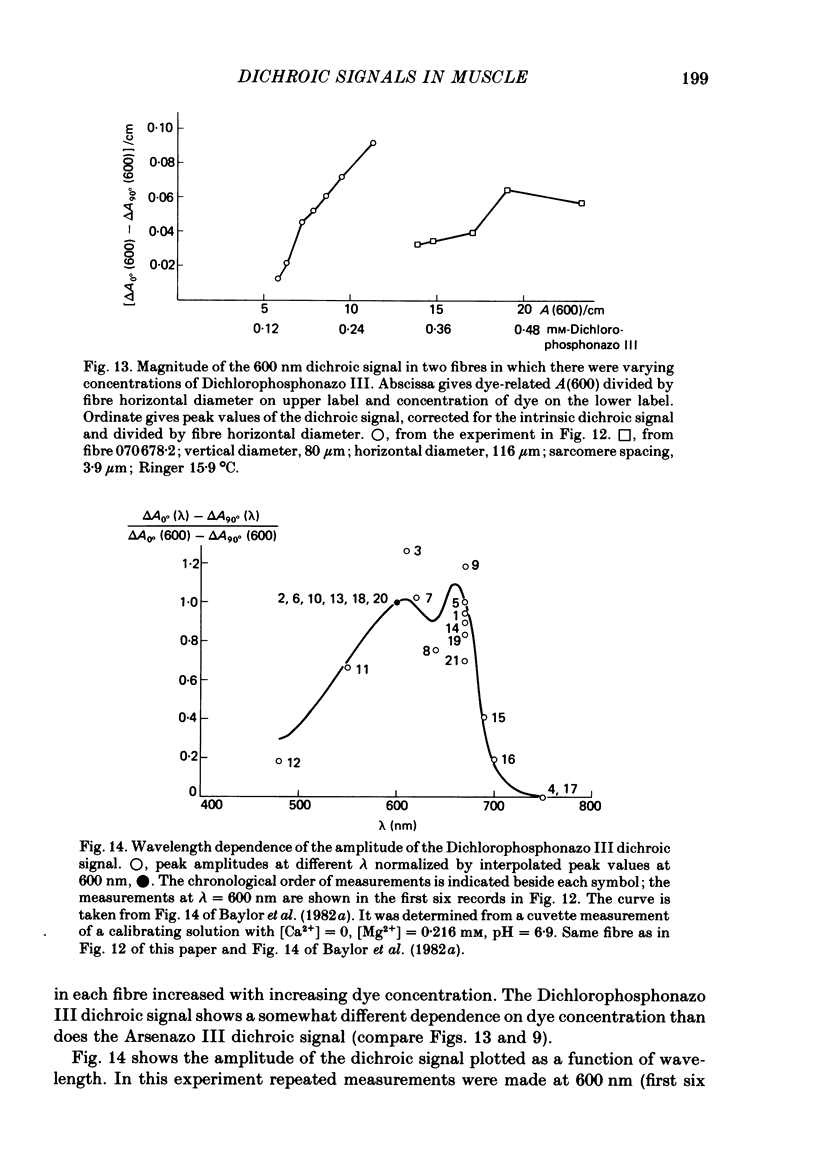
2. The time course of the dichroic signal is the same at all wavelengths employed, suggesting that a single underlying process is involved. The wavelength dependence of the magnitude of the signal is similar to that obtained for dye absorbance in a resting fibre.
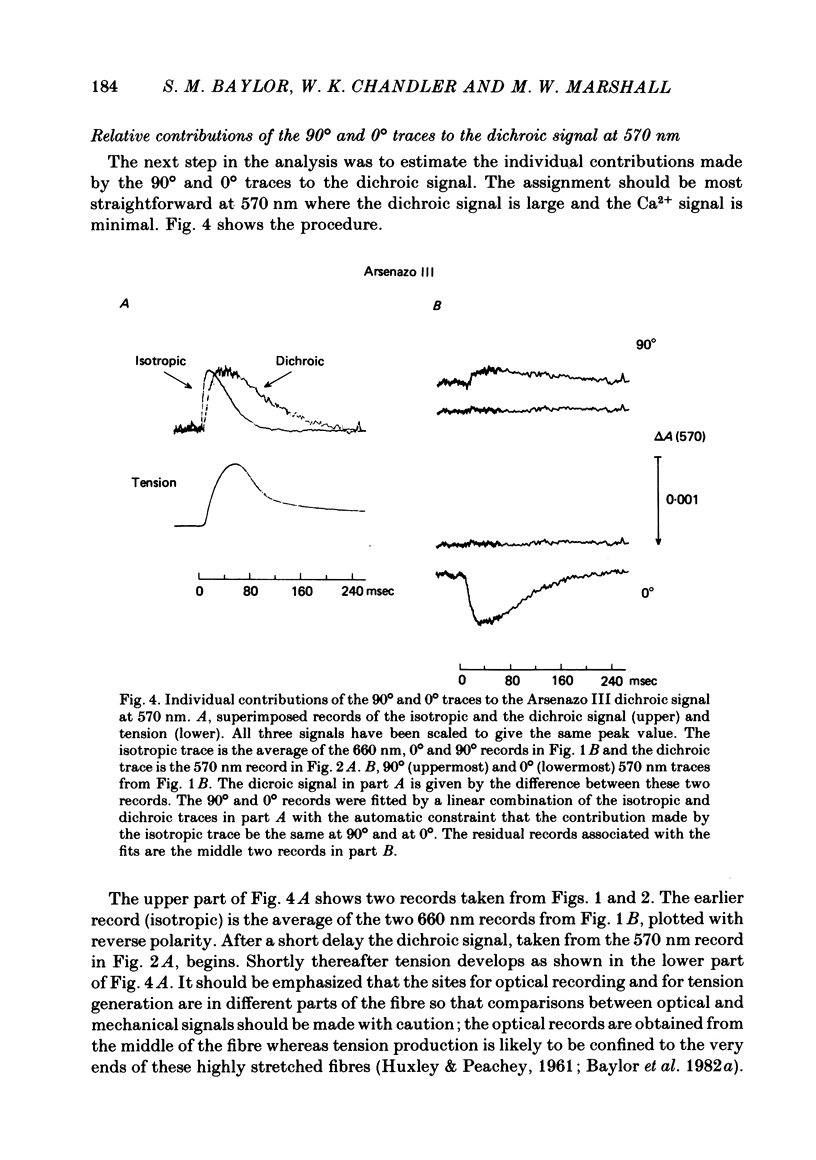
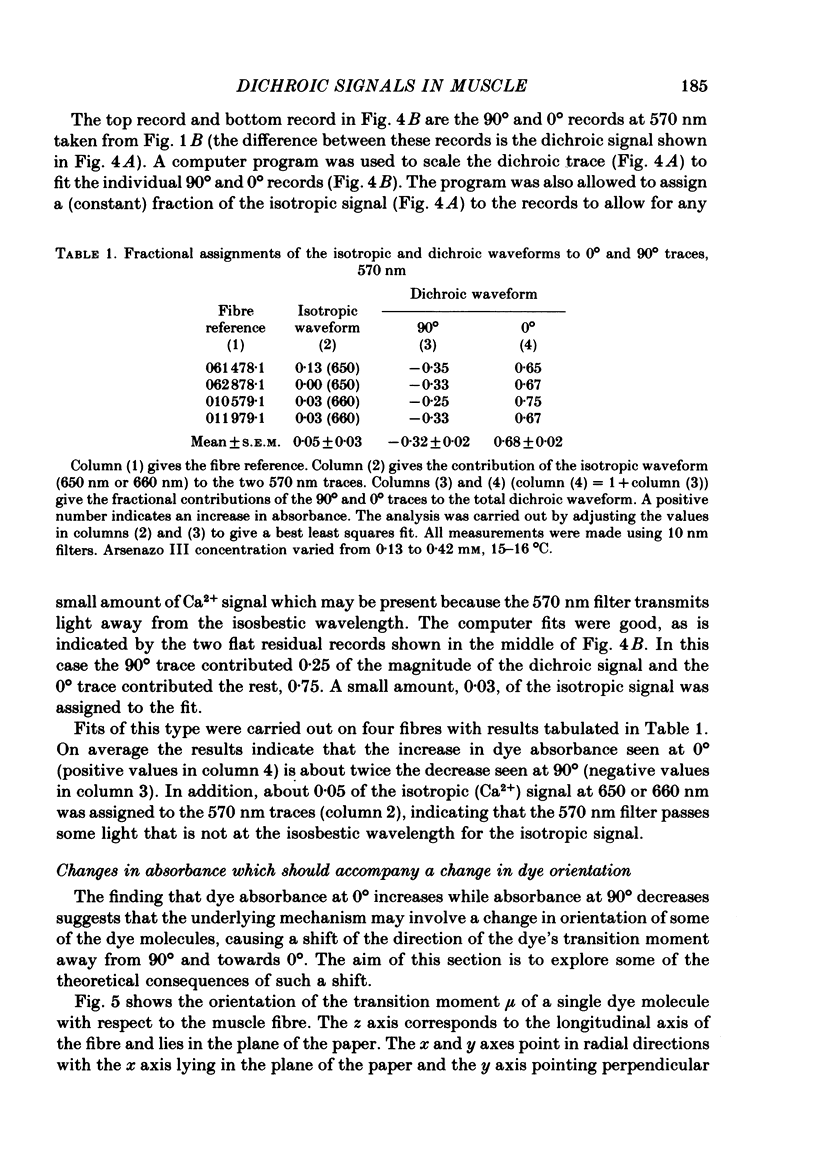
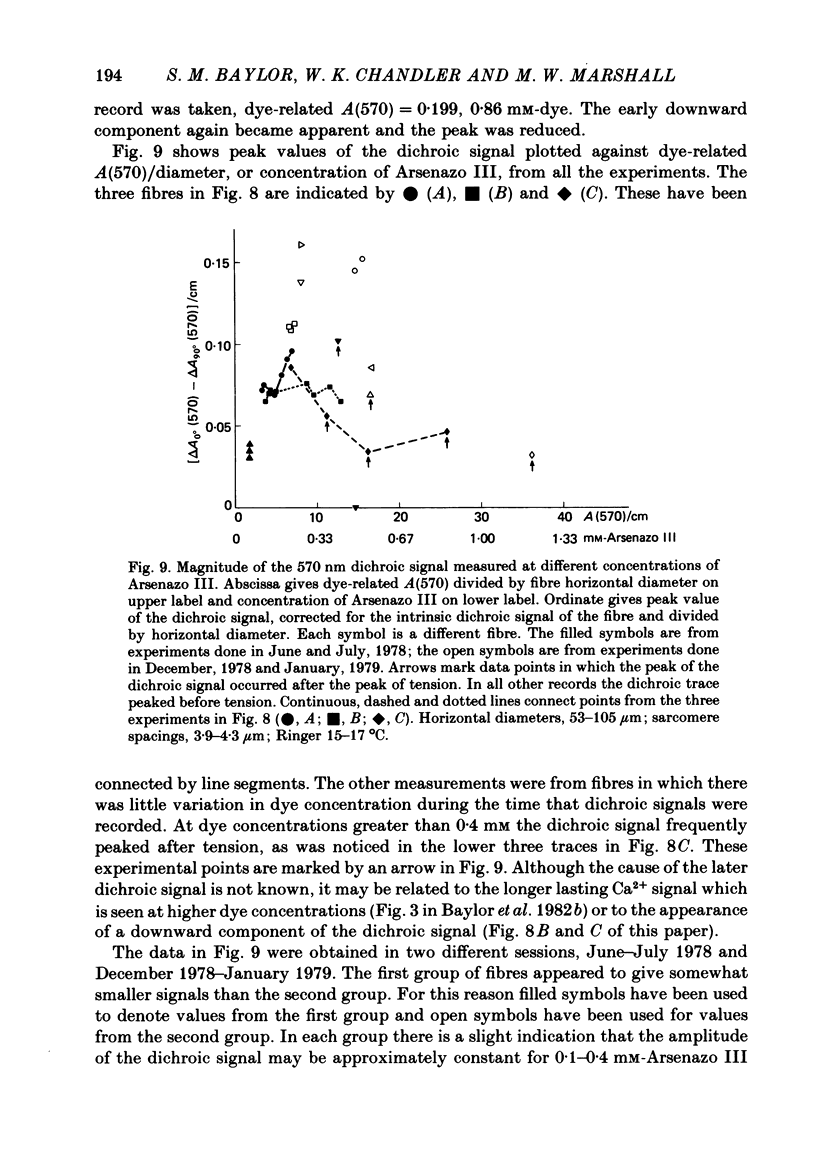
3. At 570 nm, near the isosbestic wavelength for changes in H+:dye, Mg2+:dye and Ca2+:dye, the dichroic signal is near maximal. The absorbance change with 0° light is positive and is about twice as large as the change with 90° light, which is negative. This finding is consistent with the idea that the dichroic signal arises from dye molecules which change their orientation in the radially symmetric muscle fibre. The direction of the change is for the dye's transition moment to become more aligned with the fibre axis during activity.
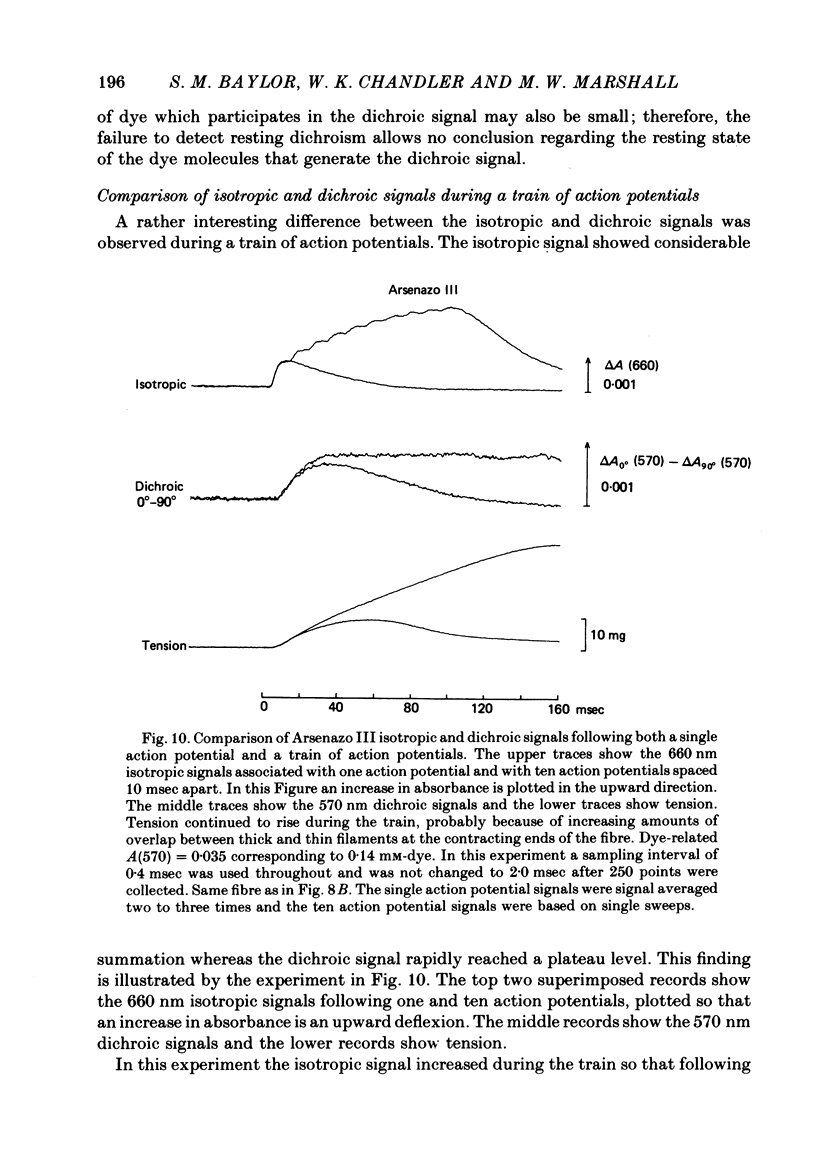
4. During a train of ten action potentials the (isotropic) Ca2+ transient increases in magnitude three-fold, whereas the dichroic waveform reaches a plateau value only 30-40% larger than the single twitch value.
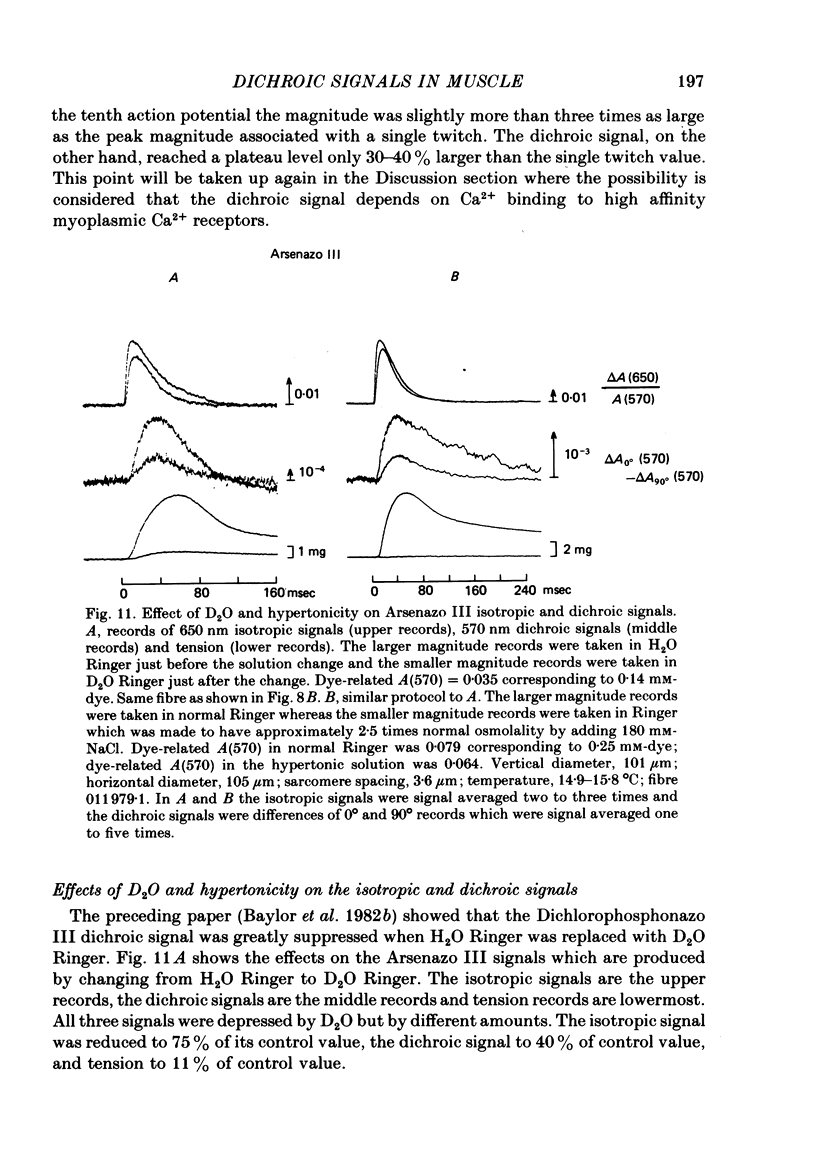
5. Replacing H2O in Ringers with D2O causes a slight reduction in the Ca2+ signal, reduces the dichroic signal to 0·4 times normal, and reduces tension to 0·1 times normal. Qualitatively similar reductions were found to accompany an increase in osmolality of H2O Ringer from 1 × to 2·5 × normal.
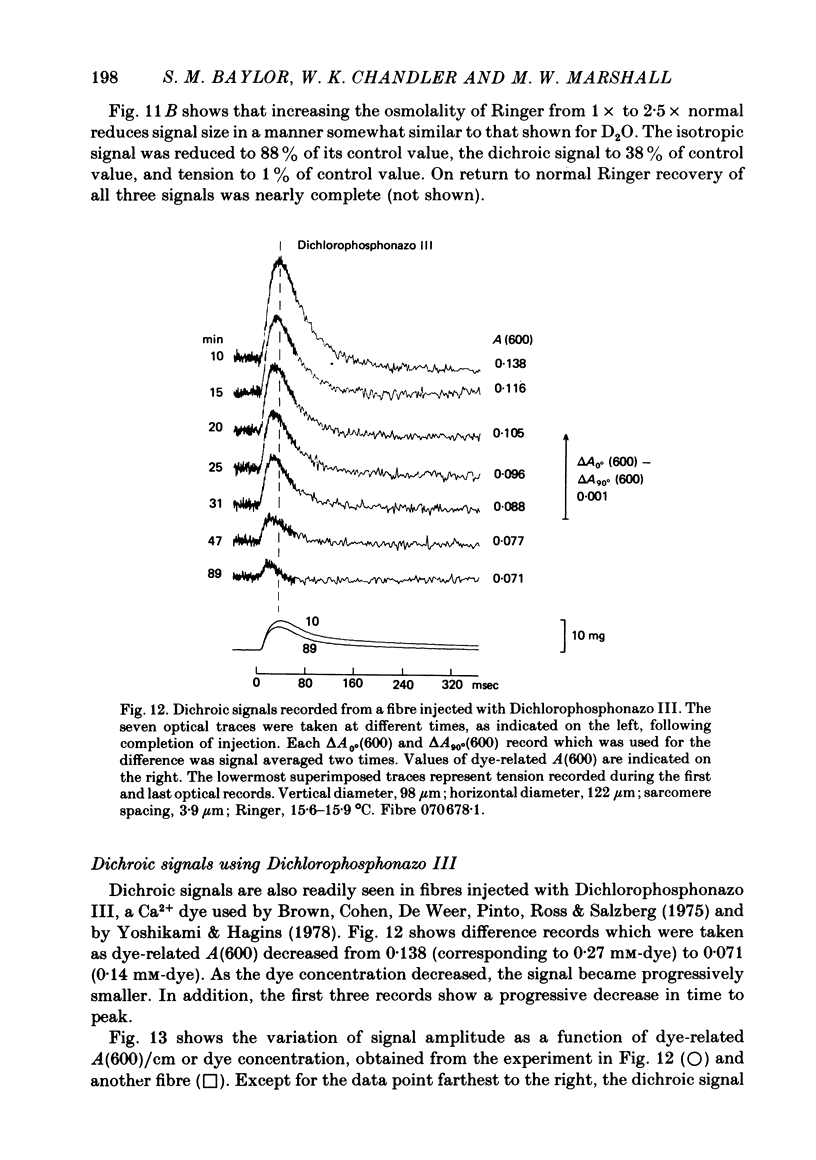
6. Dichroic signals are also observed in fibres injected with Dichlorophosphonazo III. These are similar in many respects to the Arsenazo III dichroic signals.
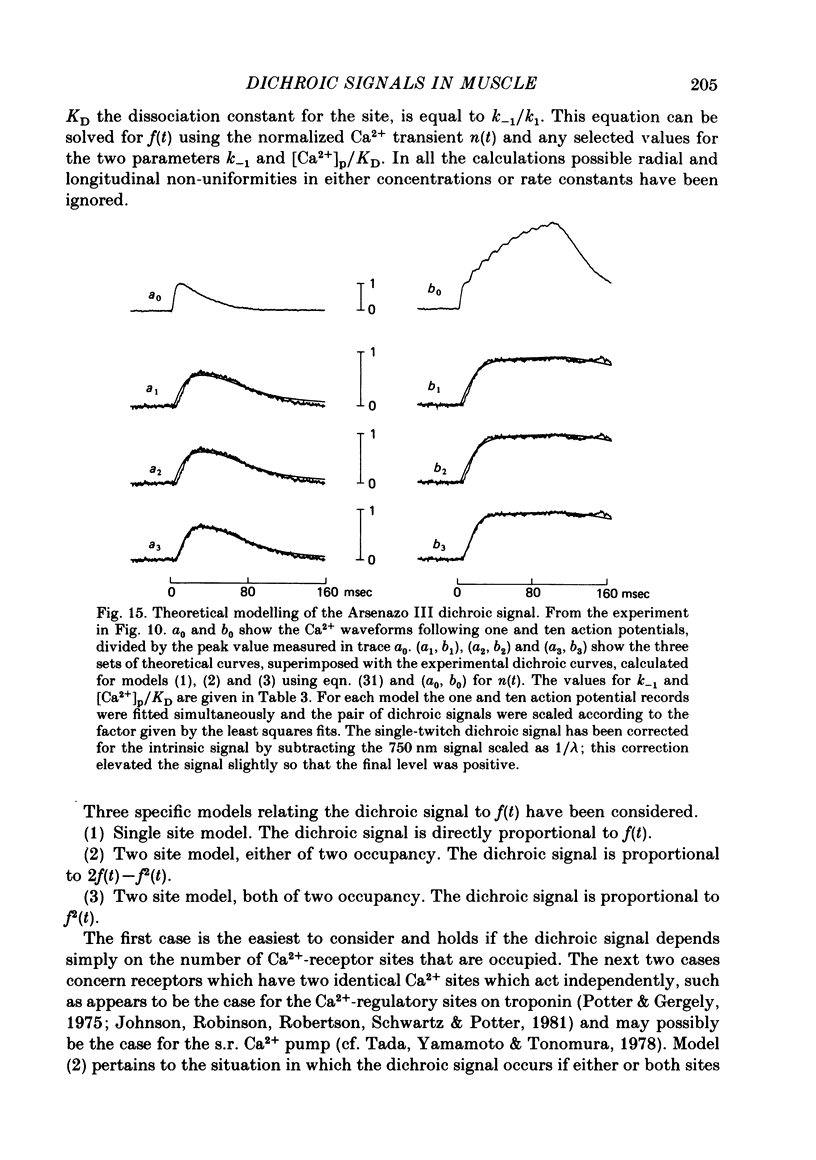
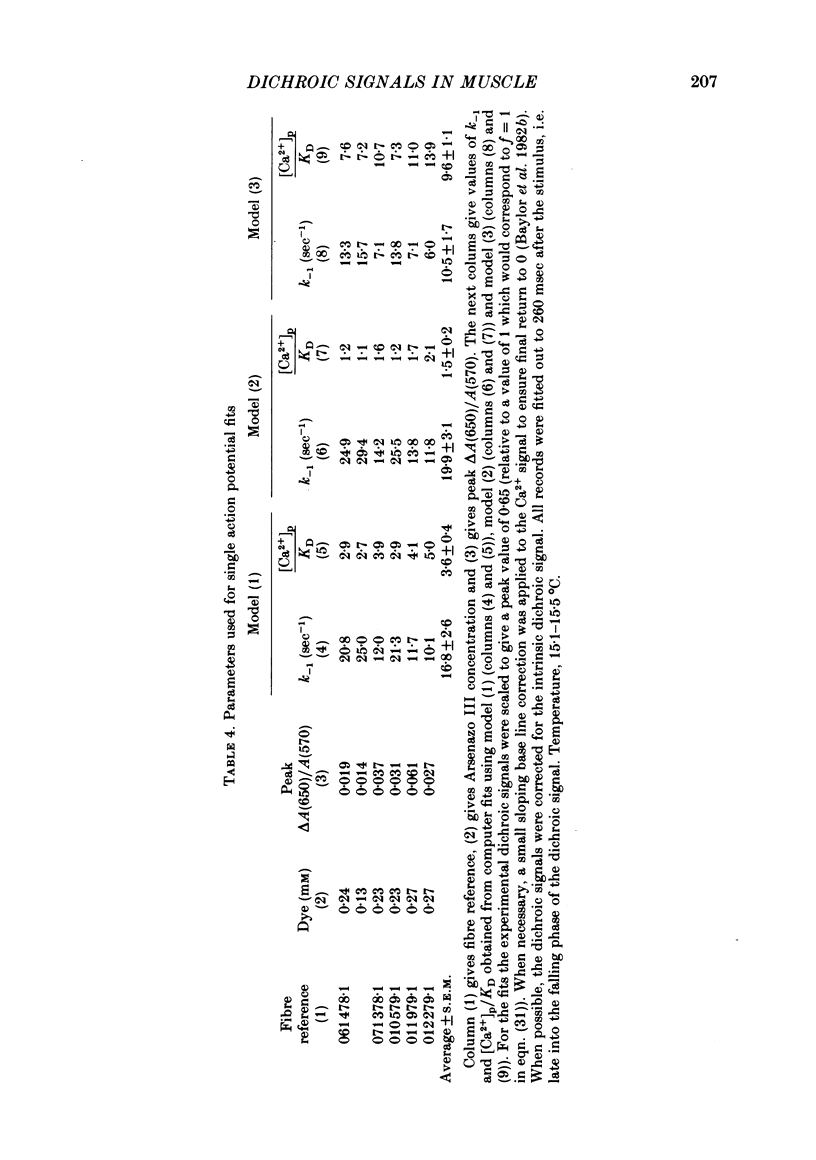
7. With Arsenazo III, the dichroic signal probably arises from a reorientation of some dye molecules which are bound to one of the oriented structures in muscle. The reorientation lags the Ca2+ transient and may be due to a change which occurs in the oriented structure itself. Using this idea, the Arsenazo III dichroic signal can be fitted by assuming that Ca2+ ions bind to receptor sites and that this binding induces the required change in the oriented structure. The analysis indicates that the hypothetical receptors have a dissociation constant for Ca2+ equal to 0·1-1 times the peak value of myoplasmic free [Ca2+] during a twitch and an `off' rate constant equal to 10-30 sec-1 at 15 °C.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor S. M., Chandler W. K., Marshall M. W. Arsenazo III signals in singly dissected frog twitch fibres [proceedings]. J Physiol. 1979 Feb;287:23P–24P. [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Optical measurements of intracellular pH and magnesium in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:105–137. doi: 10.1113/jphysiol.1982.sp014367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Use of metallochromic dyes to measure changes in myoplasmic calcium during activity in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:139–177. doi: 10.1113/jphysiol.1982.sp014368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. The optical properties of birefringence signals from single muscle fibres. J Physiol. 1977 Jan;264(1):163–198. doi: 10.1113/jphysiol.1977.sp011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler T. J., Schibeci A., Martonosi A. The binding of arsenazo III to cell components. Biochim Biophys Acta. 1980 May 7;629(2):317–327. doi: 10.1016/0304-4165(80)90104-x. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Cohen L. B., De Weer P., Pinto L. H., Ross W. N., Salzberg B. M. Rapid changes in intracellular free calcium concentration. Detection by metallochromic indicator dyes in squid giant axon. Biophys J. 1975 Nov;15(11):1155–1160. doi: 10.1016/S0006-3495(75)85891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., PEACHEY L. D. The maximum length for contraction in vertebrate straiated muscle. J Physiol. 1961 Apr;156:150–165. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick N. C., Ratzlaff R. W., Blaustein M. P. Arsenazo III as an indicator for ionized calcium in physiological salt solutions: its use for determination of the CaATP dissociation constant. Anal Biochem. 1977 Dec;83(2):433–450. doi: 10.1016/0003-2697(77)90052-5. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Measurement of calcium transients in frog muscle by the use of arsenazo III. Proc R Soc Lond B Biol Sci. 1977 Aug 22;198(1131):201–210. doi: 10.1098/rspb.1977.0094. [DOI] [PubMed] [Google Scholar]
- Potter J. D., Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1975 Jun 25;250(12):4628–4633. [PubMed] [Google Scholar]
- Rauch B., von Chak D., Hasselbach W. An estimate of the kinetics of calcium binding and dissociation of the sarcoplasmic reticulum transport ATPase. FEBS Lett. 1978 Sep 1;93(1):65–68. doi: 10.1016/0014-5793(78)80806-0. [DOI] [PubMed] [Google Scholar]
- Tada M., Yamamoto T., Tonomura Y. Molecular mechanism of active calcium transport by sarcoplasmic reticulum. Physiol Rev. 1978 Jan;58(1):1–79. doi: 10.1152/physrev.1978.58.1.1. [DOI] [PubMed] [Google Scholar]
- Yoshikami S., Hagins W. A. Calcium in excitation of vertebrate rods and cones: retinal efflux of calcium studied with dichlorophosphonazo III. Ann N Y Acad Sci. 1978 Apr 28;307:545–561. doi: 10.1111/j.1749-6632.1978.tb41981.x. [DOI] [PubMed] [Google Scholar]


