Abstract
1. Five major fibre types in chicken skeletal muscles are recognized, based upon their histochemical and morphological characteristics. A classification of these which is readily related to a commonly used classification of mammalian muscle fibre types is given.
2. Seven muscles of the chicken were analysed in recognizing this range of fibre types. The proportions of the different types in each of these were determined. In some cases a gradient of fibre type composition exists across a single muscle.
3. Measurements of muscle contraction were used in defining tonic muscles, which contain two fibre types. It was shown that in addition to the anterior latissimus dorsi (a.l.d.), previously well known to be a tonic muscle, two other muscles, the plantaris and the adductor profundus, are of the same class, but differ subtly from the a.l.d. in certain features. Gross red colouration is not a useful diagnostic feature of slow muscles, since the tonic adductor profundus, for example, is white.
4. Fibres similar histochemically to mammalian type I (slow-twitch) occur in some of the avian twitch muscles investigated. These are oxidative in character, and despite the fact that they are multiply innervated we suggest that these are avian slow-twitch fibres.
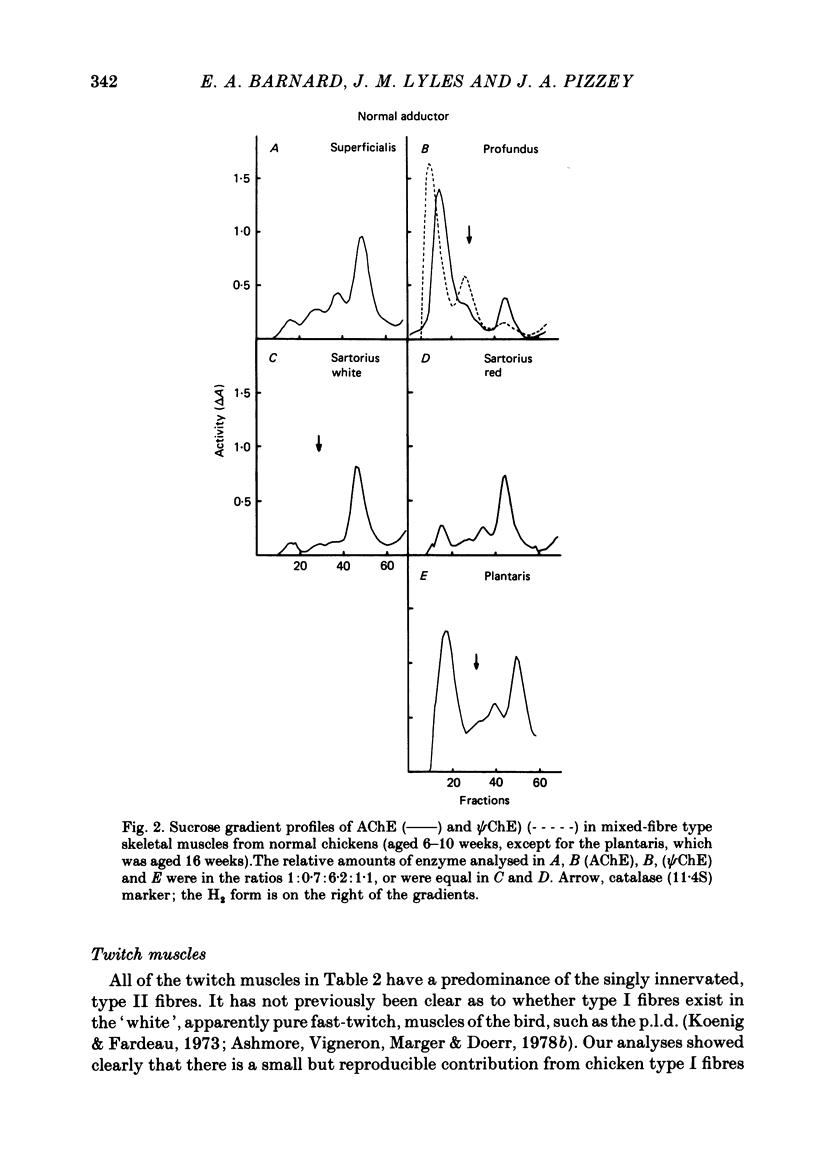
5. The patterns of cholinesterases found in a skeletal muscle correspond to its fibre type composition, with regard to both the concentrations and the proportions of the multiple forms of enzyme present. The distinctive patterns of those forms of acetylcholinesterase in the different fibre types are described.
6. The fibre type composition is changed by inherited muscular dystrophy in a characteristic manner. This change has so far been found (at the earlier stages of the disease) only in the muscles with a predominance of type II B fibres in the normal chicken. Pathological changes within the fibres occur selectively in the type II B fibres, but there are exceptions to this and the effect can be greatly modified by the type of neighbouring fibres.
Full text
PDF

























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore C. R., Doerr L. Postnatal development of fiber types in normal and dystrophic skeletal muscle of the chick. Exp Neurol. 1971 Mar;30(3):431–446. doi: 10.1016/0014-4886(71)90144-0. [DOI] [PubMed] [Google Scholar]
- Ashmore C. R., Kikuchi T., Doerr L. Some observations on the innervation patterns of different fiber types of chick muscle. Exp Neurol. 1978 Jan 15;58(2):272–284. doi: 10.1016/0014-4886(78)90140-1. [DOI] [PubMed] [Google Scholar]
- Ashmore C. R., Tompkins G., Doerr L. Comparative aspects of mitochondria isolated from W, R, and R muscle fibers of the chick. Exp Neurol. 1972 Jun;35(3):413–420. doi: 10.1016/0014-4886(72)90112-4. [DOI] [PubMed] [Google Scholar]
- Ashmore C. R., Vigneron P., Marger L., Doerr L. Simultaneous cytochemical demonstration of muscle fiber types and acetylcholinesterase in muscle fibers of dystrophic chickens. Exp Neurol. 1978 May 15;60(1):68–82. doi: 10.1016/0014-4886(78)90169-3. [DOI] [PubMed] [Google Scholar]
- Asiedu S., Shafiq S. A. Actomyosin ATPase activity of the anterior latissimus dorsi muscle of the chicken. Exp Neurol. 1972 Apr;35(1):211–213. doi: 10.1016/0014-4886(72)90070-2. [DOI] [PubMed] [Google Scholar]
- Barnard E. A., Barnard P. J. Use of genetically dystrophic animals in chemotherapy trials and application of serotonin antagonists as antidystrophic drugs. Ann N Y Acad Sci. 1979;317:374–399. [PubMed] [Google Scholar]
- Beringer T. Stereologic analysis of normal and dystrophic avian alphaW myofibers. Exp Neurol. 1978 Sep 1;61(2):380–394. doi: 10.1016/0014-4886(78)90254-6. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Cardinet G. H., 3rd, Freedland R. A., Tyler W. S., Julian L. M. Morphologic, histochemical, and quantitative enzyme study of hereditary avian muscular dystrophy. Am J Vet Res. 1972 Aug;33(8):1671–1684. [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Cosmos E. Enzymatic activity of differentiating muscle fibers. I. Development of phosphorylase in muscles of the domestic fowl. Dev Biol. 1966 Apr;13(2):163–181. doi: 10.1016/0012-1606(66)90062-5. [DOI] [PubMed] [Google Scholar]
- Cullen M. J., Harris J. B., Marshall M. W., Ward M. R. An electrophysiological and morphological study of normal and denervated chicken latissimus dorsi muscles. J Physiol. 1975 Feb;245(2):371–385. doi: 10.1113/jphysiol.1975.sp010851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedde M. R. Electrical properties and acetylcholine sensitivity of singly and multiply innervated avian muscle fibers. J Gen Physiol. 1969 May;53(5):624–637. doi: 10.1085/jgp.53.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S., Hobbs A. W. Fast and slow myosin in developing muscle fibres. Nature. 1978 Jul 6;274(5666):25–29. doi: 10.1038/274025a0. [DOI] [PubMed] [Google Scholar]
- Gleeson T. T., Putnam R. W., Bennett A. F. Histochemical, enzymatic, and contractile properties of skeletal muscle fibers in the lizard Dipsosaurus dorsalis. J Exp Zool. 1980 Dec;214(3):293–302. doi: 10.1002/jez.1402140307. [DOI] [PubMed] [Google Scholar]
- Gordon T., Perry R., Srihari T., Vrbová G. Differentiation of slow and fast muscles in chickens. Cell Tissue Res. 1977 May 16;180(2):211–222. doi: 10.1007/BF00231953. [DOI] [PubMed] [Google Scholar]
- Gordon T., Vrbová G. The influence of innervation on the differentiation of contractile speeds of developing chick muscles. Pflugers Arch. 1975 Nov 14;360(3):199–218. doi: 10.1007/BF00583716. [DOI] [PubMed] [Google Scholar]
- HESS A. Structural differences of fast and slow extrafusal muscle fibres and their nerve endings in chickens. J Physiol. 1961 Jul;157:221–231. doi: 10.1113/jphysiol.1961.sp006717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A. Vertebrate slow muscle fibers. Physiol Rev. 1970 Jan;50(1):40–62. doi: 10.1152/physrev.1970.50.1.40. [DOI] [PubMed] [Google Scholar]
- Hoekman T. B. Isometric contractile properties of the posterior latissimus dorsi muscle in normal and genetically dystrophic chickens. Exp Neurol. 1976 Dec;53(3):729–743. doi: 10.1016/0014-4886(76)90151-5. [DOI] [PubMed] [Google Scholar]
- Jedrzejczyk J., Silman I., Lyles J. M., Barnard E. A. Molecular forms of the cholinesterases inside and outside muscle endplates. Biosci Rep. 1981 Jan;1(1):45–51. doi: 10.1007/BF01115148. [DOI] [PubMed] [Google Scholar]
- Khan M. A. Histochemical sub-types of three fibre-types of avian skeletal muscle. Histochemistry. 1976 Nov 19;50(1):9–16. doi: 10.1007/BF00492781. [DOI] [PubMed] [Google Scholar]
- Koenig J., Fardeau M. Etude histochimique des muscles grands dorsaux antérieur et postérieur du poulet et des modifications observées après dénervation et réinnervation homologue ou croisée. Arch Anat Microsc Morphol Exp. 1973 Jul-Sep;62(3):249–267. [PubMed] [Google Scholar]
- Lee S. Y. A histochemical study of twitch and tonus fibers. J Morphol. 1971 Mar;133(3):253–271. doi: 10.1002/jmor.1051330302. [DOI] [PubMed] [Google Scholar]
- Libelius R., Jirmanová I., Lundquist I., Thesleff S., Barnard E. A. T-tubule endocytosis in dystrophic chicken muscle and its relation to muscle fiber degeneration. Acta Neuropathol. 1979 Oct;48(1):31–38. doi: 10.1007/BF00691788. [DOI] [PubMed] [Google Scholar]
- Lyles J. M., Barnard E. A. Disappearance of the 'endplate' form of acetylcholinesterase from a slow tonic muscle. FEBS Lett. 1980 Jan 1;109(1):9–12. doi: 10.1016/0014-5793(80)81299-3. [DOI] [PubMed] [Google Scholar]
- Lyles J. M., Silman I., Barnard E. A. Developmental changes in levels and forms of cholinesterases in muscles of normal and dystrophic chickens. J Neurochem. 1979 Sep;33(3):727–738. doi: 10.1111/j.1471-4159.1979.tb05218.x. [DOI] [PubMed] [Google Scholar]
- Lyles J. M., Silman I., Di Giamberardino L., Couraud J. Y., Barnard E. A. Comparison of the molecular forms of the cholinesterases in tissues of normal and dystrophic chickens. J Neurochem. 1982 Apr;38(4):1007–1021. doi: 10.1111/j.1471-4159.1982.tb05342.x. [DOI] [PubMed] [Google Scholar]
- McMurtry S. L., Julian L. M., Asmundson V. S. Hereditary muscular dystrophy of the chicken. Quantitative histopathological findings of the Pectoralis at 6 weeks of age. Arch Pathol. 1972 Sep;94(3):217–224. [PubMed] [Google Scholar]
- Melichna J., Gutmann E., Syrový I. Developmental changes in contraction properties, adenosine-triphosphatase activity and muscle fibre pattern of fast and slow chicken muscle. Physiol Bohemoslov. 1974;23(6):511–520. [PubMed] [Google Scholar]
- PEACHEY L. D., HUXLEY A. F. Structural identification of twitch and slow striated muscle fibers of the frog. J Cell Biol. 1962 Apr;13:177–180. doi: 10.1083/jcb.13.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. G. Structure and some contractile properties of fast and slow muscles of the chicken. J Physiol. 1969 Nov;205(1):131–145. doi: 10.1113/jphysiol.1969.sp008956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Putnam R. W., Gleeson T. T., Bennett A. F. Histochemical determination of the fiber composition of locomotory muscles in a lizard, Dipsosaurus dorsalis. J Exp Zool. 1980 Dec;214(3):303–309. doi: 10.1002/jez.1402140308. [DOI] [PubMed] [Google Scholar]
- Shafiq S. A., Askanas V., Milhorat A. T. Fiber types and preclinical changes in chicken muscular dystrophy. Arch Neurol. 1971 Dec;25(6):560–571. doi: 10.1001/archneur.1971.00490060094010. [DOI] [PubMed] [Google Scholar]
- Shear C. R., Goldspink G. Structural and physiological changes associated with the growth of avian fast and slow muscle. J Morphol. 1971 Nov;135(3):351–372. doi: 10.1002/jmor.1051350308. [DOI] [PubMed] [Google Scholar]
- Silman I., Lyles J. M., Barnard E. A. Intrinsic forms of acetylcholinesterase in skeletal muscle. FEBS Lett. 1978 Oct 1;94(1):166–170. doi: 10.1016/0014-5793(78)80929-6. [DOI] [PubMed] [Google Scholar]
- Silman I., di Giamberardino L., Lyles L., Couraud J. Y., Barnard E. A. Parallel regulation of acetylcholinesterase and pseudocholinesterase in normal, denervated and dystrophic chicken skeletal muscle. Nature. 1979 Jul 12;280(5718):160–162. doi: 10.1038/280160a0. [DOI] [PubMed] [Google Scholar]
- Talesara G. L., Goldspink G. A combined histochemical and biochemical study of myofibrillar ATPase in pectoral, leg and cardiac muscle of several species of bird. Histochem J. 1978 Nov;10(6):695–709. doi: 10.1007/BF01003119. [DOI] [PubMed] [Google Scholar]
- Toutant J. P., Toutant M. N., Renaud D., Le Douarin G. H. Histochemical differentiation of extrafusal muscle fibres of the anterior latissimus dorsi in the chick. Cell Differ. 1980 Dec;9(6):305–314. doi: 10.1016/0045-6039(80)90029-9. [DOI] [PubMed] [Google Scholar]
- Tsuji S. On the chemical basis of thiocholine methods for demonstration of acetylcholinesterase activities. Histochemistry. 1974;42(1):99–110. doi: 10.1007/BF00498482. [DOI] [PubMed] [Google Scholar]
- Wilson B. W., Linkhart S. G., Nieberg P. A. Acetylcholinesterase in singly and multiply innervated muscles of normal and dystrophic chickens. J Exp Zool. 1973 Nov;186(2):187–192. doi: 10.1002/jez.1401860209. [DOI] [PubMed] [Google Scholar]
- Wilson B. W., Montgomery M. A., Asmundson R. V. Cholinesterase activity and inherited muscular dystrophy of the cicken. Proc Soc Exp Biol Med. 1968 Oct;129(1):199–206. doi: 10.3181/00379727-129-33283. [DOI] [PubMed] [Google Scholar]
- Wilson B. W., Randall W. R., Patterson G. T., Entrikin R. K. Major physiologic and histochemical characteristics of inherited dystrophy of the chicken. Ann N Y Acad Sci. 1979;317:224–246. doi: 10.1111/j.1749-6632.1979.tb56531.x. [DOI] [PubMed] [Google Scholar]
- Yellin H. Unique intrafusal and extraocular muscle fibers exhibiting dual actomyosin ATPase activity. Exp Neurol. 1969 Sep;25(1):153–163. doi: 10.1016/0014-4886(69)90078-8. [DOI] [PubMed] [Google Scholar]