Abstract
1. Tuning properties and spontaneous discharge rate of single cochlear fibres in the anaesthetized cat were determined during short- and long-term poisoning of the cochlea by locally and systemically applied furosemide.
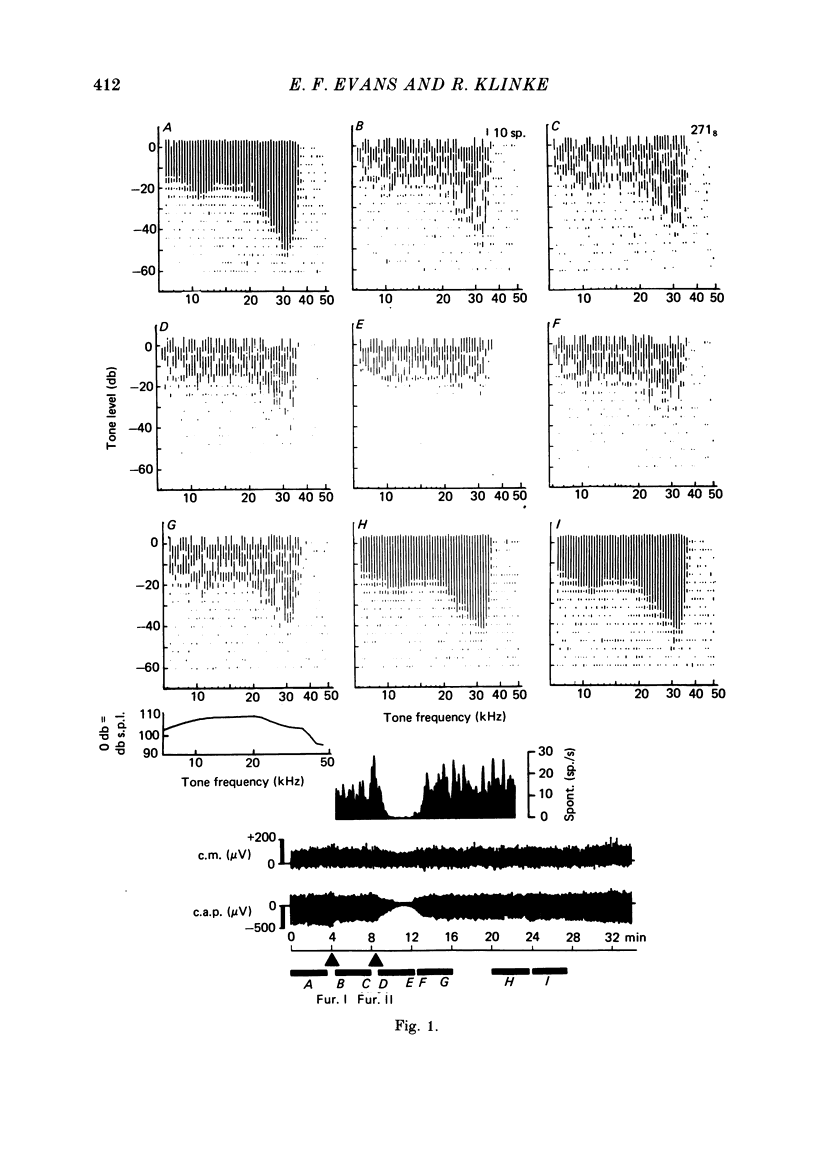
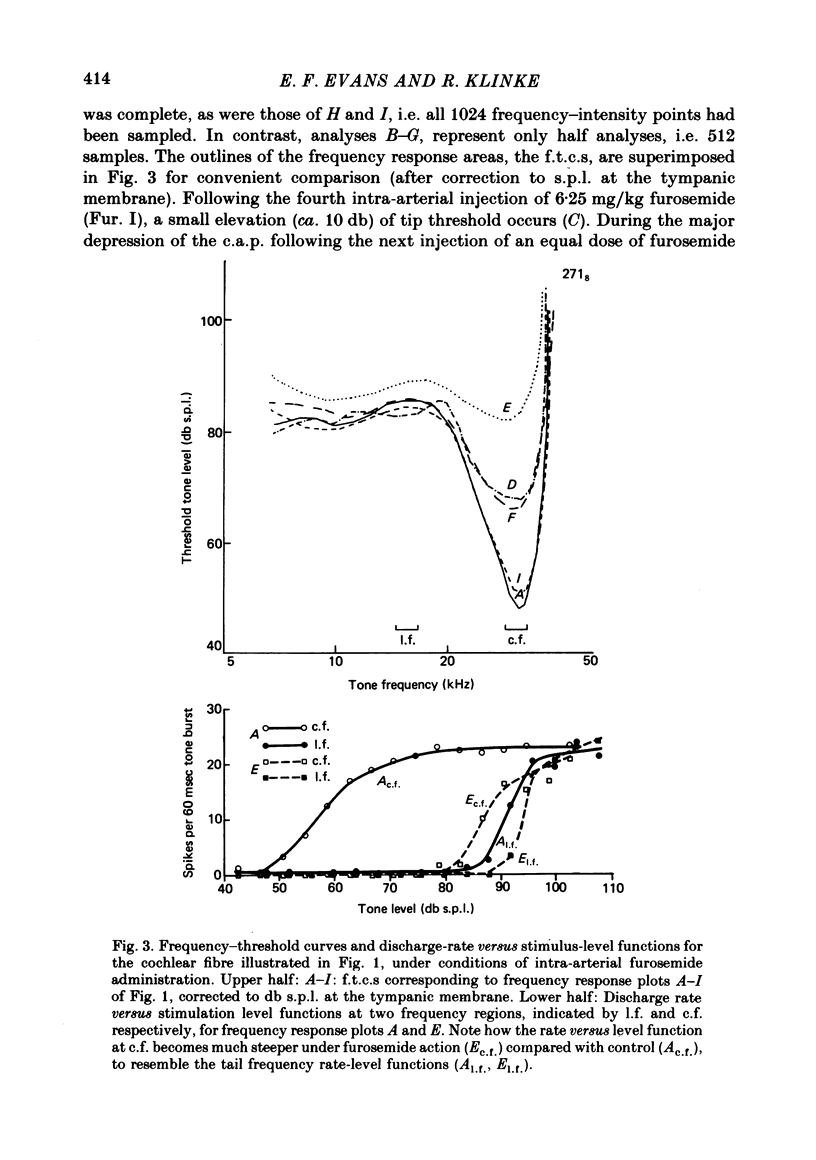
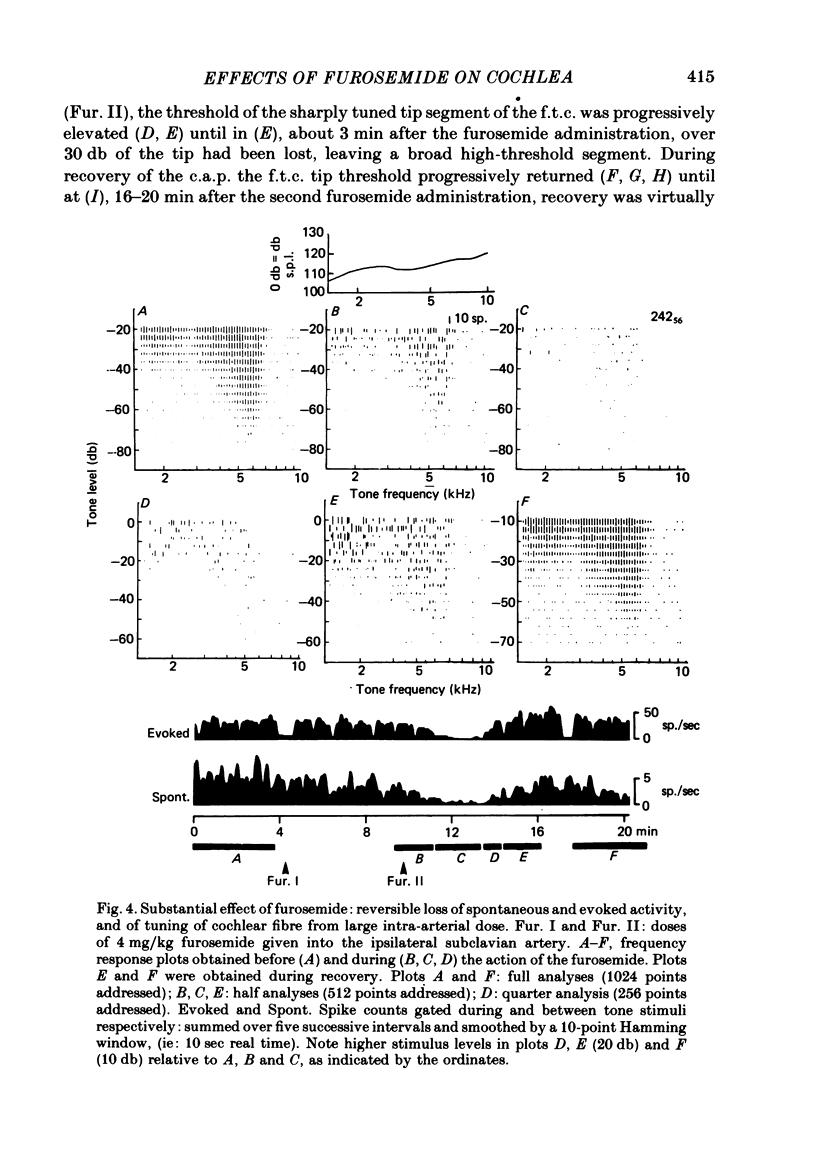
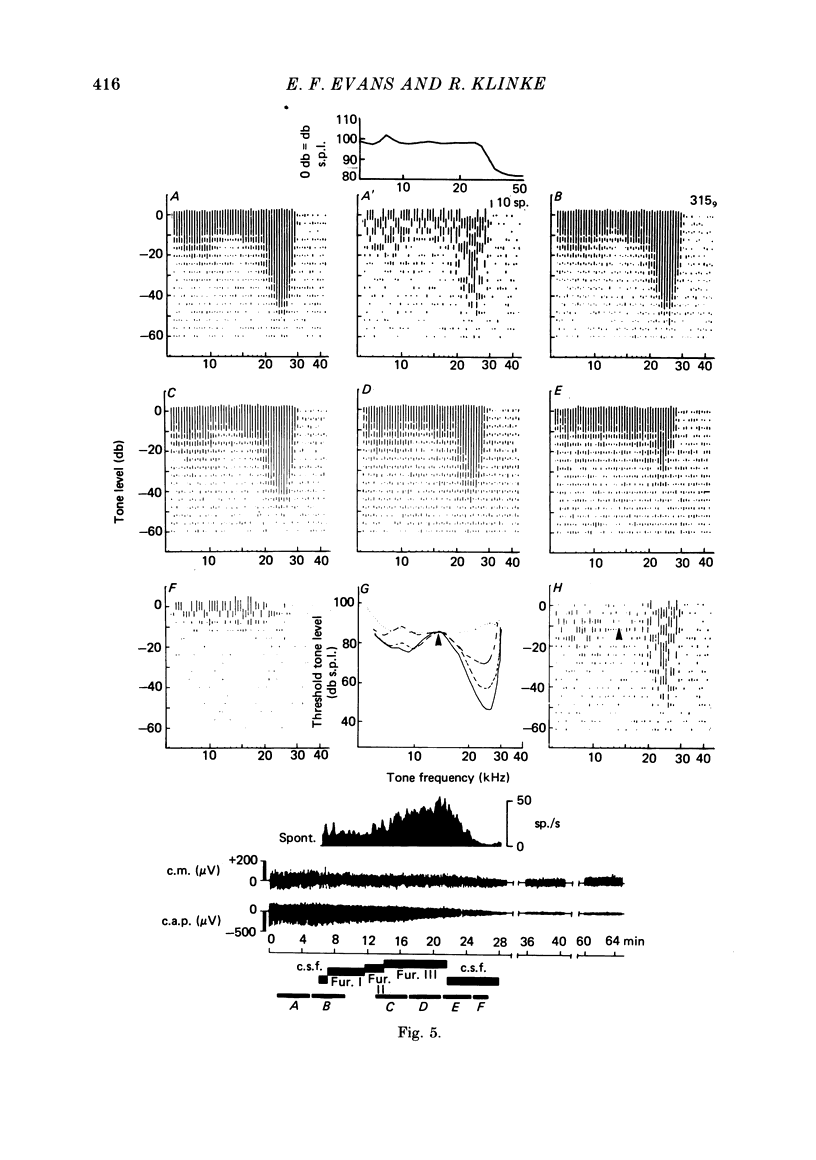
2. With intra-arterial administration of furosemide, short-term reversible elevation occurred of the low threshold sharply tuned `tip' segment of the frequency threshold (`tuning') curve (f.t.c.) by up to 40 db, without substantial changes in the threshold of the low frequency `tail' segment of the f.t.c. These changes could occur in part without changes in the spontaneous activity and entirely without changes in the maximal evoked activity. These effects were observed in all fibres examined, the characteristic frequencies of which ranged from 3·5 to 31 kHz.
3. Intracochlear administration of furosemide in 0·9 mM concentrations produced similar changes, but these were not reversible.
4. The changes correlated with the depression of the amplitude of the gross cochlear action potential. The cochlear microphonic potential, however, was either unchanged, or only slightly reduced.
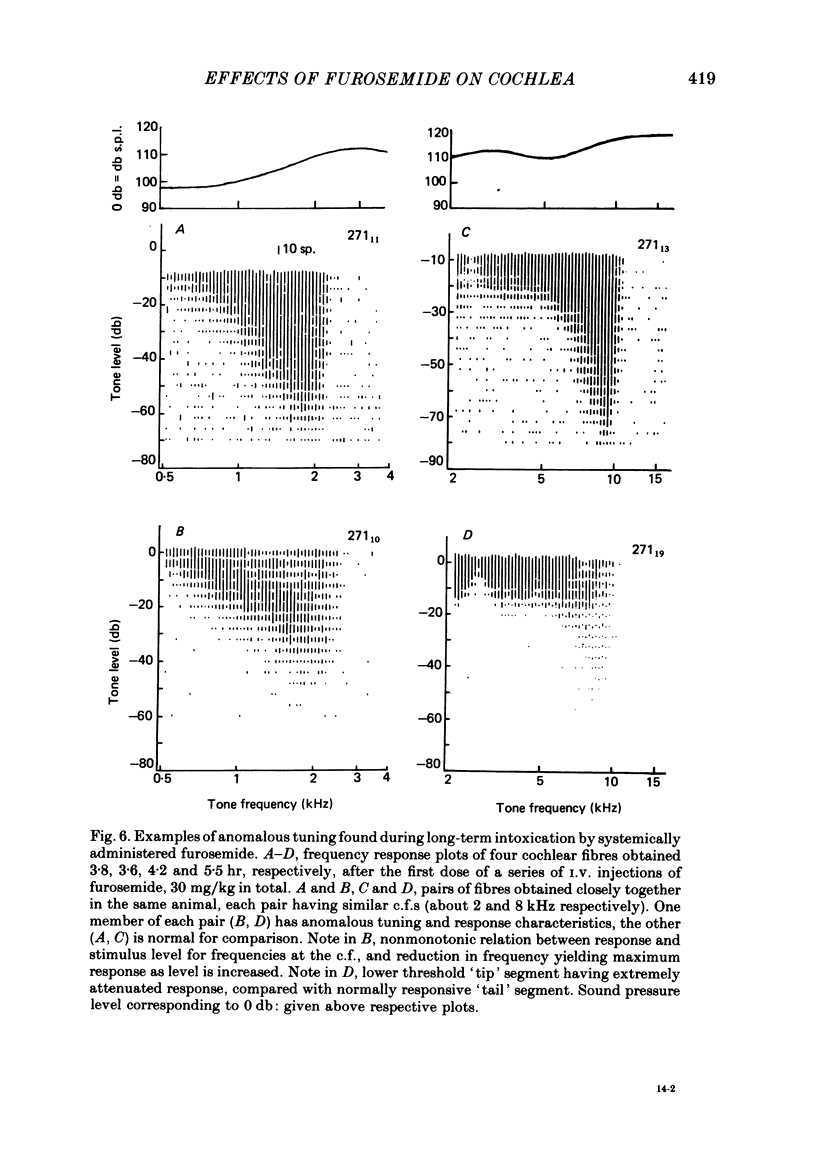
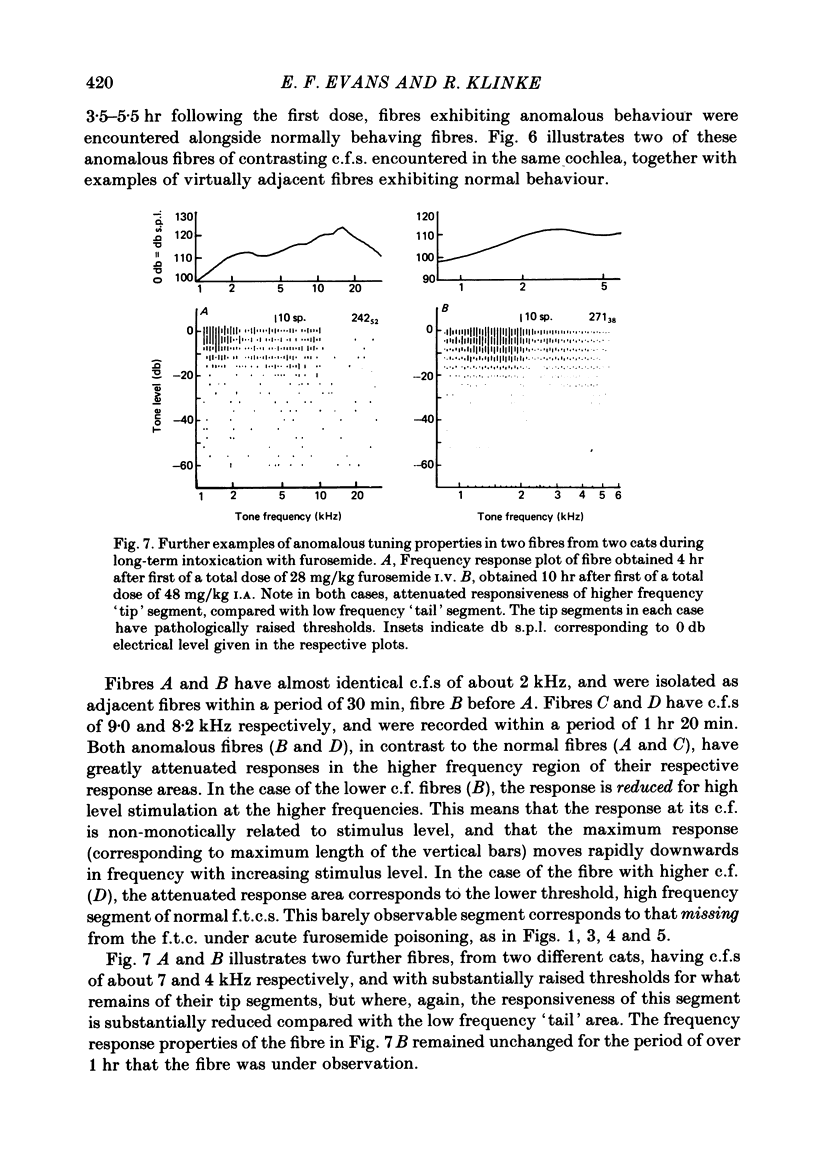
5. In long-term furosemide poisoning of the cochlea, fibres with anomalous response properties were found alongside fibres having normal tuning. The former exhibited either reduced excitability of the low threshold tip segment, or a tip segment attenuated in both excitability and threshold.
6. It is concluded that the selective effects of furosemide on the tip segment of cochlear fibre f.t.c.s offer further evidence for a physiologically vulnerable `second filter' in the cochlea. The selective influence of the furosemide on the low threshold tip segment provides support for the hypothesis that the normal f.t.c. is generated by two largely independent processes: one vulnerable, low threshold and sharply tuned, and the other less vulnerable, but high threshold and more broadly tuned.
7. The findings, obtained with an agent known to produce reversible impairment of hearing in man, provide direct physiological evidence in support of the hypothesis that in sensorineural hearing loss of cochlear origin the frequency selectivity of cochlear nerve fibres is impaired.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosher S. K., Smith C., Warren R. L. The effects of ethacrynic acid upon the cochlear endolymph and stria vascularis. A preliminary report. Acta Otolaryngol. 1973 Feb-Mar;75(2):184–191. doi: 10.3109/00016487309139694. [DOI] [PubMed] [Google Scholar]
- Bosher S. K. The nature of the negative endocochlear potentials produced by anoxia and ethacrynic acid in the rat and guinea-pig. J Physiol. 1979 Aug;293:329–345. doi: 10.1113/jphysiol.1979.sp012892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher S. K. The nature of the ototoxic actions of ethacrynic acid upon the mammalian endolymph system. I. Functional aspects. Acta Otolaryngol. 1980 May-Jun;89(5-6):407–418. doi: 10.3109/00016488009127156. [DOI] [PubMed] [Google Scholar]
- Bosher S. K. The nature of the ototoxic actions of ethacrynic acid upon the mammalian endolymph system. II. Structural-functional correlates in the stria vascularis. Acta Otolaryngol. 1980;90(1-2):40–54. doi: 10.3109/00016488009131696. [DOI] [PubMed] [Google Scholar]
- Bowman R. H., Dolgin J., Coulson R. Furosemide, ethacrynic acid, and iodoacetate on function and metabolism in perfused rat kidney. Am J Physiol. 1973 Feb;224(2):416–424. doi: 10.1152/ajplegacy.1973.224.2.416. [DOI] [PubMed] [Google Scholar]
- Brown R. D., Feldman A. M. Pharmacology of hearing and ototoxicity. Annu Rev Pharmacol Toxicol. 1978;18:233–252. doi: 10.1146/annurev.pa.18.040178.001313. [DOI] [PubMed] [Google Scholar]
- Brown R. D., McElwee T. W., Jr Effects of intra-arterially and intravenously administered ethacrynic acid and furosemide on cochlear N 1 in cats. Toxicol Appl Pharmacol. 1972 Aug;22(4):589–594. doi: 10.1016/0041-008x(72)90286-4. [DOI] [PubMed] [Google Scholar]
- Brusilow S. W., Gordes E. The mutual independence of the endolymphatic potential and the concentrations of sodium and potassium in endolymph. J Clin Invest. 1973 Oct;52(10):2517–2521. doi: 10.1172/JCI107442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M., Stoner L., Cardinal J., Green N. Furosemide effect on isolated perfused tubules. Am J Physiol. 1973 Jul;225(1):119–124. doi: 10.1152/ajplegacy.1973.225.1.119. [DOI] [PubMed] [Google Scholar]
- Candia O. A. Short-circuit current related to active transport of chloride in frog cornea: effects of furosemide and ethacrynic acid. Biochim Biophys Acta. 1973 Apr 16;298(4):1011–1014. doi: 10.1016/0005-2736(73)90407-0. [DOI] [PubMed] [Google Scholar]
- Cohn E. S., Gordes E. H., Brusilow S. W. Ethacrynic acid effect on the composition of cochlear fluids. Science. 1971 Mar 5;171(3974):910–911. doi: 10.1126/science.171.3974.910. [DOI] [PubMed] [Google Scholar]
- Desmedt J. E., Robertson D. Ionic mechanism of the efferent olivo-cochlear inhibition studied by cochlear perfusion in the cat. J Physiol. 1975 May;247(2):407–428. doi: 10.1113/jphysiol.1975.sp010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernstson S. Ethacrynic acid-induced hearing loss in guinea pigs. Acta Otolaryngol. 1972 Jun;73(6):476–483. doi: 10.3109/00016487209138968. [DOI] [PubMed] [Google Scholar]
- Evans E. F., Harrison R. V. Proceedings: Correlation between cochlear outer hair cell damage and deterioration of cochlear nerve tuning properties in the guinea-pig. J Physiol. 1976 Mar;256(1):43P–44P. [PubMed] [Google Scholar]
- Evans E. F., Klinke R. The effects of intracochlear cyanide and tetrodotoxin on the properties of single cochlear nerve fibres in the cat. J Physiol. 1982 Oct;331:385–408. doi: 10.1113/jphysiol.1982.sp014378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. F. Peripheral auditory processing in normal and abnormal ears: physiological considerations for attempts to compensate for auditory deficits by acoustic and electrical prostheses. Scand Audiol Suppl. 1978;(6):9–47. [PubMed] [Google Scholar]
- Evans E. F. Proceedings: The effects of hypoxia on the tuning of single cochlear nerve fibres. J Physiol. 1974 Apr;238(1):65P–67P. [PubMed] [Google Scholar]
- Evans E. F. The frequency response and other properties of single fibres in the guinea-pig cochlear nerve. J Physiol. 1972 Oct;226(1):263–287. doi: 10.1113/jphysiol.1972.sp009984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. F. The sharpening of cochlear frequency selectivity in the normal and abnormal cochlea. Audiology. 1975;14(5-6):419–442. doi: 10.3109/00206097509071754. [DOI] [PubMed] [Google Scholar]
- Forge A. Observations on the stria vascularis of the guinea pig cochlea and the changes resulting from the administration of the diuretic furosemide. Clin Otolaryngol Allied Sci. 1976;1(3):211–219. doi: 10.1111/j.1365-2273.1976.tb00879.x. [DOI] [PubMed] [Google Scholar]
- Geisler C. D., Rhode W. S., Kennedy D. T. Responses to tonal stimuli of single auditory nerve fibers and their relationship to basilar membrane motion in the squirrel monkey. J Neurophysiol. 1974 Nov;37(6):1156–1172. doi: 10.1152/jn.1974.37.6.1156. [DOI] [PubMed] [Google Scholar]
- Harrison R. V., Evans E. F. Cochlear fibre responses in guinea pigs with well defined cochlear lesions. Scand Audiol Suppl. 1979;(9):83–92. [PubMed] [Google Scholar]
- Kaku Y., Farmer J. C., Jr, Hudson W. R. Ototoxic drug effects on cochlear histochemistry. Arch Otolaryngol. 1973 Oct;98(4):282–286. doi: 10.1001/archotol.1973.00780020292014. [DOI] [PubMed] [Google Scholar]
- Kiang N. Y., Liberman M. C., Levine R. A. Auditory-nerve activity in cats exposed to ototoxic drugs and high-intensity sounds. Ann Otol Rhinol Laryngol. 1976 Nov-Dec;85(6 Pt 1):752–768. doi: 10.1177/000348947608500605. [DOI] [PubMed] [Google Scholar]
- Kiang N. Y., Moxon E. C., Levine R. A. Auditory-nerve activity in cats with normal and abnormal cochleas. In: Sensorineural hearing loss. Ciba Found Symp. 1970:241–273. doi: 10.1002/9780470719756.ch15. [DOI] [PubMed] [Google Scholar]
- Klahr S., Yates J., Bourgoignie J. Inhibition of glycolysis by ethacrynic acid and furosemide. Am J Physiol. 1971 Oct;221(4):1038–1043. doi: 10.1152/ajplegacy.1971.221.4.1038. [DOI] [PubMed] [Google Scholar]
- Klinke R., Oertel W. Evidence that GABA is not the afferent transmitter in the cochlea. Exp Brain Res. 1977 Jun 27;28(3-4):311–314. doi: 10.1007/BF00235712. [DOI] [PubMed] [Google Scholar]
- Koide Y., Hata A., Hando R. Vulnerability of the organ of Corti in poisoning. Acta Otolaryngol. 1966 Apr;61(4):332–344. doi: 10.3109/00016486609127069. [DOI] [PubMed] [Google Scholar]
- Kusakari J., Ise I., Comegys T. H., Thalmann I., Thalmann R. Effect of ethacrynic acid, furosemide, and ouabain upon the endolymphatic potential and upon high energy phosphates of the stria vascularis. Laryngoscope. 1978 Jan;88(1 Pt 1):12–37. doi: 10.1002/lary.1978.88.1.12. [DOI] [PubMed] [Google Scholar]
- Liberman M. C., Kiang N. Y. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl. 1978;358:1–63. [PubMed] [Google Scholar]
- Mathog R. H., Thomas W. G., Hudson W. R. Ototoxicity of new and potent diuretics. A preliminary study. Arch Otolaryngol. 1970 Jul;92(1):7–13. doi: 10.1001/archotol.1970.04310010033002. [DOI] [PubMed] [Google Scholar]
- McGahan M. C., Yorio T., Bentley P. J. The mode of action of bumetanide: inhibition of chloride transport across the amphibian cornea. J Pharmacol Exp Ther. 1977 Oct;203(1):97–102. [PubMed] [Google Scholar]
- Nakai Y. Electron microscopic study of the inner ear after ethacrynic acid intoxication. Pract Otorhinolaryngol (Basel) 1971;33(6):366–376. doi: 10.1159/000275018. [DOI] [PubMed] [Google Scholar]
- PATTERSON W. C., GULICK W. L. The effects of chloramphenicol upon the electrical activity of the ear. Ann Otol Rhinol Laryngol. 1963 Mar;72:50–55. doi: 10.1177/000348946307200104. [DOI] [PubMed] [Google Scholar]
- Quick C. A., Duvall A. J., 3rd Early changes in the cochlear duct from ethacrynic acid: an electronmicroscopie evaluation. Laryngoscope. 1970 Jun;80(6):954–965. doi: 10.1288/00005537-197006000-00009. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978 Nov;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector G. J. The ultrastructural cytochemistry of lactic dehydrogenase, succinic dehydrogenase, dihydro-nicotinamide adenine dinucleotide diaphorase and cytochrome oxidase activities in hair cell mitochondria of the guinea pig cochlea. J Histochem Cytochem. 1975 Mar;23(3):216–234. doi: 10.1177/23.3.236341. [DOI] [PubMed] [Google Scholar]
- Thalmann R., Thalmann I., Ise I., Paloheimo S. Noxious effects upon cochlear metabolism. Laryngoscope. 1977 May;87(5 Pt 1):699–721. doi: 10.1002/lary.5540870506. [DOI] [PubMed] [Google Scholar]
- Zeuthen T., Ramos M., Ellory J. C. Inhibition of active chloride transport by piretanide. Nature. 1978 Jun 22;273(5664):678–680. doi: 10.1038/273678a0. [DOI] [PubMed] [Google Scholar]


