Abstract
1. The effects of isoprenaline (10-6 M) on relaxation, unidirectional as well as net Ca2+ fluxes, and cyclic AMP levels were investigated in rabbit aorta under the condition of high-K+ depolarization in the presence of phentolamine (10-5 M).
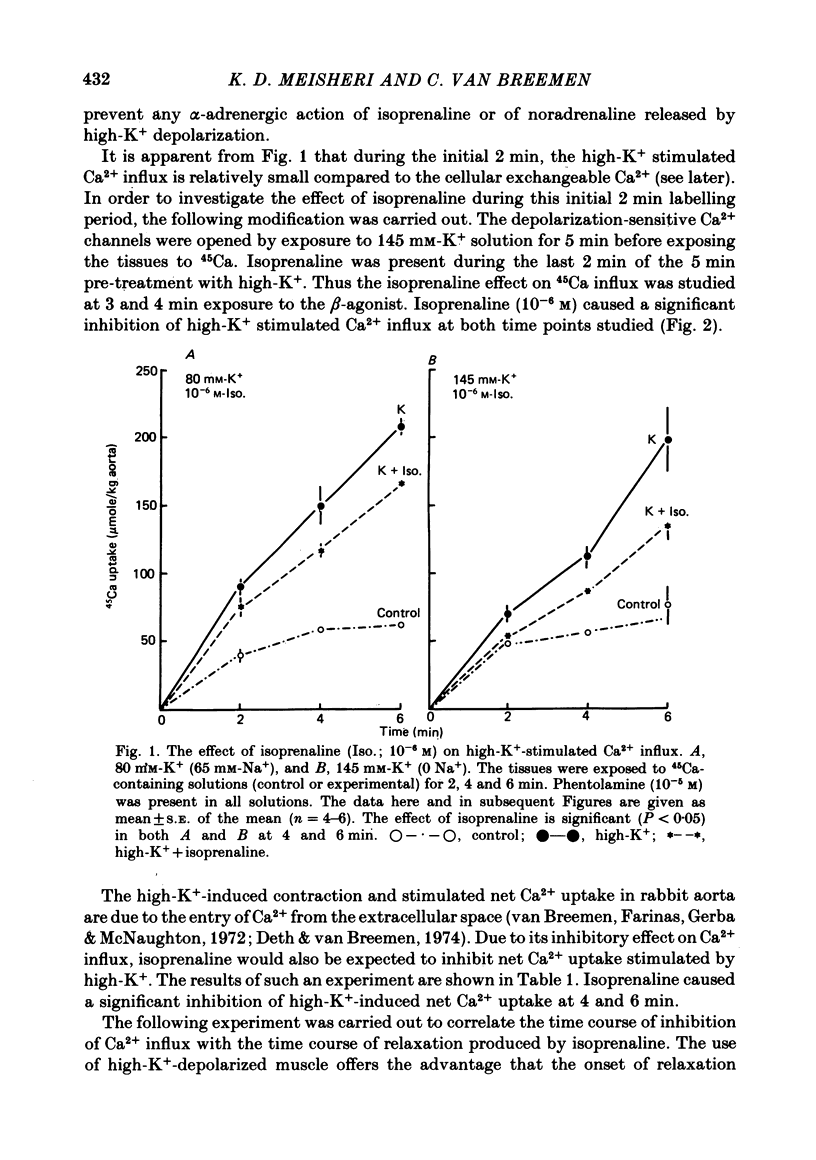
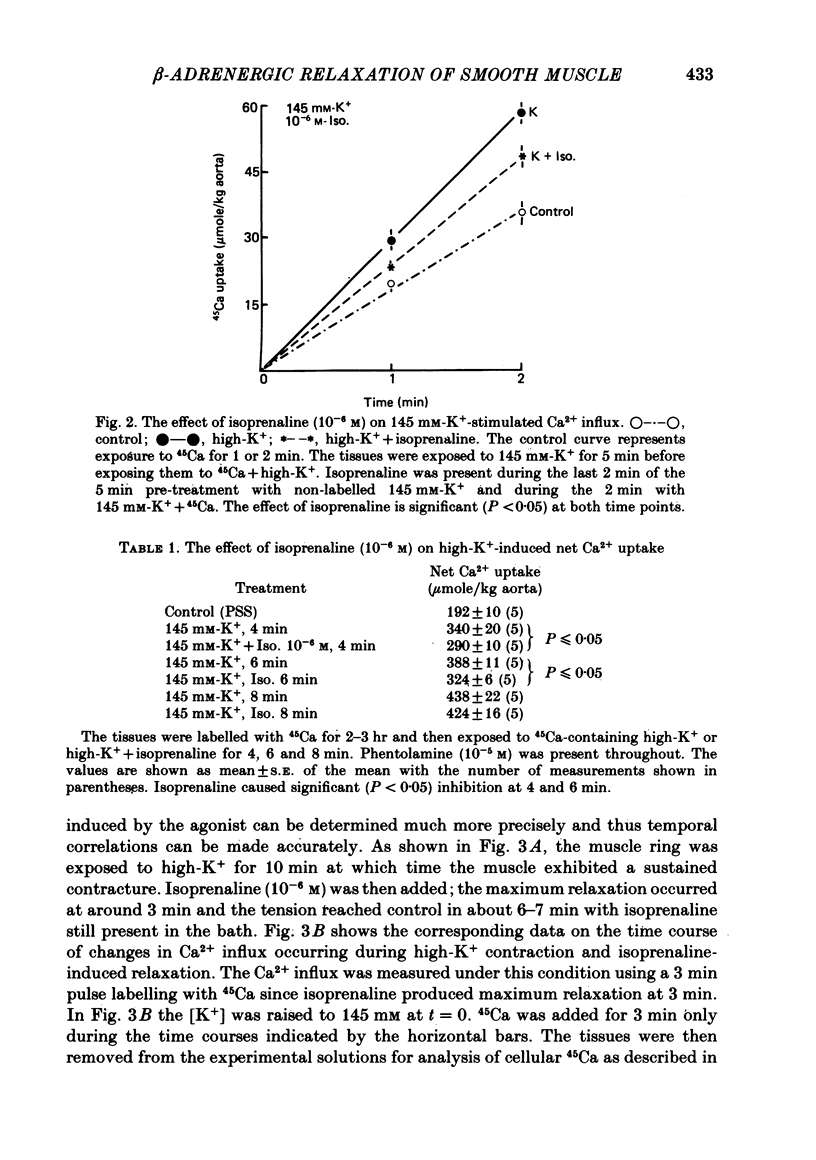
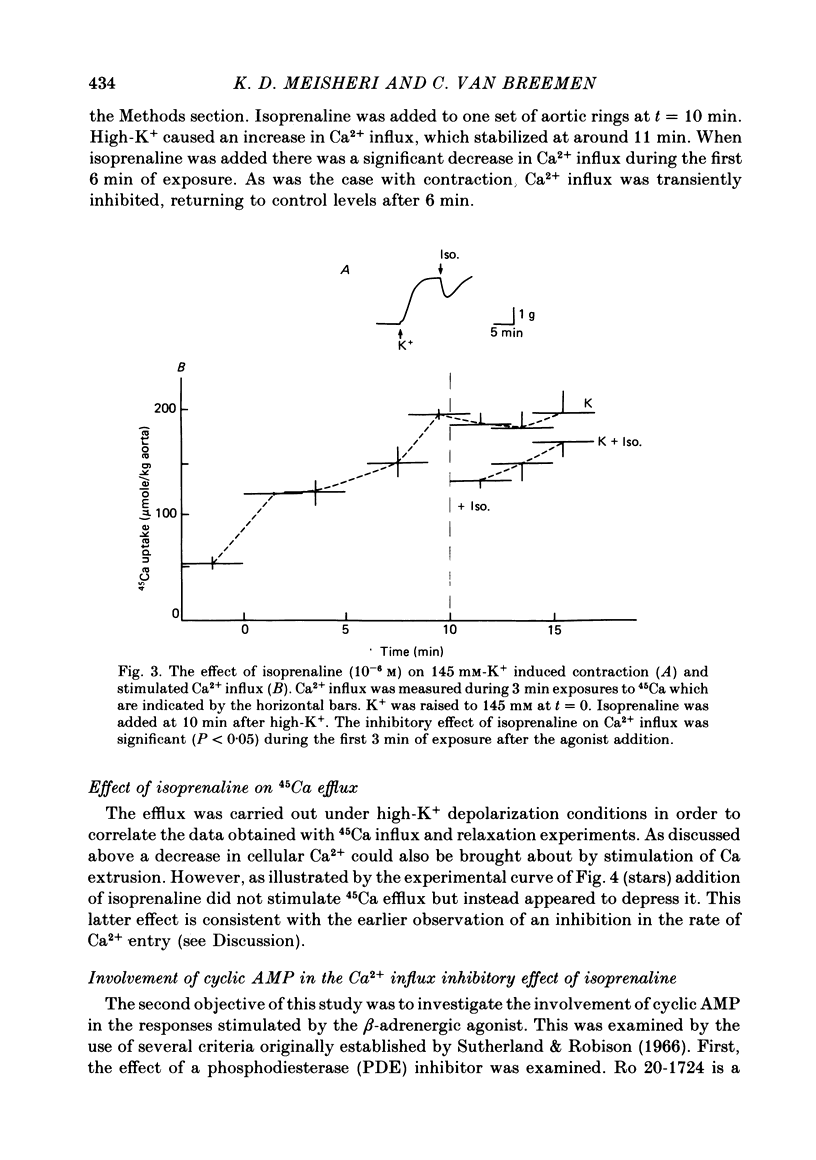
2. Isoprenaline (10-6 M) caused significant inhibition of Ca2+ influx stimulated by 145 mM-K+ (0 Na+) solution. The time courses of Ca2+ influx inhibition and relaxation by isoprenaline were parallel. Isoprenaline also caused a significant inhibition of high-K+-induced gain in net Ca2+ content.
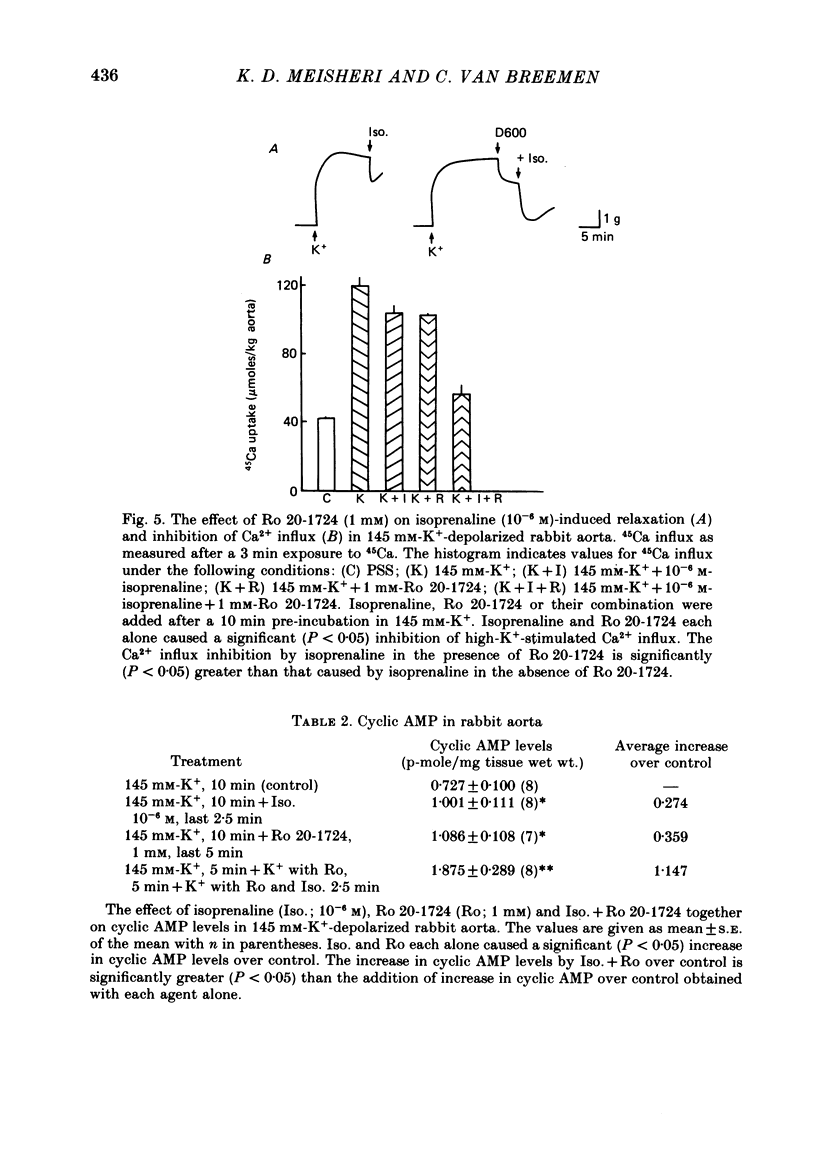
3. Ro 20-1724 (1 mM), a phosphodiesterase inhibitor, also caused relaxation and Ca2+ influx inhibition in high-K+-depolarized rabbit aorta. Pre-treatment with Ro 20-1724 potentiated isoprenaline-induced Ca2+ influx inhibition and relaxation.
4. Isoprenaline and Ro 20-1724 each alone increased cyclic AMP levels. Furthermore pre-treatment with Ro 20-1724 caused potentiation of isoprenaline-induced increases in cyclic AMP levels.
5. At submaximal concentration, D600 (10-7 M) caused partial inhibition of high-K+-stimulated Ca2+ influx and produced relaxation. However, unlike Ro 20-1724, it did not potentiate isoprenaline-induced Ca2+ influx inhibition and relaxation. D600 does not increase cyclic AMP levels in smooth muscle.
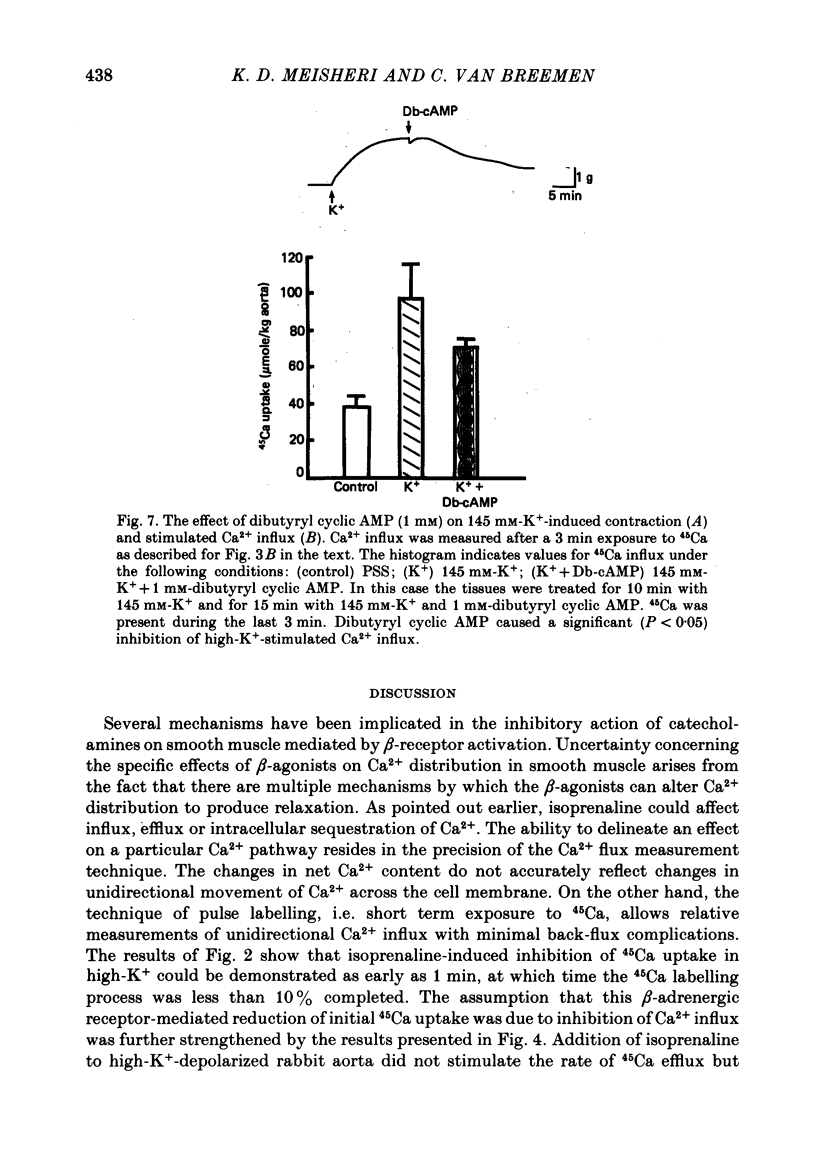
6. Dibutyryl cyclic AMP (1 mM), a lipid-soluble analogue of cyclic AMP, caused relaxation and inhibited high-K+-stimulated Ca2+ influx.
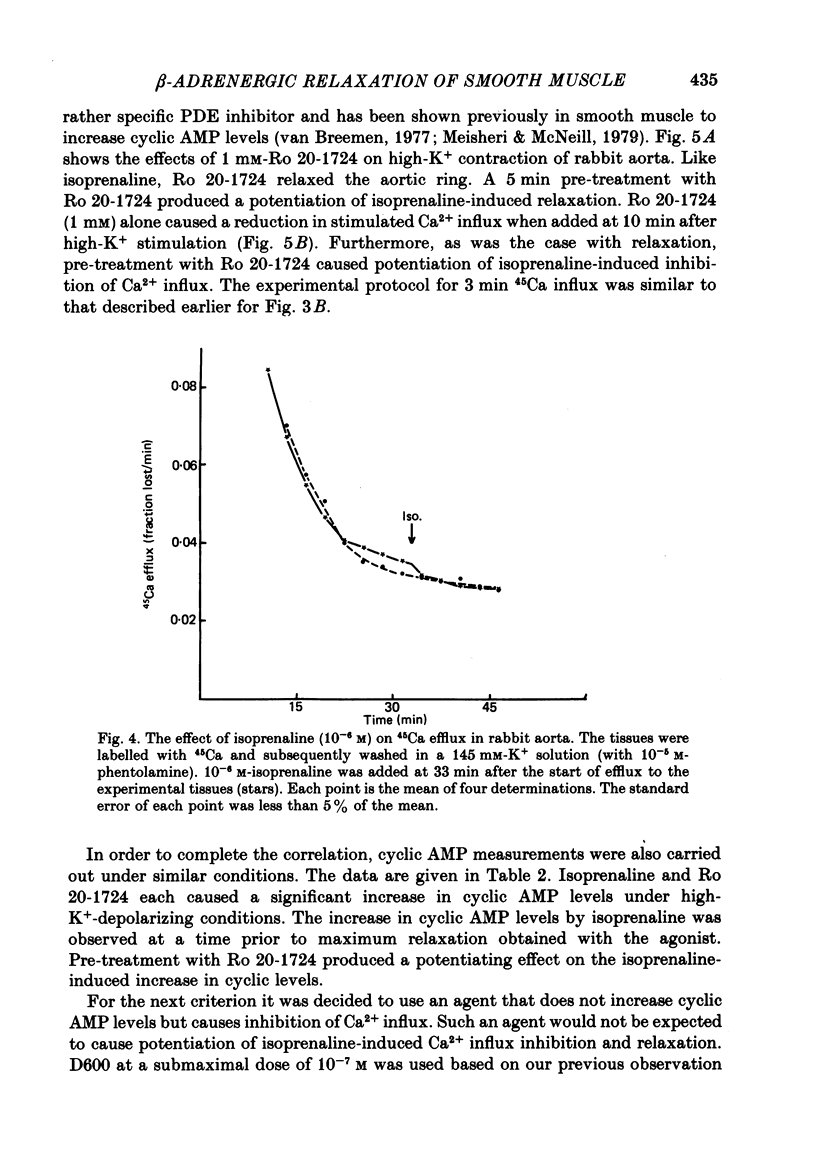
7. Isoprenaline failed to cause stimulation of Ca2+ efflux in high-K+-depolarized rabbit aorta.
8. It is concluded that the inhibition of Ca2+ influx may be one of the mechanisms by which β-receptor stimulation can reduce intracellular free Ca2+ to promote relaxation of smooth muscle. The data support the involvement of cyclic AMP in this action of the β-agonist.
9. Since the experiments were conducted in 145 mM-K+ (0 Na+) depolarizing conditions, the role of hyperpolarization or of a Na+—Ca2+ exchange mechanism in isoprenaline-induced Ca2+ influx inhibition and/or relaxation can be excluded.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson P., van Breemen C. Effects of sodium gradient manipulation upon cellular calcium, 45Ca fluxes and cellular sodium in the guinea-pig taenia coli. J Physiol. 1981;319:443–461. doi: 10.1113/jphysiol.1981.sp013920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelstein R. S., Hathaway D. R. Role of calcium and cyclic adenosine 3':5' monophosphate in regulating smooth muscle contraction. Mechanisms of excitation-contraction coupling in smooth muscle. Am J Cardiol. 1979 Oct 22;44(5):783–787. doi: 10.1016/0002-9149(79)90197-8. [DOI] [PubMed] [Google Scholar]
- Bülbring E., den Hertog A. The action of isoprenaline on the smooth muscle of the guinea-pig taenia coli. J Physiol. 1980 Jul;304:277–296. doi: 10.1113/jphysiol.1980.sp013324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis M. G., Shepherd J. T. Isoproterenol-induced relaxation of venous smooth muscle contracted by agents which mobilize different calcium pools. J Pharmacol Exp Ther. 1979 Jun;209(3):359–365. [PubMed] [Google Scholar]
- Deth R., van Breemen C. Relative contributions of Ca2+ influx and cellular Ca2+ release during drug induced activation of the rabbit aorta. Pflugers Arch. 1974 Apr 4;348(1):13–22. doi: 10.1007/BF00587735. [DOI] [PubMed] [Google Scholar]
- Diamond J. Role of cyclic nucleotides in control of smooth muscle contraction. Adv Cyclic Nucleotide Res. 1978;9:327–340. [PubMed] [Google Scholar]
- Dorevitch N. Effect of isoproterenol on adrenergic receptors in rabbit thoracic aorta. Arch Int Pharmacodyn Ther. 1968 Jul;174(1):98–107. [PubMed] [Google Scholar]
- Johansson B., Johsson O., Axelsson J., Wahlström B. Electrical and mechanical characteristics of vascular smooth muscle response to norepinephrine and isoproterenol. Circ Res. 1967 Nov;21(5):619–633. doi: 10.1161/01.res.21.5.619. [DOI] [PubMed] [Google Scholar]
- Kroeger E. A., Marshall J. M., Bianchi C. P. Effect of isoproterenol and D-600 on calcium movements in rat myometrium. J Pharmacol Exp Ther. 1975 May;193(2):309–316. [PubMed] [Google Scholar]
- Marshall J. M., Kroeger E. A. Adrenergic influences on uterine smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):135–148. doi: 10.1098/rstb.1973.0016. [DOI] [PubMed] [Google Scholar]
- Marshall J. M. Modulation of smooth muscle activity by catecholamines. Fed Proc. 1977 Sep;36(10):2450–2455. [PubMed] [Google Scholar]
- Meisheri K. D., Hwang O., van Breemen C. Evidence for two separated Ca2+ pathways in smooth muscle plasmalemma. J Membr Biol. 1981 Mar 15;59(1):19–25. doi: 10.1007/BF01870817. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., McNeill J. H., Marshall J. M. Effect of isoproterenol on the isolated pregnant rat myometrium. Eur J Pharmacol. 1979 Nov 23;60(1):1–6. doi: 10.1016/0014-2999(79)90045-1. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., McNeill J. H. Role of Ca in isoproterenol-induced increases in cAMP levels in rat uterus. Am J Physiol. 1979 Nov;237(5):C257–C263. doi: 10.1152/ajpcell.1979.237.5.C257. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., Palmer R. F., Van Breemen C. The effects of amrinone on contractility, Ca2+ uptake and cAMP in smooth muscle. Eur J Pharmacol. 1980 Jan 25;61(2):159–165. doi: 10.1016/0014-2999(80)90158-2. [DOI] [PubMed] [Google Scholar]
- Mueller E., van Breemen C. Role of intracellular Ca2+ sequestration in beta-adrenergic relaxation of a smooth muscle. Nature. 1979 Oct 25;281(5733):682–683. doi: 10.1038/281682a0. [DOI] [PubMed] [Google Scholar]
- Scheid C. R., Honeyman T. W., Fay F. S. Mechanism of beta-adrenergic relaxation of smooth muscle. Nature. 1979 Jan 4;277(5691):32–36. doi: 10.1038/277032a0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968 Jan;159(1):129–145. [PubMed] [Google Scholar]
- Sutherland E. W., Robison G. A. The role of cyclic-3',5'-AMP in responses to catecholamines and other hormones. Pharmacol Rev. 1966 Mar;18(1):145–161. [PubMed] [Google Scholar]
- Tomiyama A., Takayanagi I., Takagi K. Relaxation of intestinal smooth muscle and calcium movements. J Pharm Pharmacol. 1973 Jan;25(1):65–68. doi: 10.1111/j.2042-7158.1973.tb09117.x. [DOI] [PubMed] [Google Scholar]
- Van Breemen C. Calcium requirement for activation of intact aortic smooth muscle. J Physiol. 1977 Nov;272(2):317–329. doi: 10.1113/jphysiol.1977.sp012046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen C., Wuytack F., Casteels R., Martinelli B., Campailla E., Ferrari G. Stimulation of 45Ca efflux from smooth muscle cells by metabolic inhibition and high K depolarization. Pflugers Arch. 1975 Sep 9;359(3):183–196. doi: 10.1007/BF00587378. [DOI] [PubMed] [Google Scholar]
- Webb R. C., Bohr D. F. Relaxation of vascular smooth muscle by isoproterenol, dibutyryl-cyclic AMP and theophylline. J Pharmacol Exp Ther. 1981 Apr;217(1):26–35. [PubMed] [Google Scholar]
- van Breemen C., Aaronson P., Loutzenhiser R., Meisheri K. Ca2+ movements in smooth muscle. Chest. 1980 Jul;78(1 Suppl):157–165. doi: 10.1378/chest.78.1_supplement.157. [DOI] [PubMed] [Google Scholar]


