Abstract
Background and objectives
Middle meningeal artery embolization (MMAE) has become a pivotal intervention in managing chronic subdural hematomas (cSDHs). This systematic review synthesizes past, recent, and ongoing clinical trials to assess MMAE's role in cSDH treatment.
Methods
A systematic review was conducted using PRISMA guidelines, incorporating PubMed, ClinicalTrials.gov, and reverse bibliography searches to identify clinical trials evaluating MMAE for cSDH. Inclusion criteria included randomized and nonrandomized trials reporting outcomes, such as recurrence rates and procedural safety. Case reports, retrospective reviews, and opinion pieces were excluded.
Results
Seven published and 15 ongoing trials were identified. Landmark randomized controlled trials (RCTs), including EMBOLISE, STEM, and MAGIC-MT, demonstrated reductions in hematoma recurrence and surgical rescues with MMAE, establishing its role as both an adjunctive and standalone therapy. Ongoing trials, such as EMPROTECT and CHESS, investigate diverse embolic agents, procedural strategies, and patient populations to optimize MMAE outcomes. However, challenges remain, including variability in patient selection criteria, embolic materials, and endpoints.
Conclusion
MMAE is an innovative and minimally invasive approach that has reshaped cSDH management. Evidence supports its efficacy and safety as an adjunct to surgery and a potential standalone therapy for select patients. Future research should focus on long-term outcomes, subgroup analyses, and standardization of protocols to further refine its application and integration into clinical practice.
Keywords: MMAE, chronic subdural, embolization, middle meningeal, trials
Introduction
Chronic subdural hematoma (cSDH) is a common condition among elderly patients, and carries significant morbidity, mortality, and recurrence risks. 1 Traditional management involves burr-hole drainage and craniotomy, but although it is associated with high recurrence rates and often necessitates additional interventions. Middle meningeal artery embolization (MMAE) has emerged as a promising adjunctive therapy for cSDH. By targeting the arterial supply contributing to inflammation, angiogenesis, and recurrent bleeding in the dural layer, MMAE treats the underlying pathophysiology of cSDH and reduces recurrence rates. Initial retrospective studies demonstrated the feasibility and safety of MMAE, generating interest in its broader clinical application.
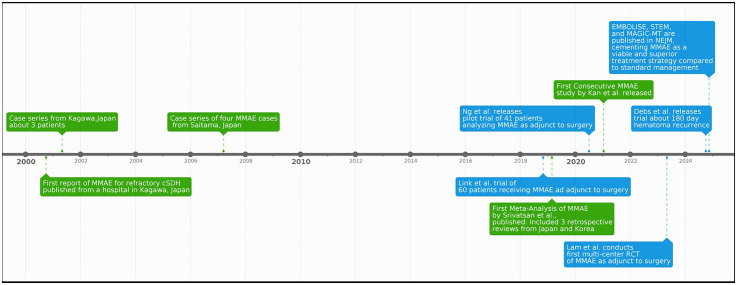
2024 marks a pivotal moment in the evolution of MMAE with the publication of results from three landmark randomized controlled trials—EMBOLISE, STEM, and MAGIC-MT—in the New England Journal of Medicine (Figure 1). EMBOLISE and STEM provided robust evidence supporting the efficacy of reducing hematoma recurrence.2,3 All three demonstrated the safety of MMAE in reducing adverse complications, sparking widespread interest in this treatment modality. Given the recent and ongoing advancements for this treatment modality, there is a need for a resource that provides a “snapshot” of the current landscape of both published and ongoing MMAE trials and their efficacy in cSDH treatment. Thus, this review aims to provide an updated and comprehensive overview of all clinical trials supporting MMAE, recent clinical breakthroughs, and ongoing research efforts to refine its role in managing cSDH.
Figure 1.
Timeline of notable MMAE studies.
Methods
The study adhered to PRISMA guidelines for systematic reviews, which do not require a protocol or registration and thus were not conducted (Figure 2). PubMed and Google Scholar were utilized to identify studies published in any language evaluating MMAE for cSDH. Keywords included “middle meningeal artery embolization,” “chronic subdural hematoma,” and “MMA embolization.” Reverse bibliography searches were performed on all included studies to ensure comprehensive coverage. Additionally, ClinicalTrials.gov and meeting abstracts were reviewed to identify ongoing trials.
Figure 2.
PRISMA flowchart.
Inclusion criteria consisted of randomized or nonrandomized trials evaluating MMAE for cSDH. Studies that provided data on adjunctive treatment status, embolic agent type, and procedural outcomes were included. Human studies that were case reports, letters of editorial opinion, or retrospective studies were excluded.
Screening was conducted using Rayyan (Rayyan Systems Inc.). Three reviewers (AAG, AN, JYC) independently screened titles and abstracts. Conflicts were resolved through open discussion with all three reviewers. Full-text articles meeting inclusion criteria were extracted using a predefined data table in Microsoft Excel (Microsoft Corporation, Redmond, WA). For each trial, we documented the trial name, start date, short name or abbreviation, principal investigator(s), clinical trial registration number, funding source, trial status, eligibility criteria regarding hematoma size, trial arms and interventions, embolic agents used, primary and secondary endpoints, enrollment targets, and the number of patients per arm.
A meta-analysis was not performed due to ongoing discussions among study investigators of trials such as EMBOLISE and STEM regarding pooled data analyses, which would provide a significantly higher level of evidence than we would be able to provide. 4 In 2015, a pivotal meta-analysis of individual patient data from five randomized trials demonstrated the efficacy of EVT, leading to a paradigm shift in guidelines. 5 These findings, published in The Lancet, demonstrated the impact of high-quality pooled analyses.5–9 Instead, a qualitative review was conducted to synthesize findings from the available literature. All extracted data are available upon reasonable request to the corresponding author.
Results
Seven published and 15 ongoing trials were identified as of November 26th, 2024.
Published trials
Seven published trials evaluating MMAE for cSDH were identified, representing a range of study designs, patient populations, and interventions (Table 1). Most are from the United States, although there were trials such as that led by Ng et al. from Montpellier, France, and another (MAGIC-MT) from Shanghai, China.10,11 Across these trials, MMAE consistently demonstrated a favorable impact on hematoma recurrence, surgical rescue rates, and functional outcomes.
Table 1.
Overview of key trials on middle meningeal artery embolization for chronic subdural hematoma.
| Name | Month | Year | Short name | First author | Last author | Journal | Clinical trial number | Funding source | Hematoma size | Trial arms/intervention | Embolic agent used | Primary endpoint | Secondary endpoint | Number of patients | Patients per arm | Key findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Embolization of the Middle Meningeal Artery for Chronic Subdural Hematoma | Nov. | 2024 | EMBOLISE | Jason M. Davies | Adnan H. Siddiqui | NEJM | NCT04402632 | Medtronic | Max 15 mm or midline shift >5 mm | Embolization + Surgery Surgery |
Onyx | Hematoma recurrence and/or surgical rescue within 90 days. | Non-inferiority assessment, readmission, radiographic features | 400 | 197 embo + surg 203 surg |
Primary outcome event occurred in 4.1% of embo group, 11.3% in control (RR, 0.36; 95% CI, 0.11–0.80; p = 0.008). |
| Adjunctive Middle Meningeal Artery Embolization for Subdural Hematoma | Nov. | 2024 | STEM | David Fiorella | Adam S. Arthur | NEJM | NCT04410146 | Balt USA | >10 mm size |
Surgical stratum: Embolization + surgery Surgery standalone Non-surgical stratum: Embolization standalone Medical management |
SQUID | Hematoma recurrence and/or surgical rescue within 180 days. | 180 day mRS | 310 |
Surgical stratum: 91 embo + surg 98 surg Non-surgical stratum: 58 embo only 63 medical management |
Surgical stratum: Primary outcome occurred in 14% of the embo + surg group, while 23% in the surg group (OR, 0.60; 95% CI, 0.27–1.35). Non-surgical stratum: Primary outcome occurred in 19% of the embo group, while 56% in the mm group (OR, 0.19; 95% CI, 0.08–0.46). No significant difference in mortality between embolization and the control for either strata. |
| Middle Meningeal Artery Embolization for Nonacute Subdural Hematoma | Nov. | 2024 | MAGIC-MT | Jianmin Liu | Ying Mao | NEJM | NCT04700345 | Shanghai Shenkang Hospital Development Center and others | Excluded patients warranting craniotomy | Embolization + medical management vs. medical management (excluded patients undergoing craniotomy) | Onyx | Symptomatic recurrence or progression within 90 days | Clinical and imaging outcomes | 722 | 360 embolization, 362 usual treatment (78.3% of all patients got burr hole drainage, most were after embo) | Symptomatic recurrence or progression was less likely in embo group (-3.3%, -7.4 to 0.8, p = 0.10). Incidence of serious adverse effects decreased in embo group (6.7% vs 11.6%, p = 0.02) |
| Middle meningeal artery embolization following surgical evacuation of symptomatic chronic subdural hematoma improves outcomes, interim results of a prospective randomized trial | Oct. | 2024 | – | Luca Debs | Scott Rahimi | JNIS | NCT04272996 | Augusta University | >10 mm size, >5 mm midline shift | Embolization + surgery vs. Surgery | Onyx | Hematoma recurrence within 180 days | embolic complications | 35 | 17 embo + surg 18 surg only |
Neurological improvement upon discharge in 71% of embo + surg and 56% of surg groups (p = 0.29). Embo + surg group maintained neurological improvements while deteriorating in surg group during follow-up (71% vs 33%*; NNT, 2.5; RR, 0.44; p = 0.03). 7 (39%) surg required reintervention, while it was needed in only 1 (6%) embo + surg (NNT, 3.0; RR, 0.15; p = 0.15). |
| The efficacy of postoperative middle meningeal artery embolization on chronic subdural hematoma—A multicentered randomized controlled trial | May | 2023 | – | Alexander Lam | Peter Mews | Surgical Neurology International | ACTRN12621000263897p | Life Health Care Group | Mean preoperative thickness was 21.2 mm vs 20.9 mm in embo and monitoring groups, respectively (p = 0.969) | Evacuation + surgery vs. Surgery | SQUID, Onyx, Phil 25%, n-BCA | Symptomatic recurrence with repeat surgical evacuation | mRS, complications, and maximal thickness of residual cSDH at 6 weeks and 3 months | 35 | 16 evacuation + embo 19 evacuation + monitoring | No recurrence was found in embolized patients compared to 3 patients without embo who needed repeat surgery (P = 0.234). No significant difference in residual hematoma thickness. Patients with embolization had lower mRS compared to non-embolized patients (P = 0.018) |
| Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: A Series of 60 Cases | Nov. | 2018 | – | Thomas Link | Jared Knopman | Neurosurgery | NCT03307395 | Weill Medical College of Cornell University | – | Embolization vs. Embolization + surgery | Polyvinyl alcohol particles | Hematoma recurrence requiring surgical rescue | Procedure-related complications, neurological deficits and hematoma size reduced by >50% | 60 | 42 embo only 8 embo for recurrent hematoma after prior surg 10 embo + surg |
Of the 50 without prior surgical history, 45 had reported data. Within this 45, only 4 (8.9%) experienced a primary outcome and 41 (91.1%) improved clinically. 31 (68.9%) experienced a reduction in size >50%, and no neurological deficits were seen. |
| Middle meningeal artery embolization as an adjuvant treatment to surgery for symptomatic chronic subdural hematoma: a pilot study assessing hematoma volume resorption | July | 2020 | – | Sam Ng | Vincent Costalat | JNIS | – | None declared | 65.2 ± 27.1 mL for embolization + surgery vs 51.2 ± 27.4 mL for surgery alone; p = 0.14 | Embolization + surgery vs. Surgery | Polyvinyl alcohol particles | Hematoma volume resorption at 3 months | Recurrence of CSDH and safety measures at 3 months | 41 | 19 embo + surg 22 surg alone | Mean difference in hematoma volume resorption was higher in embo + surg group versus surg alone by 17.5 mL (95% CI 3.87 to 31.16 mL) |
The EMBOLISE trial demonstrated that adjunctive MMAE with Onyx (Medtronic) significantly reduced 90-day recurrence rates compared to surgery alone, with recurrence observed in 4.1% of embolized patients versus 11.3% in the control group (p = 0.008). This trial highlighted the potential of MMAE to enhance surgical outcomes by addressing residual hematoma formation and improving radiographic resolution. The STEM trial demonstrated the efficacy of an alternative embolic agent, Squid (Balt USA), and similarly stratified patients into surgical and nonsurgical management cohorts. Among surgical patients, adjunctive embolization reached the primary efficacy outcome of recurrent cSDH with or without surgical rescue or major complications within 180 days (16% vs. 36%; OR = 0.36 (0.20–0.66); p = 0.001). Notably, MMAE demonstrated significant efficacy in nonsurgical patients, with a recurrence rate of 19% in the embolization group compared to 56% with medical management alone. The MAGIC-MT trial focused on patients managed medically, excluding those requiring craniotomy. Although the primary endpoint of symptomatic recurrence did not reach statistical significance (p = 0.10), serious adverse events were reduced in the embolization group (6.7% vs. 11.6%, p = 0.02).
A smaller trial by Debs et al. investigated MMAE following surgical evacuation of symptomatic cSDH. The addition of embolization was associated with sustained neurological improvement during follow-up (71% vs. 33%, p = 0.03) and a reduced need for reintervention (6% vs. 39%). Postoperative MMAE was also evaluated in a multicenter study, where embolized patients demonstrated improved functional outcomes with lower modified Rankin scale (mRS) scores (p = 0.018) and no recurrences, compared to three recurrences in the non-embolized group.
Ongoing trials
Fifteen ongoing or recently completed trials are actively investigating the role of MMAE in managing cSDH. These trials aim to further refine the role of MMAE in both surgical and nonsurgical contexts, address gaps in our knowledge of recurrence rates after MMAE, and expand upon our understanding of its impact on functional recovery and safety (Table 2).
Table 2.
Ongoing clinical trials pending per ClinicalTrials.gov.
| Name | Start month | Start year | Short name | Principal investigator(s) | Clinical trial number | Funding source | Trial status | Hematoma size | Trial arms/intervention | Embolic agent used | Primary endpoint | Secondary Endpoint | Target enrollment | Patients per arm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Embolization of the Middle Meningeal Artery for the Prevention of Chronic Subdural Hematoma Recurrence in High Risk Patients | Oct. | 2020 | EMPROTECT | Eimad Shotar | NCT04372147 | Assistance Publique-Hôpitaux de Paris | Completed* | >10 mm size, >5 mm midline shift | Embolization + surgery vs. Medical management | EmboSphere | Hematoma recurrence within 180 days | Repeated surgery, disability + dependency, mortality/complications, and hospital stay within 180 days | 342 | 171 embo + surg 171 medical management |
| Swedish Trial on Embolization of Middle Meningeal Artery Versus Surgical Evacuation in Chronic Subdural Hematoma | Mar. | 2022 | SWEMMA | Mattias Drake Johan Wasselius |
NCT05267184 | Region Skane | Recruiting | Midline shift >10 mm | Embolization vs. Surgery | Onyx or Squid or Phil | Surgical rescue within 90 days | Neurological disability (mRS), quality of life (EQ-5D), and composite endpoint between 90 and 365 days, hematoma volume and procedure related complication rate within 90 days, and technical success rate within 24 h | 288 | 144 embo alone 144 surg alone |
| Preventing Recurrences of Chronic Subdural Hematoma in Adult Patients by Middle Meningeal Artery Embolization | May | 2022 | MEMBRANE (Germany) | Alexander Hoenning | NCT05327933 | Unfallkrankenhaus Berlin | Recruiting | – | Embolization + surgery vs. Surgery | Onyx or microelectric coils | Hematoma recurrence within 90 days | mRS and hematoma recurrence or intervention complication | 154 | 77 embo + surg 77 surg alone |
| Middle Meningeal Artery Embolization for the Treatment of Subdural Hematomas With TRUFILL® n-BCA (MEMBRANE) | May | 2021 | MEMBRANE (USA) | Christopher Kellner, Ansaar Rai | NCT04816591 | Cerenovus, Part of DePuy Synthes Products, Inc. | Active, not recruiting | – | Embolization vs. Surgery vs. Embolization + Surgery, vs. Medical Management | TRUFILL n-BCA | Hematoma recurrence within 180 days | Reduction of hematoma volume, change in mRS | 376 | Not available |
| Embolization of Middle Meningeal Artery in Chronic Subdural Hematoma | Dec. | 2020 | ELIMINATE | William Vandertop | NCT04511572 | Academisch Medisch Centrum-Universiteit van Amsterdam | Recruiting | – | Embolization + surgery vs. Surgery | PVA particles | Surgical rescue for recurrent hematoma within 24 weeks | Hematoma volume reduction, mRS, complications, mNIHSS, MOCA, mortality, ADLs, short form health survey, iMCQ, EQ-5D-5L, productivity cost questionnaire, and Markwalder grading scale score within 24 weeks | 170 | Not available |
| Puerto Rico Embolization of the Middle Meningeal Artery for the Treatment of Chronic Subdural Hematoma Trial | Aug. | 2024 | PREMMA | Maria M Garcia Perez | NCT06466733 | Juan M. Ramos Acevedo | Not yet recruiting | – | Embolization vs. Surgery | PVA particles | Reoperation at 3, 6, 12 months | mRS, GCS, VAS, EQ-5D-5L, radiological outcomes, technical success of embo, procedure-related complications, adverse effects, post-operative morbidity, mortality, length of stay | 658 | Not available |
| Endovascular vs Conservative Treatment in Patients With Chronic Subdural Hematomas and Mild Symptoms | Mar. | 2024 | n/a | Lucio Castellan | NCT06274580 | Ospedale Policlinico San Martino | Not yet recruiting | <20 mm in width, <7 mm midline shift | Embolization standalone vs. Medical management | PVA particles | Incomplete (<50%) hematoma resolution or surgical rescue within 180 days | Treatment complication within 30 days or treatment success | 300 | Not available |
| Chronic Subdural Hematoma Treatment With Embolization Versus Surgery Study | Nov. | 2024 | CHESS | Peter Kan | NCT06347796 | The University of Texas Medical Branch, Galveston | Recruiting | >10 mm in thickness if unilateral, >5 mm in thickness if bilateral | Embolization vs. Surgery | CONTOUR Embolization Particles device | Need for rescue surgery or death within 180–210 days of randomization | Proportion of subjects with symptomatic ischemic stroke, serious/life threatening adverse events, worsening of neurologicla status, or development of new disabling neurological symptoms, seizures, and/or cranial neuropathy within 180 days of randomization | 520 | Not available |
| Middle Meningeal Artery (MMA) Embolization Compared to Traditional Surgical Strategies to Treat Chronic Subdural Hematomas (cSDH) | June | 2019 | n/a | Angelica Fuentes | NCT04095819 | Atlantic Health System | Unknown status | – | Embolization standalone Surgery standalone |
PVA particles and n-BCA glue | Change in size of hematoma within 180 days | Changes in neurological status within 180 days | 50 | Not available |
| Middle Meningeal Artery Embolization for Chronic Subdural Hematomas | July | 2024 | STORMM | Aria Nouri | NCT06163547 | University Hospital, Geneva | Not yet recruiting | – | Embolization + surgery vs. Surgery vs. Embolization vs. medical management | Not specified | Surgical reoperation, neurological deterioration due to cSDH after evacuation, post-operative hematomy volume of more than 90% of preoperative volume at follow-up at 6 months | GCS mRS, MGS, KPS, TDN grade, mortality rate, re-hospitalization at 6 months | 180 | Not available |
| The Onyx™ Trial For The Embolization Of The Middle Meningeal Artery For Chronic Subdural Hematoma | Oct. | 2021 | OTEMACS | Vincent Costalat | NCT04742920 | University Hospital, Montpellier | Recruiting | >10 mm in thickness, unspecified midline shift | Embolization + surgery vs. Surgery | Onyx | Hematoma recurrence in embolism group vs standard of care group within 90 days, incidence of all cause mortality at 90 days | Mortality and major disabling stroke rates at discharge within 7 days, incidence of PRSAEs and DRSAEs in 24 hr, recurrence rate requiring surgery and hematoma volume change within 90 days, length of stay, mRS, EQ-5D-5L, Barthel Index. | 440 | Not available |
| Efficacy of a Minimally Invasive Therapy Adjuvant to the Standards of Care by Cyanoacrylate Embolization | Mar. | 2023 | LEADH | Jean-Christophe Gentric | NCT05374681 | University Hospital, Brest | Recruiting | >10 mm in thickness | Embolization + surgery vs. Surgery vs. Embolization vs. medical management | n-BCA glue | CSH recurrence defined by the composite endpoint: A symptomatic CSH during the 6-month FU period A secondary surgical management during the 6-month FU period A remaining or reaccumulated hematoma on NCCT at 6 months |
Number of symptomatic cSDH, secondary surgical management, remaining or reaccumulated hematoma, mortality rate, mRS, RACE score evaluation, quality of life, Barthel scale, success rate of embolization, complication rate of embolication, volumetry of CSH, maximum thickness of CSH, and rate of adverse effects at 6 months | 550 | Not available |
| Endovascular Embolization of Chronic Subdural Hematomas After Surgery | Feb. | 2022 | ENCLOSURE | Alejandro Tomasello | NCT05220826 | Hospital Universitari Vall d'Hebron Research Institute | Unknown status | >10 mm in thickness with midline shift greater than 5 mm or neurological symptoms attributable to mass effect | Embolization + surgery vs. Surgery | Onyx, Phil, Squid, Libro | Recurrence of cSDH within 6 months | mRS, complications from embo, hematoma volume after treatment, and length of stay at 6 months | 280 | 140 surgical drainage 140 surgical drainage + embo |
| Middle Meningeal Artery Embolization for Chronic Subdural Hematoma | Sep. | 2019 | n/a | Joshua Osbun | NCT04065113 | Washington University School of Medicine | Recruiting | "Significant” midline shift | Embolization + surgery vs. Embolization vs. medical management | PVA particles | Hematoma recurrence or surgical rescue within 90 days | mNIHSS, mRS, procedure-related complication rate, and change in hematoma size within 90 days. | 600 | Not available |
| Management of CSDH With or Without EMMA- a Randomized Control Trial | Aug. | 2021 | EMMA-Can | Jai Shankar | NCT04750200 | University of Manitoba | Recruiting | >10 mm in thickness and has one or more symptoms attributable to cSDH | Embolization + surgery vs. Surgery | PVA or EVOH | Recurrence of size of cSDH on CT scan of head within 90 days from embo | Peri-procedural morbidity and mortality related to embo, reduction of size of cSDH at 90 days from embo | 200 | Not available |
Several trials, such as EMPROTECT and MEMBRANE, focus on whether adjunctive embolization reduces hematoma recurrence rates and improves long-term outcomes compared to surgery alone. For example, EMPROTECT assesses outcomes in patients with hematoma size >10 mm or midline shift >5 mm using EmboSphere particles. Similarly, the MEMBRANE trial from Berlin, Germany, evaluates adjunctive embolization with Onyx or microelectric coils, targeting recurrence within 90 days as the primary endpoint. 12 Another trial also named MEMBRANE is ongoing and is based out of the United States, uses TRUFILL n-BCA (Cerenovus, Part of DePuy Synthes Products, Inc.). In preliminary results this trial has demonstrated MMAE significantly decreased failure rates in the treatment of cSDH when compared to the standard of care, with a common odds ratio (OR) of 0.529 (90% CI: 0.308–0.909) over 6 months. In the surgical cohort, failure rates were 8.5% for patients receiving MMAE versus 20.2% for those undergoing surgery alone (OR: 0.475). 13
Conversely, trials like CHESS and SWEMMA are examining MMAE as a standalone therapy. These studies aim to identify patient populations that may benefit from minimally invasive embolization as an alternative to surgical evacuation, particularly in cases of moderate hematoma burden or high surgical risk. Both trials focus on recurrence rates and functional outcomes, with SWEMMA also assessing procedural success rates and quality of life metrics.
Ongoing research includes a diverse array of embolic materials. While established agents such as Onyx and n-BCA remain widely used, trials like ELIMINATE and CHESS investigate alternative materials, including polyvinyl alcohol (PVA) particles and Contour particles (Boston Scientific) devices, to optimize procedural safety and efficacy. This reflects a growing interest in tailoring embolization techniques to specific patient needs and procedural goals. Although hematoma recurrence is the predominant primary endpoint across trials, secondary outcomes provide a broader understanding of MMAE's impact. Many studies incorporate functional status assessments, such as mRS scores and quality of life metrics (e.g. EQ-5D).
The inclusion criteria across trials reflect efforts to stratify patients based on hematoma characteristics and symptom severity. Trials such as ENCLOSURE focus on patients with significant hematoma burden or midline shifts, while others, such as PREMMA, aim to evaluate MMAE's role in less vulnerable patient populations. This variability underscores the importance of defining optimal patient selection criteria, particularly in differentiating between candidates for standalone embolization versus adjunctive approaches.
Discussion
The evolution of cSDH management has reached a pivotal moment with the advent of middle MMAE. Over the past few years, studies have established MMAE as a promising therapeutic modality. The recent publication of landmark trials, including EMBOLISE, STEM, and MAGIC-MT, has provided the highest evidence, demonstrating MMAE's potential to potentially significantly reduce recurrence rates and surgical rescues in adjunctive and standalone roles.2,3,11
Current landscape of MMAE trials
These three trials mark a shift from feasibility and early-stage studies to large-scale, multicenter randomized controlled trials, offering robust insights into MMAE's efficacy and safety. EMBOLISE established that adjunctive MMAE significantly reduces hematoma recurrence and reoperation rates compared to surgery alone, and the results from a second observational cohort is set to be published soon. STEM demonstrated that MMAE reduces treatment failure in surgical and nonsurgical cohorts, validating its standalone efficacy in selected nonsurgical patients. Although not statistically significant, MAGIC-MT reinforced these findings by showing lower adverse event rates and a trend towards lower recurrence rates. When MMAE is combined with medical management, these findings reinforce the transformative impact of prior advancements in neurovascular care, such as the 2015 EVT trials, and position MMAE as a similarly groundbreaking innovation.
Despite these advances, understanding the trajectory of MMAE requires revisiting its origins and early evidence. MMAE was first used in a cSDH case report in 2000, where it was employed to manage a coagulopathic patient with spontaneous hematoma formation. 14 Over the decade, a few small single-center studies provided preliminary data on feasibility and safety, highlighting the potential efficacy of MMAE.15–17 Between 2016 and 2018, there was a growing interest in MMAE which led to a proliferation of prospective and observational studies and meta-analyses, which began to establish its role in reducing hematoma recurrence.10,18,19 However, these early studies faced significant limitations, including small sample sizes, heterogeneous techniques, and outcome variability, underscoring the need for rigorous randomized trials.20–23 The first large multi-center study of consecutive MMAE was led by Kan et al. in 2019, demonstrating the efficacy of MMAE across 154 consecutive embolizations, which achieved a 97.4% technical success rate, and 70.8% of patients showed over a 50% imaging improvement. 21 Within the last few years, there has been a surge of prospective, multi-center studies that have corroborated these findings.24–28
The three recent NEJM trials represent a significant advancement in the quality of evidence supporting MMAE, but critical questions persist. Patient selection criteria across these trials remain heterogeneous, particularly regarding frail or anticoagulated patients who might benefit most from MMAE but are frequently excluded from clinical studies. 29 Single-center studies have shown that up to 39% of patients with cSDH are ineligible for trials due to radiographic features or comorbidities. 29 Despite this, these studies reported 88.9% satisfactory hematoma resolution among trial-ineligible patients. Importantly, no significant differences between trial-ineligible and trial-eligible groups regarding surgical rescue rates or hematoma resolution outcomes were observed. 29 Ogoing trials such as EMPROTECT are specifically addressing higher-risk populations, including those with chronic alcoholism or liver cirrhosis, further to evaluate MMAE's role in these vulnerable groups.
The variability in embolic agents, procedural timing, and study endpoints poses significant challenges to cross-trial comparisons in MMAE research. 30 Some studies, such as EMPROTECT, focus narrowly on recurrence and reoperation rates, while others, like PREMMA, adopt a broader perspective by incorporating metrics such as quality of life, functional independence, and healthcare utilization. 31 This diversity highlights the evolving priorities in evaluating MMAE, reflecting clinical and patient-centered outcomes. Efforts to standardize procedural techniques and optimize embolic agent selection are central to advancing the field. The usage of other agents, including Contour PVA particles (Boston Scientific) and EmboSphere (MeritMedical), aims to improve safety profiles and procedural efficacy while reducing outcome variability.
Recent trials also emphasize the need for comprehensive patient centric endpoints beyond technical success. Evaluations of neurological outcomes, long-term functional recovery, and cost-effectiveness are gaining prominence, reflecting the multifaceted impact of MMAE on patient care. These ongoing trials are poised to provide critical evidence on the efficacy, safety, and cost-effectiveness of MMAE. By addressing variability in embolic agents, procedural strategies, and patient populations, these studies are expected to clarify the role of embolic agents in MMAE for cSDH management, and guide future standardization efforts.32–34 By integrating these broader measures, ongoing studies strive to balance clinical effectiveness with considerations of quality of life and healthcare resource utilization, ultimately enhancing the relevance and applicability of MMAE findings to diverse patient populations and clinical settings.
Future directions
The evidence base for MMAE is now highly compelling, but it is not without limitations. While the three landmark NEJM trials provided high-quality data, long-term outcomes still need to be explored. Most studies focus on short- to medium-term endpoints, leaving questions about the durability of MMAE and its impact on long-term functional outcomes needing to be answered. Additionally, the underrepresentation of specific patient populations, such as those with significant comorbidities or bleeding diatheses, limits the generalizability of current findings. However, current single- and multi-center studies have demonstrated strong efficacy among these patients, and ongoing trials such as EMPROTECT are continuing to answer these questions.35–38
MMAE is now firmly established as a valuable adjunct to surgery, particularly for reducing recurrence and reoperation rates in high-risk patients. It is also emerging as a standalone option for selected nonsurgical candidates, offering a less invasive alternative to traditional approaches. The challenge lies in tailoring MMAE strategies to individual patient profiles, considering factors such as hematoma size, symptom severity, and comorbidities to maximize therapeutic benefit. As ongoing trials refine its indications, MMAE is poised to become a cornerstone of cSDH management, reshaping treatment paradigms and improving outcomes for this complex and heterogeneous condition.
Conclusion
The recent advancements in MMAE have solidified its position as a transformative intervention in cSDH management. The recent landmark trials, including EMBOLISE, STEM, and MAGIC-MT, have provided evidence supporting MMAE's efficacy and safety, establishing it as a valuable adjunct to surgical evacuation and a promising standalone treatment for selected patients. These findings mark a pivotal moment in the evolution of cSDH treatment, shifting the paradigm from traditional surgical approaches to a more nuanced, minimally invasive strategy. Despite the substantial progress, challenges remain, including variability in patient selection criteria, procedural techniques, and outcome measures. Ongoing trials aim to address these gaps by exploring innovative embolic agents, optimizing procedural timing, and broadening the scope of MMAE's application in diverse patient populations. Future research must prioritize subgroup analyses, long-term outcome studies, and comparative trials to refine MMAE's role further and ensure its integration into standardized care protocols. As the evidence base grows, MMAE is poised to redefine cSDH treatment, offering improved outcomes and a path forward for high-risk and nonsurgical patients.
Abbreviations
- MMAE
middle meningeal artery embolization
- cSDH
chronic subdural hematoma
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- mRS
modified Rankin scale
- PVA
polyvinyl alcohol
- n-BCA
N-butyl cyanoacrylate adhesive
- EQ-5D
EuroQol-5 dimensions
- EMPROTECT
Embolization of the Middle Meningeal Artery for the Prevention of Chronic Subdural Hematoma Recurrence in High Risk Patients
- STEM
Adjunctive Middle Meningeal Artery Embolization for Subdural Hematoma
- MAGIC-MT
Middle Meningeal Artery Embolization for Nonacute Subdural Hematoma
- MEMBRANE (Germany)
Preventing Recurrences of Chronic Subdural Hematoma in Adult Patients by Middle Meningeal Artery Embolization
- MEMBRANE (USA)
Middle Meningeal Artery Embolization for the Treatment of Subdural Hematomas with TRUFILL® n-BCA
- CHESS
Chronic Subdural Hematoma Treatment With Embolization Versus Surgery Study
- SWEMMA
Swedish Trial on Embolization of Middle Meningeal Artery Versus Surgical Evacuation in Chronic Subdural Hematoma
- ELIMINATE
Embolization of Middle Meningeal Artery in Chronic Subdural Hematoma
- PREMMA
Puerto Rico Embolization of the Middle Meningeal Artery for the Treatment of Chronic Subdural Hematoma Trial
- STORMM
Middle Meningeal Artery Embolization for Chronic Subdural Hematomas
- OTEMACS
The Onyx™ Trial for the Embolization of the Middle Meningeal Artery for Chronic Subdural Hematoma
- LEADH
Efficacy of a Minimally Invasive Therapy Adjuvant to the Standards of Care by Cyanoacrylate Embolization
- ENCLOSURE
Endovascular Embolization of Chronic Subdural Hematomas After Surgery
- EMMA-Can
Management of CSDH With or Without EMMA – a Randomized Control Trial.
Footnotes
Author contributions: Conceptualization, methodology and project administration were done by AAG and ARP. Data curation was done by AAG, AN, and JYC. Formal analysis, visualization, and writing—original draft were done by AAG. Supervision was done by ARP. Validation was accomplished by AAG, AC, ASB, JCD, NCF, and ARP. Writing—reviewing and editing was done by all authors. All authors did investigation and approved the final manuscript draft.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ARP is a consultant for Microvention, IRRAS, Penumbra, Medtronic, and NICO. Remaining authors had no conflicting interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Avi A Gajjar https://orcid.org/0000-0003-3686-9413
Ali Naqvi https://orcid.org/0000-0002-8794-4607
John Y Chen https://orcid.org/0009-0002-6646-3166
Nicholas C Field https://orcid.org/0000-0003-4735-196X
Alexandra R Paul https://orcid.org/0000-0002-8315-5870
References
- 1.Kan P, Maragkos GA, Srivatsan A, et al. Middle meningeal artery embolization for chronic subdural hematoma: a multi-center experience of 154 consecutive embolizations. Neurosurgery 2021; 88: 268–277. [DOI] [PubMed] [Google Scholar]
- 2.Davies JM, Knopman J, Mokin M, et al. Adjunctive middle meningeal artery embolization for subdural hematoma. N Engl J Med 2024; 391: 1890–1900. [DOI] [PubMed] [Google Scholar]
- 3.Fiorella D, Monteith SJ, Hanel R, et al. Embolization of the middle meningeal artery for chronic subdural hematoma. N Engl J Med 2024; 392: 855–864. [DOI] [PubMed] [Google Scholar]
- 4.Bell J. Recent breakthroughs in cSDH intervention may lead to “HERMES-style collaborative.” NeuroNews International, https://neuronewsinternational.com/recent-breakthroughs-in-csdh-intervention-may-lead-to-hermes-style-collaborative/ (2024, accessed 2 December 2024).
- 5.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 6.Palaniswami M, Yan B. Mechanical thrombectomy is now the gold standard for acute ischemic stroke: implications for routine clinical practice. Interv Neurol 2015; 4: 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016; 316: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 8.Campbell BCV, van Zwam WH, Goyal M, et al. Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: a meta-analysis of individual patient data. Lancet Neurol 2018; 17: 47–53. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka K, Brown S, Goyal M, et al. HERMES-24 score derivation and validation for simple and robust outcome prediction after large vessel occlusion treatment. Stroke 2024; 55: 1982–1990. [DOI] [PubMed] [Google Scholar]
- 10.Ng S, Derraz I, Boetto J, et al. Middle meningeal artery embolization as an adjuvant treatment to surgery for symptomatic chronic subdural hematoma: a pilot study assessing hematoma volume resorption. J Neurointerv Surg 2020; 12: 695–699. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Ni W, Zuo Q, et al. Middle meningeal artery embolization for nonacute subdural hematoma. N Engl J Med 2024; 391: 1901–1912. [DOI] [PubMed] [Google Scholar]
- 12.Hoenning A, Lemcke J, Rot S, et al. Middle meningeal artery embolization minimizes burdensome recurrence rates after newly diagnosed chronic subdural hematoma evacuation (MEMBRANE): study protocol for a randomized controlled trial. Trials 2022; 23: 03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell J. MEMBRANE becomes latest RCT to demonstrate MMA embolisation’s benefits in cSDH treatment. NeuroNews International, https://neuronewsinternational.com/membrane-becomes-latest-rct-to-demonstrate-mma-embolisations-benefits-in-csdh-treatment/ (2024, accessed 2 December 2024).
- 14.Mandai S, Sakurai M, Matsumoto Y. Middle meningeal artery embolization for refractory chronic subdural hematoma: case report. J Neurosurg 2000; 93: 686–688. [DOI] [PubMed] [Google Scholar]
- 15.Mino M, Nishimura S, Hori E, et al. Efficacy of middle meningeal artery embolization in the treatment of refractory chronic subdural hematoma. Surg Neurol Int 2010; 1: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsukamoto Y, Oishi M, Shinbo Jet al. et al. Transarterial embolisation for refractory bilateral chronic subdural hematomas in a case with dentatorubral-pallidoluysian atrophy. Acta Neurochir 2011; 153: 1145–1147. [DOI] [PubMed] [Google Scholar]
- 17.Hirai S, Ono J, Odaki M, et al. Embolization of the middle meningeal artery for refractory chronic subdural haematoma. Usefulness for patients under anticoagulant therapy. Interv Neuroradiol 2004; 10: 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosaka T, Ikeda N, Furuse M, et al. Refractory chronic subdural hematoma associated with dural metastasis of lung adenocarcinoma treated with endovascular embolization for the middle meningeal artery: a case report and review of the literature. World Neurosurg 2020; 133: 256–259. [DOI] [PubMed] [Google Scholar]
- 19.Court J, Touchette CJ, Iorio-Morin C, et al. Embolization of the middle meningeal artery in chronic subdural hematoma—a systematic review. Clin Neurol Neurosurg 2019; 186: 105464. [DOI] [PubMed] [Google Scholar]
- 20.Sattur MG, Spiotta AM. Anomalous “middle” meningeal artery from basilar artery and implications for neuroendovascular surgery: case report and review of literature. World Neurosurg 2020; 133: 84–89. [DOI] [PubMed] [Google Scholar]
- 21.Starnoni D, Giammattei L, Messerer Met al. et al. Letter to the editor regarding: “middle meningeal artery embolization for chronic subdural hematoma: meta-analysis and systematic review”. World Neurosurg 2019; 124: 480–481. [DOI] [PubMed] [Google Scholar]
- 22.Link TW, Knopman J. In reply: middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases. Neurosurgery 2019; 85: E393. [DOI] [PubMed] [Google Scholar]
- 23.Entezami P, Boulos A, Paul A, et al. Contrast enhancement of chronic subdural hematomas after embolization of the middle meningeal artery. Interv Neuroradiol 2019; 25: 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salem MM, Helal A, Gajjar AA, et al. Embolic materials’ comparison in meningeal artery embolization for chronic subdural hematomas: multicenter propensity score-matched analysis of 1070 cases. Neurosurgery 2024. doi: 10.1227/neu.0000000000003218 [DOI] [PubMed] [Google Scholar]
- 25.Salem MM, Sioutas GS, Gajjar A, et al. Femoral versus radial access for middle meningeal artery embolization for chronic subdural hematomas: multicenter propensity score matched study. J Neurointerv Surg 2024: jnis-2024-021880. doi: 10.1136/jnis-2024-021880 [DOI] [PubMed] [Google Scholar]
- 26.Salem MM, Sioutas GS, Khalife J, et al. General versus nongeneral anesthesia for middle meningeal artery embolization for chronic subdural hematomas: multicenter propensity score matched study. Neurosurgery 2024. doi: 10.1227/neu.0000000000002874 [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Salem MM, Colasurdo M, et al. Standalone middle meningeal artery embolization versus middle meningeal artery embolization with concurrent surgical evacuation for chronic subdural hematomas: a multicenter propensity score matched analysis of clinical and radiographic outcomes. J Neurointerv Surg 2024; 16: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 28.Salah WK, Baker C, Scoville JP, et al. Middle meningeal artery embolization as a perioperative adjunct to surgical evacuation of nonacute subdural hematomas: an multicenter analysis of safety and efficacy. Interv Neuroradiol 2023: 15910199231162665. doi: 10.1177/15910199231162665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma L, Hoz SS, Doheim MF, et al. Middle meningeal artery embolization for “trial-ineligible” chronic subdural hematomas. Neurosurgery 2024. doi: 10.1227/neu.0000000000003136 [DOI] [PubMed] [Google Scholar]
- 30.Bell J. Recent breakthroughs in cSDH intervention may lead to “HERMES-style collaborative.” NeuroNews International, https://neuronewsinternational.com/recent-breakthroughs-in-csdh-intervention-may-lead-to-hermes-style-collaborative/ (2024, accessed 2 December 2024).
- 31.Acevedo JMR. Puerto Rico Embolization of the Middle Meningeal Artery for the Treatment of Chronic Subdural Hematoma Trial (PREMMA). clinicaltrials.gov, https://clinicaltrials.gov/study/NCT06466733 (2024, accessed 2 December 2024).
- 32.NeuroNews [@NN_publishing]. EMPROTECT study results, presented by Eimad Shotar at #ESMINT2024, indicate comparable six-month post-surgery cSDH recurrence rates and similar adverse event rates between MMA embolisation using EMBOSPHERE particles (@MeritMedical) and best medical treatment @esmintsociety https://t.co/t9oxiXCePp. Twitter, https://x.com/NN_publishing/status/1831275702630375695 (2024, accessed 2 December 2024).
- 33.Spahn J. Chronic Subdural Hematoma Treatment With Embolization Versus Surgery Study. clinicaltrials.gov, https://clinicaltrials.gov/study/NCT06347796 (2024, accessed 2 December 2024).
- 34.Gentric J-C. LEADH: Efficacy of a Minimally Invasive Therapy Adjuvant to the Standards of Care by Cyanoacrylate Embolization : Liquid Embolic Agent for the Treatment of Chronic subDural Hematoma a Randomized Control Study. clinicaltrials.gov, https://clinicaltrials.gov/study/NCT05374681 (2024, accessed 2 December 2024).
- 35.Lee S, Srivatsan A, Srinivasan VM, et al. Middle meningeal artery embolization for chronic subdural hematoma in cancer patients with refractory thrombocytopenia. J Neurosurg 2022; 136: 1273–1277. [DOI] [PubMed] [Google Scholar]
- 36.Shotar E, Mathon B, Rouchaud A, et al. Embolization of the middle meningeal artery for the prevention of chronic subdural hematoma recurrence in high-risk patients: a randomized controlled trial-the EMPROTECT study protocol. J Neurointerv Surg 2024; 17: e172. [DOI] [PubMed] [Google Scholar]
- 37.O’Gorman J, Geevarghese R, Bodard S, et al. Embolization of middle meningeal arteries for symptomatic subacute subdural hematoma in patients with cancer. Acad Radiol 2024; 31: 4196–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salah WK, Findlay MC, Baker CM, et al. The influence of coagulopathy on radiographic and clinical outcomes in patients undergoing middle meningeal artery embolization as standalone treatment for non-acute subdural hematomas. J Neurotrauma 2024; 41: 1375–1383. [DOI] [PubMed] [Google Scholar]