Abstract
Ascaris lumbricoides infections represent a major global health challenge, affecting both humans and non-human primates. In non-human primates, A. lumbricoides infections pose a significant threat to conservation efforts, as they can impact both captive and wild populations, causing complications ranging from mild to severe. This study reports the case of two black sakis (Chiroptes utahickae) kept under human care in a zoo—a male and a female—presented for routine examination due to weight loss. Ultrasonography revealed a substantial intestinal infestation of large roundworms. The female was treated with pyrantel pamoate, and the male with fenbendazole, both with successful outcomes. The worms were sent for morphological and molecular identification, and the animals were submitted for new examinations. This case underscores the importance of preventive veterinary check-ups in zoos, particularly for parasites that pose zoonotic risks, to ensure both animal and human health.
Keywords: parasitism, primates, zoonosis
Resumo
Infecções por Ascaris lumbricoides representam um grande desafio para a saúde global, afetando tanto humanos quanto primatas não humanos. Em primatas não humanos, essas infecções representam uma ameaça significativa para os esforços de conservação, pois podem impactar tanto populações cativas quanto selvagens, causando complicações que variam de leves a graves. Este estudo relata o caso de dois cuxiús-pretos (Chiropotes utahickae) mantidos sob cuidados humanos em um zoológico—um macho e uma fêmea—apresentados para exame de rotina devido à perda de peso. A ultrassonografia evidenciou uma infestação intestinal substancial por vermes redondos de grande porte. A fêmea foi tratada com pamoato de pirantel e o macho com fenbendazol, ambos com sucesso. Os helmintos foram enviados para identificação morfológica e molecular, e os animais foram submetidos a novos exames. Este caso destaca a importância de check-ups veterinários preventivos em zoológicos, especialmente para parasitas com potencial zoonótico, a fim de garantir a saúde humana e animal.
Palavras-chave: parasitismo, primatas, zoonose
Introduction
Ascaridiasis, caused by nematodes of the genus Ascaris, is a prevalent parasitic infection that affects both human and non-human primates, particularly in tropical and subtropical regions (Kightlinger et al., 1995; Caraballo & Acevedo, 2011; Mbaya & Udendeye, 2011; Medkour et al., 2020; Rondon Robayo et al., 2022). This parasitic infection, considered as a neglected tropical disease (NTD), is one of the most common and widely distributed helminthic diseases, causing substantial morbidity across diverse geographic regions (Brooker & Pullan, 2013). In humans, A. lumbricoides can lead to a range of health problems, including abdominal pain, malnutrition, and intestinal obstruction due to their large size (Akgun, 1996; Villamizar et al., 1996). Similarly, non-human primates, monkeys and apes, can be affected by this parasite (Michaud et al., 2003; Cooper & Redmond, 2016; Stephens et al., 2017; de la Hoz et al., 2020).
Ascaris lumbricoides male worms measure between 15 and 31 cm in length; the posterior end is curved ventrally and features a blunt tail. Females range from 20 to 49 cm long; the vulva is located at the anterior end, which corresponds to about one-third of the body length. The developmental cycle is direct (Hall et al., 1992; Taylor et al., 2017). The eggs are highly resistant to extreme temperatures and they can remain viable on the soil for many years ( Hall et al., 1992; Taylor et al., 2017; Nisha et al., 2019). After ingestion, the larvated egg hatches in the small intestine, the L3 larva penetrates the intestinal mucosa, and then travels to the liver. The larva then moves from the bloodstream to the lungs and subsequently to the small intestine, via the bronchi, trachea, and pharynx. In the intestine, it undergoes its final molt and the young adult worms establish themselves in the lumen of the small intestine. Eggs are then shed, restarting the cycle by contaminating new hosts through the ingestion of contaminated water or food. Reinfection depends on constant exposure to A. lumbricoides eggs (Hall et al., 1992; Schulz & Kroeger, 1992; Taylor et al., 2017).
Reports indicate that A. lumbricoides infections have been documented in zoo environments (Dawet et al., 2013). The proximity of these animals to one another, combined with potential flaws in the sanitation practices, can facilitate the spread of the parasite within these controlled settings (Medkour et al., 2020).
Treatment of A. lumbricoides commonly involves the use of anthelmintic medications such as benzimidazole derivatives, pyrantel pamoate, levamisole, and ivermectin. In some communities, plant extracts are also utilized as part of the treatment regimen (Massara & Enk, 2004; Hagel & Giusti, 2010). While these medications are effective in eliminating the parasites from the host's system, their administration must be meticulously managed to ensure complete eradication and to prevent recurrence (Massara & Enk, 2004).
Diagnostic imaging, particularly ultrasound, plays a crucial role in identifying and assessing roundworm infestations (Montórfano, 1999; Wigger et al., 2007; Corda et al., 2022). Ultrasound can provide detailed visualization of the intestinal tract, enabling the accurate detection of parasites and the assessment of infestation severity. This non-invasive technique is essential for guiding treatment decisions and monitoring the effectiveness of therapeutic interventions (Montórfano, 1999; Wigger et al., 2007; Soni et al., 2015; Corda et al., 2022). To date, there are no documented reports of the ultrasound being used to diagnose A. lumbricoides parasitism in a non-human primate specimen.
Furthermore, the significance of regular health check-ups extends beyond the zoo animals themselves to include the human caretakers who work in close contact with them (Oh et al., 2002; Souza, 2011; Dawet et al., 2013). The zoonotic transmission of A. lumbricoides can pose health risks to those involved in animal care, highlighting the need for comprehensive health surveillance and preventative measures. Ensuring both animal and human health through routine veterinary and medical check-ups is essential in managing and mitigating the impacts of parasitic infections, particularly zoonotic diseases (Deem, 2007; Souza, 2011). In this context, this study aims to report a case of A. lumbricoides infection in two black sakis (Chiroptes utahickae) and to discuss the diagnostic methods and treatments employed, highlighting its implications as a zoonotic threat.
Case presentation
Two black sakis (Chiroptes utahickae), a male and a female, housed for over 11 years under human care in a zoo in Rio de Janeiro, Brazil, were brought in for a veterinary examination due to reports from their keepers of deterioration in body condition. Their enclosure housed only these two individuals as the sole primate species. Both animals were transported to the veterinary hospital, where they were pre-anesthetized with intramuscular ketamine (7 mg/kg) and midazolam (0.6 mg/kg). Anesthesia was then induced and maintained with isoflurane vaporized through a tracheal tube. A comprehensive evaluation was performed, including physical examination, abdominal ultrasound, thoracic radiographs, echocardiography, blood collection, analysis of fecal samples, and weighing. The male exhibited a poor body condition score of 2/5, while the female’s condition was within the normal range (3/5). Both echocardiograms showed no signs of vascular or cardiac disease, and lung auscultation revealed normal findings in both animals. The x-rays displayed a slight bronchial pattern consistent with normal age-related changes in both patients (Figure 1). The primates exhibited no signs of respiratory disease prior to the examination day. Abdominal ultrasounds were performed using a GE® Logiq E in two-dimensional mode with a high-frequency linear multi frequency transducer ranging from 10 to 22 MHz. The examination revealed intraluminal parasite-like structures which were characterized as elongated structures with 3 to 4 parallel echogenic lines on the longitudinal scan, and in the transverse scan, their appearance resembled a cylindrical “donut.” The duodenal walls were thickened in both patients and had a corrugated appearance, measuring at least 0.25 cm, while the jejunum in both measured between 0.08 cm and 0.15 cm with no wall disturbance. Peristalsis and layer pattern remained unaltered in both animals (Figure 2). Based on the ultrasound findings, deworming was carried out while both the male and female sakis were still under anesthesia. The male was treated with fenbendazole (20 mg/kg), and the female received pyrantel pamoate (10 mg/kg). Both medications were administered in a suspension formulation directly into the stomachs using a lubricated disposable gastric tube (n.12). After administration, the feeding tube was rinsed with 10 ml of water while still in the esophagus of both patients to ensure complete delivery of the medication. Hematological and biochemical analyses were conducted and no noteworthy abnormalities were observed. The coproparasitological examination by sucrose flotation technique (Sheather, 1923) revealed nematode eggs (++), which were oval to round in shape with a thick outer shell (Figure 3).
Figure 1. Thoracic radiographs of anesthetized male (A and B) and female (C and D) Chiroptes utahickae kept under human care in a zoo showing age-related slight bronchial patterns. Ventrodorsal views (A and C) and lateral views (B and D).
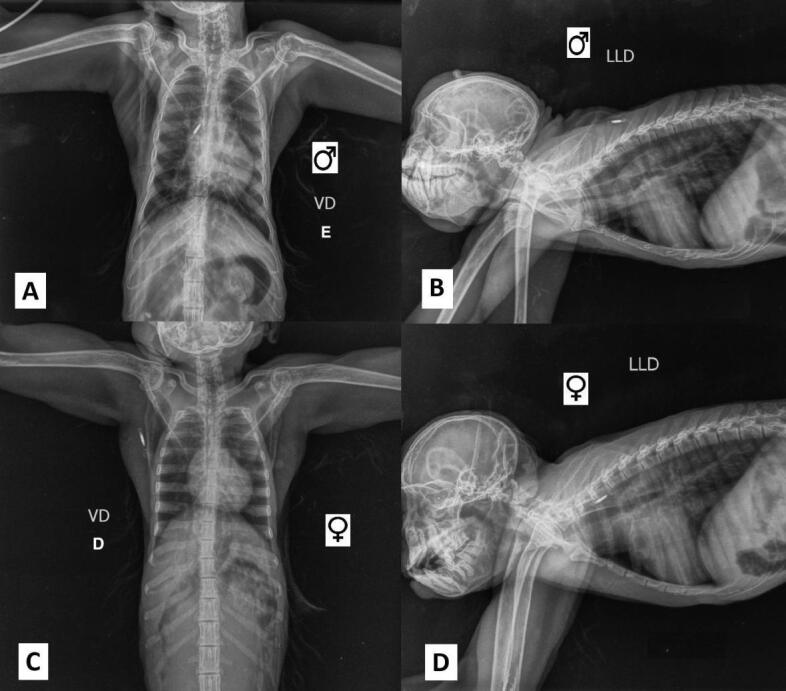
Figure 2. Ultrasonographic images of intestinal nematodes in transverse section (red arrowhead) and longitudinal section (yellow arrowhead) in jejunal segments of two black sakis. Female (A) and male (B).
Figure 3. Eggs of Ascaris lumbricoides in a fresh Chiroptes utahickae fecal sample, coproparasitological examination (Faust technique). Optical microscopy, 40x magnification (+2x digital zoom from a cell phone camera).

After recovering smoothly from anesthesia, both animals were kept separately in controlled cages with slotted bottoms to closely monitor parasite elimination, detect any signs of abdominal discomfort, and prevent recontamination, as feces would fall onto clean sheets placed at the bottom of the cages. Simultaneously, their original enclosure was thoroughly cleaned with a fire broom, and all traces of feces were removed.
The female saki expelled four roundworms within 24 hours, ranging in size from 15.5 cm to 21 cm. In contrast, the male saki took three days to eliminate the first seven roundworms and four days to expel the last one. In total, the male saki expelled seven parasites, ranging from 11 cm to 25 cm. The adult worms were preserved in 70% alcohol and sent to the Parasitological Diseases Laboratory of the Universidade Federal Fluminense for morphological identification according to Vicente et al. (1997) (Figure 4 and 5). Subsequently, molecular analysis was performed at the Molecular Epidemiology Laboratory at the same university, confirming A. lumbricoides infection.
Figure 4. Adults of Ascaris lumbricoides preserved in ethanol 70% for morphological identification.
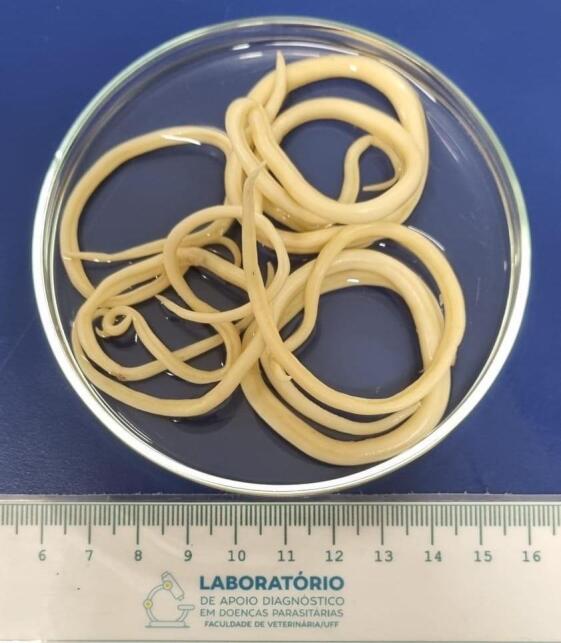
Figure 5. Evidentiation of the trilabial anterior extremity of an adult Ascaris lumbricoides specimen.

Using the morphological aspects described by the aforementioned author, the following diagnostic keys were observed for identifying the family: a claviform esophagus without an esophageal bulb, prominent lips, an excretory system shaped like a tuning fork, an excretory pore at the level of the nerve ring, and a non-spiny cuticle. For species suggestion, we observed the absence of cervical alae, a mouth surrounded by three large lips with rows of denticles, no interlabia, and a claviform esophagus without a ventricle. Males had a conical tail without a caudal ala, numerous pre-anal papillae, few post-anal papillae, equal non-winged spicules, and no gubernaculum. Females had a vulva located anterior to the mid-body, a posteriorly directed vagina, and parallel uterine branches.
In order to confirm the diagnostics of A. lumbricoides, molecular analysis was performed using conventional polymerase chain reaction (PCR). The sample was preserved in 70% ethanol solution and stored in a refrigerator (2 to 8 °C) until processing. A fragment of the sample, approximately 2 cm, was removed from the ethanol, washed in 1X phosphate-buffered saline (PBS), and ground using a mortar and pestle. The sample was then processed in duplicate and subjected to DNA extraction using the phenol-chloroform method. The DNA quantification in nanograms per microliter was calculated based on the absorbance at 260 nm (A260), and the purity was determined by the A260/230 and A260/A280 ratios. The DNA was stored at -20 °C until use.
The samples were subjected to conventional PCR targeting the internal transcribed spacer (ITS) regions of ribosomal DNA (rDNA) from Ascaris spp. following Ishiwata et al. (2004). For the ITS1 region, the forward primer was F2662, 5′-GGCAAAAGTCGTAACAAGGT-3′, and the reverse primer was R3214, 5′-CTGCAATTCGCACTATTTATCG-3′. For the ITS2 region, the forward primer was F3207, 5′-CGAGTATCGATGAAGAACGCAGC-3′, and the reverse primer was R3720, 5′-ATATGCTTAAGTTCAGCGGG-3′. Conventional PCR was performed in a total volume of 25 μL, including PCR buffer (1X, Promega), deoxynucleotide triphosphate solution (dNTPs) (0.2 mM, Ludwig), forward and reverse primers (0.2 μM, Thermofisher), Taq DNA polymerase (0.5 U/µL, Promega), PCR-grade water, and template DNA (600 ng) or water (negative control). The thermocycler reactions (Bio-Rad) were programmed as follows: initial incubation incubation at 95°C for five minutes, followed by 35 cycles of 1 minute at 94 °C, 1 minute at 60 °C, and 1 minute at 72 °C, with a final extension period at 72°C for 10 minutes. PCR products were analyzed via 1.5% agarose gel electrophoresis stained with ethidium bromide and visualized under UV transillumination (Figure 6). Amplicons were purified using the ReliaPrep DNA Clean-up and Concentration System (Promega) and sequenced using the same primers as in the amplification reactions. The nucleotide sequences obtained were analyzed using BioEdit software (Ibis Biosciences) and compared with sequences deposited in the NCBI database for Ascaris spp. The DNA extracted from the Ascaris specimen yielded concentrations of 137.8 ng/μL and 197.8 ng/μL. The purity ratios were 1.738 and 1.893 for A260/230 and 1.708 and 1.749 for A260/A280. PCR amplification of the ITS-1 and ITS-2 regions produced fragments of 580 bp and 456 bp, respectively, confirming the sample’s identification as Ascaris spp. (Figure 6).
Figure 6. Electrophoresis gel showing PCR results for Ascaris lumbricoides detection through the internal transcribed spacer (ITS) region. (A) Lanes 1 and 2 display amplification of the ITS1 region from Ascaris samples, with lane 3 being the negative control; (B) Lanes 1 and 2 show amplification of the ITS2 region from Ascaris samples, with lane 3 as the negative control. Molecular weight markers (100 bp ladder, Ludwig) indicate the 500-base pair position highlighted in the figure.

A phylogenetic tree was constructed using the MEGA X software with the Maximum Likelihood method and Kimura’s two-parameter model (Kimura, 1980). The bootstrap consensus tree inferred from 1,000 replicates was used to determine the phylogeny of the analyzed taxa, and branches corresponding to partitions reproduced in less than 50% of the bootstrap replicates were collapsed.
In the ITS-1 region analysis (Figure 7), the sequence (GenBank accession number PQ882781) formed a highly supported clade (bootstrap = 100) with A. lumbricoides sequences isolated from Homo sapiens and Pongo abelii. Similarly, in the ITS-2 region (Figure 8), the sample from this study (GenBank accession number PQ882782) was also grouped with an A. lumbricoides sequence.
Figure 7. Phylogenetic tree of Ascaris spp. using the partial sequence of the ITS-1 gene. All accession numbers correspond to different isolates, followed by their host and GenBank database accession number. The newly generated sequence from this study is marked with a solid black circle.
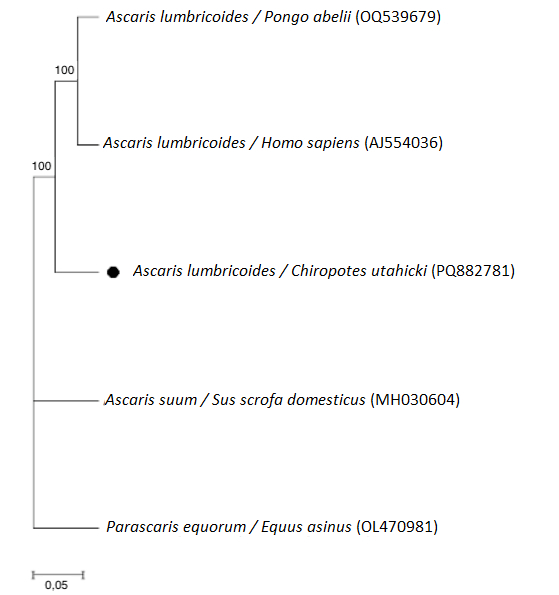
Figure 8. Phylogenetic tree of Ascaris spp. using the partial sequence of the ITS-2 gene. All accession numbers correspond to different isolates, followed by their host and GenBank database accession number. The newly generated sequence from this study is marked with a solid black circle.

As for the host primates, anesthesia and deworming were repeated according to the same protocol after 21 days for both animals. Subsequent coproparasitological examinations showed no eggs, and ultrasound scans revealed no remaining roundworms. Given these results, both animals were returned to their original enclosure. Throughout their stay in the hospital, neither animal exhibited signs of hyporexia, diarrhea, or apathy.
Discussion
Lilly et al. (2002) performed faecal examinations on indigenous tribes, active researchers and wild local Apes in the Central African Republic. Their study revealed a high prevalence of zoonotic nematodes, suggesting that due to inadequate hygiene practices within these communities, cross-infection between humans and non-human primates could occur. Similarly, Medkour et al. (2020) found homologue findings in the Republic of Congo and Senegal.
This suggests that the geographical proximity between non-human primates (NHPs) and humans can serve as a risk factor for the transmission of parasitic diseases, specially under favorable conditions, such as the weather (tropical or subtropical), high humidity and the deficiency of sanitary conditions (Tshikuka et al., 1995; Walker et al., 2011; Yang et al., 2018). It is important to point out that the zoo that is being discussed in this study is located adjacent to a low-income community with many informal housing, where a significant portion of residents rely on rainwater harvesting tanks as their main source. Additionally, Rio de Janeiro has a tropical climate with frequent rainfall. Given that the sakis’ enclosure is an outdoor space, the soil remains consistently humid, creating an environment conducive to the viability of Ascaris spp. eggs (Brooker & Michael, 2000; Lilly et al., 2002).
Considering the unexpected diagnosis of the NHP of this study, it is important to note that the ultrasound played a crucial role in the initial suspicion of ascaridiasis and in assessing the clinical condition of both patients. It guided the veterinarians in the decision to administer antiparasitic treatment with drugs capable of addressing the disease while the animals were still under anesthesia, ensuring a more favorable outcome and full intake of the medication, as it was administered directly into their stomachs. Moreover, this approach allowed for a more comprehensive assessment of the parasite's impact on the hosts, serving as a valuable complement to coproparasitological examination. In our case, the coproparasitological examination was performed after the ultrasound confirmed the presence of parasites, demonstrating the essential complementarity of both diagnostic methods in detection of the disease. In humans, the ultrasound has been used as an important diagnostic tool in ascaridiasis cases, also aiding in the evaluation of other organs that may be affected by the larvae migration, such as the biliary tract and liver (Price & Leung, 1988; Montórfano, 1998; Wu, 2009; Soni et al., 2015). To the best of the authors’ knowledge, there have been no previous report of this diagnostic tool being used to detect ascaridiasis in a neotropical NHP.
There is an ongoing debate in the literature regarding whether A. lumbricoides and Ascaris suum, a nematode that primarily infect pigs, represent distinct species or variants of the same species (Leles et al., 2012; Zhou et al., 2020). This discussion stems from the significant genetic similarities shared between the two, as well as their morphological overlap. While traditionally classified based on host specificity, with A. lumbricoides infecting primates and A. suum primarily associated with pigs, molecular evidence suggests frequent gene flow between populations, indicating that cross transmission can occur, thus blurring the lines of differentiation (Leles et al., 2012; Alves et al., 2016; Zhou et al., 2020).
Thus, the molecular analysis of the helminths in this case is of critical importance. Due to the substantial morphological similarities between A. lumbricoides and A. suum, relying solely on morphological characteristics can lead to misidentification. Traditional morphological methods are essential for approximating species-level identification; however, they lack the precision necessary to differentiate these two closely related species, given their minimal differences (Maung, 1973; Bacelar et al., 2023). Performing molecular analysis, such as PCR targeting specific genomic regions, provides species confirmation and also plays a crucial role in improving our understanding of host-parasite relationships (Zhou et al., 2020).
The sample from this study (GenBank accession number PQ882781) was identified as A. lumbricoides through analysis of the ITS-1 region, reinforcing the utility of this region for species identification within the Ascaris genus, as previously reported by Sadaow et al. (2018). This approach can be applied to cases involving non-human primates, such as C. utahickae. Meanwhile, the analysis of the ITS-2 region emphasized the genetic proximity to A. lumbricoides and the formation of a closely related cluster with A. suum, which has been previously reported in other studies due to the genetic similarity between these species.
The treatment for both cases of ascaridiasis was effective with both fenbendazole and pyrantel pamoate, although the time required to start eliminating parasites varied between individuals. This difference in response could be attributed to the different parasitic loads between the two sakis observed on the ultrasounds, but it is more likely due to the distinct mechanisms of the medications used. Fenbendazole, a broad-spectrum benzimidazole, disrupts microtubule formation in nematodes, leading to their death. It typically begins to show effects within a few days to a week (Fennell et al., 2008; Taylor et al., 2017). In contrast, pyrantel pamoate causes paralysis of the worms by affecting the neuromuscular junction, which leads to their expulsion from the host’s body through normal peristaltic movements. Pyrantel pamoate generally acts more quickly to kill parasites (Massara & Enk, 2004; Taylor et al., 2017), which was consistent with our observations.
Regarding treatment, it is crucial to note that treating the infected animals alone will not prevent reinfection, as Ascaris eggs are highly resistant in the soil and can lead to recurring infections (Hlaing et al., 1987; Henry, 1988; Hall et al., 1992). Therefore, in addition to administering chemical treatment to the affected animals, environmental management and ensuring the quality of water and food is essential. This includes implementing proper soil drainage, providing clean water and food, removing old feces from the enclosure, and conducting regular stool testing for both the zoo staff and the animals. Such measures are vital for maintaining a parasite-free environment (Pérez Cordón et al., 2008; Panayotova-Pencheva, 2013; Bais et al., 2017; Moreno Mañas et al., 2019; Martelli & Krishnasamy, 2023). This comprehensive approach not only enhances the well-being of the animals but also protects the health of the zoo staff and the surrounding community, creating a safer and healthier environment for all. It aligns with the One Health approach and contributes to wildlife conservation efforts.
Conclusion
In conclusion, the importance of conducting multiple complementary diagnostic tests cannot be overstated when addressing parasitic diseases. In the case of the two black sakis (C. utahickae) infected with A. lumbricoides, ultrasound proved to be an invaluable tool for accurately assessing the extent of the infection and guiding treatment decisions. Additionally, this case reports the successful treatment of both cases with fenbendazole and pyrantel pamoate, with a slight difference in the time required for elimination of adult parasites. Finally, considering the importance of an One Health approach for wildlife veterinarians, this paper underscores the importance of vigilant monitoring and effective management of parasitic infections in zoo environments.
Acknowledgements
Our sincere gratitude to BioParque do Rio Zoo for their invaluable support in making this case report possible. We also thank the esteemed Dr. Luciano Antunes Barros from the Parasitological Diseases Laboratory of the Universidade Federal Fluminense for his dedicated assistance to wildlife professionals throughout Brazil in identifying challenging parasites that without him would remain nameless.
Footnotes
How to cite: Barbosa, B. E. P., Alves, A. C. T., Miranda, B. P., Santos, B. A., Belsito, A. C., Magalhães, I. F. B., & Barros, L. A. (2025). Ascaris lumbricoides infection in captive black sakis (Chiroptes utahickae): a case report and implications for zoonotic transmission. Brazilian Journal of Veterinary Medicine, 47, e008924. https://doi.org/10.29374/2527-2179.bjvm008924
Ethics statement: All examinations and treatment were performed due to a medical need.
Financial support: None.
Availability of complementary results: There is no complementary information suggested.
The study was carried out at BioParque do Rio and the Laboratório de Doenças Parasitárias of the Universidade Federal Fluminense, Niterói, RJ, Brazil.
References
- Akgun Y. Intestinal obstruction caused by Ascaris lumbricoides. Diseases of the Colon and Rectum. 1996;39(10):1159–1163. doi: 10.1007/BF02081419. [DOI] [PubMed] [Google Scholar]
- Alves E. B. D. S., Conceição M. J., Leles D. Ascaris lumbricoides, Ascaris suum, or “Ascaris lumbrisuum”? The Journal of Infectious Diseases. 2016;213(8):1355. doi: 10.1093/infdis/jiw027. [DOI] [PubMed] [Google Scholar]
- Bacelar P. A. A., Santos J. P., Calegar D. A., Silva D. A., Leal D. N., Evangelista B. B. C., Reis E. R. C., Mallet J. R. S., Carvalho-Costa F. A., Jaeger L. H., Monteiro K. J. L. A molecular and morphological study of Ascaris suum in a human-pig contact scenario in northeastern Brazil. Revista Brasileira de Parasitologia Veterinária. 2023;32(3):e005623. doi: 10.1590/s1984-29612023057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais B., Tak L., Mahla S. Study of preventive health measures for wildlife in captivity: A review of management approaches. International Journal of Avian & Wildlife Biology. 2017;2(3):73–75. [Google Scholar]
- Brooker S. J., Pullan R. L. In: Ascaris: The neglected parasite. Holland C., editor. New York: Elsevier; 2013. Ascaris lumbricoides and ascariasis: Estimating numbers infected and burden of disease. pp. 343–362. [DOI] [Google Scholar]
- Brooker S., Michael E. The potential of geographical information systems and remote sensing in the epidemiology and control of human helminth infections. Advances in Parasitology. 2000;47:245–288. doi: 10.1016/S0065-308X(00)47011-9. [DOI] [PubMed] [Google Scholar]
- Caraballo L., Acevedo N. New allergens of relevance in tropical regions: The impact of Ascaris lumbricoides infections. The World Allergy Organization Journal. 2011;4(5):77–84. doi: 10.1097/WOX.0b013e3182167e04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J., Redmond I. In: Gorilla pathology and health: With a catalogue of preserved materials. Hull G., Cooper J. E., editors. London: Academic Press; 2016. Alimentary tract and associated organs. pp. 154–174. 11. [Google Scholar]
- Corda A., Corda F., Secchi V., Pentcheva P., Tamponi C., Tilocca L., Varcasia A., Scala A. Ultrasonography of parasitic diseases in domestic animals: A systematic review. Animals. 2022;12(10):1252. doi: 10.3390/ani12101252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawet A., Yakubu D. P., Butu H. M. Survey of gastrointestinal parasites of non-human primates in Jos Zoological Garden. Nigeria: University of Jos; 2013. [Google Scholar]
- de la Hoz D. M. E., Cañate González A. S., Vergel E. F., Payares Ramírez K. J., Morales López S. E. Parasitic and fungal agents in Aotus sp., Alouatta seniculus and Cebus albifrons in the Colombian Caribbean. Revista de Investigaciones Veterinarias del Perú. 2020;31:1. [Google Scholar]
- Deem S. L. Role of the zoo veterinarian in the conservation of captive and free-ranging wildlife. International Zoo Yearbook. 2007;41(1):3–11. doi: 10.1111/j.1748-1090.2007.00020.x. [DOI] [Google Scholar]
- Fennell B., Naughton J., Barlow J., Brennan G., Fairweather I., Hoey E., McFerran N., Trudgett A., Bell A. Microtubules as antiparasitic drug targets. Expert Opinion on Drug Discovery. 2008;3(5):501–518. doi: 10.1517/17460441.3.5.501. [DOI] [PubMed] [Google Scholar]
- Hagel I., Giusti T. Ascaris lumbricoides: An overview of therapeutic targets. Infectious Disorders Drug Targets. 2010;10(5):349–367. doi: 10.2174/187152610793180876. [DOI] [PubMed] [Google Scholar]
- Hall A., Anwar K. S., Tomkins A. M. Intensity of reinfection with Ascaris lumbricoides and its implications for parasite control. Lancet. 1992;339(8804):1253–1257. doi: 10.1016/0140-6736(92)91593-W. [DOI] [PubMed] [Google Scholar]
- Henry F. J. Reinfection with Ascaris lumbricoides after chemotherapy: A comparative study in three villages with varying sanitation. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1988;82(3):460–464. doi: 10.1016/0035-9203(88)90162-9. [DOI] [PubMed] [Google Scholar]
- Hlaing T., Saw T., Lwin M. Reinfection of people with Ascaris lumbricoides following single, 6-month, and 12-month interval mass chemotherapy in Okpo village, rural Burma. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987;81(1):140–146. doi: 10.1016/0035-9203(87)90306-3. [DOI] [PubMed] [Google Scholar]
- Ishiwata K., Shinohara A., Yagi K., Horii Y., Tsuchiya K., Nawa Y. Identification of tissue-embedded ascarid larvae by ribosomal DNA sequencing. Parasitology Research. 2004;92(1):50–52. doi: 10.1007/s00436-003-1010-7. [DOI] [PubMed] [Google Scholar]
- Kightlinger L. K., Seed J. R., Kightlinger M. B. The epidemiology of Ascaris lumbricoides, Trichuris trichiura, and hookworm in children in the Ranomafana rainforest, Madagascar. The Journal of Parasitology. 1995;81(2):159–169. doi: 10.2307/3283914. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution. 1980;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Leles D., Gardner S. L., Reinhard K., Iñiguez A., Araujo A. Are Ascaris lumbricoides and Ascaris suum a single species? Parasites & Vectors. 2012;5(1):42. doi: 10.1186/1756-3305-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly A. A., Mehlman P. T., Doran D. Intestinal parasites in gorillas, chimpanzees, and humans at Mondika research site, Dzanga-Ndoki National Park, Central African Republic. International Journal of Primatology. 2002;23(3):555–573. doi: 10.1023/A:1014969617036. [DOI] [Google Scholar]
- Martelli P., Krishnasamy K. The role of preventative medicine programs in animal welfare and wellbeing in zoological institutions. Animals. 2023;13(14):2299. doi: 10.3390/ani13142299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massara C. L., Enk M. J. Treatment options in the management of Ascaris lumbricoides. Expert Opinion on Pharmacotherapy. 2004;5(3):529–539. doi: 10.1517/14656566.5.3.529. [DOI] [PubMed] [Google Scholar]
- Maung M. Ascaris lumbricoides Linné, 1758 and Ascaris suum Goeze, 1782: Morphological differences between specimens obtained from man and pig. The Southeast Asian Journal of Tropical Medicine and Public Health. 1973;4(1):41–45. [PubMed] [Google Scholar]
- Mbaya A. W., Udendeye U. J. Gastrointestinal parasites of captive and free-roaming primates at the Afi Mountain Primate Conservation Area in Calabar, Nigeria and their zoonotic implications. Pakistan Journal of Biological Sciences. 2011;14(13):709–714. doi: 10.3923/pjbs.2011.709.714. [DOI] [PubMed] [Google Scholar]
- Medkour H., Amona I., Laidoudi Y., Davoust B., Bitam I., Levasseur A., Akiana J., Diatta G., Pacheco L., Gorsane S., Sokhna C., Hernandez-Aguilar R. A., Barciela A., Fenollar F., Raoult D., Mediannikov O. Parasitic infections in African humans and non-human primates. Pathogens. 2020;9(7):561. doi: 10.3390/pathogens9070561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud C., Tantalean M., Ique C., Montoya E., Gozalo A. A survey for helminth parasites in feral New World non-human primate populations and its comparison with parasitological data from man in the region. Journal of Medical Primatology. 2003;32(6):341–345. doi: 10.1046/j.1600-0684.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- Montórfano M. Ascaris lumbricoides: Role of ultrasound in diagnosis and management of abdominal manifestation. Journal d’échographie et de Médecine Ultra-sonore. 1998;19:394–398. [Google Scholar]
- Montórfano M. Ultrasound and ascariasis. Ultrasound Quarterly. 1999;15(4):206–209. doi: 10.1097/00013644-199912000-00005. [DOI] [Google Scholar]
- Moreno Mañas E., Gonzálvez Juan M., Ruiz de Ybáñez Carnero M. R., Gilbert T., Ortiz J., Espeso G., Benzal J., Ibáñez B., Valera Hernández F. Survey of husbandry practices for bovidae in zoos: The importance of parasite management for reintroduction programmes. The Veterinary Record. 2019;184(9):282. doi: 10.1136/vr.104985. [DOI] [PubMed] [Google Scholar]
- Nisha M., Amira N. A., Nadiah N., Davamani F. Detection of Ascaris lumbricoides and Trichuris trichiura in various soil types from an indigenous village in Malaysia. Tropical Biomedicine. 2019;36(1):201–208. [PubMed] [Google Scholar]
- Oh P., Granich R., Scott J., Sun B., Joseph M., Stringfield C., Thisdell S., Staley J., Workman-Malcolm D., Borenstein L., Lehnkering E., Ryan P., Soukup J., Nitta A., Flood J. Human exposure following Mycobacterium tuberculosis infection of multiple animal species in a metropolitan zoo. Emerging Infectious Diseases. 2002;8(11):1290–1293. doi: 10.3201/eid0811.020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotova-Pencheva M. S. Parasites in captive animals: A review of studies in some European zoos. Der Zoologische Garten. 2013;82(1-2):60–71. doi: 10.1016/j.zoolgart.2013.04.005. [DOI] [Google Scholar]
- Pérez Cordón G., Hitos Prados A., Romero D., Sánchez Moreno M., Pontes A., Osuna A., Rosales M. J. Intestinal parasitism in the animals of the zoological garden “Peña Escrita” (Almuñecar, Spain) Veterinary Parasitology. 2008;156(3-4):302–309. doi: 10.1016/j.vetpar.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Price J., Leung J. W. C. Ultrasound diagnosis of Ascaris lumbricoides in the pancreatic duct: The “four-lines” sign. The British Journal of Radiology. 1988;61(725):411–413. doi: 10.1259/0007-1285-61-725-411. [DOI] [PubMed] [Google Scholar]
- Rondon Robayo S. Y., Ramirez A. D., Sandoval A. B. Intestinal parasites infecting squirrel monkeys (Saimiri cassiquiarensis) in a human-nonhuman primate interface in Colombia.; Annali XXXII Congresso Nazionale della Società Italiana di Parassitologia.; Roma: Sapienza Università di Roma; 2022. [Google Scholar]
- Sadaow L., Sanpool O., Phosuk I., Rodpai R., Thanchomnang T., Wijit A., Anamnart W., Laymanivong S., Aung W. P. P., Janwan P., Maleewong W., Intapan P. M. Molecular identification of Ascaris lumbricoides and Ascaris suum recovered from humans and pigs in Thailand, Lao PDR, and Myanmar. Parasitology Research. 2018;117(8):2427–2436. doi: 10.1007/s00436-018-5931-6. [DOI] [PubMed] [Google Scholar]
- Schulz S., Kroeger A. Soil contamination with Ascaris lumbricoides eggs as an indicator of environmental hygiene in urban areas of north-east Brazil. The Journal of Tropical Medicine and Hygiene. 1992;95(2):95–103. [PubMed] [Google Scholar]
- Sheather A. L. The detection of intestinal protozoa and mange parasites by a flotation technique. The Journal of Comparative Pathology and Therapeutics. 1923;36:266–275. doi: 10.1016/S0368-1742(23)80052-2. [DOI] [Google Scholar]
- Soni S., Singh S., Kaur H., Gupta S., Sharma R. Should intensivist do routine abdominal ultrasound? Indian Journal of Critical Care Medicine. 2015;19(9):560–562. doi: 10.4103/0972-5229.164813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza M. J. One health: Zoonoses in the exotic animal practice. The Veterinary Clinics of North America. Exotic Animal Practice. 2011;14(3):421–426. doi: 10.1016/j.cvex.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P. R., Pappalardo P., Huang S., Byers J. E., Farrell M. J., Gehman A., Ghai R. R., Haas S. E., Han B., Park A. W., Schmidt J. P., Altizer S., Ezenwa V. O., Nunn C. L. Global Mammal Parasite Database version 2.0. Ecology. 2017;98(5) doi: 10.1002/ecy.1799. 1476. [DOI] [PubMed] [Google Scholar]
- Taylor M. A., Coop R. L., Wall R. L. Parasitologia veterinária. 4ª. Rio de Janeiro: Guanabara Koogan; 2017. [Google Scholar]
- Tshikuka J.-G., Scott M. E., Gray-Donald K. Ascaris lumbricoides infection and environmental risk factors in an urban African setting. Annals of Tropical Medicine and Parasitology. 1995;89(5):505–514. doi: 10.1080/00034983.1995.11812983. [DOI] [PubMed] [Google Scholar]
- Vicente J. V., Rodrigues H. O., Gomes D. C., Pinto R. M. Nematóides do Brasil: Parte V: Nematóides de mamíferos. Revista Brasileira de Biologia. 1997;(1):1–452. [Google Scholar]
- Villamizar E., Méndez M., Bonilla E., Varon H., de Ontra S. Ascaris lumbricoides infestation as a cause of intestinal obstruction in children: Experience with 87 cases. Journal of Pediatric Surgery. 1996;31(1):201–205. doi: 10.1016/S0022-3468(96)90348-6. [DOI] [PubMed] [Google Scholar]
- Walker M., Hall A., Basáñez M.-G. Individual predisposition, household clustering, and risk factors for human infection with Ascaris lumbricoides: New epidemiological insights. PLoS Neglected Tropical Diseases. 2011;5(4):e1047. doi: 10.1371/journal.pntd.0001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigger A., Peppler C., Kramer M. appearance of intestinal roundworms in a dog and a cat. The Veterinary Record. 2007;161(6):200–201. doi: 10.1136/vr.161.6.200. [DOI] [PubMed] [Google Scholar]
- Wu S. Sonographic findings of Ascaris lumbricoides in the gastrointestinal and biliary tracts. Ultrasound Quarterly. 2009;25(4):207–209. doi: 10.1097/RUQ.0b013e3181c47a2d. [DOI] [PubMed] [Google Scholar]
- Yang D., Yang Y., Wang Y., Yang Y., Dong S., Chen Y., Jiang Q., Zhou Y. Prevalence and risk factors of Ascaris lumbricoides, Trichuris trichiura, and Cryptosporidium infections in elementary school children in southwestern China: A school-based cross-sectional study. International Journal of Environmental Research and Public Health. 2018;15(9):1809. doi: 10.3390/ijerph15091809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Chen J., Niu H., Ouyang S., Wu X. Study on the population evolution of Ascaris lumbricoides and Ascaris suum based on whole genome resequencing. Veterinary Parasitology. 2020;279:109062. doi: 10.1016/j.vetpar.2020.109062. [DOI] [PubMed] [Google Scholar]



