Abstract
This work aimed to elucidate the anti-PF mechanism of ECC-JHF.The effects of ECC-JHF on lung fibrosis and fibroblast activation were investigated by establishing a BLM-induced PF rat model and a transforming growth factor-beta (TGF-β)-induced fibroblast activation model. Furthermore, the effects of ECC-JHF on Nrf2 signaling and mitophagy were explored both in vivo and in vitro. In the PF model rats, ECC-JHF mitigated pathological damage, reduced collagen deposition, decreased levels of malondialdehyde (MDA) and P62, and increased levels of total superoxide dismutase (T-SOD) as well as the expression of Nrf2, HO-1, PINK1, PARK2, and LC3B in lung tissues. These results suggest that the anti-PF mechanism of ECC-JHF may be associated with the inhibition of oxidative stress and the enhancement of mitophagy. The medium dose of ECC-JHF and pirfenidone were similar in improving pulmonary fibrosis in rats. In the TGF-β-induced lung fibroblast activation, ECC-JHF inhibited fibroblast activation by downregulating the levels of fibronectin, alpha-smooth muscle actin (α-SMA), and collagen I. Additionally, ECC-JHF upregulated the level of Nrf2 and its target proteins, including HO-1 and NQO1, as well as mitophagy-related proteins PINK1, PARK2, and LC3B. This led to an increase in the co-localization of TOM20 and LC3, thereby enhancing mitochondrial autophagy. The application of Nrf2 siRNA and Nrf2 inhibitors significantly diminished the effects of ECC-JHF on Nrf2 signaling, PINK1/PARK2-mediated mitophagy, and fibroblast activation. ECC-JHF exerts a protective effect against PF by suppressing fibroblast activation through the upregulation of Nrf2 and PINK1/PARK2-mediated mitophagy, it provides a new target and strategy for the treatment of pulmonary fibrosis.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-95175-8.
Keywords: Pulmonary fibrosis, Effective-compound combination, Fibroblasts activation, Nrf2, Mitophagy
Subject terms: Medical research, Molecular medicine, Pathogenesis
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive interstitial pneumonia characterized by a median survival of only 3 to 5 years following diagnosis1,2. Pirfenidone (PFD) and nintedanib, two antifibrotic agents approved by the Food and Drug Administration (FDA), can slow the decline in lung function; however, they do not reverse lung structural damage and are associated with notable adverse effects, including nausea and diarrhea1,3,4. Therefore, there is an urgent need to identify new therapeutic targets and agents. Current research indicates that fibroblasts play a pivotal role as effector cells in the fibrotic process5,6. The abnormal microenvironment resulting from epithelial cell injury and endothelial cell activation triggers fibroblast proliferation and their differentiation into myofibroblasts2. These myofibroblasts, which exhibit irreversible contractility and resistance to apoptosis, secrete abundant collagen, leading to extensive extracellular matrix (ECM) deposition that promotes the advancement of fibrosis7,8. Consequently, the aberrant activation of fibroblasts represents a critical pathological mechanism in the development of IPF.
Mitophagy represents a distinct type of autophagy that specifically targets and removes dysfunctional mitochondria. The PTEN-induced kinase 1 (PINK1)-Parkin pathway represents a key mechanism facilitating mitophagy. PINK1, acting as a sensor for damaged mitochondria, accumulates on the outer mitochondrial membrane (OMM) and phosphorylates Parkin, thereby recruiting and activating it. Activated Parkin ubiquitinates OMM proteins, ultimately leading to the degradation of damaged mitochondria9. Consequently, mitophagy effectively prevents the accumulation of damaged mitochondria, minimizes the excessive generation of reactive oxygen species (ROS), maintains cellular homeostasis, and protects against cell damage and apoptosis. Impaired mitophagy contributes to the conversion of lung fibroblasts into myofibroblasts, which in turn facilitates the progression of pulmonary fibrosis (PF)10. Insufficient mitophagy mediated by Park2 leads to excessive ROS production, which activates the platelet-derived growth factor receptor (PDGFR)/mammalian target of rapamycin (mTOR) signaling pathway, resulting in the differentiation and proliferation of myofibroblasts and ultimately contributing to fibrosis11. Thus, mitophagy serves as an adaptive response and is a crucial pathway for maintaining normal fibroblast function and inhibiting their transformation into myofibroblasts. Additionally, the nuclear factor erythroid 2-related factor 2 (Nrf2) acts as a crucial regulator of cellular redox balance. Under standard physiological conditions, it maintains stability with its primary repressor, Kelch-like ECH-associated protein 1 (Keap1), within the cytosol12.
Under stressful conditions, a change in a cysteine residue within Keap1 triggers Nrf2 to translocate to the nucleus. In this location, Nrf2 binds to the antioxidant response element (ARE) to regulate downstream target genes, including heme oxygenase 1 (HO-1) and NADPH quinone oxidoreductase 1 (NQO1)10,13,14. Studies indicate that activating Nrf2 can enhance mitophagy and improve renal fibrosis15. Additionally, knockdown of Nrf2 partially abolishes PINK1/Parkin-mediated mitophagy activated by farrerol pretreatment in mice16. Furthermore, Nrf2 activation can inhibit fibroblast activation by reducing intracellular ROS, thereby alleviating PF. Consequently, we suggest that the suppression of aberrant fibroblast stimulation via the stimulation of the Nrf2-mitophagy pathway could serve as an innovative therapeutic strategy for PF. Jinshui Huanxian Formula (JHF; ZL.201610877761.4) is a traditional Chinese medicine (TCM) prescription designed to treat patients with idiopathic pulmonary fibrosis (IPF) characterized by lung and kidney qi deficiency syndrome, based on TCM theories. The formula comprises Ginseng radix et rhizome (GRRa), Rehmanniae radix praeparata (RRP), Ophiopogonis radix (OR), Trichosanthis fructus (TF), Fritillariae thunbergii bulbus (FTB), Moutan cortex (MC), Citri reticulatae pericarpium (CRP), Glycyrrhizae radix et rhizome (GRRb), Epimedii folium (EF), and Ginkgo semen (GS). Clinical studies have demonstrated that JHF can effectively alleviate clinical symptoms of IPF, slow disease progression, and improve quality of life. Five active compounds—icariin, isoliquiritigenin, nobiletin, peimine, and paeoniflorin—were identified from JHF and combined to form the effective-component compatibility of JHF II (ECC-JHF II), which exhibits effects similar to those of JHF. However, ECC-JHF has clear advantages over JHF, controllable quality, higher safety, and convenient clinical application. Although previous studies have indicated that ECC-JHF can ameliorate bleomycin (BLM)-induced pulmonary fibrosis (PF) in rats, the underlying mechanisms require further investigation.
This study reveals a novel mechanism by which ECC-JHF improves pulmonary fibrosis by inhibiting fibroblast activation through activation of the Nrf2 signaling pathway and PINK1/ Park2-mediated mitochondrial autophagy. This discovery not only provides a new target and strategy for the treatment of pulmonary fibrosis, but also expands the application potential of traditional Chinese medicine in modern medicine.
Materials and methods
Chemicals and animals
Bleomycin (BLM) hydrochloride was acquired from Hanhui Pharmaceutical Co.Ltd. (lot No. 19033911, Shanghai, China). Pirfenidone (PFD) was purchased from Beijing Kangdini Pharmaceutical Co. Ltd (lot No. 20201001, Beijing, China). Icariin (CAS: 489-32-7), paeoniflorin (CAS: 23180-57-6), peimine (CAS: 23496-41-5), isoliquiritigenin (CAS: 961-29-5) and nobiletin (CAS: 478-01-3) were obtained from Chengdu Manster Biotechnology Co.Ltd (Chengdu, China). The purity of the above compounds is more than 98%. The Sprague-Dawley rats (license number: SCXK (Beijing) 2016-0006) were supplied by Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). All experimental procedures involving animals received ethical approval (YFYDW2017013) from the First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, China. And in accordance with the animal experiment guidelines of the Chinese Society of Experimental Animals. his study was conducted in accordance with the ARRIVE guidelines and the Animal Experimental Guide of the Chinese Laboratory Animal Society.
PF model and intervention
PF was induced in rats through intratracheal instillation of BLM at a dose of 5 mg/kg, dissolved in saline. On day 0, all rats, with the exception of those designated as controls, underwent an intratracheal administration of BLM, while the control group was administered an equivalent volume of saline. From day 29 to day 42 following bleomycin induction, ECC-JHF and PFD were administered by gavage once daily. Both the control and BLM groups of rats received identical volumes of solvent, specifically a 0.5% CMC-Na solution. On day 42, rats were anesthetized with an intraperitoneal injection of sodium pentobarbitone (15 mg / kg). Blood was collected from the venae cava inferior.
In the first experiment, the rats were randomly divided into six groups: (1) control group (0.5 mL/100 g, qd); (2) BLM group (0.5 mL/100 g, qd); (3) low-dose ECC-JHF group (ECC-JHF L, 2.36 mg/kg/d, 0.5 mL/100 g, qd); (4) medium-dose ECC-JHF group (ECC-JHF M, 4.72 mg/kg/d, 0.5 mL/100 g, qd); (5) high-dose ECC-JHF group (ECC-JHF H, 9.44 mg/kg/d, 0.5 mL/100 g, qd): (6) the PFD group (50 mg/kg/d, 0.5 mL/100 g, qd). In the second experiment, the rats were randomly divided into six groups: (1) control group (0.5 mL/100 g, qd); (2) BLM group (0.5 mL/100 g, qd); (3) ECC-JHF group (4.72 mg/kg/d, 0.5 mL/100 g, qd); (4) brusatol group (50 mg/kg/d, 0.5 mL/100 g, qd); (5) ECC-JHF + brusatol group (4.72 mg/kg/d, 0.5 mL/100 g, qd; 50 mg/kg/d, 0.5 mL/100 g, qd); (6) the PFD group (50 mg/kg/d, 0.5 mL/100 g, qd).
Histopathological assessment
After fixation in 4% paraformaldehyde, the left lung tissue was embedded in paraffin, sectioned to a thickness of 4 μm, deparaffinized in water, and subsequently stained with hematoxylin and eosin (HE) and Masson’s trichrome. The staining results were examined using an optical microscope (Olympus, Tokyo, Japan). The severity of alveolitis and fibrosis was assessed using the Szapiel score17 and the Ashcroft score18, respectively.
Immunohistochemical analysis
Tissue sections were deparaffinized and underwent antigen retrieval before being incubated with 5% bovine serum albumin to inhibit non-specific binding. The samples were then placed in an incubator at 4 °C overnight with primary antibodies targeting collagen III (COL3), α-smooth muscle actin (α-SMA), Nrf2, heme oxygenase-1 (HO-1), PTEN-induced putative kinase 1 (PINK1), Parkin (Park2), P62, and LC3B. The next day, the tissue sections were incubated with their respective secondary antibodies. The integrated optical density (IOD) values of the images were quantified using the Image-Pro Plus 6.0 professional imaging acquisition and analysis system.
T-SOD and MDA measurements
Weigh approximately 20 mg of tissue and cut it into small pieces with scissors. Subsequently, incorporate three steel balls and introduce phosphate-buffered saline (PBS) at a weight-to-volume ratio of 1:9. The resultant mixture is then subjected to homogenization followed by centrifugation at 4 °C for a duration of 10 min at a rotation speed of 12,000 rpm. After extracting the supernatant, the levels of total superoxide dismutase (T-SOD) and malondialdehyde (MDA) were quantified in accordance with the guidelines provided by the manufacturer (E-BC-K019-M; E-BC-K025-M, Elabscience, China).
Electron microscope
Fresh lung tissue was sectioned into approximately 1 mm³ blocks and promptly fixed in 2.5% glutaraldehyde. Following dehydration, embedding, ultrathin sectioning, and staining, the morphology of mitochondria and autophagy in fibroblasts was examined using transmission electron microscopy.
Cell culture and treatment
Human fetal lung fibroblast 1 (HFL1) cells sourced from Wuhan Procell Life Science & Technology Co, were maintained in a complete medium comprising Ham’s F-12 K (Procell), enriched with 10% fetal bovine serum and 1% penicillin-streptomycin solution, within a 5% CO2 incubator set at 37 °C.For all experiments, HFL1 cells were incubated with ECC-JHF at concentrations of 30.63 µg/mL and 61.25 µg/mL for 3 h prior to exposure to TGF-β1 (PeproTech) at a concentration of 5 ng/mL.
Real-time polymerase chain reaction (RT-PCR) assay
Total RNA was isolated from HFL1 cells utilizing Qiazol reagent. cDNA synthesis was performed with the HiScript II Q RT SuperMix. Then real-time Q-PCR were performd by diluted cDNA using qPCR SYBR Green Master Mix (Vazyme, Nanjing, China). The specific primer sequences were displayed in Table 1. Relative expression was calculated using the comparative Ct method (ΔΔCt).
Table 1.
The primer sequences amplified used for RT-PCR.
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| COL1A1 | GAGGGCCAAGACGAAGACATC | GAGGGCCAAGACGAAGACATC |
| ACTA2 | AAAAGACAGCTACGTGGGTGA | GCCATGTTCTATCGGGTACTTC |
| FN1 | CGGTGGCTGTCAGTCAAAG | AAACCTCGGCTTCCTCCATAA |
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
Immunofluorescent staining
The cells were subjected to fixation using 4% paraformaldehyde for 15 min, subsequently permeabilized with 0.3% Triton X-100 for 30 min, and blocked with a phosphate-buffered saline (PBS) solution supplemented with 10% normal goat serum (NGS) for a duration of 2 h at ambient temperature. Subsequently, the primary antibodies—fibronectin (FN) (1:100 dilution), α-smooth muscle actin (α-SMA) (1:100 dilution), TOM20 (1:200 dilution), and LC3 (1:500 dilution)—were incubated with the cells overnight at 4 °C. The following day, the cells were treated with the appropriate secondary antibodies for 2 h and counterstained with DAPI for 2 min. Images were captured using an Olympus UTBI90 fluorescence microscope and analyzed with ImageJ software.
Western blotting
Protein extracted from lung tissue or cells using RIPA buffer was quantified with a BCA protein assay kit. Equal quantities of protein samples were subsequently resolved using 10% or 12.5% SDS-PAGE, followed by transfer to PVDF membranes. Following a 1-hour blocking step with 5% skim milk in TBST, the membranes were incubated overnight at 4 °C with primary antibodies, followed by a 1-hour incubation with secondary antibodies the next day. Finally, the protein bands were detected employing the Bio-Rad Imaging System.
Transfection with Nrf2 SiRNA
When the cell density reached 60-80%, Nrf2 siRNAs were transfected into HFL1 cells using TranslT-X2 (Mirus, USA) reagent according to the manufacturer’s instructions, and the transfection was allowed to proceed for 24 h. Subsequently, the cells underwent treatment with ECC-JHF at concentrations of 30.625–61.250 µg/mL for a duration of 3 h. followed by stimulation with 5 ng/mL TGF-β1 for an additional 24 h. Finally, the cells were harvested for subsequent experiments.
Statistical analysis
The experimental data were evaluated utilizing IBM SPSS version 22.0 statistical analysis software and presented as mean ± standard error (SE). The significance between the groups was conducted using one-way ANOVA. P-values < 0.05 was considered statistically significant.
Results
ECC-JHF attenuates BLM-induced PF in rats
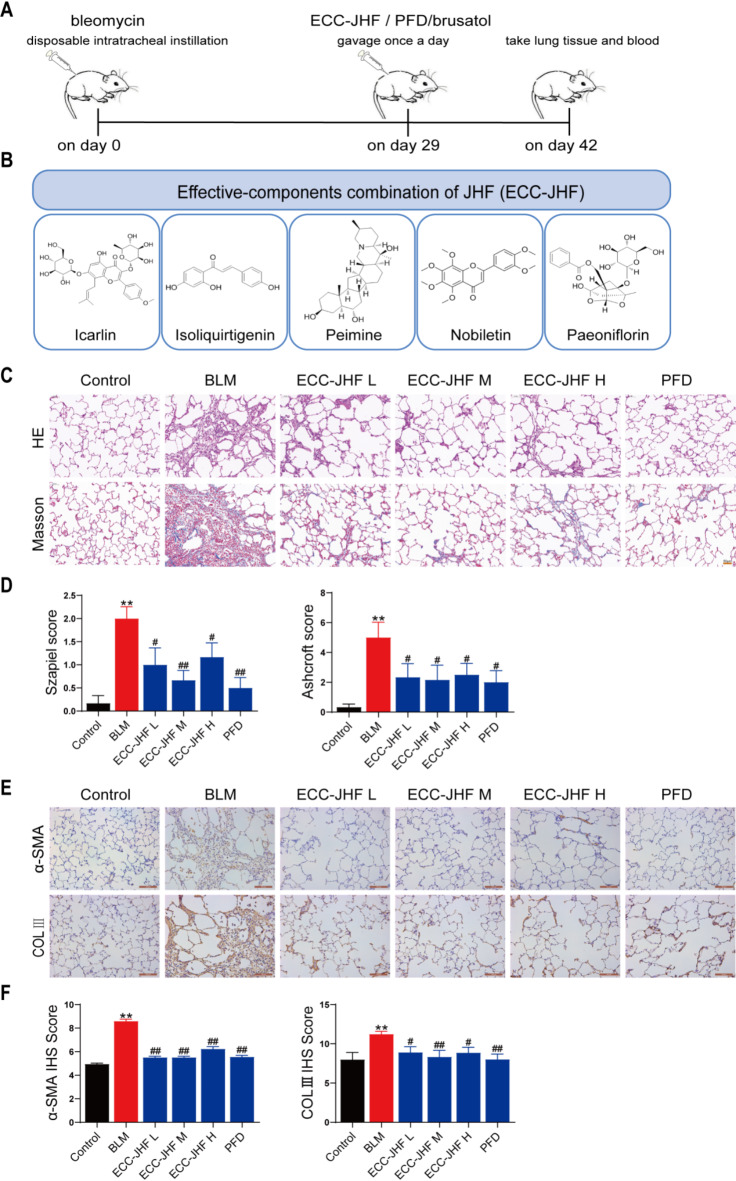
Various doses of ECC-JHF were administered to the BLM-injected rats from day 29 to day 42 to evaluate the therapeutic effects on pulmonary fibrosis (PF) (Fig. 1A). The compounds of ECC-JHF and their chemical structure (Fig. 1B). The lungs of BLM-induced rats exhibited destruction of alveolar structures, rupture and thickening of the alveolar walls, infiltration of inflammatory cells, and deposition of collagen fibers. Both ECC-JHF and PFD treatments effectively ameliorated these pathological changes and significantly reduced Szapiel and Ashcroft scores (Fig. 1C, D). Furthermore, ECC-JHF and PFD also inhibited the BLM-induced elevation of α-SMA and COL3 protein levels (Fig. 1E, F). In conclusion, ECC-JHF protects against PF by suppressing the inflammatory response and collagen deposition. Notably, the medium dose of ECC-JHF demonstrated superior improvement in pulmonary fibrosis compared to both the low-dose and high-dose groups. Therefore, we selected the medium-dose ECC-JHF for subsequent mechanistic investigations.
Fig. 1.
The therapeutic effect of ECC-JHF on BLM-induced PF in rats. (A) The general process of animal experiments. (B) The compounds of ECC-JHF and their chemical structure. (C) HE and Masson staining (magnification, × 200). (D) The Szapiel and Ashcroft scores of lung tissue (Alveolitis and fibrosis scores). (E-F) Immunohistochemical analysis of α-SMA, Col III and IHS score assessment (magnification, × 200). All data were expressed as mean ± SE (n = 6)., **p < 0.01, vs. control group. #p < 0.05, ##p < 0.01, vs. BLM group.
ECC-JHF inhibits oxidative stress in BLM-induced PF in rats
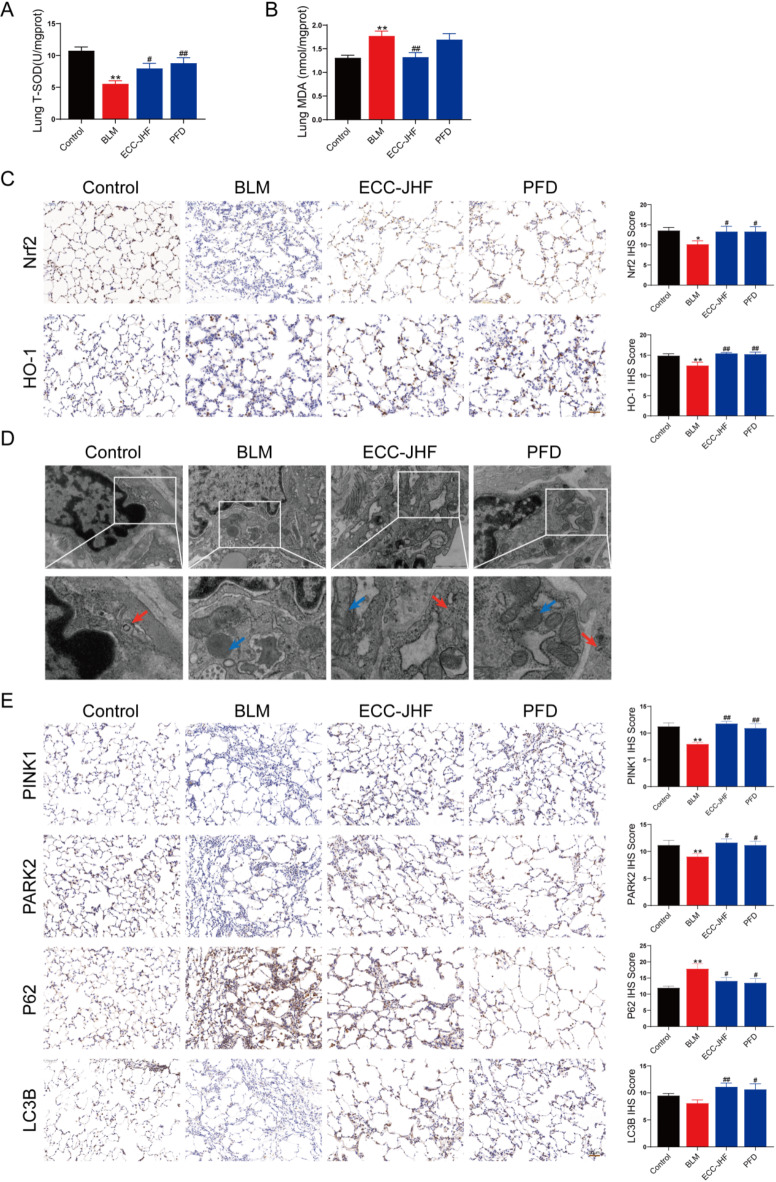
Oxidative stress is strongly associated with the progression of pulmonary fibrosis (PF)12. To evaluate the efficacy of ECC-JHF in scavenging oxygen free radicals and mitigating peroxidative damage in lung tissue, we assessed the activity of total superoxide dismutase (T-SOD) and the levels of malondialdehyde (MDA). In comparison to the control group, the T-SOD activity was significantly reduced, and MDA levels were elevated in the BLM group. Notably, following ECC-JHF treatment, these alterations were reversed (Fig. 2A-B). Additionally, ECC-JHF treatment led to a significant upregulation of Nrf2 and HO-1 expression (Fig. 2C). These findings suggest that ECC-JHF exerts its protective effects against OS by activating the Nrf2 signaling pathway.
Fig. 2.
ECC-JHF suppresses oxidative stress and enhances mitochondrial autophagy in lung tissue. (A) The activity of T-SOD. (B) The content of MDA. (C) Immunohistochemical analysis of Nrf2, HO-1, and IHS score assessment (magnification, × 200). (D) Transmission electron microscopy: the red arrows indicate autophagosomes and the blue indicate mitochondria (magnification, × 30000). (E) Immunohistochemical analysis of PINK1, Park2, p62 and LC3B and IHS score assessment (magnification, × 200). All data were expressed as mean ± SE (n = 6). *p < 0.05, **p < 0.01, vs. control group. #p < 0.05, ##p < 0.01, vs. BLM group.
ECC-JHF improves mitophagy deficiency in BLM-induced pulmonary fibrosis in rats
Inadequate mitophagy has been identified as a significant factor contributing to the accumulation of damaged mitochondria, which promotes pulmonary fibrosis (PF)10. Therefore, we evaluated the levels of mitophagy in lung tissue. Immunohistochemical analysis demonstrated that ECC-JHF treatment effectively restored the BLM-induced reductions in PINK1, PARK2, and LC3B expression, while also decreasing P62 expression (Fig. 2E). Furthermore, transmission electron microscopy revealed that ECC-JHF treatment alleviated mitochondrial swelling, ridge breakage, and even disappearance, as well as mitigating autophagy insufficiency (Fig. 2D). These findings suggest that ECC-JHF enhances mitophagy mediated by the PINK1/PARK2 pathway.
ECC-JHF inhibits TGF-β1-induced fibroblast-myofibroblast differentiation (FMD)
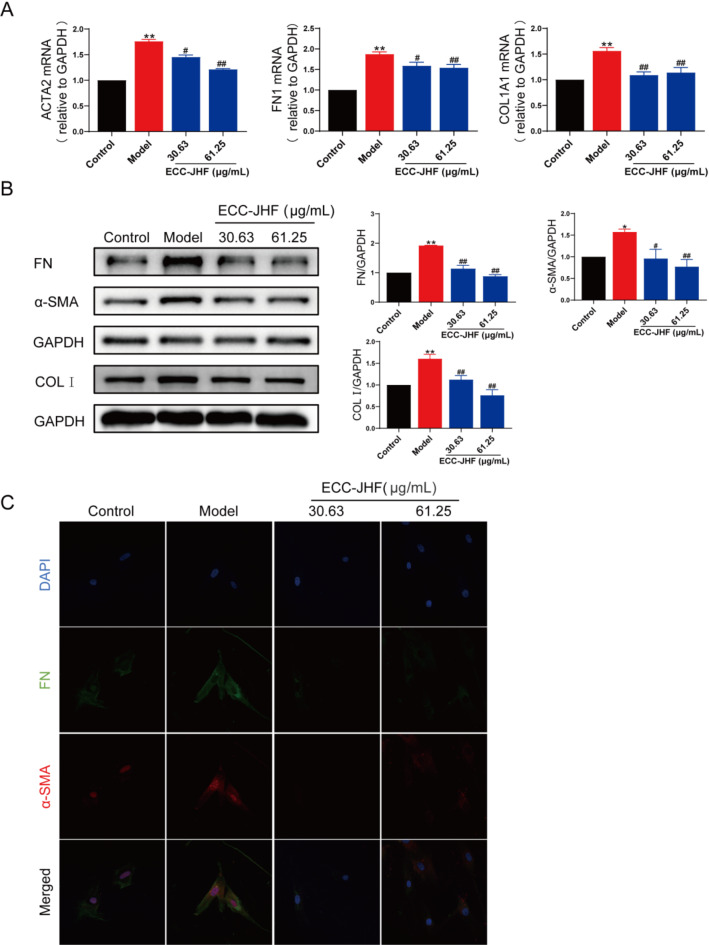
To assess the impact of ECC-JHF on fibroblast activation, we established an in vitro fibroblast-myofibroblast differentiation model using TGF-β1-treated HFL1 cells. ECC-JHF (30.63, 61.25 µg/ml) significantly inhibited the TGF-β-induced upregulation of FN, α-SMA, and COL1 (Fig. 3A & B), as corroborated by immunofluorescence analysis (Fig. 3C). These findings suggest that ECC-JHF effectively suppresses fibroblast activation.
Fig. 3.
Inhibitory effect of ECC-JHF on fibroblast activation. (A) Relative mRNA levels of ACTA2, FN1 and COL1A1. (B) The protein expression levels of α-SMA, FN and COL1 were detected by western blotting. (C) Immunofluorescent staining of the protein expression levels of α-SMA and FN (magnification, × 200). All data were expressed as mean ± SE. *p < 0.05, **p < 0.01, vs. control group. #p < 0.05, ##p < 0.01, vs. model group.
ECC-JHF enhances mitophagy via activating Nrf2 signaling to inhibit FMD
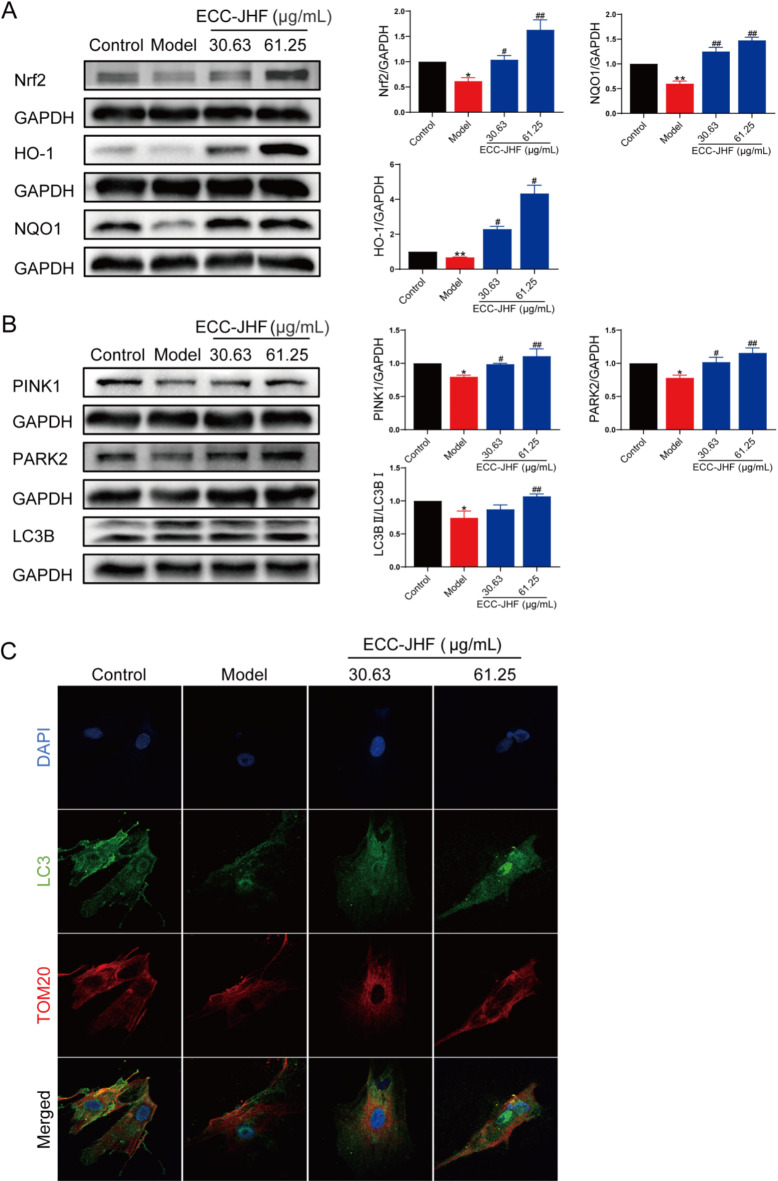
Both Nrf2 signaling and mitophagy play crucial roles in fibrotic disease. Using western blotting, we demonstrated that ECC-JHF effectively activates Nrf2 and upregulates the expression of its downstream target proteins, NQO1 and HO-1 (Fig. 4A). Furthermore, ECC-JHF treatment enhanced PINK1/PARK2-mediated mitophagy and increased elevated the conversion rate of LC3-I to LC3-II (Fig. 4B). Immunofluorescence analysis revealed an increased co-localization of TOM20 and LC3 in the ECC-JHF-treated cells (Fig. 4C).
Fig. 4.
Effect of ECC-JHF on Nrf2 signaling and mitophagy in TGFβ1-induced HFL1 cells. (A) The protein expression levels of Nrf2, HO-1 and NQO1. (B) The protein expression levels of PINK1, PARK2 and LC3B. (C) Immunofluorescent staining of the co-localization of TOM20 and LC3 (magnification, × 400). All data were expressed as mean ± SE. *p < 0.05, **p < 0.01, vs. control group. #p < 0.05, ##p < 0.01, vs. model group.
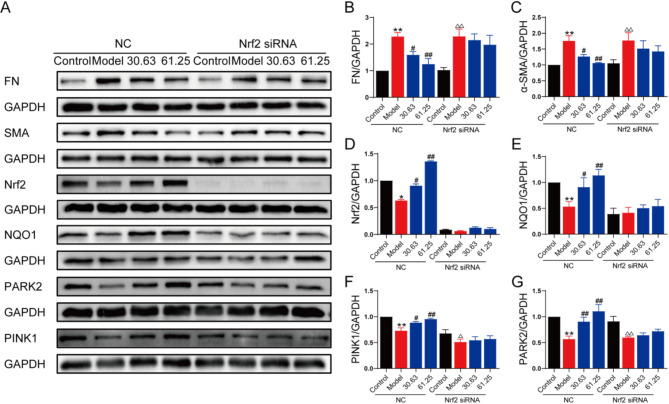
To elucidate the role of Nrf2 in ECC-JHF-enhanced mitophagy and ECC-JHF-mediated inhibition of fibroblast-myofibroblast differentiation, we conducted Nrf2 siRNA (siNrf2) transfection. HLF1 cells transfected with siNrf2 exhibited a significant reduction in the expression of Nrf2 and NQO1. Moreover, siNrf2 reversed the inhibitory effects of ECC-JHF on α-SMA and FN expression in HLF1 cells (Fig. 5A-E). Additionally, siNrf2 inhibited ECC-JHF-induced expression of PINK1 and PARK2 (Fig. 4A&F-G). These findings indicate that ECC-JHF enhances mitophagy by activating the Nrf2 signaling pathway, thereby inhibiting fibroblast-myofibroblast differentiation.
Fig. 5.
ECC-JHF inhibit TGF-β1-induced fibroblast activation by activting Nrf2 singaling, strengthening mitophagy. Effect of Nrf2 in ECC -treated HFL1 cells. After transfection with siNrf2 for 24 h, HFL1 cells were treated with ECC-JHF (30.63 and 61.25 µg/ml) and TGF-β1 (5 ng/ml) for 24 h. Then, cells were collected for western blotting. (A-G)The protein expression levels of α-SMA, FN, Nrf2, HO-1, and PINK1, PARK2. All data were expressed as mean ± SE. *p < 0.05, **p < 0.01, vs. control group in normal cells. #p < 0.05, ##p < 0.01, vs. model group in normal cells. △p < 0.05, △△p < 0.01, vs. control group in Nrf2 siRNA cells.
Effect of the Nrf2 on ECC-JHF alleviates BLM-induced PF in rats
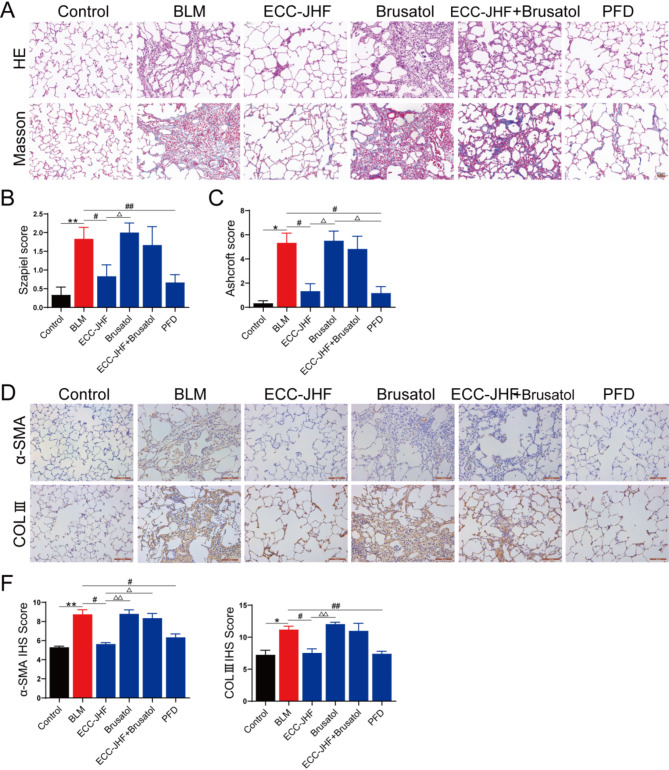
To further investigate the role of Nrf2 in the antifibrotic effects of ECC-JHF, we administered brusatol (an Nrf2 inhibitor) and/or ECC-JHF to the rats via gavage. As depicted in Fig. 6, brusatol exacerbated BLM-induced lung pathological damage and collagen deposition. Moreover, co-treatment with brusatol attenuated the protective effects of ECC-JHF against BLM-induced lung fibrosis.
Fig. 6.
Nrf2-dependence for the anti-PF effect of ECC-JHF. (A) HE and Masson staining (magnification, × 200). (B-C) The Szapiel and Ashcroft scores of lung tissue (Alveolitis and fibrosis scores). (D-F) Immunohistochemical analysis of α-SMA, Col III and IHS score assessment (magnification, × 200). All data were expressed as mean ± SE (n = 6). *p < 0.05, **p < 0.01, vs. control group. #p < 0.05, ##p < 0.01, vs.BLM group. △p < 0.05, △△p < 0.01, vs. ECC-JHF group.
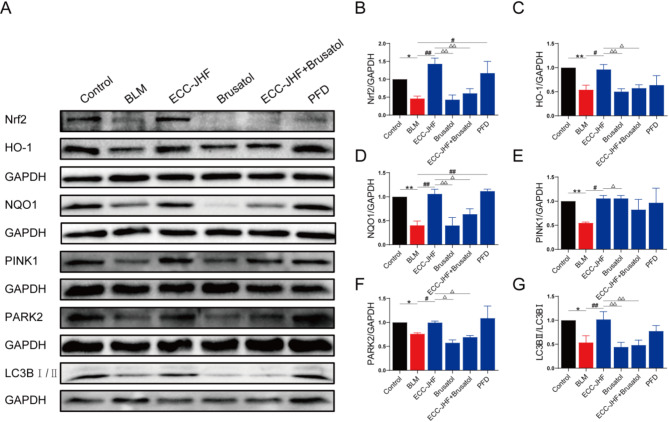
Additionally, we assessed the expression of Nrf2 pathway and mitophagy-related proteins in lung tissues. Results presented in Fig. 7 indicate that the protein levels of Nrf2, HO-1, NQO1, PINK1, PARK2, and LCB in the ECC-JHF combined with brusatol group were significantly reduced compared to those in the ECC-JHF group. Collectively, these findings suggest that ECC-JHF may contribute to the inhibition of pulmonary fibrosis progression by enhancing mitophagy through Nrf2 signaling.
Fig. 7.
ECC-JHF improves BLM-induced PF in rats by activating Nrf2, enhancing mitophagy. (A-G)The protein expression levels of Nrf2, HO-1, NQO1, PINK1, PARK2 and LC3B. All data were expressed as mean ± SE (n = 3). *p < 0.05, **p < 0.01, versus the control group. #p < 0.05, ##p < 0.01, vs. BLM group. △p < 0.05, △△p < 0.01, vs. ECC-JHF group.
Discussion
IPF is a fatal pulmonary interstitial disease in which abnormal activation of fibroblasts and excessive deposition of extracellular matrix (ECM) lead to fibrosis remodeling, scar tissue formation, progressive loss of lung function, and eventually death from respiratory failure2,19. However, there is a lack of effective treatment for IPF in clinic. In recent years, traditional Chinese medicine has been widely used to prevent and treat PF, and JHF is one of them. However, due to the complexity and diversity of JHF components, the underlying mechanisms of its anti-PF is difficult to explore. ECC-JHF, a mixture of five effective components derived from JHF with the optimal ratio, is equivalent to JHF. Here, we established a PF rat model via BLM intratracheal instillation, and demonstrated that ECC-JHF can effectively improve PF by inhibiting fibroblasts activation and collagen deposition.
OS is a primary mechanism in PF, with ROS being the main byproducts. Anecdotal evidence suggests that ROS can accelerate the apoptosis of alveolar epithelial cells, triggering inflammatory responses and promoting collagen deposition through the regulation of specific cytokines and growth factors13,20,21. Furthermore, some studies indicate that excessive ROS production can also enhance the proliferation and differentiation of myofibroblasts. Nrf2 is a crucial transcription factor that regulates redox balance and exerts antioxidant effects by reducing intracellular ROS accumulation22,23. For instance, DMI activates Nrf2, inhibiting the ROS/TXNIP signaling pathway and further preventing the conversion of fibroblasts into myofibroblasts24. Our study demonstrated that ECC-JHF increased the levels of the antioxidant enzyme superoxide dismutase (SOD) and decreased the levels of malondialdehyde (MDA) in the lung tissue of BLM-induced pulmonary fibrosis rats. Additionally, ECC-JHF promotes the expression of downstream antioxidant proteins, such as HO-1 and NQO1, by activating the Nrf2 signaling pathway to mitigate oxidative stress, thereby inhibiting fibroblast activation and alleviating BLM-induced PF in rats.
Mitochondria are a primary source of reactive oxygen species (ROS)25. Mitophagy prevents the pathological accumulation of mitochondrial ROS and protects cells from oxidative damage by eliminating damaged mitochondria26,27. Recent research indicates that mitophagy contributes to the pathogenesis of pulmonary fibrosis (PF) by regulating the senescence of alveolar epithelial cells (AECs), apoptosis of macrophages, and differentiation of myofibroblasts28. Treatment with TGF-β1 reduces the expression levels of PINK1 and PARK2 in lung fibroblasts, leading to myofibroblast proliferation and differentiation29. Silencing either the PINK1 or PARK2 gene can induce α-smooth muscle actin (α-SMA) expression in fibroblasts29. As an antifibrotic agent, pirfenidone promotes mitophagy to inhibit fibroblast activation by increasing PARK2 protein levels30. In this study, we observed a decrease in mitophagy in bleomycin (BLM)-induced rats, which aligns with previous reports. Furthermore, our research indicates that ECC-JHF enhances mitophagyby increasing the levels of PINK1, PARK2, and LC3B-II in the lung tissues of rats treated with BLM, thereby exerting an antifibrotic effect.
Myofibroblasts serve as the principal cell types that synthesize extracellular matrix proteins31,32. Fibroblasts undergo transformation into myofibroblasts in response to pro-fibrotic mediators33. This study demonstrates that ECC-JHF inhibits the activation of fibroblasts and reduces mitophagy. Furthermore, ECC-JHF significantly enhanced the expression of Nrf2 and NQO1 proteins, which are critical components of cellular defense mechanisms. Notably, the upregulation of these proteins, along with PARK2 and PINK1, and the downregulation of FN and α-SMA, could be reversed by the administration of Nrf2 siRNA. These findings indicate that the Nrf2 pathway is crucial in mediating the therapeutic effects of ECC-JHF. Additionally, the protective action of ECC-JHF against BLM-induced pulmonary fibrosis in rats diminished upon the administration of an Nrf2 inhibitor. This emphasizes the importance of the Nrf2 pathway in the therapeutic potential of ECC-JHF and its role in counteracting fibrotic processes.
This study found that ECC-JHF has a protective effect against PF in rats by inhibiting oxidative stress and enhancing mitochondrial phagocytosis.” This effect is associated with inhibiting fibroblast activation by activating the Nrf2 signaling pathway, which in turn promotes PINK1/ Park2-mediated mitochondrial phagocytosis. These results reveal that ECC-JHF significantly improves BLM-induced pulmonary fibrosis through multiple mechanisms, providing new ideas and evidence for the application of traditional Chinese medicine in the treatment of pulmonary fibrosis. It provides specific prospects for drug development and clinical trial design.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to express their gratitude to Henan Key Laboratory of Chinese Medicine for Respiratory Disease for providing equipment, technical expertise, and experimental support.
Abbreviations
- ARE
Antioxidant response element
- AECs
Alveolar epithelial cells
- BLM
Bleomycin
- COL3
Collagen III
- CRP
Citri reticulatae pericarpium
- ECC-JHF
Effective-component compatibility of Jinshui Huanxian formula
- EF
Epimedii folium
- FMD
Fibroblast-myofibroblast differentiation
- FTB
Fritillariae thunbergii bulbusGRRb
- GRRa
Ginseng radix et rhizome
- GRRb
Glycyrrhizae radix et rhizome
- GS
Ginkgo semen
- HO-1
Heme oxygenase 1
- HFL1
Human fetal lung fibroblast 1
- IPF
Idiopathic pulmonary fibrosis
- JHF
Jinshui huanxian formula
- MC
Moutan cortex
- mTOR
Mammalian target of rapamycin
- Nrf2
Ophiopogonis radix
- OR
Nuclear factor erythroid 2-related factor 2
- OMM
Outer mitochondrial membrane
- OS
Oxidative stress
- PF
Pulmonary fibrosis
- PFD
Pirfenidone
- PINK1
PTEN-induced kinase 1
- PDGFR
Platelet-derived growth factor receptor
- Park2
Parkinson disease protein 2
- ROS
Reactive oxygen species
- RRP
Rehmanniae radix praeparata
- RT-PCR
Real-time polymerase chain reaction
- siNrf2
Nrf2 siRNA
- T-SOD
Total superoxide dismutase
- TEM
Transmission electron microscopy
- TF
Trichosanthis fructus
- α-SMA
α-smooth muscle actin
Author contributions
B: Visualization, Project administration, Writing – review & editing. Y: Methodology, Validation, Writing – original draft. Both authors are designated as joint first authors. Z: Methodology, Writing – review & editing. S: Validation, Writing – review & editing. S: Methodology. W: Validation. Z: Visualization, Project administration, Writing – review. L: Visualization, Project administration, Writing – review. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work, ensuring integrity and accuracy.
Funding
This study received funding from National Natural Science Fund of China (grant number U23A20503, 81904170), and Chinese inheritance and innovation team of Chinese medicine (ZYYCXTD-C-202206).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Sprague-Dawley rats (license number: SCXK (Beijing) 2016-0006) were supplied by Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). All experimental procedures received approval from the First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China (YFYDW2017013).
Consent for publication
In this study, all participants provided written informed consent for their data to be used in publications.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yunping Bai and Xiaohong Yin contributed equally to this work.
References
- 1.Raghu, G. et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. S Respir Crit. Care Med.205 (9), e18–e47 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sgalla, G. et al. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res.19 (1), 32 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossmann, M. P. et al. Mitochondrial function in development and disease. Dis. Model. Mech.14(6). (2021). [DOI] [PMC free article] [PubMed]
- 4.Chianese, M. et al. Pirfenidone and nintedanib in pulmonary fibrosis: lights and shadows. Pharmaceuticals (Basel). 17 (6), 709 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu, X. et al. HER2 drives lung fibrosis by activating a metastatic cancer signature in invasive lung fibroblasts. J. Exp. Med.219 (10), e20220126 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaeger, B. et al. Airway basal cells show a dedifferentiated KRT17highPhenotype and promote fibrosis in idiopathic pulmonary fibrosis. Nat. Commun.13 (1), 5637 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geng, Y. et al. Author correction: PEAR1 regulates expansion of activated fibroblasts and deposition of extracellular matrix in pulmonary fibrosis. Nat. Commun.13 (1), 7749 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao, W. et al. Endothelial cell-derived MMP19 promotes pulmonary fibrosis by inducing E(nd)MT and monocyte infiltration. Cell. Commun. Signal.21 (1), 56 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narendra, D. P. & Youle, R. J. The role of PINK1-Parkin in mitochondrial quality control. Nat. Cell. Biol.26 (10), 1639–1651 (2024). [DOI] [PubMed] [Google Scholar]
- 10.ArLin, Q. et al. ACSL1 improves pulmonary fibrosis by reducing mitochondrial damage and activating PINK1/Parkin mediated mitophagy. Sci. Rep.14 (1), 26504 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi, K. et al. Involvement of PARK2-Mediated mitophagy in idiopathic pulmonary fibrosis pathogenesis. J. Immunol.197 (2), 504–516 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Baird, L. & Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol.40 (13), e00099–e00020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otoupalova, E. et al. Oxidative stress in pulmonary fibrosis. Compr. Physiol.10 (2), 509–547 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Shaw, P. & Chattopadhyay, A. Nrf2-ARE signaling in cellular protection: mechanism of action and the regulatory mechanisms. J. Cell. Physiol.235 (4), 3119–3130 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Bueno, M. et al. ATF3 represses PINK1 gene transcription in lung epithelial cells to control mitochondrial homeostasis. Aging Cell.17 (2), e12720 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma, N., Wei, Z., Hu, J., Gu, W. & Ci, X. Farrerol ameliorated Cisplatin-Induced chronic kidney disease through mitophagy induction via Nrf2/PINK1 pathway. Front. Pharmacol.12, 768700 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szapiel, S. V., Elson, N. A., Fulmer, J. D., Hunninghake, G. W. & Crystal, R. G. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am. Rev. Respir Dis.120 (4), 893–899 (1979). [DOI] [PubMed] [Google Scholar]
- 18.Ashcroft, T., Simpson, J. M. & Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol.41 (4), 467–470 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathai, S. K. & Schwartz, D. A. Translational research in pulmonary fibrosis. Transl Res.209, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao, N., Li, K., Liu, J., Fan, G. & Sun, T. Liproxstatin-1 alleviates bleomycin-induced alveolar epithelial cells injury and mice pulmonary fibrosis via attenuating inflammation, reshaping redox equilibrium, and suppressing ROS/p53/α-SMA pathway. Biochem. Biophys. Res. Commun.551, 133–139 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Liu, W., Han, X., Li, Q., Sun, L. & Wang, J. Iguratimod ameliorates bleomycin-induced pulmonary fibrosis by inhibiting the EMT process and NLRP3 inflammasome activation. Biomed. Pharmacother. 153, 113460 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Liu, Q., Gao, Y. & Ci, X. Role of Nrf2 and Its activators in respiratory diseases. Oxid. Med. Cell Longev 2019 7090534. (2019). [DOI] [PMC free article] [PubMed]
- 23.Kasai, S., Shimizu, S., Tatara, Y., Mimura, J. & Itoh, K. Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules10 (2), 320 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han, Y. Y. et al. Protective effect of dimethyl Itaconate against fibroblast-myofibroblast differentiation during pulmonary fibrosis by inhibiting TXNIP. J. Cell. Physiol.236 (11), 7734–7744 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Chen, S. et al. New insights into the role of mitochondrial dynamics in oxidative stress-induced diseases. Biomed. Pharmacother. 178, 117084 (2024). [DOI] [PubMed] [Google Scholar]
- 26.Zhong, Y. et al. The interplay between mitophagy and mitochondrial ROS in acute lung injury. Mitochondrion78, 101920 (2024). [DOI] [PubMed] [Google Scholar]
- 27.Lu, Y. et al. Cellular mitophagy: mechanism, roles in diseases and small molecule Pharmacological regulation. Theranostics13 (2), 736–766 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bueno, M., Calyeca, J., Rojas, M. & Mora, A. L. Mitochondria dysfunction and metabolic reprogramming as drivers of idiopathic pulmonary fibrosis. Redox Biol.33, 101509 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sosulski, M. L. et al. Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFβ1. Aging Cell.14 (5), 774–783 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurita, Y. et al. Pirfenidone inhibits myofibroblast differentiation and lung fibrosis development during insufficient mitophagy. Respir Res.18 (1), 114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang, D., Dey, T. & Liu, G. Recent developments in the pathobiology of lung myofibroblasts. Expert Rev. Respir Med.15 (2), 239–247 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zabihi, M. et al. Understanding myofibroblast origin in the fibrotic lung. Chin. Med. J. Pulm Crit. Care Med.2 (3), 142–150 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacho, D., Rabino, A., Garcia-Mata, R. & Yildirim-Ayan, E. Mechanoresponsive regulation of fibroblast-to-myofibroblast transition in three-dimensional tissue analogues: mechanical strain amplitude dependency of fibrosis. Sci. Rep.12 (1), 16832 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.