Abstract
Drought stress at jointing and booting stages of plant development directly affects plant growth and productivity in rice. Jointing and booting stages may overlap in high-latitude areas where water deficits occur. However, little is known about the effects of photosynthesis on grain sucrose metabolism and the differences of sucrose metabolism strategies between superior and inferior grains under different drought stress was unclear. In this study, rice plants were subjected to drought stress for 15 days at jointing-booting. Drought stress affected normal leaf growth, and decreased the leaf area index linearly. Short-term mild drought stress had positive effects on photosynthesis, but long-term drought stress reduced the transpiration rate, stomatal conductance, and intercellular CO2 concentration. Stomatal conductance increased with drought stress duration but increased intercellular CO2 concentration did not prevent decrease in net photosynthetic rate. Vacuolar invertase activity was important for panicle development (where its activity differed between superior and inferior grains), but not for rice grain filling. Vacuolar invertase activity of drought-sensitive rice varieties superior grains increased by 111.24%~118.46% under drought stress. Drought stress reduced sucrose-phosphate synthase activities in superior and inferior grains. SuSase activity of inferior grains affected sucrose content significantly.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-85598-8.
Keywords: Jointing-booting stages, Gradient drought, Superior and inferior grain, Photosynthesis, Sucrose metabolism
Subject terms: Plant physiology, Drought
Introduction
Worldwide, drought has become the main cause of reductions in crop yields. For example, China suffered an agricultural drought in 2016, and the affected area totaled 9872.7 thousand hectares according to the National Bureau of Statistics China (NBSC)1. Given that a decrease in crop production caused by drought has the potential to cause significant economic disruption, demand for the development of drought-tolerant crops is increasing2. Locally adapted traits of drought stress tolerance are required to achieve maximal crop yield potential3.
In 2020, the area cultivated for rice (Oryza sativa L.) totaled 30080 thousand hectares in China, producing a yield of 211.9 million tons4. Three to five thousand liters of water are required for the production of 1 kg of rice seed, while other crops, such as maize or wheat require less than half of that5. Therefore, understanding the range of physiological changes initiated by drought stress is important for developing supportive measures to enhance drought resistance.
Under drought stress, photosynthesis is limited and leaf area decreases6,7. As a response of rice to drought stress, leaf rolling is one of the causes of leaf area reduction under drought stress, which help to maintain the water balance inside the cells8–10. Drought stress causes the numbers of leaves per plant to decrease which also decreases the leaf area. This phenomenon may occur following the rapid decline in cell division and leaf elongation under drought conditions11.
It is generally believed that maintaining a high chlorophyll content for a long time after heading is one way to increase rice yield. However, there is some evidence that higher chlorophyll content in leaves does not significantly improve photosynthetic rate, and lower chlorophyll content does not affect photosynthetic electron transfer12. However, the chlorophyll content of okra (Abelmoschus esculentus L.), increased significantly under mild drought stress because mild drought stress promoted chlorophyll synthesis, although severe drought did not13. Other studies suggest that the effect of drought stress on chlorophyll is related to the duration of drought. For example, under short-term drought stress, the chlorophyll content of rice leaves was not significantly affected by mild or severe drought stress, and even increased, whereas under long-term drought stress, the chlorophyll content of rice leaves decreased under both mild and severe drought stress14.
Under drought stress, plants tend to close their stomata and reduce the flow of CO2 into the leaves. Consequently, more electrons form reactive oxygen species15,16. Many studies have shown that drought stress can significantly reduce the net photosynthetic rate in rice17–19. The limitations of photosynthesis are caused by the low concentration of CO2 caused by early stomatal closure, decreased photosynthase activity, biochemical reactions related to phosphotriose formation, and decreased photochemical efficiency of PSII. Stomatal conductance (gs) and mesophyll conductance (gm) are also significantly reduced under drought stress20,21, resulting in a decrease in CO2 transport capacity. The limited photosynthetic carbon assimilation capacity and high intensity of mitochondrial respiration eventually lead to a reduction in yield.
Both stomatal conductance and photosynthetic rate decrease in the early stage of drought stress, but both increase significantly after prolonged or continuous drought stress22. However, the decrease in photosynthetic rate is caused not only by stomatal conductance but also by non-stomatal limiting factors. In early or mild drought stress the stomatal openings decrease or the stomata even close. With increasing levels of drought stress, the concentration of intercellular CO2 stops decreasing and even increases, indicating that the inhibition of photosynthesis is not caused by stomatal conductance, but by other causes23,24. Because RuBisCO is one of the key enzymes in photosynthesis, decrease in RuBisCO activity is considered one of the non-stomatal limiting factors25,26. The synthesis of RuBisCO is limited under drought stress27, and there is an S-shaped relationship between photosynthetic rate and RuBisCO activity in leaves28.
The sucrose concentration in the leaves of plants under drought stress can be increased by regulating Sucrose synthase (SuSase) and sucrose-phosphate synthase (SPS) activities29,30. Research suggests that Sucrose synthase prefers the sucrose cracking reaction31,32. Under drought stress, SPS activity in corn (Zea mays), potato (Solanum tuberosum), soybean (Glycine max), and some other crops decreased or remained unchanged33. However, some data showed that SPS activity increased in rice and wheat leaves under drought stress34–36. Yang et al. also showed that SuSase and SPS activities increased significantly under dehydration and osmotic stress37–39. Invertase (Inv) can irreversibly catalyze the hydrolysis of sucrose into two hexose molecules, thus doubling the osmotic pressure40,41. Soluble acid invertase, also known as vacuolar invertase (VIN), regulates sugar accumulation and sucrose utilization in plant cell vacuoles. The activity of sucrose invertase is associated with cell division, tissue growth, and development. High invertase activity is beneficial for carbon and energy metabolism32. The accumulation of sucrose in cells is mainly determined by sucrose synthase, sucrose phosphate synthase, and sucrose acid invertase42,43.
In higher latitudes, rice experiences a shorter growing season due to the limited frost-free period. Post-flowering, temperatures decline, leaving a constrained window for grain filling. Consequently, rice in these regions is characterized by a high degree of overlap in growth phases, with the jointing and booting stages occurring concurrently. Following flowering, the period for grain filling is brief, yet the intensity of grain filling is elevated. This condition accentuates the disparity between robust and less vigorous grains. Most prior research has focused on rice varieties that have a prolonged grain filling duration. Addressing the unique agricultural challenges in high latitude areas, this study comprehensively examines the impact of drought stress on photosynthesis and sucrose metabolism during the water-sensitive stages of rice with overlapping growth periods. It further explores the metabolic strategies of different grain types under drought conditions, using the sucrose metabolism of superior and inferior grains as focal points. In this study, we selected two typical rice varieties from cold regions with overlapping growth stages. We set three different degrees of drought stress treatments at the jointing-booting stage: mild, moderate, and severe drought stress, to explore how changes in chlorophyll content, stomatal conductance, and transpiration rate affected the photosynthetic rate and sucrose metabolism of superior and inferior grains under different water deficit conditions.
Results
Effects of drought stress on leaf photosynthesis at jointing- booting stage
Leaf area index
Compared with the control, the leaf area index (LAI) of the two cultivars did not significantly decrease after five days of mild drought stress treatment (Fig. 1). The leaf area indexes under mild and moderate drought stress reached their maximum values at D15; the LAI of SJ6 under mild and moderate drought stress respectively decreased by 5.70% and 11.94% compared with the control, and those of DN425 by 10.04% and 19.96%. Severe drought stress significantly reduced the LAI. The LAI of SJ6 and DN425 under severe drought stress respectively decreased by 9.07% and 10.17% at D5, and by 17.00% and 23.03% at D15.
Fig. 1.
Effect of drought stress at booting stage on leaf area index of two rice varieties with overlapping growth stages. A0 and B0, control treatments (0 kPa) of SJ6 and DN425; A1 and B1, mild drought stress treatments (-10 kPa); A2 and B2, moderate drought stress treatments (-25 kPa); A3 and B3, severe drought stress (-40 kPa). Vertical bars represent standard deviation. Values for the same day and the same varieties followed by different letters are significantly different at p = 0.05. Days after drought stress were recorded as Dx days, and days after restoration of irrigation were recorded as Rx days (similarly hereinafter).
The LAI of plants under the mild drought stress treatment recovered to the control level at R10. The rate of decline in LAI of SJ6 plants under moderate drought stress was significantly greater than those in other treatments. In contrast, the LAI of DN425 plants under moderate drought stress was significantly lower than that under severe drought stress. Interestingly, under severe drought stress the LAI of both cultivars continued to increase after resuming irrigation and reached maximum values at R10 when the full heading stage had already passed, and grain filling had proceeded for several days.
Leaf SPAD value
The leaf SPAD value is an important index of leaf chlorophyll content. There was no significant difference in leaf SPAD values between plants under mild and moderate drought stress compared with controls (Fig. 2). The leaf SPAD values at D15 under severe drought stress were significantly lower than that of the control; leaf SPAD values for SJ6 and DN425 decreased by 8.90% and 8.14%, respectively.
Fig. 2.
Effect of drought stress at booting stage on leaf SPAD values of two rice varieties with overlapping growth stages.
After the restoration of irrigation, the moderate drought stress treatment resulted in a greater rate of decline in leaf SPAD values, and the leaf SPAD value at R30 was significantly lower than those of the other treatments. Under moderate drought stress, leaf SPAD values of SJ6 and DN425 respectively decreased by 22.47% and 19.52% from R10 to R30. After the restoration of irrigation to plants under severe drought stress, the leaf SPAD value initially increased, during D15 to R10, and then decreased again. The leaf SPAD value at R20 was significantly lower than that in the control.
Stomatal factors affecting photosynthesis
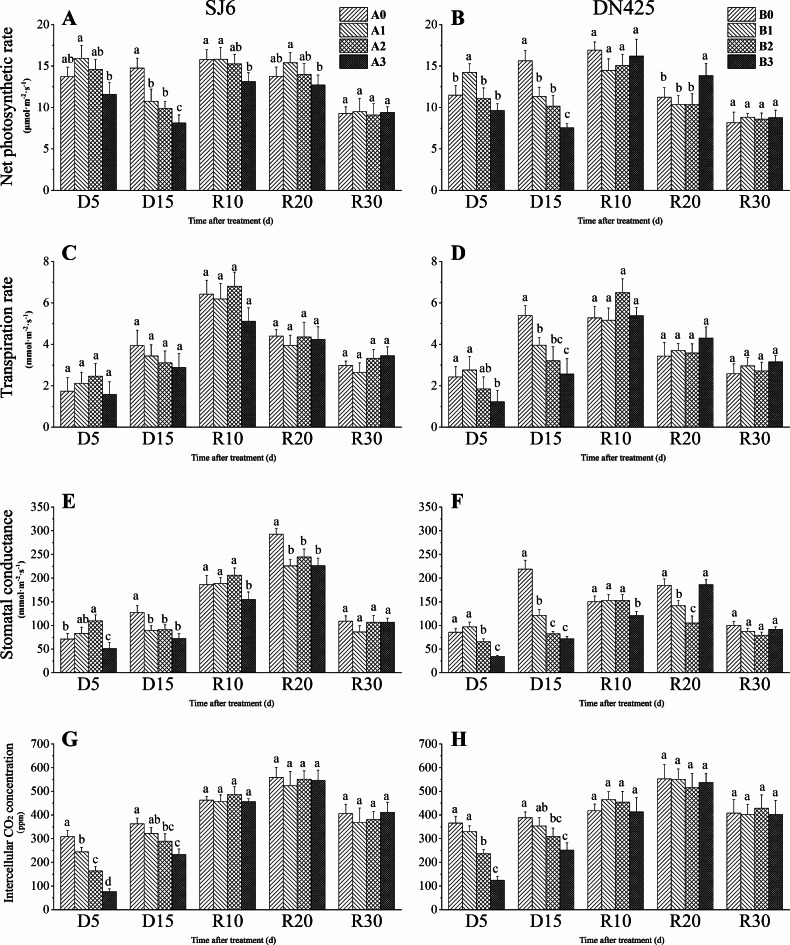
The net photosynthetic rate of the two cultivars decreased significantly in D15 days under drought stress (Fig. 3A, B). Compared with that of the control, the net photosynthetic rate of the two cultivars decreased by 27.24% and 27.51% under mild drought stress, 33.06% and 34.97% under moderate drought stress, and 44.89% and 51.70% under severe drought stress, respectively. After the restoration of irrigation, DN425 (Fig. 3B) showed a stronger recovery ability than that of SJ6 (Fig. 3A) under severe drought stress. Compared with D15, the net photosynthetic rate of the two cultivars under severe drought stress increased by 61.24% and 114.66% in R10 days, respectively. The net photosynthetic rate of DN425 under severe drought stress at R20 was significantly higher than that of the control (net photosynthetic rate data have been published separately)44.
Fig. 3.
Effect of drought stress at booting stage on photosynthetic parameters of two rice varieties with overlapping growth stages. (A, B) Net photosynthetic rate, (C, D) Transpiration rate, (E, F) Stomatal conductance, (G, H) Intercellular CO2 concentration.
The transpiration rate of DN425 under moderate drought was not significantly different from that of the control at D5, but showed a downward trend (Fig. 3D). The transpiration rate of DN425 under severe drought was significantly lower than that of the control at D5. The transpiration rate of DN425 plants under all drought stress treatments was significantly lower than that of the control at D15. After irrigation resumed, the transpiration rate of each treatment continued to increase and reached a maximum value at R5, with no significant differences among treatments. However, the transpiration rate of SJ6 under severe drought treatment was lower than that under other treatments at R5 (Fig. 3C).
The stomatal conductance of SJ6 under moderate drought conditions was significantly higher than that of the control, whereas that of DN425 was significantly lower than that of the control (Fig. 3E, F). Under severe drought stress, stomatal conductances of the two cultivars were significantly lower than that of the control at D15. The stomatal conductance of the two cultivars under severe drought stress increased significantly at D15 compared with D5, increasing by 42.05% and 108.15% in SJ6 and DN425, respectively. After the restoration of irrigation, the stomatal conductance of SJ6 under severe drought treatment was lower than that of the control treatment before R20. The stomatal conductance of DN425 under mild and moderate drought stress recovered to the control level at R10 but were significantly lower than that of the control at R20. Stomatal conductance of both varieties under severe drought stress reached its maximum value at R20.
Drought stress significantly reduced intercellular CO2 concentrations, and the more severe the drought stress, the greater the reduction (Fig. 3G, H). The intercellular CO2 concentration of SJ6 under moderate and severe drought conditions decreased by 46.89% and 75.43%, respectively, compared to the control. In DN425 plants under moderate and severe drought treatments, intercellular CO2 concentration decreased by 35.46% and 66.07% respectively at D5, and decreased by 20.47% and 35.28% respectively at D15. After irrigation resumed, the intercellular CO2 concentration of each treatment continued to increase and recovered to the control level by day R10.
Response of sucrose metabolism in rice grain to drought stress
Sucrose content in panicle
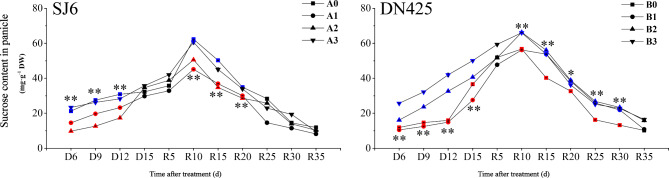
Panicles of SJ6 plants under mild and moderate drought treatments had significantly lower sucrose content than the control during D6-D12, whereas there was no significant difference between panicles under the severe drought treatment and those of the control (Fig. 4). There was no significant difference in the sucrose content of panicles between the DN425 mild drought treatment and the control at the early stage of drought stress, and the sucrose content at D15 was significantly lower than that of the control. During the drought stress period, the sucrose contents of the panicles in the moderate and severe drought treatments were significantly higher than in those in the control. After resuming irrigation, the sucrose content of each treatment continued to increase, reaching the maximum value at R10 and then gradually decreasing. The maximum values of sucrose content in the panicles of SJ6 under mild and moderate drought stress were significantly lower than that of the control (27.61% and 18.94% lower, respectively). Compared to the control, there was no significant difference in the sucrose content of the panicles under severe drought conditions after resuming irrigation. The sucrose contents of panicles of SJ6 under mild and moderate drought treatments at R10-R20 were significantly lower than those of the control. The maximum values of sucrose content in the panicles of DN425 under moderate and severe drought were significantly higher than that of the control (16.65% and 16.41% higher, respectively), whereas there was no significant difference between sucrose contents of DN425 under mild drought and control. The sucrose content in the panicles of DN425 plants under drought stress before R30 was significantly higher than that in the control.
Fig. 4.
Effect of drought stress at booting stage on sucrose content in panicles of two rice varieties. *, ** represent significance at p < 0.05 and p < 0.01, respectively. Vertical bars represent standard deviation. Values for the same time and the same variety marked blue is significantly higher than the value marked red (similarly hereinafter).
Sucrose synthase
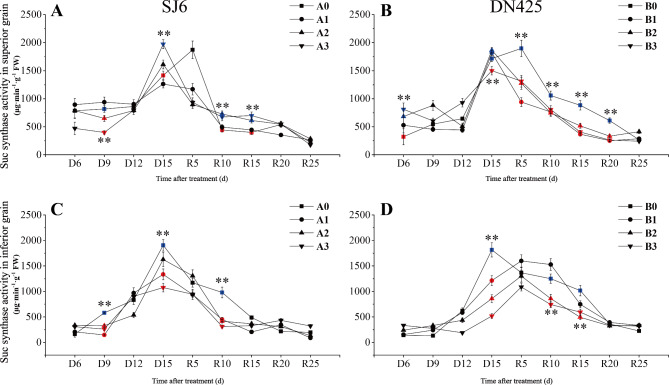
The SuSase activity of superior grains under drought stress reached a maximum at D15, earlier than that of the control (Fig. 5A). The SuSase activities of SJ6 under moderate and severe drought treatments were significantly lower than that of the control at D9. The SuSase activity of superior grains at D15 under the severe drought treatment was 39.43% higher than that in the control. The activities of SuSase in DN425 superior grains under moderate and severe drought stress at D6 were significantly higher than that in the control (Fig. 5B). However, the maximum value of SuSase activity in DN425 under the severe drought treatment was 12.61% lower than that in the control at D15, while there were no significant differences between plants under mild and moderate drought stress and the control. After irrigation resumed, the SuSase activity of superior grains of SJ6 under moderate and severe drought stress was significantly higher at R10-R15 than that of the control, whereas there was no significant difference between plants under mild drought and control treatments. In contrast to SJ6, the SuSase activity of DN425 superior grain was significantly lower than that of the control at R5, but there were no significant differences among drought stress treatments.
Fig. 5.
Effect of drought stress at booting stage on sucrose synthetase activity in superior (A, B) and inferior (C, D) grain of two rice varieties.
The SuSase activity of SJ6 inferior grains under drought stress was significantly lower than that of the control at D9; the SuSase activity then increased rapidly and reached the maximum value at D15 (Fig. 5C). Under mild and severe drought, the SuSase activity of inferior grains decreased by 29.91% and 43.52%, respectively, compared with the control. The SuSase activities of DN425 inferior grains were 33.17%, 52.75%, and 71.45% lower than that of the control, respectively, with increasing drought intensity (Fig. 5D). After the restoration of irrigation, the SuSase activity of SJ6 inferior grains under drought stress showed an increased rate of decline. The SuSase activities of plants under all drought stress treatments at R10 were significantly lower than that of the control. The maximum activity of SuSase in DN425 under drought stress decreased by 12.00%, 28.33%, and 40.13% compared with the control respectively with increasing drought intensity. The activity of SuSase decreased faster in DN425 plants under moderate and severe drought stress at R5-R15.
Sucrose phosphate synthase
Drought stress significantly reduced SPS activity in the superior grains (Fig. 6A, B). The SPS activity of the superior grains of SJ6 was significantly lower than that of the control at D9, with no significant difference among treatments. Compared with the control, SPS activity at D15 decreased by 46.05%, 53.12%, and 47.66%, with increasing drought intensity. Similar to SJ6, the SPS activity of DN425 superior grains was significantly lower than that of the control at D9, and the SPS activity of DN425 superior grains at D15 decreased by 76.91%, 66.49%, and 57.52% with increasing drought intensity. After the resumption of irrigation, SPS activity under the severe drought treatment reached its maximum value at R5. The SPS activity of SJ6 under drought stress treatments at R5 was still significantly lower than that of the control. The SPS activity of DN425 superior grains under drought stress reached a maximum value at R5, and all treatments were significantly lower than the control level. The SPS activity of plants under the drought stress treatments at R20 remained significantly lower than that of the control.
Fig. 6.
Effect of drought stress at booting stage on the activity of sucrose phosphate synthetase in superior (A, B) and inferior (C, D) grain of two rice varieties.
The SPS activity of inferior grains in the SJ6 treatment decreased from D6 to D12 (Fig. 6C). The decrease in SPS activities in plants under the drought stress treatments were significantly greater than that in the control treatment. With the increasing drought intensity, SPS activity decreased by 73.21%, 72.49%, and 86.16% from D6 to D12. The SPS activity of inferior grains at D15 decreased by 29.42%, 73.68%, and 81.31% with increasing drought intensity compared with the control. With increasing drought intensity, the SPS activity of DN425 decreased by 36.71%, 73.66%, and 59.89% at D12 compared with D6 (Fig. 6D). The SPS activity in plants under the drought stress treatment at D15 was significantly lower than that in the control (48.76%, 30.82% and 35.09%, respectively). After the resumption of irrigation, the SPS activity of SJ6 inferior grains reached the maximum value at R5. With increasing drought intensity, the maximum activities of SPS decreased by 34.00%, 14.22%, and 18.16% compared to the control. The SPS activities of the inferior grains under drought stress at R25 were significantly higher than that of the control. The maximum SPS activity in DN425 under mild drought conditions was significantly lower than that of the control; however, the severe drought treatment resulted in a lesser rate of decrease in SPS activity.
Vacuolar invertase
The VIN activities in the superior grains of the two varieties was already high at D6 (Fig. 7A, B). The VIN activity of the superior grains of SJ6 under the drought stress treatments at D6 was significantly higher than those of the control. The maximum VIN activity was reached at D9. The maximum VIN activities of plants under the drought stress treatments were significantly higher than that of the control, (increased by 118.46%, 111.24%, and 115.03%, respectively). The VIN activity of plants under the moderate and severe drought treatments remained low after D15 days, while that of plants under the mild drought treatment remained low after R5 days. The VIN activity of DN425 reached the maximum value at D9, and there was no significant difference between mild and moderate drought stress treatments and the control, while VIN activity of plants under the severe drought stress treatment decreased 24.52% compared with the control. Notably, the maximum VIN activity of DN425 was higher than that of SJ6. The VIN activity of plants under the mild and moderate drought treatments remained low at D15, while that of plants under the severe drought treatment remained low at R5.
Fig. 7.
Effect of drought stress at booting stage on vacuolar invertase activity in superior (A, B) and inferior (C, D) grains of two rice varieties.
Similar to the superior grains, the VIN activity of the inferior grains was already at a high level at D6 in both varieties (Fig. 7C, D). The VIN activities of SJ6 at D6 under moderate and severe drought conditions were significantly higher than that of the control. The VIN activity of the SJ6 control treatment reached the maximum value at D9, while that of the drought stress treatment reached the maximum value at D12. The VIN activity of the drought stress treatment was significantly higher than that of the control from D12 to D15. The VIN activity of DN425 was significantly higher than that of the control at D6, and there was no significant difference in VIN activity at D15.
After the resumption of irrigation, the VIN activity of inferior grains of SJ6 continued to decrease and remained at a low level after R5 days, with no significant difference among treatments. The VIN activity in the DN425 treatment remained low after R15 days.
AGPase
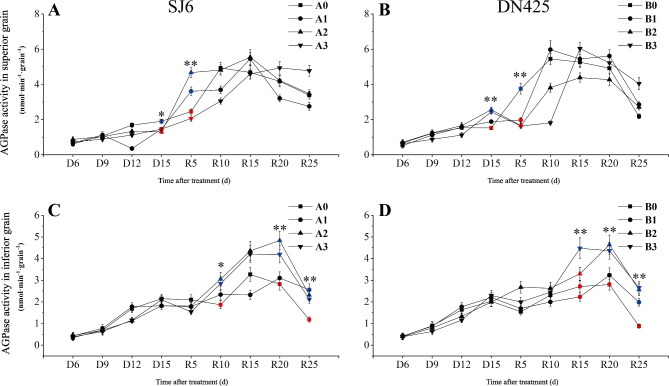
The ultimate goal of sucrose degradation in grains is to provide raw materials for starch synthesis; therefore, we measured the activity of AGPase, a key enzyme in starch synthesis. The results showed that the AGPase activity of superior grains of SJ6 under drought stress was significantly lower than that of the control at D15, with no significant difference between treatments (Fig. 8A). The AGPase activity of DN425 at D15 under moderate and severe drought conditions was significantly higher than that of the control (Fig. 8B). After irrigation resumed, AGPase activity increased faster in SJ6 under mild and moderate drought stress; it was significantly higher than that in the control at R5, and reached the maximum value at R15. There was no significant difference in the maximum value of AGPase activity between the drought stress treatments and the control. The AGPase activity of DN425 under mild and moderate drought conditions reached maximum values at R10 and R15, respectively. AGPase activity of plants under the severe drought treatment reached their maximum value at R15.
Fig. 8.
Effect of drought stress at booting stage on AGPase activity in superior (A, B) and inferior (C, D) grain of two rice varieties.
After resuming irrigation, the AGPase activities in plants under the moderate and severe drought treatments were significantly higher than that of the control at R10 and reached a maximum at R20 (Fig. 8C, D). The maximum AGPase activities of plants under the moderate and severe drought treatments were 47.87% and 28.34% respectively higher than that of the control. There was no significant difference in the maximum AGPase activity between the mild drought treatment and control groups. The AGPase activity of SJ6 under drought stress was significantly higher than that of the control at R25. The AGPase activity of DN425 under severe drought reached its maximum value at R15, and was significantly higher than those of other treatments in the same period. AGPase activities of plants under the mild and moderate treatments reached the maximum value at R20. The AGPase activity of DN425 under moderate and severe drought treatments were significantly higher than that of the control at R20 (increased by 65.63% and 59.93%, respectively). The AGPase activity of DN425 was significantly higher than that of the control at R25.
Discussion
Indicators related to photosynthesis
Photosynthesis is the energy source for all physiological activities of crops. Drought stress significantly reduces the photosynthetic capacity and photosynthetic products in rice22. The effect of drought stress on photosynthesis is mainly reflected in the decrease in leaf area, closure of stomata45,46 and inhibition of photosynthetic activity of mesophyll cells or chloroplasts47,48. In this study, the rate of increase of LAI under mild and moderate drought stress was significantly slower than that of the control during drought stress, and the maximum value of LAI decreased compared with that of the control. This indicated that drought stress significantly affected the normal growth of leaves. The LAI continued to increase after the restoration of irrigation following severe drought stress, indicating that leaf growth was greatly inhibited under severe drought stress and could not meet the requirements of photosynthetic production after the restoration of irrigation. Therefore, the leaves continued to grow to maintain the necessary photosynthesis. Related research also found that drought stress prolonged the nutritional growth period and the reproductive growth period was shortened49.
There is a close relationship between leaf SPAD value and chlorophyll content50. The leaf SPAD value is an important index of chlorophyll content. Drought stress during different periods reduced leaf SPAD values51. The leaf SPAD value of plants subjected to moderate drought stress remained stable or slightly increased in the short-term following the restoration of irrigation, but then showed a large decline. These results indicated that moderate drought stress caused premature leaf senescence, and this premature leaf senescence could not be alleviated by restoration of the water supply. Previous research found that severe drought stress at booting stage significantly reduced leaf chlorophyll content, but the chlorophyll content after restoration irrigation was not studied52. We found that severe drought stress significantly reduced the leaf SPAD value, which then increased after the restoration of irrigation. This may be because chlorophyll decomposition under severe drought stress could not meet the requirements of photosynthetic production after the restoration of irrigation, so a large amount of chlorophyll synthesis was needed to maintain the necessary photosynthesis. Unlike the leaf SPAD values of plants subjected to moderate drought stress, the leaf SPAD value of plants subjected to severe drought stress did not show a relatively high decay rate after a short rise, which may be due to the lack of photosynthate reserves caused by a large amount of chlorophyll degradation during the stress stage, and a large amount of photosynthate needed to complete grain filling after the restoration of irrigation. A subsequent study provided evidence in indirect support of this point of view by significantly prolonging the grain-filling active period under severe drought conditions53.
In the early stages of drought stress, stomatal closure of leaves had a positive effect on reducing transpiration and excessive water loss54,55. However, stomatal closure can also lead to intercellular CO2 insufficiency, so the capacity for photosynthetic carbon sequestration decreased21. In this study, mild drought stress increased stomatal conductance at the early stage of drought stress (D5), and then increased the transpiration rate, thus increasing the net photosynthetic rate, which is inconsistent with previous research results21,54,55. This may be due to an increase in the chlorophyll content in leaves under mild drought stress. Stomatal conductance increased over time, which directly led to the corresponding change in transpiration rate during drought stress. However, our previous study found that the net photosynthetic rate did not increase accordingly44. The variation in the net photosynthetic rate during drought stress was the same as that of the leaf SPAD values. Therefore, we believe that the change in net photosynthetic rate under drought stress cannot be solely attributed to the change in stomatal conductance, transpiration rate, or chlorophyll content but is the result of a combination of many factors; similar conclusions have been reached in related studies25,56. Under drought stress, the change in stomatal conductance caused a difference in the net photosynthetic rate among different treatments, but with the worsening of drought stress, LAI and chlorophyll content decreased significantly, and the net photosynthetic rate was restricted by both photosynthetic reaction sites and regulatory mechanisms. This also explains why the net photosynthetic rate decreased significantly, rather than increased, with an increase in stomatal conductance during drought stress. Path analysis further showed that leaf SPAD value and stomatal conductance were the main factors affecting the net photosynthetic rate of leaves, and LAI mainly affected the net photosynthetic rate by affecting the leaf SPAD value (Table S1). After irrigation resumed, the photosynthetic parameters under mild and moderate drought stress were restored to the control level at R5. However, the stomatal conductance and transpiration rate of SJ6 under severe drought stress did not recover to the control level at R5, and the leaf SPAD value was still lower than that of the control, although the difference was not significant. This may be the main reason why the net photosynthetic rate failed to recover to the control level at R5 after the restoration of irrigation.
We noted that stomatal conductance and the transpiration rate increased during drought stress. Considering also the results of our earlier study on the RuBisCO activity, we found that the activity of RuBisCO increased rapidly during the early stage of drought stress and reached its first peak at D9 (Figure S3)44. Therefore, we believe that after the onset of drought stress, leaf water loss and chloroplast degradation decreased, leading to reduced CO2 transport capacity and photosynthetic rate, activation of a large amount of RuBisCO, and increased stomatal conductance to achieve increased transpiration rate and CO2 concentration. These measures compensated for the decreased capacity for photosynthetic carbon sequestration caused by degradation of chloroplasts. However, with further water loss caused by drought stress, the mesophyll conductance decreased, and the CO2 transport capacity was significantly reduced, which greatly reduced the carboxylation capacity of RuBisCO. This also explains why stomatal conductance, transpiration rate, and chlorophyll content under mild and moderate drought stress were not significantly different from those of the control at D15, but the net photosynthetic rate was significantly decreased, which was consistent with Ding’s research21. Path analysis further showed that stomatal conductance affected the net photosynthetic rate by affecting RuBisCO activity under drought stress, and the direct effect of RuBisCO activity on the net photosynthetic rate increased under severe drought stress (Table S1), which confirmed the above deduction.
Sucrose metabolism
None of the plants under drought stress treatment reached the heading stage at D6, and the panicles were still at the end of development. At this time, the VIN activities of the superior and inferior grains were higher and they continued to increase in the following days. Therefore, it is reasonable to believe that VIN is involved in the formation of young panicles, as is consistent with other studies that have reported that invertase is involved in cell division and tissue development57. The VIN activity of superior grains of DN425 was significantly higher than that of SJ6, which is of positive significance for the development of DN425 panicle under drought stress. The VIN activity decreased significantly in all treatments at the later stages of drought stress and did not increase thereafter, indicating that VIN is not a key enzyme in the grain-filling process. The VIN activity decreased rapidly after the SuSase activity began to increase, which is consistent with this deduction. SuSase and SPS co-regulate the sucrose content in panicles, and SPS plays a key role in sucrose resynthesis in panicles58. After a period of drought stress, SPS activities in the superior grains of both cultivars were significantly lower than that of the control. The SPS activity of the superior grains of SJ6 was not significantly different from that of the control at R15, and that of DN425 was always significantly lower than that of the control, indicating that the sucrose resynthesis ability in the DN425 panicles was lower than that in the control. The SPS activity in the inferior grains of the two varieties decreased gradually before D12, while the VIN activity in the inferior grains was at a high level at this time. The results indicated that at his time, the inferior grain was in the late stage of development, and that a large amount of sucrose was used for decomposition to complete the construction of the “sink”, which did not require a high sucrose resynthesis ability. The SPS activity increased again in the inferior grains, indicating the beginning of grain filling. After grain filling, the SPS activity in the inferior grains of DN425 did not differ significantly from that of the control, nor did the SPS activity in SJ6 differ significantly from that in the control except that the maximum value was lower. These results indicated that there was no significant difference in the sucrose resynthesis ability between the inferior grains and the control after resumed irrigation. Considering the SuSase activity of the inferior grains, we believed that the sucrose synthesis ability of the inferior grains of SJ6 did not differ from that of the control during the critical grain-filling period after the resumption of irrigation, but the decomposition ability decreased. There was no significant difference in the sucrose synthesis ability of inferior grain between DN425 under drought stress and the control, and the sucrose decomposition ability decreased under moderate and severe drought stress. Path analysis was used to explore the internal relationship between sucrose metabolism-related enzymes and sucrose content in the superior and inferior grains (Table S2). The SuSase activity, especially inferior grain SuSase activity which directly influences the sucrose content of grains, was the main factor determining grain sucrose content. The other traits affected the sucrose content of grains indirectly through SuSase activity. The SuSase activity in the DN425 superior and inferior grains were always lower than that of the control, which also explains why the sucrose contents of DN425 grains under drought stress were higher than that of the control. The SuSase activity of SJ6 inferior grains was significantly lower than that of the control, and the SuSase activity of superior grains was significantly higher than that of the control by ten days after the resumption of irrigation. Therefore, the sucrose content of grains under drought stress was lower than that in the control at R10. Our earlier study found that there was a complex interaction between SuSase, SPS, and VIN, and that the three enzymes worked together to determine sucrose content. The mechanisms of internal interactions among these three enzymes require further study44.
The effects of drought stress on rice grain filling may be completely opposite during different growth periods. Some studies have shown that the activities of SuSase, soluble starch synthase and starch branching enzyme in grains were significantly increased at the grain-filling stage during moderate drought which promoted starch synthesis37,38; however, there are opposing views59,60. Studies on drought stress during the rice booting stage have shown that it has a great impact on rice yield61–63. In the present study, the AGPase activity of the superior and inferior grains increased rapidly after grain filling (after resuming irrigation). The AGPase activity of superior grains of SJ6 under severe drought stress was significantly lower than that of the control before reaching the maximum value. The AGPase activity of the superior grains of DN425 under drought stress was higher than that of the control, and AGPase activity under moderate drought stress was significantly lower than that of the control. The maximum AGPase activities of inferior grains under moderate and severe drought stress were significantly higher than that of the control. The above results indicate that under moderate and severe drought stress, the ability of inferior grains to synthesize starch was significantly improved to compensate for the yield loss caused by the decrease in ability of superior grains under drought stress to synthesize starch. The study by Xu reached a similar conclusion64.
Materials and methods
Plant material and growth conditions
The research was conducted in the rainproof shelter of the A’Cheng experimental site of the Northeast Agricultural University in Harbin City, China (126°40E, 45°10 N) during the rice-growing season in 2016. The frost-free period is short at high latitudes and the suitable season for rice growth is only from May to October. The average daily temperature during the growing season at the experimental site is shown in Figure S2. Two typical japonica rice varieties, Oryza sativa subsp. Japonica with overlapping growth periods, Songjing 6 (SJ6, grain number type, 135 days growth period and 2500 °C effective accumulated temperature), and Dongnong 425 (DN425, grain weight type, 140 days growth period and 2550 °C effective accumulated temperature) were used as experimental materials. In mid-April, rice seeds were sown in a seedbed and transplanted in mid-May with a hill spacing of 30 × 10 cm, with three plants per hill in the field experiment. The standard fertilization was composed of nitrogen (150 kg per ha as urea), phosphorus (100 kg per ha as diammonium phosphate), and potassium (75 kg per ha as potassium sulfate). Urea was also used (100 kg per ha) as a top dressing at the mid-tillering stage.
Experimental design
Drought stress with different soil-water levels at the booting stage was set as a whole-plot factor with three levels: mild drought stress (-10 kPa), moderate (-25 kPa), and severe drought stress (-40 kPa); irrigation at 3 cm depth was used as a control (0 kPa). Two rice cultivars SJ6 (Songjing 6) and DN425 (Dongnong 425) were grown in soil with different soil-water potential. The soil-water potentials of SJ6 are denoted as A0 (0 kPa), A1 (-10 kPa), A2 (-25 kPa), and A3 (-40 kPa), and those of DN425 are denoted as B0 (0 kPa), B1 (-10 kPa), B2 (-25 kPa), and B3 (-40 kPa). The booting stage was determined by manually dissecting and visually observing the stem. The subplot factor was the rice variety, and the area of each subplot was 35 m2. A large soil ridge was used to separate plots (Figure S1). The booting stage was defined as 50% of the plants having panicles that were visible to the naked eye as a tiny and transparent growth of < 2 mm in length buried within the leaf sheaths near the base of the plant, approximately two weeks before heading. The drought stress treatment lasted for 15 d. Irrigation was controlled before the drought stress to gradually reduce the soil-water potential. A small amount of water was added to maintain the potential of the design if there was a water shortage during the comparable stress period. All treatments were placed under a rainproof shelter during drought stress. Eight soil tensiometers were placed evenly in two rows in each plot (Figure S1). A soil tensiometer (Institute of Soil Science, Chinese Academy of Sciences) was used to monitor soil-water potential at a depth of 20 cm. The soil potential was monitored daily at 06:00, 12:00, and 18:00. When the soil-water potential was low, an appropriate water supply was used to maintain it within ± 3 kPa of the designed soil-water potential. Regular irrigation was restored immediately after the drought stress. The soil-water potential was rapidly restored to 0 kPa and the water depth was maintained at 3 cm until maturity. For convenience of description, days after drought stress were recorded as Dx days, and days after restoration of irrigation were recorded as Rx days.
Photosynthetic characteristics
Five hills were selected from plants with the consistent growth every 10 days, and all green leaves were collected. A total of 15 hills were collected in three replicates. The leaf area was measured by drying and weighing method (punching weighing method), and the leaf area index was calculated. Before the onset of drought stress, 5 hills of consistent plants were selected and labeled. Leaf SPAD values were measured every 10 days after drought stress. The labeled plants were selected, and the top 6 fully developed leaves of each hill were selected. The SPAD values of the upper, middle and lower parts of each leaf was measured by SPAD502 chlorophyll analyzer. The mean SPAD value of all 6 fully developed leaves was calculated and recorded as the SPAD value of the leaves of one hill, and 5 hills were measured. After drought stress, the labeled plants were selected every 10 days, and the top 3 fully developed leaves of each hill were selected. The net photosynthetic rate, transpiration rate, stomatal conductance and intercellular CO2 concentration of the leaves were measured by CI-340 portable photosynthesis system, and 5 hills were measured.
Determination of physiological indicators
Every 3 days after drought stress and every 5 days after restoration irrigation, three hills of plants with consistent growth were selected, and the panicles were collected from each treatment (they were not sampled before D6 days because of their small size). A total of 9 hills were collected in three replicates. Panicles were selected and killed out at 105 °C for 30 min and dried to constant weight at 80 °C to determine sucrose content in panicle. Panicles of another 3 hills plants were selected, and three grains (spikelets) at the top of the upper three primary branches were the superior grains and three grains (spikelets) at the end of the lower three secondary branches were the inferior grains. Superior and inferior grains were selected and stored in liquid nitrogen to determine the physiological indicators. A total of 9 hills were collected in three replicates. All physiological measurements were performed in triplicate with technical replicates.
Sucrose content was measured using the resorcinol method65, with some modifications. 0.1 g grains were ground with a mortar and pestle in liquid nitrogen and homogenized in 8 mL 80% ethanol, followed by an 80 °C water bath for 40 min. Repeated extraction once and merged the extract. Activated carbon was added to the extracting solution, decolorized by an 80 °C water bath for 30 min. The extracting solution was a crude enzyme solution with a constant volume of 20 mL. One hundred microliters of 2 mol·L-1 NaOH were added to a 4 mL crude enzyme solution, boiled in a water bath for 5 min. Seven milliliters of 30% hydrochloric acid and 2 mL 0.1% resorcinol were added successively, followed by an 80 °C water bath for 10 min, and then the absorbance was measured at 480 nm.
The activity of vacuolar invertase (VIN) was determined as previously described66, with some modifications. 0.1 g grains were ground with a mortar and pestle in liquid nitrogen and homogenized in 1 mL extracting solution (50 mM HEPES-NaOH (pH 7.5), 50 mM MgCl2, 2 mM EDTA, 0.2% BSA, 2% PVP, 10 mM DTT). The homogenate was centrifuged at 12000 × g for 10 min at 4 °C. The supernatant was a crude enzyme solution. Fifty microliters of crude enzyme solution were added to the determination and control tubes. Then, 200 µL 8% sucrose and 200 µL acetic acid-sodium acetate buffer solution was added to the determination and control tubes, respectively. Water baths were run at 37 ℃ for 30 min, followed by a 95 °C water bath for 10 min. One hundred twenty-five microliters of 20 mM dinitrosalicylic acid were added to the determination and control tubes, followed by a 95 °C water bath for 10 min, and then the absorbance was measured at 510 nm. The catalyzed production of 1 µg reducing sugar per minute per gram leaf is defined as one unit of enzyme activity.
The activity of sucrose phosphate synthase (SPS) and sucrose synthase (SuSase) was determined as previously described67, with some modifications. 0.5 g grains were ground with a mortar and pestle in liquid nitrogen and homogenized in 3 mL extracting solution (50 mM HEPES-NaOH (pH 7.5), 50 mM MgCl2, 2 mM EDTA, 0.2% BSA, 2% PVP). The homogenate was centrifuged at 10000 × g for 10 min at 4 °C. The supernatant was a crude enzyme solution. Fifty microliters of HEPES-NaOH, 20µL 20mM MgCl2, 20 µL 100 mM UDPG and 20 µL 100 mM fructose-6-phosphate (sucrose synthase determination with fructose added) were added to 50 µL crude enzyme solution, respectively. Then, water baths were run at 37 ℃ for 30 min. Then, 200 µL 2 M NaOH was added to stop the reaction, boiled in a water bath for 10 min. One and a half milliliters of 30% hydrochloric acid and 0.5 mL 0.1% resorcinol were added successively, followed by an 80 °C water bath for 10 min, and then the absorbance was measured at 480 nm. The catalyzed production of 1 µg sucrose per minute per gram leaf is defined as one unit of enzyme activity.
The activity of AGPase was determined by referring to Choix68. 5–10 grains were added with 1mL extract (50mM HEPES-NaOH (pH 7.5), 50mM MgCl2, 10 mM DTT, 2% PVP), ground in ice bath, centrifuged at 10000 × g for 10 min at 4℃, and the supernatant was crude enzyme solution. Add 50µL HEPES-NaOH, 80µL reaction solution (1.2mM ADPG, 5mM MgCl2, 3mM pyrophosphoric acid), 10µL crude enzyme solution successively, after mixing, take water bath at 30℃ for 15 min, boiling water bath for 1 min, then take ice bath quickly, centrifuge at 4000 × g at 4℃ for 5 min. Take 80µL supernatant, add 85µL HEPES-NaOH, 35µL working solution (0.3 g·mL-1 NADP, 0.8U hexose phosphate mutase, 1U glucose-6-phosphate dehydrogenase), mix all, and then the coloration is at 340 nm. The catalytic production of 1 nmol NADPH per grain per minute was defined as one unit of enzymatic activity.
Path analysis
In order to find the trait that has the greatest effect on the target trait from numerous path relationships, the decision coefficients of related traits were calculated after path analysis, and the decision coefficients were used to represent the comprehensive determining effect of each variable on the dependent variable69,70. The calculation formula is as follows:
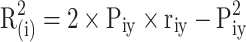 |
In the formula, 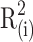 is the decision coefficient of trait xi on target trait y,
is the decision coefficient of trait xi on target trait y,  is the direct path coefficient of trait xi and target trait y, and
is the direct path coefficient of trait xi and target trait y, and 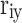 is the correlation coefficient of trait xi and target trait y. The variable with the largest decision coefficient is the decision variable, and the variable with the smallest and negative decision coefficient is the limiting variable.
is the correlation coefficient of trait xi and target trait y. The variable with the largest decision coefficient is the decision variable, and the variable with the smallest and negative decision coefficient is the limiting variable.
Statistical analysis
Data analyses were performed using the SPSS 18.0 (Chicago, IL) software package. Analysis of variance (ANOVA) was used to analyze all data and differences among treatments. Results are reported as the mean ± standard deviation (SD) values of three independent experiments, by measuring at least three different replicates (plants) in each experiment. SD was calculated directly from crude data. The levels of significance in the figures are given by ns for not significant, * and ** for significance at p < 0.05, and p < 0.01, respectively.
Conclusion
This study systematically investigated the impact of drought stress on rice growth, physiological traits, and sugar metabolism, providing comprehensive insights into plant responses under varying degrees of water scarcity. The leaf area index (LAI) decreased under drought stress, and the more severe the drought stress, the greater the decrease in LAI. The effects of mild and moderate drought stress on the SPAD values, indicative of chlorophyll content, during overlapping growth phases were minimal. However, a notable decrease in SPAD values was observed in rice leaves after re-irrigation following moderate drought stress, while severe drought stress led to a significant reduction in these values during the stress period itself. This suggests a possible inability to recover chlorophyll synthesis promptly after re-watering in the case of moderate stress. Drought stress significantly reduced the transpiration rate, stomatal conductance, and intercellular CO2 concentration; the more severe the drought stress, the greater the decrease. After the resumption of irrigation, the photosynthetic parameters of rice under the mild and moderate drought stress treatments recovered to the control level, while the time required for recovery was longer for rice under the severe drought stress treatment. This highlights the potential irreversible damage or long-term impacts of severe drought on rice photosynthesis. VIN is involved in the formation of young panicles, and higher VIN activity in the panicles during stress had a positive effect on resistance to drought stress. Drought stress significantly reduced the SuSase activity of superior and inferior grains and SPS activity of superior grains. The SuSase activity of inferior grains had the most significant effect on sucrose content. In conclusion, our findings elucidate the multifaceted effects of drought stress on rice, highlighting the physiological and metabolic adjustments plants undergo to cope with water scarcity. These insights are crucial for developing strategies to enhance drought resilience in crops, particularly in the context of global climate variability.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Author contributions
W.X. writing original draft, investigation, funding acquisition and methodology. L.H., M.H., W.J., D.Y., H.Y. investigation and methodology. W.J., L.H. project administration. W.X., Z.D., Z.H. re-view and editing, supervision, and funding acquisition. Z.C., L.A. prepared figures. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by National Natural Science Foundation of China (32101823).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Bureau of Statistics of China. China Statistical Yearbook. 8–27 (2017).
- 2.Todaka, D., Shinozaki, K. & Yamaguchi-Shinozaki, K. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front. Plant Sci.6, 84. 10.3389/fpls.2015.00084 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenham, K. et al. Temporal network analysis identifies early physiological and transcriptomic indicators of mild drought in Brassica rapa. eLife6, e29655 (2017). 10.7554/eLife.29655.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Bureau of Statistics of China. China Statistical Yearbook. 12–10 (2020).
- 5.Singh, A. K., Choudhury, B. U. & Bouman, B. A. M. in The International Workshop on water-wise rice production. (ed H. Hengsdijk, B. A. M. Bouman, B. Hardy, P. S. Bindraban, T. P. Tuong, and J. K. Ladha) 237–248.
- 6.Pandey, V. & Shukla, A. Acclimation and tolerance strategies of rice under drought stress. Rice Sci.22, 147–161. 10.1016/j.rsci.2015.04.001 (2015). [Google Scholar]
- 7.Bhandari, U. et al. Morpho-physiological and biochemical response of rice (Oryza sativa L.) to drought stress: A review. Heliyon9, e13744. 10.1016/j.heliyon.2023.e13744 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latif, A., Ying, S., Cuixia, P. & Ali, N. Rice curled its leaves either adaxially or abaxially to combat drought stress. Rice Sci.30, 405–416. 10.1016/j.rsci.2023.04.002 (2023). [Google Scholar]
- 9.Yavas, I. et al. Drought-induced changes in leaf morphology and anatomy: overview, implications and perspectives. 33 (2024).
- 10.Terzi, K. R. A dehydration avoidance mechanism: Leaf rolling. Bot. Rev.73, 290–302 (2007). [Google Scholar]
- 11.Mukamuhirwa, A. et al. Effect of intermittent drought on grain yield and quality of rice (Oryza sativa L.) grown in Rwanda. 206, 252–262 (2020).
- 12.Gu, J. et al. Rice (Oryza sativa L.) with reduced chlorophyll content exhibit higher photosynthetic rate and efficiency, improved canopy light distribution, and greater yields than normally pigmented plants. Field Crops Res.200, 58–70. 10.1016/j.fcr.2016.10.008 (2017). [Google Scholar]
- 13.Wang, J. et al. Effect of water stress on the physiological and photosynthesis characteristic of Okra (Abloschus Esculentus L). Chin. J. Trop. Crops. 38, 6 (2017). [Google Scholar]
- 14.Hao, S. R., Guo, X. P., Wang, W. M., Zhang, L. J. & Wang, Q. Effects of water stress and rewatering at shooting stage of rice on chlorophyll pigments in leaves. J. Hohai Univ. (Natural Sciences). 34, 397–406 (2006). [Google Scholar]
- 15.Qu, M. et al. Alterations in stomatal response to fluctuating light increase biomass and yield of rice under drought conditions. 104, 1334–1347 (2020). [DOI] [PubMed]
- 16.Bhandari, U. et al. Morpho-physiological and biochemical response of rice (Oryza sativa L.) to drought stress: A review. 9 (2023). [DOI] [PMC free article] [PubMed]
- 17.Lauteri, M., Haworth, M., Serraj, R., Monteverdi, M. C. & Centritto, M. Photosynthetic diffusional constraints affect yield in drought stressed rice cultivars during flowering. Plos One. 9, e109054. 10.1371/journal.pone.0109054 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang, P. M., Huang, Q. C., Qin, G. Y., Zhao, S. P. & Zhou, J. G. Different drought-stress responses in photosynthesis and reactive oxygen metabolism between autotetraploid and diploid rice. Photosynthetica52, 193–202. 10.1007/s11099-014-0020-2 (2014). [Google Scholar]
- 19.Ostmeyer, T. et al. Impacts of heat, drought, and their interaction with nutrients on physiology, grain yield, and quality in field crops. 25, 549–568 (2020).
- 20.Centritto, M., Lauteri, M., Monteverdi, M. C. & Serraj, R. Leaf gas exchange, carbon isotope discrimination, and grain yield in contrasting rice genotypes subjected to water deficits during the reproductive stage. J. Exp. Bot.60, 2325–2339. 10.1093/jxb/erp123 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Ding, L., Ying-Rui, L. I., Yong, L. I., Shen, Q. R. & Guo, S. W. Effects of drought stress on photosynthesis and water status of rice leaves. Chin. J. Rice Sci.28, 65–70 (2014). [Google Scholar]
- 22.Lu, C. M., Zhang, Q. D., Kuang, T. Y., Wang, Z. & Gao, Y. Z. The mechanism for the inhibition of photosynthesis in rice by water stress. Acta Agron. Sinica. 20, 601–604 (1994). [Google Scholar]
- 23.Wang, B. F. et al. Advances on inhibition mechanism of crop photosynthesis by drought stress. Hubei Agricultural Sci.53, 28–32 (2014). [Google Scholar]
- 24.Smirnoff, N. & Colombé, S. V. Drought influences the activity of enzymes of the chloroplast hydrogen peroxide scavenging system. J. Exp. Bot.39, 1097–1108 (1988). [Google Scholar]
- 25.Parry, M. A. J., Andralojc, P. J., Khan, S., Lea, P. J. & Keys, A. J. Rubisco activity: Effects of drought stress. Ann. Botany. 89, 833–839. 10.1093/aob/mcf103 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawlor, D. W. & Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant. Cell. Environ.25, 275–294 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Huang, G. et al. Temperature responses of photosynthesis and stomatal conductance in rice and wheat plants. 300, 108322 (2021).
- 28.Gunasekera, D. & Berkowitz, G. A. Use of transgenic plants with ribulose-1,5-bisphosphate carboxylase/oxygenase antisense DNA to evaluate the rate limitation of photosynthesis under water stress. Plant Physiol.103, 629–635 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zahoor, R. et al. Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environ. Exp. Bot.137, 73–83. 10.1016/j.envexpbot.2017.02.002 (2017). [Google Scholar]
- 30.Thomas, A. et al. Changes in sucrose metabolic enzymes to water stress in contrasting rice genotypes. Plant. Stress. 5, 100088. 10.1016/j.stress.2022.100088 (2022). [Google Scholar]
- 31.Geigenberger, P. & Stitt, M. Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta189, 329–339. 10.1007/bf00194429 (1993). [DOI] [PubMed] [Google Scholar]
- 32.Heim, U., Weber, H., Baumlein, H. & Wobus, U. A sucrose-synthase gene of Vicia faba L.: Expression pattern in developing seeds in relation to starch synthesis and metabolic regulation. Planta191, 394–401 (1993). [DOI] [PubMed] [Google Scholar]
- 33.Vassey, T. L., Quick, W. P., Sharkey, T. D. & Stitt, M. Water stress, carbon dioxide, and light effects on sucrosephosphate synthase activity in Phaseolus vulgaris. Physiol. Plant.81, 37–44 (2010). [Google Scholar]
- 34.Yang, J., Zhang, J., Wang, Z., Zhu, Q. & Liu, L. Abscisic acid and cytokinins in the root exudates and leaves and their relationship to senescence and remobilization of carbon reserves in rice subjected to water stress during grain filling. Planta215, 645–652. 10.1007/s00425-002-0789-2 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Niedzwiedz-Siegien, I., Bogatek-Leszczynska, R., Come, D. & Corbineau, F. Effects of drying rate on dehydration sensitivity of excised wheat seedling shoots as related to sucrose metabolism and antioxidant enzyme activities. Plant Sci.167, 879–888. 10.1016/j.plantsci.2004.05.042 (2004). [Google Scholar]
- 36.Du, Y. et al. Effect of drought stress during soybean R2–R6 growth stages on sucrose metabolism in leaf and seed. 21, 618 (2020). [DOI] [PMC free article] [PubMed]
- 37.Yang, J. C., Zhang, J. H., Wang, Z. Q., Zhu, Q. S. & Liu, L. Activities of enzymes involved in sucrose-to-starch metabolism in rice grains subjected to water stress during filling. Field Crops Res.81, 69–81. 10.1016/s0378-4290(02)00214-9 (2003). [Google Scholar]
- 38.Cai, Y. et al. Effects of water stress on the main characters of superior and inferior grains quality and the properties of RVA profile during grain-filling stage. ACTA Agron. SINICA. 30, 241–247 (2004). [Google Scholar]
- 39.Yang, J. C., Zhang, J., Wang, Z. & Zhu, Q. Activities of starch hydrolytic enzymes and sucrose-phosphate synthase in the stems of rice subjected to water stress during grain filling. J. Exp. Bot.52, 2169–2179. 10.1093/jexbot/52.364.2169 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Roitsch, T. & Gonzalez, M. C. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci.9, 606–613. 10.1016/j.tplants.2004.10.009 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Sergeeva, L. I. et al. Vacuolar invertase regulates elongation of Arabidopsis thaliana roots as revealed by QTL and mutant analysis. Proc. Natl. Acad. Sci. U.S.A.103, 2994–2999. 10.1073/pnas.0511015103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pollock, C. J. Fructan metabolism in grasses and cereals. Annu. Rev. Plant Biol.42, 77–101 (1991). [Google Scholar]
- 43.Misra, V., Mall, A., Ansari, S. A. & Ansari, M. I. Sugar transporters, sugar-metabolizing enzymes, and their interaction with phytohormones in sugarcane. J. Plant Growth Regul. 42, 4975–4988 (2023). [Google Scholar]
- 44.Wang, X. et al. Photosynthetic carbon fixation and sucrose metabolism supplemented by weighted gene co-expression network analysis in response to water stress in rice with overlapping growth stages. Front. Plant Sci.13, 864605. 10.3389/fpls.2022.864605 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dale, J. E. & Milthorpe, F. L. The Growth and Functioning of Leaves. (The Growth and functioning of leaves 1983).
- 46.Golfam, R. et al. A review of drought stress on wheat (Triticum aestivum L.) starch. 6, 47–57 (2021).
- 47.Kozlowski, T. T. Water deficits and plant growth. 342–351 (Academic Press, 1976).
- 48.Havrlentová, M., Kraic, J., Gregusová, V. & Kovácsová, B. J. A. Drought stress in cereals–a review. 67, 47–60 (2021).
- 49.Mao, Z. Effects of Drought Stress on dry Matter Distribution and Yield Formation in Hybrid and Inbred rice (Huazhong Agricultural University, 2023).
- 50.Chen, J. & Pan, J. Analysis on correlation between leaf SPAD value and chlorophyll content of different grape varieties. North. Hortic.19, 50–54 (2015). [Google Scholar]
- 51.Li, S. et al. Young panicle formation stage after water stress on the production and transport of photosynthate in rice. Acta Agriculturae Boreali-Sinica. 28, 133–147 (2013). [Google Scholar]
- 52.Wang, B. Effects of Drought Stress on Photosynthetic Characteristics and Physiological Mechanism of Different Drought Resistance rice (Huazhong Agricultural University, 2023).
- 53.Wang, X. et al. Response of rice with overlapping growth stages to water stress by assimilates accumulation and transport and starch synthesis of superior and inferior grains. Int. J. Mol. Sci.23, 11157 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jia, W. & Davies, W. J. Modification of leaf apoplastic pH in relation to stomatal sensitivity to root-sourced abscisic acid signals. Plant Physiol.143, 68–77. 10.1104/pp.106.089110 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilkinson, S. & Davies, W. J. Xylem Sap pH increase: A drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiol.113, 559–573. 10.1104/pp.113.2.559 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gabriel & Cornic. Drought stress inhibits photosynthesis by decreasing stomatal aperture – not by affecting ATP synthesis. Trends Plant Sci.5, 187–198 (2000). [Google Scholar]
- 57.Wobus, B. U. Sugar import and metabolism during seed development. Trends Plant Sci.2, 169–174 (1997). [Google Scholar]
- 58.Liu, L., Shen, F., Lu, H., Han, Q. & Liu, Y. Research advance on sucrose phosphate synthase in sucrose metabolism. Mol. Plant. Breed.3, 275–281 (2005). [Google Scholar]
- 59.Tabbal, D. F., Bouman, B., Bhuiyan, S. I., Sibayan, E. B. & Sattar, M. A. On-farm strategies for reducing water input in irrigated rice; case studies in the Philippines. Agric. Water Manage.56, 93–112 (2002). [Google Scholar]
- 60.Belder, P. et al. Effect of water-saving irrigation on rice yield and water use in typical lowland conditions in Asia. Agric. Water Manage.65, 193–210. 10.1016/j.agwat.2003.09.002 (2004). [Google Scholar]
- 61.Zhao, H. W. et al. Effect of drought stress at booting stage on grain nitrogen formation and yield of rice in cold-region. J. Northeast Agricultural Univ.48, 1–10 (2017). [Google Scholar]
- 62.Chen, L., Wang, B., Jiang, Y., Cao, C. & Ping, L. I. Effects of drought and re-watering on rice physiological and biochemical indexes of leaves and grain yield at booting stage. China Rice. 22, 59–64 (2016). [Google Scholar]
- 63.Dietz, K. J., Zörb, C. & Geilfus, C. M. J. Drought and crop yield. P B. 23, 881–893 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Xu, Y. J. et al. Effect of alternate wetting and drying irrigation on post-anthesis remobilization of assimilates and grain filling of rice. Acta Agron. Sinica. 44, 554 (2018). [Google Scholar]
- 65.Du, X., Zhang, X., Xi, M. & Kong, L. Split application enhances sweetpotato starch production by regulating the conversion of sucrose to starch under reduced nitrogen supply. Plant. Physiol. Biochem.151, 743–750. 10.1016/j.plaphy.2020.04.027 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Yu, P., Liu, S., Han, K., Guan, S. & Zhou, D. Conversion of cropland to forage land and grassland increases soil labile carbon and enzyme activities in northeastern China. Agric. Ecosyst. Environ.245, 83–91. 10.1016/j.agee.2017.05.013 (2017). [Google Scholar]
- 67.Yu, L. et al. Effects of hot air and methyl jasmonate treatment on the metabolism of soluble sugars in peach fruit during cold storage. Postharvest Biol. Technol.113, 8–16. 10.1016/j.postharvbio.2015.10.013 (2016). [Google Scholar]
- 68.Choix, F. J., Bashan, Y., Mendoza, A. & de-Bashan, L. E. Enhanced activity of ADP glucose pyrophosphorylase and formation of starch induced by Azospirillum brasilense in Chlorella vulgaris. J. Biotechnol.177, 22–34. 10.1016/j.jbiotec.2014.02.014 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Yuan, Z. F., Zhou, J. Y., Guo, M. C., Lei, X. Q. & Xie, X. L. Decision coefficient-the decision index of path analysis. J. Northwest. Sci-Tech Univ. Agric. Forestry. 29, 131–133 (2001). [Google Scholar]
- 70.Xu, C. H., Zhang, H., Zhang, L. & Kang, Y. R. Factors influencing photosynthesis of three typical plant species in Beishan Mountain of Lanzhou based on path analysis. Chin. J. Ecol.34, 1289–1294 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.