Full text
PDF






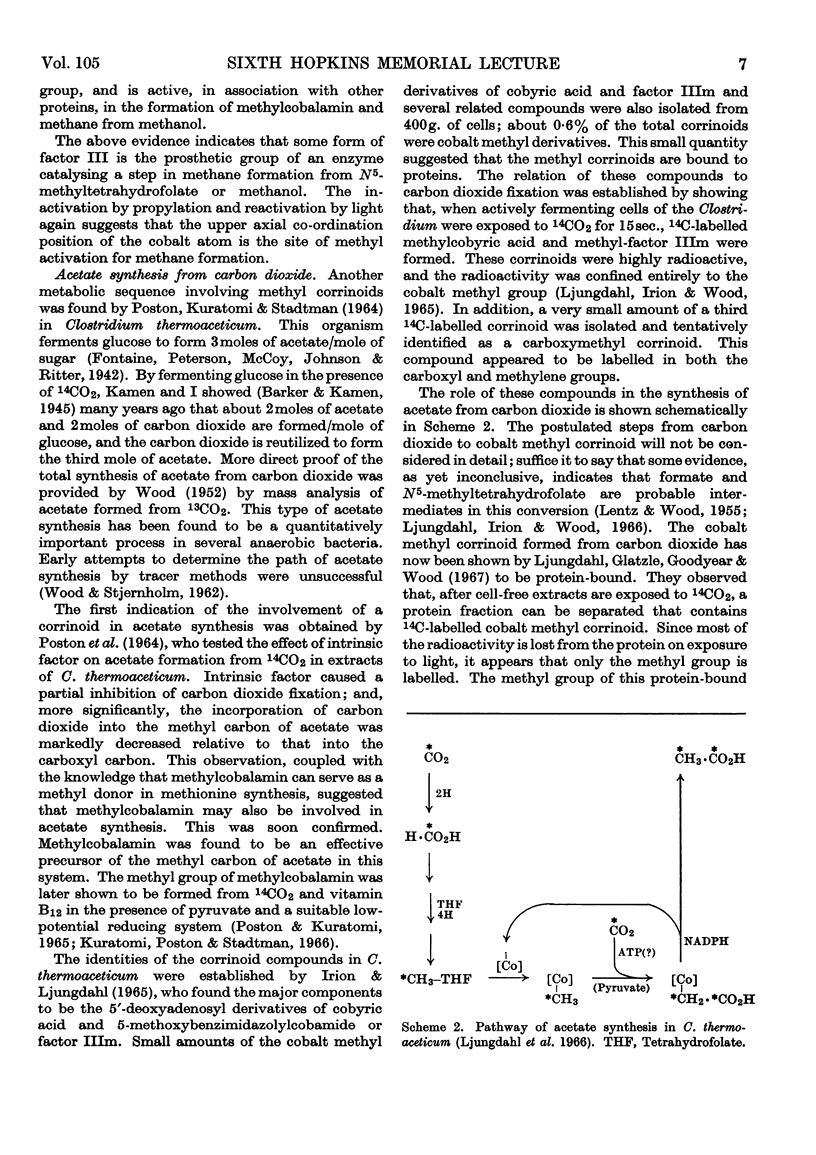
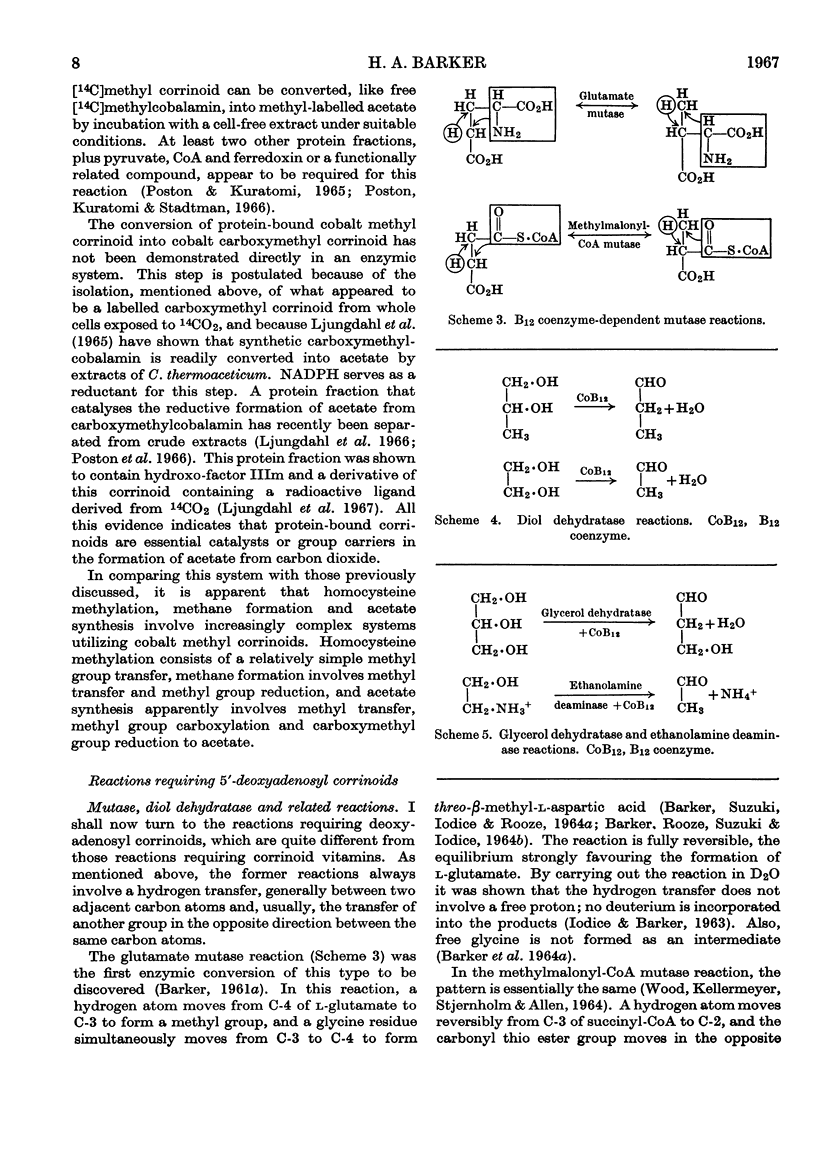







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELES R. H., LEE H. A., Jr An intramolecular oxidation-reduction requiring a cobamide coenzyme. J Biol Chem. 1961 Aug;236:2347–2350. [PubMed] [Google Scholar]
- ABELES R. H., LEE H. A., Jr Studies on diol dehydrase, an enzyme requiring a cobamide coenzyme. Brookhaven Symp Biol. 1962 Dec;15:310–319. [PubMed] [Google Scholar]
- Abeles R. H., Frey P. A. Role of B12 coenzymes in the dioldehydrase reaction. Fed Proc. 1966 Nov-Dec;25(6):1639–1641. [PubMed] [Google Scholar]
- Abeles R. H., Zagalak B. The nature of the hydrogen transfer in the dimethylbenzimidazolylcobamide coenzyme-catalyzed conversion of 1,2-propanediol to propionaldehyde. J Biol Chem. 1966 Mar 10;241(5):1245–1246. [PubMed] [Google Scholar]
- BARKER H. A., ROOZE V., SUZUKI F., IODICE A. A. THE GLUTAMATE MUTASE SYSTEM. ASSAYS AND PROPERTIES. J Biol Chem. 1964 Oct;239:3260–3266. [PubMed] [Google Scholar]
- BARKER H. A., SMYTH R. D., WAWSZKIEWICZ E. J., LEE M. N., WILSON R. M. Enzymic preparation and characterization of an alpha-L-beta-methylaspartic acid. Arch Biochem Biophys. 1958 Dec;78(2):468–476. doi: 10.1016/0003-9861(58)90371-0. [DOI] [PubMed] [Google Scholar]
- BARKER H. A., SMYTH R. D., WEISSBACH H., TOOHEY J. I., LADD J. N., VOLCANI B. E. Isolation and properties of crystalline cobamide coenzymes containing benzimidazole or 5, 6-dimethylbenzimidazole. J Biol Chem. 1960 Feb;235:480–488. [PubMed] [Google Scholar]
- BARKER H. A., SMYTH R. D., WILSON R. M., WEISSBACH H. The purification and properties of beta-methylaspartase. J Biol Chem. 1959 Feb;234(2):320–328. [PubMed] [Google Scholar]
- BARKER H. A., SUZUKI F., IODICE A., ROOZE V. GLUTAMATE MUTASE REACTION. Ann N Y Acad Sci. 1964 Apr 24;112:644–654. doi: 10.1111/j.1749-6632.1964.tb45041.x. [DOI] [PubMed] [Google Scholar]
- BARKER H. A. Structure and function of cobamide coenzymes. Fed Proc. 1961 Dec;20:956–961. [PubMed] [Google Scholar]
- BLAKLEY R. L. COBAMIDES AND RIBONUCLEOTIDE REDUCTION. I. COBAMIDE STIMULATION OF RIBONUCLEOTIDE REDUCTION IN EXTRACTS OF LACTOBACILLUS LEICHMANNII. J Biol Chem. 1965 May;240:2173–2180. [PubMed] [Google Scholar]
- BRADY R. O., CASTANERA E. G., BARKER H. A. The enzymatic synthesis of cobamide coenzymes. J Biol Chem. 1962 Jul;237:2325–2332. [PubMed] [Google Scholar]
- BROT N., WEISSBACH H. ENZYMATIC SYNTHESIS OF METHIONINE. CHEMICAL ALKYLATION OF THE ENZYME-BOUND COBAMIDE. J Biol Chem. 1965 Jul;240:3064–3070. [PubMed] [Google Scholar]
- BUCHANAN J. M., ELFORD H. L., LOUGHLIN R. E., MCDOUGALL B. M., ROSENTHAL S. THE ROLE OF VITAMIN B12 IN METHYL TRANSFER TO HOMOCYSTEINE. Ann N Y Acad Sci. 1964 Apr 24;112:756–773. doi: 10.1111/j.1749-6632.1964.tb45053.x. [DOI] [PubMed] [Google Scholar]
- Barker H. A., Kamen M. D. Carbon Dioxide Utilization in the Synthesis of Acetic Acid by Clostridium Thermoaceticum. Proc Natl Acad Sci U S A. 1945 Aug;31(8):219–225. doi: 10.1073/pnas.31.8.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A., Weissbach H., Smyth R. D. A COENZYME CONTAINING PSEUDOVITAMIN B(12). Proc Natl Acad Sci U S A. 1958 Nov 15;44(11):1093–1097. doi: 10.1073/pnas.44.11.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham T. J., Ghambeer R. K., Blakley R. L., Brownson C. Cobamides and ribonucleotide reduction. IV. Sterochemistry of hydrogen transfer to the deoxyribonucleotide. Biochemistry. 1967 Apr;6(4):1203–1208. doi: 10.1021/bi00856a033. [DOI] [PubMed] [Google Scholar]
- Beck W. S., Abeles R. H., Robinson W. G. Transfer of hydrogen from cobamide coenzyme to water during enzymatic ribonucleotide reduction. Biochem Biophys Res Commun. 1966 Nov 22;25(4):421–425. doi: 10.1016/0006-291x(66)90222-1. [DOI] [PubMed] [Google Scholar]
- Beck W. S., Goulian M., Larsson A., Reichard P. Hydrogen donor specificity of cobamide-dependent ribonucleotide reductase and allosteric regulation of substrate specificity. J Biol Chem. 1966 May 10;241(9):2177–2179. [PubMed] [Google Scholar]
- Blakley R. L. B12-dependent synthesis of deoxyribonucleotides. Fed Proc. 1966 Nov-Dec;25(6):1633–1638. [PubMed] [Google Scholar]
- Blakley R. L., Barker H. A. Cobamide stimulation of the reduction of ribotides to deoxyribotides in Lactobacillus leichmannii. Biochem Biophys Res Commun. 1964 Jul 27;16(5):391–397. doi: 10.1016/0006-291x(64)90363-8. [DOI] [PubMed] [Google Scholar]
- Blakley R. L. Cobamides and ribonucleotide reduction. II. Estimation of the enzymic formation of purine and pyrimidine deoxyribonucleotides by the use of the diphenylamine reagent. J Biol Chem. 1966 Jan 10;241(1):176–179. [PubMed] [Google Scholar]
- Blakley R. L., Ghambeer R. K., Batterham T. J., Brownson C. Studies with hydrogen isotopes on the mechanism of action of cobamide-dependent ribonucleotide reductase. Biochem Biophys Res Commun. 1966 Aug 12;24(3):418–426. doi: 10.1016/0006-291x(66)90176-8. [DOI] [PubMed] [Google Scholar]
- Blakley R. L., Ghambeer R. K., Nixon P. F., Vitols E. The cobamide-dependent ribonucleoside triphosphate reductase of lactobacilli. Biochem Biophys Res Commun. 1965 Aug 16;20(4):439–445. doi: 10.1016/0006-291x(65)90597-8. [DOI] [PubMed] [Google Scholar]
- Blaylock B. A., Stadtman T. C. Methane biosynthesis by Methanosarcina barkeri. Properties of the soluble enzyme system. Arch Biochem Biophys. 1966 Sep 26;116(1):138–152. doi: 10.1016/0003-9861(66)90022-1. [DOI] [PubMed] [Google Scholar]
- Bradbeer C. The clostridial fermentations of choline and ethanolamine. II. Requirement for a cobamide coenzyme by an ethanolamine deaminase. J Biol Chem. 1965 Dec;240(12):4675–4681. [PubMed] [Google Scholar]
- Brot N., Weissbach H. The role of cobamides in methionine synthesis. Enzymatic formation of holoenzyme. J Biol Chem. 1966 May 10;241(9):2024–2028. [PubMed] [Google Scholar]
- Costilow R. N., Rochovansky O. M., Barker H. A. Isolation and identification of beta-lysine as an intermediate in lysine fermentation. J Biol Chem. 1966 Apr 10;241(7):1573–1580. [PubMed] [Google Scholar]
- DICKERMAN H. W., REDFIELD B. G., BIERI J. G., WEISSBACH H. STUDIES ON THE ROLE OF VITAMIN B12 FOR THE SYNTHESIS OF METHIONINE IN LIVER. Ann N Y Acad Sci. 1964 Apr 24;112:791–798. doi: 10.1111/j.1749-6632.1964.tb45055.x. [DOI] [PubMed] [Google Scholar]
- DOLPHIN D., JOHNSON A. W., RODRIGO R. SOME REACTIONS OF THE VITAMIN B 12 COENZYME AND ITS ALKYL ANALOGUES. Ann N Y Acad Sci. 1964 Apr 24;112:590–600. doi: 10.1111/j.1749-6632.1964.tb45035.x. [DOI] [PubMed] [Google Scholar]
- FOSTER M. A., DILWORTH M. J., WOODS D. D. COBALAMIN AND THE SYNTHESIS OF METHIONINE BY ESCHERICHIA COLI. Nature. 1964 Jan 4;201:39–42. doi: 10.1038/201039a0. [DOI] [PubMed] [Google Scholar]
- FOSTER M. A., JONES K. M., WOODS D. D. The purification and properties of a factor containing vitamin B12 concerned in the synthesis of methionine by Escherichia coli. Biochem J. 1961 Sep;80:519–531. doi: 10.1042/bj0800519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREY P. A., KARABATSOS G. L., ABELESS R. H. THE STEREOCHEMISTRY OF CONVERSION OF PROPANEDIOL TO PROPIONALDHYDE. Biochem Biophys Res Commun. 1965 Feb 17;18:551–556. doi: 10.1016/0006-291x(65)90789-8. [DOI] [PubMed] [Google Scholar]
- Fontaine F. E., Peterson W. H., McCoy E., Johnson M. J., Ritter G. J. A New Type of Glucose Fermentation by Clostridium thermoaceticum. J Bacteriol. 1942 Jun;43(6):701–715. doi: 10.1128/jb.43.6.701-715.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey P. A., Abeles R. H. The role of the B12 coenzyme in the conversion of 1,2-propanediol to propionaldehyde. J Biol Chem. 1966 Jun 10;241(11):2732–2733. [PubMed] [Google Scholar]
- GUEST J. R., FRIEDMAN S., DILWORTH M. J., WOODS D. D. METHYLCOBALAMIN AS A SOURCE OF THE METHYL GROUP OF METHIONINE. Ann N Y Acad Sci. 1964 Apr 24;112:774–790. doi: 10.1111/j.1749-6632.1964.tb45054.x. [DOI] [PubMed] [Google Scholar]
- GUEST J. R., FRIEDMAN S., WOODS D. D., SMITH E. L. A methyl analogue of cobamide coenzyme in relation to methionine synthesis by bacteria. Nature. 1962 Jul 28;195:340–342. doi: 10.1038/195340a0. [DOI] [PubMed] [Google Scholar]
- GUEST J. R., HELLEINER C. W., CROSS M. J., WOODS D. D. Cobalamin and the synthesis of methionine by ultrasonic extracts of Escherichia coli. Biochem J. 1960 Aug;76:396–405. doi: 10.1042/bj0760396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Beck W. S. Transfer of hydrogen in the cobamide-dependent ribonucleotide reductase reaction. Biochem Biophys Res Commun. 1966 Aug 12;24(3):353–359. doi: 10.1016/0006-291x(66)90163-x. [DOI] [PubMed] [Google Scholar]
- Goulian M., Beck W. S. Purification and properties of cobamide-dependent ribonucleotide reductase from Lactobacillus leichmannii. J Biol Chem. 1966 Sep 25;241(18):4233–4242. [PubMed] [Google Scholar]
- HATCH F. T., LARRABEE A. R., CATHOU R. E., BUCHANAN J. M. Enzymatic synthesis of the methyl group of methionine. I. Identification of the enzymes and cofactors involved in the system isolated from Escherichia coli. J Biol Chem. 1961 Apr;236:1095–1101. [PubMed] [Google Scholar]
- INGRAHAM L. L. B12 COENZYMES: BIOLOGICAL GRIGNARD REAGENTS. Ann N Y Acad Sci. 1964 Apr 24;112:713–720. doi: 10.1111/j.1749-6632.1964.tb45049.x. [DOI] [PubMed] [Google Scholar]
- IODICE A. A., BARKER H. A. The glutamate isomerase reaction: studies on the incorporation of solvent hydrogen. J Biol Chem. 1963 Jun;238:2094–2097. [PubMed] [Google Scholar]
- KISLIUK R. L. Further studies on the relationship of vitamin B12 to methionine synthesis in extracts of Escherichia coli. J Biol Chem. 1961 Mar;236:817–822. [PubMed] [Google Scholar]
- KISLIUK R. L., WOODS D. D. Interrelationships between folic acid and cobalamin in the synthesis of methionine by extracts of Escherichia coli. Biochem J. 1960 Jun;75:467–477. doi: 10.1042/bj0750467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwar S. S., Mangum J. H., Scrimgeour K. G., Brodie J. D., Huennekens F. M. Interrelationship of adenosyl methionine and methyl-B12 in the biosynthesis of methionine. Arch Biochem Biophys. 1966 Sep 26;116(1):305–318. doi: 10.1016/0003-9861(66)90037-3. [DOI] [PubMed] [Google Scholar]
- Kuratomi K., Poston J. M., Stadtman E. R. Synthesis of Co-methyl cobalamin by cell-free extracts of Clostridium thermoaceticum. Biochem Biophys Res Commun. 1966 Jun 13;23(5):691–695. doi: 10.1016/0006-291x(66)90455-4. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., MOORE E. C., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. IV. ISOLATION AND CHARACTERIZATION OF THIOREDOXIN, THE HYDROGEN DONOR FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3436–3444. [PubMed] [Google Scholar]
- LEE H. A., Jr, ABELES R. H. Purification and properties of dioldehydrase, and enzyme requiring a cobamide coenzyme. J Biol Chem. 1963 Jul;238:2367–2373. [PubMed] [Google Scholar]
- LENHERT P. G., HODGKIN D. C. Structure of the 5,6-dimethyl-benzimidazolylcobamide coenzyme. Nature. 1961 Dec 9;192:937–938. doi: 10.1038/192937a0. [DOI] [PubMed] [Google Scholar]
- LENTZ K., WOOD H. G. Synthesis of acetate from formate and carbon dioxide by Clostridium thermoaceticum. J Biol Chem. 1955 Aug;215(2):645–654. [PubMed] [Google Scholar]
- LEZIUS A. G., BARKER H. A. CORRINOID COMPOUNDS OF METHANOBACILLUS OMELIANSKII. I. FRACTIONATION OF THE CORRINOID COMPOUNDS AND IDENTIFICATION OF FACTOR 3 AND FACTOR 3 COENZYME. Biochemistry. 1965 Mar;4:510–518. doi: 10.1021/bi00879a021. [DOI] [PubMed] [Google Scholar]
- LINDSTRAND K. ISOLATION OF METHYLCOBALAMIN FROM NATURAL SOURCE MATERIAL. Nature. 1964 Oct 10;204:188–189. doi: 10.1038/204188a0. [DOI] [PubMed] [Google Scholar]
- LINDSTRAND K. ON VITAMIN B12 FORMS IN HUMAN PLASMA. Acta Med Scand. 1963 Dec;174:665–669. doi: 10.1111/j.0954-6820.1963.tb16534.x. [DOI] [PubMed] [Google Scholar]
- LOUGHLIN R. E., ELFORD H. L., BUCHANAN J. M. ENZYMATIC SYNTHESIS OF THE METHYL GROUP OF METHIONINE. VII. ISOLATION OF A COBALAMIN-CONTAINING TRANSMETHYLASE (5-METHYLTETRAHYDRO-FOLATE-HOMOCYSTEINE) FROM MAMMALIAN LIVER. J Biol Chem. 1964 Sep;239:2888–2895. [PubMed] [Google Scholar]
- Ljungdahl L., Irion E., Wood H. G. Role of corrinoids in the total synthesis of acetate from CO-2 by Clostridium thermoaceticum. Fed Proc. 1966 Nov-Dec;25(6):1642–1648. [PubMed] [Google Scholar]
- Ljungdahl L., Irion E., Wood H. G. Total synthesis of acetate from CO2. I. Co-methylcobyric acid and CO-(methyl)-5-methoxybenzimidazolylcobamide as intermediates with Clostridium thermoaceticum. Biochemistry. 1965 Dec;4(12):2771–2780. doi: 10.1021/bi00888a030. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L., Irion E., Wood H. G. Total synthesis of acetate from CO2. I. Co-methylcobyric acid and CO-(methyl)-5-methoxybenzimidazolylcobamide as intermediates with Clostridium thermoaceticum. Biochemistry. 1965 Dec;4(12):2771–2780. doi: 10.1021/bi00888a030. [DOI] [PubMed] [Google Scholar]
- MANSON L. A. Vitamin B12 and deoxyribose synthesis in Lactobacillus leichmannii. J Biol Chem. 1960 Oct;235:2955–2958. [PubMed] [Google Scholar]
- Marsh C. A. Metabolism of D-glucuronolactone in mammalian systems. Inhibitory properties of the products of D-glucuronolactone-dehydrogenase action. Biochem J. 1966 Apr;99(1):22–27. doi: 10.1042/bj0990022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OVERATH P., KELLERMAN G. M., LYNEN F., FRITZ H. P., KELLER H. J. [On the mechanism of the rearrangement of methylmalonyl-Co A into succinyl-Co A. II. Experiments on the mechanism of action of methylmalonyl-Co A isomerase and methylmalonyl-Co A racemase]. Biochem Z. 1962;335:500–518. [PubMed] [Google Scholar]
- Orr M. D., Vitols E. Thioredoxin from Lactobacillus leichmannii and its role as hydrogen donor for ribonucleoside triphosphate reductase. Biochem Biophys Res Commun. 1966 Oct 5;25(1):109–115. doi: 10.1016/0006-291x(66)90647-4. [DOI] [PubMed] [Google Scholar]
- PETERKOFSKY A., WEISSBACH H. ENZYMATIC SYNTHESIS OF COENZYME B12. Ann N Y Acad Sci. 1964 Apr 24;112:622–637. doi: 10.1111/j.1749-6632.1964.tb45038.x. [DOI] [PubMed] [Google Scholar]
- POSTON J. M., KURATOMI K., STADTMAN E. R. METHYL-VITAMIN B12 AS A SOURCE OF METHYL GROUPS FOR THE SYNTHESIS OF ACETATE BY CELL-FREE EXTRACTS OF CLOSTRIDIUM THERMOACETICUM. Ann N Y Acad Sci. 1964 Apr 24;112:804–806. doi: 10.1111/j.1749-6632.1964.tb45057.x. [DOI] [PubMed] [Google Scholar]
- Poston J. M., Kuratomi K., Stadtman E. R. The conversion of carbon dioxide to acetate. I. The use of cobalt-methylcobalamin as a source of methyl groups for the synthesis of acetate by cell-free extracts of Clostridium thermoaceticum. J Biol Chem. 1966 Sep 25;241(18):4209–4216. [PubMed] [Google Scholar]
- REICHARD P. Enzymatic synthesis of deoxyribonucleotides. I. Formation of deoxycytidine diphosphate from cytidine diphosphate with enzymes from Escherichia coli. J Biol Chem. 1962 Nov;237:3513–3519. [PubMed] [Google Scholar]
- ROSENTHAL S., SMITH L. C., BUCHANAN J. M. ENZYMATIC SYNTHESIS OF THE METHYL GROUP OF METHIONINE. IX. TRANSMETHYLATION FROM S-ADENOSYLMETHIONINE AND 5-METHYLTETRAHYDROFOLATE TO 2-MERCAPTOETHANOL AND HOMOCYSTEINE. J Biol Chem. 1965 Feb;240:836–843. [PubMed] [Google Scholar]
- Rétey J., Arigoni D. Coenzym B12 als gemeinsamer Wasserstoffüberträger der Dioldehydrase- und der Methylmalonyl-CoA-Mutase-Reaktion. Experientia. 1966 Dec 15;22(12):783–784. doi: 10.1007/BF01897408. [DOI] [PubMed] [Google Scholar]
- Rétey J., Umani-Ronchi A., Arigoni D. Zur Stereochemie der Propandioldehydrase-Reaktion. Experientia. 1966 Feb 15;22(2):72–73. doi: 10.1007/BF01900153. [DOI] [PubMed] [Google Scholar]
- Rétey J., Umani-Ronchi A., Sebl J., Arigoni D. Zum Mechanismus der Propandioldehydrase-Reaktion. Experientia. 1966 Aug 15;22(8):502–503. doi: 10.1007/BF01898652. [DOI] [PubMed] [Google Scholar]
- SMILEY K. L., SOBOLOV M. A cobamide-requiring glycerol dehydrase from an acrolein-forming Lactobacillus. Arch Biochem Biophys. 1962 Jun;97:538–543. doi: 10.1016/0003-9861(62)90118-2. [DOI] [PubMed] [Google Scholar]
- SMILEY K. L., SOBOLOV M. SOME PROPERTIES OF THE GLYCEROL DEHYDRASE SYSTEM FROM A SPECIES OF LACTOBACILLUS. Ann N Y Acad Sci. 1964 Apr 24;112:706–712. doi: 10.1111/j.1749-6632.1964.tb45048.x. [DOI] [PubMed] [Google Scholar]
- SMITH E. L., MERVYN L., JOHNSON A. W., SHAW N. Partial synthesis of vitamin B 12 coenzymes and analogues. Nature. 1962 Jun 23;194:1175–1175. doi: 10.1038/1941175a0. [DOI] [PubMed] [Google Scholar]
- STADTMAN T. C. ANAEROBIC DEGRADATION OF LYSINE. II. COFACTOR REQUIREMENTS AND PROPERTIES OF THE SOLUBLE ENZYME SYSTEM. J Biol Chem. 1963 Aug;238:2766–2773. [PubMed] [Google Scholar]
- STADTMAN T. C. COBAMIDE COENZYME REQUIREMENT FOR THE ANAEROBIC DEGRADATION OF LYSINE. Ann N Y Acad Sci. 1964 Apr 24;112:728–734. doi: 10.1111/j.1749-6632.1964.tb45051.x. [DOI] [PubMed] [Google Scholar]
- Schneider Z., Pawelkiewicz J. The properties of glycerol dehydratase isolated from Aerobacter aerogenes, and the properties of the apoenzyme subunits. Acta Biochim Pol. 1966;13(4):311–328. [PubMed] [Google Scholar]
- Sprecher M., Clark M. J., Sprinson D. B. The absolute configuration of methylmalonyl coenzyme A and stereochemistry of the methymalonyl coenzyme A mutase reaction. J Biol Chem. 1966 Feb 25;241(4):872–877. [PubMed] [Google Scholar]
- Sprecher M., Switzer R. L., Sprinson D. B. Stereochemistry of the glutamate mutase reaction. J Biol Chem. 1966 Feb 25;241(4):864–867. [PubMed] [Google Scholar]
- Stadtman T. C., Blaylock B. A. Role of B12 compounds in methane formation. Fed Proc. 1966 Nov-Dec;25(6):1657–1661. [PubMed] [Google Scholar]
- Suzuki F., Barker H. A. Purification and properties of component E of glutamate mutase. J Biol Chem. 1966 Feb 25;241(4):878–888. [PubMed] [Google Scholar]
- Switzer R. L., Barker H. A. Purification and characterization of component S of glutamate mutase. J Biol Chem. 1967 Jun 10;242(11):2658–2674. [PubMed] [Google Scholar]
- TAKEYAMA S., BUCHANAN J. M. Enzymatic synthesis of the methyl group of methionine. III. Spectral and electrophoretic studies of the prosthetic group of the B12 enzyme. J Biochem. 1961 Jun;49:578–588. doi: 10.1093/oxfordjournals.jbchem.a127347. [DOI] [PubMed] [Google Scholar]
- TAKEYAMA S., HATCH F. T., BUCHANAN J. M. Enzymatic synthesis of the methyl group of methionine. II. Involvement of vitamin B12. J Biol Chem. 1961 Apr;236:1102–1108. [PubMed] [Google Scholar]
- TOOHEY J. I., BARKER H. A. Isolation of coenzyme B12 from liver. J Biol Chem. 1961 Feb;236:560–563. [PubMed] [Google Scholar]
- Taylor R. T., Weissbach H. N5-methyltetrahydrofolate-homocysteine transmethylase. Partial purification and properties. J Biol Chem. 1967 Apr 10;242(7):1502–1508. [PubMed] [Google Scholar]
- Taylor R. T., Weissbach H. N5-methyltetrahydrofolate-homocysteine transmethylase. Propylation characteristics with the use of a chemical reducing system and purified enzyme. J Biol Chem. 1967 Apr 10;242(7):1509–1516. [PubMed] [Google Scholar]
- VOLCANI B. E., TOOHEY J. I., BARKER H. A. Detection of cobamide coenzymes in microorganisms by the ionophoretic bioautographic method. Arch Biochem Biophys. 1961 Mar;92:381–391. doi: 10.1016/0003-9861(61)90376-9. [DOI] [PubMed] [Google Scholar]
- Vitols E., Blakley R. L. Hydrogen-donor specificity of ribonucleoside triphosphate reductase from Lactobacillus leichmannii. Biochem Biophys Res Commun. 1965 Dec 9;21(5):466–472. doi: 10.1016/0006-291x(65)90406-7. [DOI] [PubMed] [Google Scholar]
- Vitols E., Brownson C., Gardiner W., Blakley R. L. Cobamides and ribonucleotide reduction. V. A kinetic study of the ribonucleoside triphosphate reductase of Lactobacillus leichmannii. J Biol Chem. 1967 Jul 10;242(13):3035–3041. [PubMed] [Google Scholar]
- Vitols E., Walker G. A., Huennekens F. M. Enzymatic conversion of vitamin B-12s to a cobamide coenzyme, alpha-(5,6-dimethylbenzimidazolyl)deoxyadenosylcobamide (adenosyl-B-12). J Biol Chem. 1966 Apr 10;241(7):1455–1461. [PubMed] [Google Scholar]
- WACHSMAN J. T., BARKER H. A. Tracer experiments on glutamate fermentation by Clostridium tetanomorphum. J Biol Chem. 1955 Dec;217(2):695–702. [PubMed] [Google Scholar]
- WACKER A., KIRSCHFELD S., TRAGER L. Die Biosynthese der Desoxyribose bei Bakterien. Z Naturforsch B. 1959 Mar;14B(3):145–150. [PubMed] [Google Scholar]
- WEISSBACH H., LADD J. N., VOLCANI B. E., SMYTH R. D., BARKER H. A. Structure of the adenylcobamide coenzyme: degradation by cyanide, acid, and light. J Biol Chem. 1960 May;235:1462–1473. [PubMed] [Google Scholar]
- WEISSBACH H., PETERKOFSKY A., REDFIELD B. G., DICKERMAN H. STUDIES ON THE TERMINAL REACTION IN THE BIOSYNTHESIS OF METHIONINE. J Biol Chem. 1963 Oct;238:3318–3324. [PubMed] [Google Scholar]
- WEISSBACH H., REDFIELD B. G., DICKERMAN H., BROT N. STUDIES ON METHIONINE BIOSYNTHESIS. EFFECT OF ALKYLCOBAMIDE DERIVATIVES ON THE FORMATION OF HOLOENZYME. J Biol Chem. 1965 Feb;240:856–862. [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- WOOD H. G. A study of carbon dioxide fixation by mass determination of the types of C13-acetate. J Biol Chem. 1952 Feb;194(2):905–931. [PubMed] [Google Scholar]
- WOOD J. M., WOLFE R. S. THE FORMATION OF CH4 FROM N-5-METHYLTETRAHYDROFOLATE MONOGLUTAMATE BY CELL-FREE EXTRACTS OF METHANOBACILLUS OMELIANSKII. Biochem Biophys Res Commun. 1965 Apr 23;19:306–311. doi: 10.1016/0006-291x(65)90459-6. [DOI] [PubMed] [Google Scholar]
- Wagner O. W., Lee H. A., Jr, Frey P. A., Abeles R. H. Studies on the mechanism of action of cobamide coenzymes. Chemical properties of the enzyme-coenzyme complex. J Biol Chem. 1966 Apr 25;241(8):1751–1762. [PubMed] [Google Scholar]
- Weissbach H., Brot N., Lovenberg W. Reduction of cobamides by reduced ferredoxin. J Biol Chem. 1966 Jan 25;241(2):317–321. [PubMed] [Google Scholar]
- Weissbach H., Taylor R. Role of vitamin B12 in methionine synthesis. Fed Proc. 1966 Nov-Dec;25(6):1649–1656. [PubMed] [Google Scholar]
- Weissbach H., Toohey J., Barker H. A. ISOLATION AND PROPERTIES OF B(12) COENZYMES CONTAINING BENZIMIDAZOLE OR DIMETHYLBENZIMIDAZOLE. Proc Natl Acad Sci U S A. 1959 Apr;45(4):521–525. doi: 10.1073/pnas.45.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin M. J., Wolin E. A., Wolfe R. S. The cobalamin product of the conversion of methylcobalamin to CH4 by extracts of methanobacillus omelianskii. Biochem Biophys Res Commun. 1964 Apr 22;15(5):420–423. doi: 10.1016/0006-291x(64)90478-4. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Allam A. M., Brill W. J., Wolfe R. S. Formation of methane from serine by cell-free extracts of Methanobacillus omelianskii. J Biol Chem. 1965 Dec;240(12):4564–4569. [PubMed] [Google Scholar]
- Wood J. M., Wolfe R. S. Alkylation of an enzyme in the methane-forming system of Methanobacillus omelianskii. Biochem Biophys Res Commun. 1966 Jan 4;22(1):119–123. doi: 10.1016/0006-291x(66)90612-7. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Wolfe R. S. Components required for the formation of CH-4 from methylcobalamin by extracts of Methanobacillus omelianskii. J Bacteriol. 1966 Sep;92(3):696–700. doi: 10.1128/jb.92.3.696-700.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M., Wolfe R. S. Propylation and purification of a B12 enzyme involved in methane formation. Biochemistry. 1966 Nov;5(11):3598–3603. doi: 10.1021/bi00875a031. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Wolin M. J., Wolfe R. S. Formation of methane from methyl factor B and methyl factor 3 by cell-free extracts of Methanobacillus omelianskii. Biochemistry. 1966 Jul;5(7):2381–2384. doi: 10.1021/bi00871a030. [DOI] [PubMed] [Google Scholar]
- Zagalak B., Frey P. A., Karabatsos G. L., Abeles R. H. The stereochemistry of the conversion of D and L 1,2-propanediols to propionaldehyde. J Biol Chem. 1966 Jul 10;241(13):3028–3035. [PubMed] [Google Scholar]



