Abstract
Objectives: Metabolic syndrome (MS) is a cluster of metabolic disorders characterized by damage to multiple organs. Platelet distribution width (PDW) has been used to assess the progression of several metabolic disorders, including left ventricular hypertrophy (LVH) and diabetic nephropathy (DN). Therefore, this study aimed to evaluate the predictive value of PDW in relation to organ damage in patients with MS. Methods: The study included 151 patients with MS and 113 healthy controls. Clinicopathological data, including sex, age, abdominal circumference, blood pressure, and body mass index (BMI), were collected. The predictive potential of PDW was assessed by analyzing its correlation with MS progression, LVH, atherosclerosis, and kidney function. Results: The analysis revealed that patients in the MS group had higher levels of BMI, abdominal circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), triglycerides (TG), and fasting plasma glucose (FPG), and lower levels of high-density lipoprotein cholesterol (HDL-C), compared with controls. PDW was positively correlated with BMI, abdominal circumference, SBP, DBP, and FPG, and negatively correlated with HDL-C. FPG, SBP, and HDL-C were identified as independent parameters contributing to changes in PDW. Furthermore, heart function was positively related to PDW levels, while kidney function was negatively related. Logistic regression analysis further demonstrated that PDW was an independent risk factor for LVH, atherosclerosis, and kidney dysfunction. Conclusions: PDW could serve as a promising predictive indicator for organ damage associated with the progression of MS.
Keywords: Atherosclerosis, kidney dysfunction, left ventricular hypertrophy, metabolic syndrome, platelet distribution width
Introduction
Metabolic syndrome (MS) is defined as a cluster of metabolic disorders, including being overweight, obesity, hypertension, high triglycerides, low lipoprotein cholesterol (HDL), and hyperglycemia or diabetes [1,2]. In recent years, numerous studies have shown that the prevalence of MS is rising annually [3] and it affects approximately one-quarter to one-third of adults worldwide [4]. For example, the prevalence of MS in the United States is 34.7% among individuals over 18 years old [5]. In developing countries like China, the overall prevalence of MS is 24.5% among people aged 15 and older [6], with the rate increasing with age [7,8]. Consequently, the growing population with MS has become a significant public health concern, making it critical to reduce the incidence of MS and manage its progression effectively.
Platelet distribution width (PDW) is a commonly used index that reflects the variability in platelet size. A decrease in PDW indicates uniformity in platelet size, while an increase suggests more active platelets with variable sizes [9]. Typically, elevated PDW levels are associated with platelet activation and are closely linked to the onset of various diseases [10]. For instance, studies have demonstrated that patients with hypertension, diabetes, obesity, and dyslipidemia exhibit increased consumption of peripheral platelets, low systemic inflammation, oxidative stress, and insulin resistance. In response to these changes in platelet function, the body compensates by producing larger, more active platelets [10]. Given that the pathophysiology of MS involves inflammation, insulin resistance, oxidative stress, and endothelial dysfunction - factors that can trigger platelet hyperactivity [11] - PDW may hold predictive value for the progression of MS. A similar conclusion was preliminarily drawn by Yang et al. [12], who observed that PDW levels were elevated in older adults with MS. However, their study explored various platelet parameters and did not focus specifically on PDW, leaving its precise role in MS progression unclear.
Recent studies have connected PDW levels with organ damage related to MS. A cross-sectional study by Yarlioglues et al. demonstrated that platelet activation was independently associated with the left ventricular mass index [13]. Similarly, Fujita et al. [14] found a correlation between the degree of left ventricular hypertrophy (LVH) and PDW levels. Yuan et al. [15] also reported significantly higher PDW levels in ischemic stroke patients compared to healthy controls. Additionally, Cui et al. [16] found that patients with diabetic nephropathy had higher PDW levels than those with diabetes alone, suggesting that PDW changes are closely related to the progression of diabetic nephropathy. Taken together, it is reasonable to speculate that there may be an association between organ damage and changes in PDW in patients with MS.
In light of these studies, we conducted a retrospective study involving 151 MS patients to evaluate the potential relationship between PDW and organ damage in MS. Clinicopathological data, PDW, and organ function indicators from both patients and healthy controls were collected and analyzed. The findings of this study may provide valuable information for clinicians in assessing organ damage related to MS.
Materials and methods
Patients and clinicopathological information collection
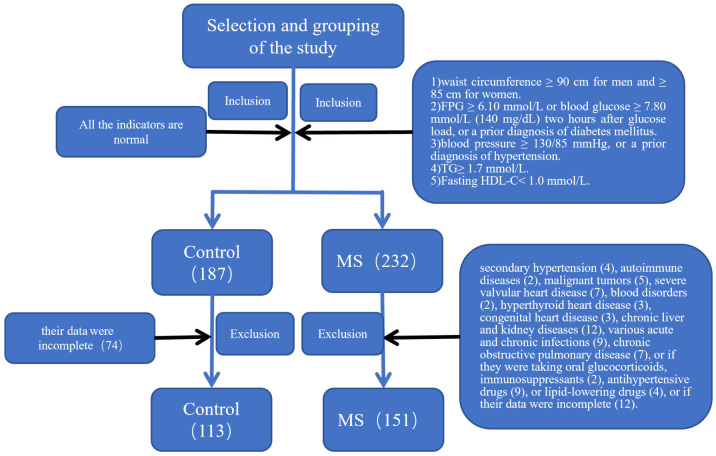
A total of 151 MS patients and 113 healthy controls were enrolled between November 2019 and November 2021 at the General Hospital of Ningxia Medical University (Figure 1). Based on the clinical data from our institute, the estimated probability of elevated platelet distribution width (PDW) in the control group was 15%-20%. Assuming an expected odds ratio (OR) of 2.0, with α = 0.05 and β = 0.2, the required sample size for both the case and control groups was calculated using PASS 11 software, yielding N1 = N2 = 85. Considering a 10% non-response rate, the adjusted required sample size was N1 = N2 = 85 ÷ 0.9 = 95. Since the sample sizes for both the control and case groups in this study exceeded 95, the sample size was deemed sufficient.
Figure 1.
Flow chart for inclusion and exclusion of the patients.
Metabolic syndrome (MS) was diagnosed according to the following criteria [17]: 1) Central and/or abdominal obesity: waist circumference ≥ 90 cm for men and ≥ 85 cm for women. 2) Fasting plasma glucose (FPG) ≥ 6.10 mmol/L (110 mg/dL) or blood glucose ≥ 7.80 mmol/L (140 mg/dL) two hours after glucose load, or a prior diagnosis of diabetes mellitus. 3) Hypertension: blood pressure ≥ 130/85 mmHg, or a prior diagnosis of hypertension. 4) Fasting triglycerides (TG) ≥ 1.7 mmol/L (150 mg/dL). 5) Fasting high-density lipoprotein cholesterol (HDL-C) < 1.0 mmol/L (40 mg/dL). A diagnosis of MS required the presence of three or more of the above criteria. Patients were excluded if they met any of the following conditions: secondary hypertension, autoimmune diseases, malignant tumors, severe valvular heart disease, blood disorders, hyperthyroid heart disease, congenital heart disease, chronic liver and kidney diseases, various acute and chronic infections, chronic obstructive pulmonary disease, or if they were taking oral glucocorticoids, immunosuppressants, antihypertensive drugs, or lipid-lowering drugs, or if their data were incomplete.
Additionally, general clinicopathological information, including sex, age, abdominal circumference, blood pressure, and body mass index (BMI), was collected. All data collection and analysis were approved by the Ethics Committee of the General Hospital of Ningxia Medical University and were conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Routine blood testing
All participants fasted for more than 8 hours prior to blood collection. The following morning, 5 ml of peripheral venous blood was collected for the measurement of PDW, mean platelet volume (MPV), platelet count (PLT), fasting plasma glucose (FPG), serum creatinine (Scr), triglycerides (TG), total cholesterol (TC), high-density HDL-C, and low-density lipoprotein cholesterol (LDL-C). A 95% cut-off value was used as the reference for PDW elevation, with any PDW value above this threshold considered indicative of an increase in platelet distribution width. In this study, the upper limit of the 95% reference value for PDW was 12.15 fL.
Assessment of organ damage
The potential damage to organs associated with metabolic syndrome (MS) was assessed using various strategies. For the heart, the left ventricular diastolic diameter (LVDd), interventricular septal thickness (IVST), left ventricular posterior wall thickness (LVPWT), left ventricular ejection fraction (LVEF), and left ventricular mass index (LVMI) were measured using a GE Vivid 7 cardiac ultrasound system. For the kidneys, the glomerular filtration rate (GFR) was calculated using a simplified MDRD formula [18]: for males, estimated glomerular filtration rate (eGFR) = 175 × Scr (mg/dL)^(-1.234) × age^(-0.179); for females, eGFR = 175 × Scr (mg/dL)^(-1.234) × age^(-0.179) × 0.79. Patients with eGFR < 90 mL/min/m2 were diagnosed with reduced glomerular filtration rate. For the cervical vessels, carotid intima-media thickness (CIMT) was measured using a Philips HD15 color Doppler ultrasound system. The criteria for carotid intima-media thickening were as follows: normal < 1.0 mm, thickening ≥ 1.0 mm, and plaque formation ≥ 1.5 mm.
Statistical analysis
Categorical data were expressed as counts and proportions, and differences between groups were analyzed using chi-square analysis. Normally distributed continuous data were expressed as mean ± standard deviation (SD), while abnormally distributed continuous data were expressed as median (interquartile range). Differences between groups were analyzed using the t-test for normally distributed data and the Wilcoxon rank-sum test for abnormally distributed data. The relationship between PDW and organ damage was analyzed using Pearson correlation analysis for normally distributed data and Spearman correlation analysis for abnormally distributed data. The logistic regression and receiver operating curve (ROC) methods were employed to assess the predictive value of PDW regarding the organ damage of MS patients. For logistic analysis, the 95% percentile was used the reference for elevated PDW, with any PDW value above this threshold considered an indicator of increased platelet distribution width. All statistical analyses and graph plotting were conducted using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, California, USA, www.graphpad.com), with a significance level set at 0.05.
Results
Clinicopathological information
The current analysis included 151 cases of metabolic syndrome (MS) and 113 healthy controls. The MS group comprised 89 males and 62 females, with an average age of 52.07 ± 8.68 years, while the control group included 55 males and 58 females, with an average age of 50.43 ± 8.28 years. There was no significant difference in the male proportion or age between the two groups. Detailed information on other clinicopathological parameters, such as BMI and abdominal circumference, is presented in Table 1. Based on the analysis, patients in the MS group had higher levels of BMI, abdominal circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), TG, and FPG, as well as lower levels of HDL-C compared to the controls (P < 0.001) (Table 1). Moreover, PDW was elevated in the MS group, and this difference was statistically significant (P < 0.001).
Table 1.
Clinicopathological information
| Parameters | Control (n = 113) | MS (n = 151) | F/t/z value | P value |
|---|---|---|---|---|
| Male [n (%)] | 55 (48.67%) | 89 (58.94%) | 2.75 | 0.097 |
| Age (Year) | 50.43 ± 8.28 | 52.07 ± 8.68 | 1.55 | 0.122 |
| BMI (kg/m2) | 22.95 ± 2.43 | 26.27 ± 3.74 | 8.22 | < 0.001 |
| Abdominal circumference (cm) | 80.85 ± 8.01 | 94.11 ± 9.50 | 11.98 | < 0.001 |
| SBP (mmHg) | 119.59 ± 10.83 | 137.09 ± 17.29 | 9.45 | < 0.001 |
| DBP (mmHg) | 73.62 ± 7.98 | 84.77 ± 10.81 | 9.24 | < 0.001 |
| TG (mmol/l) | 1.27 (0.85, 1.74) | 2.11 (1.48, 2.94) | -7.15 | < 0.001 |
| TC (mmol/l) | 4.52 ± 0.90 | 4.52 ± 1.35 | -0.01 | 0.993 |
| LDL-C (mmol/l) | 2.63 ± 0.73 | 2.47 ± 0.94 | -1.57 | 0.120 |
| HDL-C (mmol/l) | 1.29 ± 0.27 | 0.93 ± 0.28 | -10.47 | < 0.001 |
| FPG (umol/l) | 4.98 ± 0.60 | 8.28 ± 1.42 | 11.43 | < 0.001 |
| PDW (%) | 11.50 ± 1.23 | 13.96 ± 2.34 | 10.53 | < 0.001 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; FPG, fasting plasma glucose; PDW, platelet distribution width.
Association between platelet parameters and progression of MS
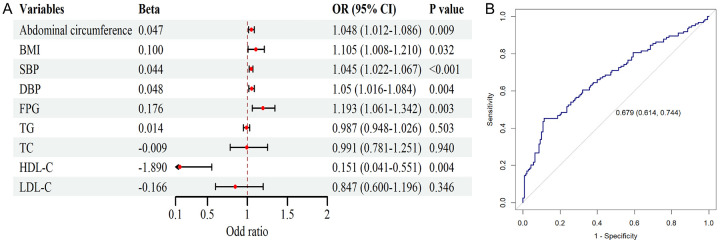
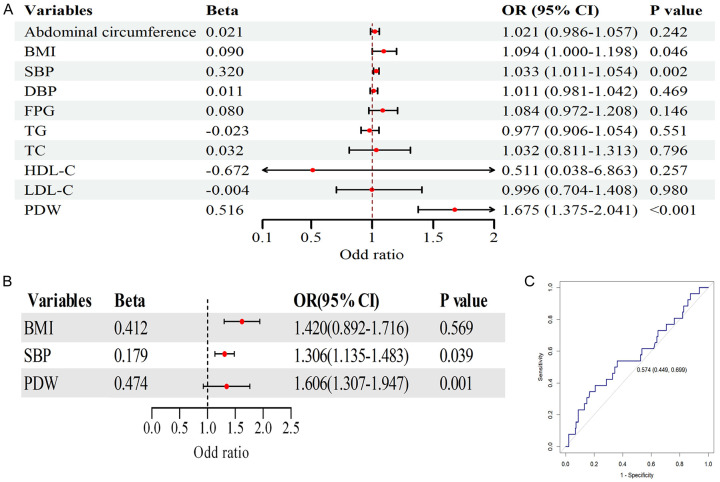
Further logistic regression analysis revealed that, in the univariate analysis with PDW as the response variable, BMI, abdominal circumference, SBP, DBP, FPG, and HDL-C significantly influenced the elevation of PDW (P < 0.05) (Figure 2A). However, in the multivariate analysis including these parameters, only FPG, SBP, and HDL-C were identified as independent factors contributing to the changes in PDW (P < 0.05) (Table 2). The predictive value of PDW regarding organ damage in MS patients was further analyzed using ROC analysis, and the result showed that the model’s AUC was 0.679 (95% CI: 0.614-0.744), with a specificity of 75% and a sensitivity of 62% (Figure 2B). This indicates that the model can effectively identify most cases of the disease while minimizing false positives to the greatest extent possible, which provides an economical and effective approach for screening MS patients in community settings.
Figure 2.
Univariate logistic regression analysis of factors influencing MS progression and ROC analysis of the predictive value of platelet distribution width (PDW) regarding metabolic syndrome (MS) progression. A. Forest plot of the logistic analysis. B. ROC analysis of PDW the predictive value of PDW regarding MS progression. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Table 2.
Multivariate logistic regression analysis of clinicopathological parameters and platelet distribution width (PDW)
| Parameter | B | S.E. | Wald | P value | OR value |
|---|---|---|---|---|---|
| FPG | 0.170 | 0.068 | 6.183 | 0.013* | 1.186 |
| HLD-C | -1.898 | 0.746 | 6.480 | 0.011* | 0.150 |
| SBP | 0.039 | 0.014 | 7.546 | 0.006* | 1.400 |
FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; SBP, systolic blood pressure.
represents P < 0.05.
Association between PDW and organ damags in MS patients
The subsequent correlation analysis demonstrated that LVDd, IVST, LVPWT, LVMI, CIMT, and Scr were positively correlated with PDW levels, while ejection fraction (EF) and eGFR were negatively correlated with PDW levels (Table 3). This indicates that changes in PDW correspond to changes in overall organ function.
Table 3.
Association between platelet distribution width (PDW) and organ damages detected by correlation analysis
| Parameter | R value | P value |
|---|---|---|
| LVDd | 0.361 | < 0.001 |
| IVST | 0.258 | 0.014 |
| LVPWT | 0.276 | 0.031 |
| LVMI | 0.299 | < 0.001 |
| EF | -0.391 | < 0.001 |
| CIMT | 0.438 | < 0.001 |
| Scr | 0.396 | < 0.001 |
| eGFR | -0.309 | < 0.001 |
LVDd, left ventricular diastolic diameter; IVST, interventricular septal thickness; LVPWT, left ventricular posterior wall thickness; LVMI, left ventricular mass index; Scr, serum creatinine; eGFR, estimated glomerular filtration rate.
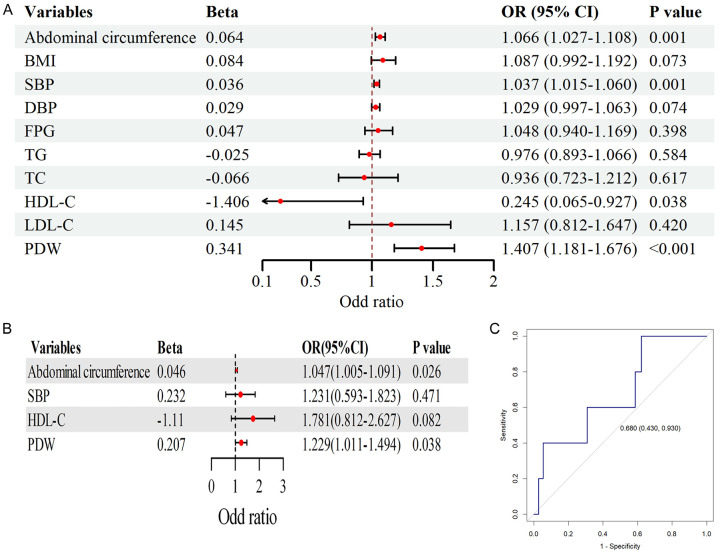
To further investigate the predictive value of PDW for target organ damage in MS patients, we performed logistic regression analysis combined with ROC curve analysis. For LVH, univariate logistic regression analysis (Figure 3A) revealed that waist circumference, SBP, HDL-C, and PDW were significantly associated with elevated LVMI (P < 0.05). The multivariate logistic regression analysis (Figure 3B) confirmed PDW as an independent predictor of LVMI (P < 0.05), consistent with the ROC curve analysis (Figure 3C), which showed good predictive performance (AUC = 0.680, 95% CI: 0.430-0.930).
Figure 3.
Univariate logistic regression analysis of factors influencing left ventricular hypertrophy (LVH), multivariate logistic regression analysis of LVH in relation to platelet distribution width (PDW) and other factors, and receiver operating curve (ROC) analysis of the predictive value of PDW for LVH. A. Forest plot of univariate logistic analysis. B. Forest plot of multivariate logistic analysis. C. ROC analysis of PDW, demonstrating the predictive value of PDW for LVH. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; FPG, fasting plasma glucose.
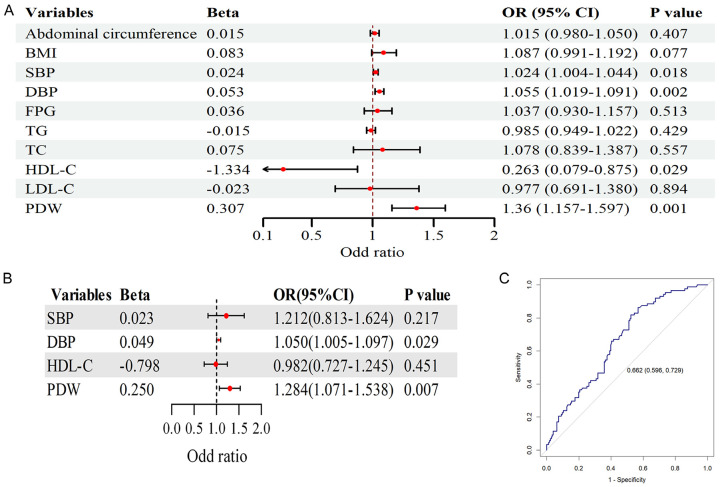
Similarly, for CIMT, representing atherosclerosis, univariate logistic regression analysis (Figure 4A) showed significant associations between elevated CIMT and SBP, DBP, HDL-C, and PDW (P < 0.05). Multivariate logistic regression analysis (Figure 4B) also identified PDW as an independent predictor of elevated CIMT (P < 0.05), although the ROC curve (Figure 4C) indicated moderate predictive ability (AUC = 0.662, 95% CI: 0.596-0.729).
Figure 4.
Univariate logistic regression analysis of factors influencing atherosclerosis, multivariate logistic regression analysis of atherosclerosis in relation to platelet distribution width (PDW) and other factors, and receiver operating curve (ROC) analysis of the predictive value of PDW for atherosclerosis. A. Forest plot of the univariate logistic analysis. B. Forest plot of the multivariate logistic analysis. C. ROC analysis showing the predictive value of PDW for atherosclerosis. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; FPG, fasting plasma glucose.
Finally, regarding renal function, univariate logistic regression analysis (Figure 5A) demonstrated significant associations between eGFR and BMI, SBP, and PDW (P < 0.05). Multivariate logistic regression analysis (Figure 5B) confirmed PDW as an independent predictor of eGFR (P < 0.05), and the ROC curve (Figure 5C) displayed moderate predictive performance (AUC = 0.574, 95% CI: 0.449-0.699). These findings consistently suggest that PDW is an independent predictor of target organ damage in MS patients.
Figure 5.
Univariate logistic regression analysis of factors influencing renal dysfunction, multivariate logistic regression analysis of renal dysfunction in relation to platelet distribution width (PDW) and other factors, and receiver operating curve (ROC) analysis of the predictive value of PDW for atherosclerosis. A. Forest plot of the univariate logistic analysis. B. Forest plot of the multivariate logistic analysis. C. ROC analysis of PDW, demonstrating the predictive value of PDW for renal insufficiency. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; FPG, fasting plasma glucose.
Discussion
Metabolic syndrome (MS) is a multifactorial disorder, characterized by a combination of several metabolic risk factors including abdominal obesity, hypertension, dyslipidemia, and insulin resistance [19,20]. It significantly increases the risk of developing cardiovascular diseases, type 2 diabetes, and chronic kidney disease, contributing to a substantial burden on public health globally [2,3,5]. As the prevalence of MS continues to rise, particularly in aging populations and in countries undergoing rapid industrialization like China, identifying effective biomarkers for early diagnosis and prediction of disease progression has become crucial for mitigating its long-term impact [7]. Multiple studies have confirmed that MS is a risk factor for target organ damage, including atherosclerotic disease, chronic kidney disease, and ventricular hypertrophy [21-23]. The long-term course of MS increases the risk of poor prognosis and mortality rates among MS patients. Therefore, early and accurate prediction of MS is crucial for effective clinical management of the disorder.
In the current study, we employed PDW as a potential predictive indicator for the progression of MS. PDW, a marker of platelet heterogeneity, has been recognized as a reflection of platelet activation, which is known to play a pivotal role in inflammatory processes, vascular injury, and thrombosis [10]. Elevated PDW is commonly seen in conditions characterized by systemic inflammation, such as obesity, diabetes, hypertension, and dyslipidemia - all of which are integral components of MS [11]. We analyzed data from 264 patients, and the results showed PDW levels were significantly elevated in MS patients and was in a positive correlation with impaired function in the arteries, heart, and kidneys. This observation is consistent with previous studies that have suggested a strong association between platelet activation and MS-related factors, such as insulin resistance, oxidative stress, and endothelial dysfunction [10,11,24]. In particular, insulin resistance, a hallmark of MS, has been shown to induce platelet hyperactivity by altering platelet function and enhancing platelet aggregation [25]. These pathophysiological mechanisms are thought to contribute to increased platelet turnover, resulting in larger and more heterogeneous platelets, thereby elevating PDW. Our findings support this link and suggest that PDW may serve as a novel biomarker to monitor the metabolic disturbances underlying MS and predict its progression. Furthermore, we observed that PDW levels correlated with clinical markers of MS progression, including abdominal circumference, BMI, FPG, and TG. These results underline the central role of metabolic dysregulation in driving platelet activation and implicate PDW as a useful tool for both early detection and continuous monitoring of MS-related complications. Furthermore, ROC curve analysis indicated that PDW was an independent risk factor for the progression of MS and could be employed as a promising predictor regarding target organ damage in MS patients. Specifically, the control group data allow us to demonstrate a significant difference in the mean PDW between patients with metabolic syndrome and healthy controls. This comparison strengthens our conclusion that elevated PDW is characteristic of metabolic syndrome, rather than being a result of potential confounding factors.
With the long-term effects of MS progression, LVDd, IVST, LVPWT, LVMI, MT, and Scr levels were significantly increased in patients, while EF and eGFR were significantly decreased [26-29]. Our data suggest that patients with MS in the current study exhibited damage to organ function in the heart, brain, and kidneys as verified by these the previous studies. Additionally, the impairments in these indicators were all associated with changes in PDW, and logistic regression analyses showed that PDW was an independent risk factor for left ventricular hypertrophy, renal function decline, and carotid atherosclerosis. The study by Fujita et al. demonstrated that in patients with cardiovascular disorders, PDW was positively correlated with left ventricular hypertrophy [14]. The research by Gary et al. indicated that platelet activation led to increased blood viscosity and inflammation, closely related to atherosclerotic plaques [30,31]. Other studies have shown that elevated PDW levels could induce avascular necrosis of renal tissue, contributing to decreased renal function [32,33]. These findings support our results that elevated PDW can predict the progression of MS.
Nevertheless, the biological mechanisms underlying the relationship between MS progression and elevated PDW have not been fully elucidated, although several possibilities have been proposed. Generally, MS-induced impairments lead to abnormal function of vascular endothelial cells, resulting in roughened blood vessel walls. This dysfunction in the vascular system facilitates platelet adhesion and aggregation, ultimately causing excessive platelet consumption in peripheral blood, which leads to uneven platelet volume and increased levels of PDW and mean platelet volume (MVP). It is well recognized that platelet activation and aggregation play key roles in vascular intimal injury, atherosclerosis, and thrombosis. Under the influence of multiple factors, platelets in MS patients exhibit hyperfunction, leading to inflammation and resulting in pathological reactions such as left ventricular hypertrophy, carotid atherosclerosis, and decreased glomerular filtration rate [34]. Additionally, a significant amount of oxygen radicals and lipid peroxidation in MS patients can also lead to the production of excessive platelet aggregators in MS patients, resulting in elevated PDW levels. Other mechanisms, such as insulin resistance-induced PDW elevation [25] and nitric oxide-induced PDW elevation, have also been proposed and verified by different studies [35].
In the current study, aside from PDW, the level of platelet count (PLT) also significantly increased, consistent with previously published works [36,37], suggests that obesity, abnormal glucose metabolism, abnormal lipid metabolism, and hypertension associated with MS can further aggravate endothelial cell damage and disrupt normal platelet function [38]. Consequently, the decreased number of platelets in peripheral blood diminishes feedback stimulation, leading to compensatory proliferation of new platelets in the bone marrow and ultimately increasing platelet production and elevating PDW levels.
Despite the promising findings, there are several limitations to this study. First, the cross-sectional design prevents us from drawing causal inferences regarding the relationship between PDW and organ damage in MS patients. Longitudinal studies are needed to confirm whether elevated PDW is a causative factor in the development of organ damage or merely a consequence of the metabolic disturbances in MS. Future research should aim to track PDW levels over time and assess their ability to predict the onset and progression of cardiovascular, renal, and cerebrovascular complications in MS. Second, although we found significant associations between PDW and organ damage markers, the underlying mechanisms driving this relationship remain unclear. While platelet activation is well established as a key factor in the development of vascular and renal damage, more research is needed to elucidate the molecular pathways that link PDW elevation to target organ injury in MS patients. Investigating the role of platelet-derived mediators, such as growth factors and inflammatory cytokines, could provide a deeper understanding of how PDW contributes to the pathophysiology of MS-related organ damage. Third, while our study was conducted at a single center with a well-defined cohort of MS patients, future studies should aim to validate these findings in larger, more diverse populations. The generalizability of our results may be limited by the sample size and the demographic characteristics of our patient population. Multicenter studies with larger sample sizes and more diverse populations would help confirm the role of PDW as a predictive biomarker for MS and its complications.
Conclusion
Collectively, this retrospective analysis involving 151 MS patients at a single center demonstrates that PDW could serve as a promising predictive indicator for organ damage associated with MS progression. Additionally, given that PDW differs from traditional risk factors for MS, combining the two strategies may improve the predictive accuracy of MS. However, this analysis has limitations, including selection bias and a relatively small sample size. Further multicenter studies with larger sample sizes are needed to validate these conclusions.
Disclosure of conflict of interest
None.
References
- 1.Martemucci G, Fracchiolla G, Muraglia M, Tardugno R, Dibenedetto RS, D’Alessandro AG. Metabolic syndrome: a narrative review from the oxidative stress to the management of related diseases. Antioxidants (Basel) 2023;12:2091. doi: 10.3390/antiox12122091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geerling E, Hameed M, Weger-Lucarelli J, Pinto AK. Metabolic syndrome and aberrant immune responses to viral infection and vaccination: insights from small animal models. Front Immunol. 2022;13:1015563. doi: 10.3389/fimmu.2022.1015563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young DR, Hivert MF, Alhassan S, Camhi SM, Ferguson JF, Katzmarzyk PT, Lewis CE, Owen N, Perry CK, Siddique J, Yong CM Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; and Stroke Council. Sedentary behavior and cardiovascular morbidity and mortality: a science advisory from the American Heart Association. Circulation. 2016;134:e262–279. doi: 10.1161/CIR.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 5.Scuteri A, Laurent S, Cucca F, Cockcroft J, Cunha PG, Mañas LR, Mattace Raso FU, Muiesan ML, Ryliškytė L, Rietzschel E, Strait J, Vlachopoulos C, Völzke H, Lakatta EG, Nilsson PM Metabolic Syndrome and Arteries Research (MARE) Consortium. Metabolic syndrome across Europe: different clusters of risk factors. Eur J Prev Cardiol. 2015;22:486–491. doi: 10.1177/2047487314525529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. 2020;323:2526–2528. doi: 10.1001/jama.2020.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li R, Li W, Lun Z, Zhang H, Sun Z, Kanu JS, Qiu S, Cheng Y, Liu Y. Prevalence of metabolic syndrome in Mainland China: a meta-analysis of published studies. BMC Public Health. 2016;16:296. doi: 10.1186/s12889-016-2870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bankoski A, Harris TB, McClain JJ, Brychta RJ, Caserotti P, Chen KY, Berrigan D, Troiano RP, Koster A. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care. 2011;34:497–503. doi: 10.2337/dc10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yilmaz Z, Eralp O, Ilcol YO. Evaluation of platelet count and its association with plateletcrit, mean platelet volume, and platelet size distribution width in a canine model of endotoxemia. Vet Clin Pathol. 2008;37:159–163. doi: 10.1111/j.1939-165X.2008.00023.x. [DOI] [PubMed] [Google Scholar]
- 10.Batista TR, Figueiredo RC, Rios DRA. Platelets volume indexes and cardiovascular risk factors. Rev Assoc Med Bras (1992) 2018;64:554–559. doi: 10.1590/1806-9282.64.06.554. [DOI] [PubMed] [Google Scholar]
- 11.Santilli F, Vazzana N, Liani R, Guagnano MT, Davì G. Platelet activation in obesity and metabolic syndrome. Obes Rev. 2012;13:27–42. doi: 10.1111/j.1467-789X.2011.00930.x. [DOI] [PubMed] [Google Scholar]
- 12.Yang XJ, Zhang LY, Ma QH, Sun HP, Xu Y, Chen X, Pan CW. Platelet parameters in Chinese older adults with metabolic syndrome. Endocr Connect. 2020;9:696–704. doi: 10.1530/EC-20-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarlioglues M, Kaya MG, Ardic I, Dogdu O, Kasapkara HA, Gunturk E, Akpek M, Kalay N, Dogan A, Ozdogru I, Oguzhan A. Relationship between mean platelet volume levels and subclinical target organ damage in newly diagnosed hypertensive patients. Blood Press. 2011;20:92–97. doi: 10.3109/08037051.2010.532317. [DOI] [PubMed] [Google Scholar]
- 14.Fujita S, Takeda Y, Kizawa S, Ito T, Sakane K, Ikemoto T, Okada Y, Sohmiya K, Hoshiga M, Ishizaka N. Platelet volume indices are associated with systolic and diastolic cardiac dysfunction, and left ventricular hypertrophy. BMC Cardiovasc Disord. 2015;15:52. doi: 10.1186/s12872-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou L, Yuan YH, Jiang H, Shen LY, Zeng GL. Analysis of changes in platelet volume and distribution width in patients with ischemic stroke (translated) Contemporary Medical Symposium. 2019;17:83–83. [Google Scholar]
- 16.Cui XQ. Changes and significance of blood lipid and platelet parameters in patients with type 2 diabetic nephropathy (translated) Clinical Medicine. 2006;08:79–80. [Google Scholar]
- 17.Ge JB, Xu YJ. Internal Medicine. Beijing: People’s Medical Publishing House; 2018. [Google Scholar]
- 18.Microvascular Complications Group DS, Chinese Medical Association. Expert in prevention and treatment of kidney disease of sugar urine disease. Chinese Journal of Diabetes Mellitus. 2018;6:792–801. [Google Scholar]
- 19.Wu L, Han D, Xue Y, He S, Ma Z, Su S, Li P, Liu S, Zhou H. Association between the C-reactive protein-albumin-lymphocyte index and metabolic syndrome: evidence from the 2003-2010 national health and nutrition examination survey. Diabetol Metab Syndr. 2025;17:39. doi: 10.1186/s13098-025-01609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilar G, Suhandi C, Fukunaga K, Shigeno M, Kawahata I, Abdulah R, Sasaki T. Effects of nanocurcumin supplementation on metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2025:107641. doi: 10.1016/j.phrs.2025.107641. [DOI] [PubMed] [Google Scholar]
- 21.Luo X, Wang Y, Wang L, Shen Y, Ren M. Association between female androgen levels, metabolic syndrome, and cardiovascular disease: an NHANES analysis (2013-2016) Int J Womens Health. 2024;16:2087–2101. doi: 10.2147/IJWH.S475149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostić S, Tasić I, Stojanović N, Rakočević J, Deljanin Ilić M, Đorđević D, Stoičkov V, Tasić I. Impact of obesity on target organ damage in patients with metabolic syndrome. Diagnostics (Basel) 2024;14:1569. doi: 10.3390/diagnostics14141569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petramala L, Gigante A, Sarlo F, Servello A, Circosta F, Marino L, Ciccarelli A, Cavallaro G, Letizia C. Relevance of obesity-related organ damage and metabolic syndrome classification in cardiovascular and renal risk stratification in patients with essential hypertension. Front Cardiovasc Med. 2024;11:1369090. doi: 10.3389/fcvm.2024.1369090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T, Xu J, Fu L, Li L. Hypertriglyceridemia is associated with platelet hyperactivation in metabolic syndrome patients. Int J Clin Pract. 2020;74:e13508. doi: 10.1111/ijcp.13508. [DOI] [PubMed] [Google Scholar]
- 25.Trovati M, Anfossi G. Influence of insulin and of insulin resistance on platelet and vascular smooth muscle cell function. J Diabetes Complications. 2002;16:35–40. doi: 10.1016/s1056-8727(01)00196-9. [DOI] [PubMed] [Google Scholar]
- 26.Smith DO, LeRoith D. Insulin resistance syndrome, pre-diabetes, and the prevention of type 2 diabetes mellitus. Clin Cornerstone. 2004;6:7–6. doi: 10.1016/s1098-3597(04)80050-4. [DOI] [PubMed] [Google Scholar]
- 27.Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodés-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 29.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 30.Gary T, Pichler M, Belaj K, Hafner F, Gerger A, Froehlich H, Eller P, Rief P, Hackl G, Pilger E, Brodmann M. Platelet-to-lymphocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS One. 2013;8:e67688. doi: 10.1371/journal.pone.0067688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balta S, Demırkol S, Kucuk U. The platelet lymphocyte ratio may be useful inflammatory indicator in clinical practice. Hemodial Int. 2013;17:668–669. doi: 10.1111/hdi.12058. [DOI] [PubMed] [Google Scholar]
- 32.Song J, Li Y, Han T, Wu J, Lou T, Zhang J, Ye Z, Peng H. The difference between nocturnal dipping status and morning blood pressure surge for target organ damage in patients with chronic kidney disease. J Clin Hypertens (Greenwich) 2020;22:2025–2034. doi: 10.1111/jch.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turak O, Afsar B, Siriopol D, Ozcan F, Cagli K, Yayla C, Oksuz F, Mendi MA, Kario K, Covic A, Kanbay M. Morning blood pressure surge as a predictor of development of chronic kidney disease. J Clin Hypertens (Greenwich) 2016;18:444–448. doi: 10.1111/jch.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badimon L, Vilahur G. Platelets, arterial thrombosis and cerebral ischemia. Cerebrovasc Dis. 2007;24(Suppl 1):30–39. doi: 10.1159/000107377. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira IA, Mocking AI, Feijge MA, Gorter G, van Haeften TW, Heemskerk JW, Akkerman JW. Platelet inhibition by insulin is absent in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2006;26:417–422. doi: 10.1161/01.ATV.0000199519.37089.a0. [DOI] [PubMed] [Google Scholar]
- 36.Wu ZR, Yang K, Ping Z, Guo XH, Tao LX, Cao K, Guo J. A study of the association between changes in platelet count and metabolic syndrome and its components (translated) Chinese Journal of Convalescent Medicine. 2016;25:225–228. [Google Scholar]
- 37.Zhao YJ, Wang X, Lin XQ, Cheng Y, Chi FY. Relationship between white blood cell, platelet and metabolic syndrome in Fuzhou area. China Health Standard Management. 2021;11:12–14. [Google Scholar]
- 38.Zhang HY, Zhang T, Zhang XG. Study on the relationship between platelet parameters and vaso-complication of type two diabetes. Modern Preventive Medicine. 2013;39:6455–6457. [Google Scholar]