Abstract
Objective: To investigate the diagnostic value of electroencephalogram (EEG) combined with neuron-specific enolase (NSE) detection in differentiating febrile seizures and to evaluate their predictive value for brain function prognosis in pediatric patients. Methods: A retrospective analysis was performed on the medical records of 95 pediatric patients with febrile seizures treated at the Second People’s Hospital of Jingdezhen City from January 2021 to December 2023. Of these, 40 cases of simple febrile seizure (SFS) patients were categorized into Group A, and 55 cases of complex febrile seizure (CFS) patients were included in Group B. Brainwave data was collected within 72 hours of the seizure, and NSE levels were measured at 12 and 48 hours post-seizure, as well as immediately after treatment. Venous blood (1-2 mL) was drawn and tested within 2 hours. The number and incidence of abnormal EEG findings, along with NSE levels, were recorded. The diagnostic value of EEG, NSE, and their combined application in febrile seizures, as well as their predictive value for brain function prognosis, were analyzed. Results: The incidence of abnormal EEG in Group B was notably higher than that in Group A (P=0.038). Additionally, the NSE level in Group B was consistently higher at all time points compared to Group A (all P<0.05). The area under the curve (AUC) for EEG, NSE, and their combined detection in differential diagnosis of febrile seizures was 0.600, 0.807, and 0.814, respectively. The specificity for these measures was 80.00%, 85.00%, and 75.00%, while the sensitivity was 40.00%, 72.73%, and 78.18%, respectively. The AUCs of EEG, NSE and their combined detection for predicting the prognosis of febrile seizures in children was 0.745, 0.878 and 0.951, with the specificity of 66.67%, 58.72% and 81.48%, and the sensitivity of 82.35%, 73.53% and 100.00%, respectively. Logistic multivariate analysis revealed that EEG findings, febrile seizure type, perinatal abnormalities, and NSE levels were independent risk factors affecting post-seizure sequelae in pediatric patients with febrile seizures. Conclusion: The combination of EEG and NSE is valuable for the differential diagnosis of febrile seizures and offers strong predictive power for brain function prognosis. Their combined detection enhances diagnostic accuracy and offers substantial practical benefits.
Keywords: Electroencephalogram, neuron-specific enolase, febrile seizure
Introduction
Febrile seizures are among the most common types of neurological disorders in children, and strongly associated with high fever [1,2]. Research indicates that febrile seizures may be linked to genetic factors, infectious diseases, the severity of fever, and family history [3,4]. In addition, age and gender may also influence the occurrence of febrile seizures [5]. While most febrile seizures have favorable outcome, they can lead to long-term neurological consequences in some children [6]. Therefore, accurate diagnosis and reliable prediction of brain function prognosis in children with febrile seizures holds significant clinical significance.
Electroencephalogram (EEG) is a commonly used neuroelectrophysiological tool for assessing nervous system function and injury [7]. As a non-invasive detection method, EEG monitors the brain’s electrophysiological state by recording electrical signals from the scalp [8]. It offers insights into the spatiotemporal distribution and spectral characteristics of brain electrical activity, making it crucial for diagnosing febrile seizures and assessing prognosis [9].
Neuron-specific enolase (NSE) is an enzymatic protein mainly present in the cytoplasm of neurons [10]. It is considered a key neuronal marker, with its concentration level reflecting the metabolic activity and extent of neuronal damage [11]. In cases of neurological injury, the release of NSE typically increases. Therefore, measuring NSE levels in the serum or cerebrospinal fluid can provide valuable information about the extent of neuronal damage [12].
There may be limitations in using EEG or NSE alone as assessment markers. This study aims to evaluate the combined detection of EEG and NSE in differential diagnosis of children with febrile seizures and assess their predictive value for brain function prognosis, thereby providing more reliable evidence for clinical decision-making.
Patients and methods
Study cohort
This retrospective study was conducted at the Second People’s Hospital of Jingdezhen after being approved by the hospital’s Ethics Committee. Medical records of 95 pediatric patients with febrile seizures who were treated in the outpatient and inpatient departments between January 2021 and December 2023 were analyzed.
Inclusive criteria: 1. Diagnosis of Febrile Seizures: All patients met the clinical criteria for febrile seizures based on established guidelines [13]. 2. Age Range: Children aged between 1 and 5 years. 3. Fever: Presence of fever (temperature ≥38°C) associated with the seizure episode. 4. Seizure Characteristics: Seizures were generalized and short, lasting less than 15 minutes without recurrence within 24 hours for simple febrile seizures. For complex febrile seizures, patients met criteria such as focal onset, lasting longer than 15 minutes, or recurring within 24 hours.
Exclusive criteria: 1. Systemic Metabolic Disorders: Children with known systemic metabolic disorders were excluded to avoid confounding factors that might affect seizure activity. 2. Intracranial Infections: Children with intracranial infections (e.g., meningitis, encephalitis) were excluded to ensure that the seizures were truly febrile and not due to a primary central nervous system infection. 3. Hepatic and Renal Insufficiency: Patients with significant hepatic or renal insufficiency were excluded, as these conditions could influence seizure activity and overall health. 4. Neurological Dysfunction: Children with pre-existing neurological dysfunction or developmental delays were excluded to eliminate other potential causes of seizures. 5. History of Trauma: Children with recent head trauma were excluded to rule out seizures secondary to traumatic brain injury. 6. Other Underlying Conditions: Children with chronic conditions or treatments (e.g., ongoing chemotherapy, immunosuppressive therapy) that could influence their overall health or seizure susceptibility were excluded.
Febrile seizures primarily involve two common types: simple febrile seizure (SFS) and complex febrile seizure (CFS). The clinical characteristics of these two types are detailed in Table 1.
Table 1.
Clinical characteristics of different types of febrile seizures
| Simple febrile seizure (SFS) | Complex febrile seizure (CFS) | |
|---|---|---|
| Type of seizure | Generalized seizures with short duration, usually lasting a few seconds to several minutes. | Seizures last more than 15 minutes and may present as partial seizures or multiple episodes. |
| Fever characteristics | Typically associated with high fever (≥38°C/100.4°F), often caused by upper respiratory tract infections or other infectious diseases. | May develop with low-grade fever or without significantly high temperatures. |
| State of consciousness | Children often experience unconsciousness or confusion during the episode. | Children may show alterations in consciousness, appearing dull, sluggish, or displaying psychiatric disorder. |
| Systemic features/local features | Limb convulsions, tonic-clonic seizures, or paroxysmal seizures affecting the entire body. | Partial seizures, involving specific body part, such as the face or limbs, rather than generalized convulsions. |
| Prognosis | Generally favorable, with rare occurrence of neurological sequelae. | Relatively poorer prognosis, possibly associated with underlying nervous system abnormalities or other conditions. |
Data collection
Number and incidence of abnormal EEG findings
We recorded the frequency and incidence of abnormal EEG findings from patients’ medical records. EEG abnormalities encompass various abnormal waveforms, frequency deviations, and temporal or spatial anomalies, among others. The number of individuals with abnormal EEG findings refers to the count of individuals within the sample who exhibited such abnormalities; while the incidence of refers to the proportion or percentage of these individuals with abnormal EEG findings in the entire sample. These parameters reflect the extent and frequency of EEG abnormalities in the study population. EEG examinations were conducted within 72 hours after the febrile seizure episodes. During the procedure, the child remained awake, with eyes closed, and seated quietly. Scalp electrodes were placed according to the international 10-20 system, using the earlobes as reference electrodes. A monopolar lead was used for recording, with a high-frequency filter set at 30 Hz and a notch filter at 50 Hz. The examination included evoked response tests such as eye-opening/closing and hyperventilation. The EEG data were collected using the SOLAR ROVER 7000n Neurocentral Analysis System (a 70000B multi-parameter dynamic EEG monitor) manufactured by Beijing Solar Electronic Technologies Co., Ltd. The brainwave data for each selected patient were collected within 72 hours of the seizure.
NSE level
NSE is an enzymatic protein found in cells of the nervous system, which is elevated in conditions such as brain injuries, brain tumors, or neurological disorders. In this study, we recorded NSE levels to reflect neuronal damage or cell death. The Quantitative Determination Reagent Kit for 21-1 (produced by Roche Diagnostics (Shanghai) Ltd.), was used for the detection, employing an ELISA and electrochemiluminescence method. Although the kit is often associated with non-small cell lung cancer detection, it is also effective in assessing NSE levels in neurological contexts. NSE levels were measured at 12 and 48 hours after the seizure, as well as immediately after the treatment. For all the selected patients, 1-2 mL of venous blood was drawn and tested within 2 hours.
Other related outcome assessments
The diagnostic values of EEG, NSE, and their combined application in children with febrile seizures were analyzed.
Further, the children were sub-grouped into a favorable outcome group (n=68) and an unfavorable outcome group (n=27) based on whether the children experienced residual sequelae. The predictive values of EEG, NSE, and their combined application for brain function prognosis in children with febrile seizure were analyzed.
Statistical analysis
SPSS 26.0 and GraphPad Prism 8 were utilized for statistical analysis and visual presentation of results in this study. Count data, expressed as percentages (%), were compared between two groups using the chi-square test (χ2). Measurement data, expressed as Means ± SD, were compared between the two groups using independent sample t-test. The receiver operating characteristic (ROC) curve was utilized to evaluate the diagnostic values of EEG, NSE and their combined detection in differentiating children with febrile seizures and their effectiveness in predicting brain function prognosis. Logistic regression analysis was utilized to analyze the risk factors influencing patient prognosis. A P<0.05 was considered statistically significant in all analyses.
Results
Patient characteristics
A total of 118 patients with medical records were screened. After applying patient selection criteria, 95 patients of febrile seizure were included in the final study population: 40 cases of SFS (Group A) and 55 cases of CFS (Group B). In Group A, the age of the patients ranged from 1 to 6 years, with an average age of (3.68±1.19) years. There were 22 males and 18 females in group A. In Group B, the age of the patients ranged from 1 to 10 years, with an average age of (4.31±1.79) years. There were 29 males and 26 females in group B (Table 2).
Table 2.
Comparison of the general data between the two groups
| Group A (n=40) | Group B (n=55) | t/χ2 | P | |
|---|---|---|---|---|
| Average age | 3.68±1.19 | 4.31±1.79 | 1.947 | 0.055 |
| Sex (male/female) | 22/18 | 29/26 | 0.048 | 0.826 |
Comparison of incidence of abnormal EEG findings between the two groups
The electroencephalogram (EEG) showed a significantly higher incidence of abnormalities in Group B compared to Group A (*P*=0.038, Table 3).
Table 3.
Comparison of incidence of abnormal electroencephalogram (EEG) findings between the two groups
| n | Abnormality | Percentage (%) | χ2 | P | |
|---|---|---|---|---|---|
| Group A | 40 | 8 | 20.00 | 4.287 | 0.038 |
| Group B | 55 | 22 | 40.00 |
Comparison of NSE levels between the two groups
The comparison of NSE levels at 12 hours and 48 hours after febrile seizure onset, as well as post-treatment, revealed that the NSE levels in Group B were notably higher than those in Group A across all time points (all P<0.05), the details are shown in Table 4 and Figures 1, 2.
Table 4.
NSE levels in the two groups at 12, 48 hours after febrile seizure and after treatment
| n | Minimum value | Maximum value | Median | Mean | SD | SE | ||
|---|---|---|---|---|---|---|---|---|
| Group A | 12 h after febrile seizure | 40 | 7.80 | 21.47 | 14.72 | 14.46 | 3.23 | 0.51 |
| Group B | 12 h after febrile seizure | 55 | 9.12 | 22.34 | 15.93 | 15.83 | 2.50 | 0.34 |
| Group A | 48 h after febrile seizure | 40 | 5.20 | 15.25 | 11.155 | 10.91 | 1.94 | 0.31 |
| Group B | 48 h after febrile seizure | 55 | 8.11 | 20.89 | 13.68 | 13.90 | 3.02 | 0.41 |
| Group A | Post-treatment febrile seizure | 40 | 6.46 | 12.96 | 9.42 | 9.33 | 1.65 | 0.26 |
| Group B | Post-treatment febrile seizure | 55 | 6.21 | 14.54 | 10.70 | 10.43 | 1.83 | 0.25 |
Note: NSE, neuron-specific enolase.
Figure 1.
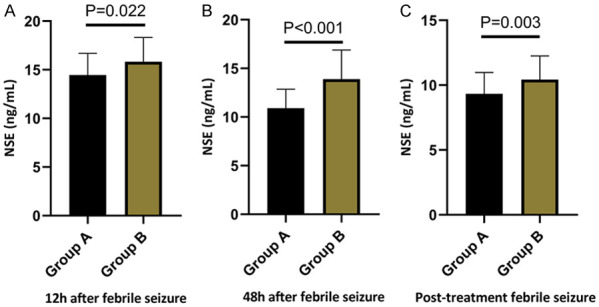
Comparison of NSE levels between the two groups at 12, 48 hours post-seizure and post-treatment. A: Comparison of NSE levels between the two groups at 12 h after febrile seizure. B: Comparison of NSE levels between the two groups at 48 h after febrile seizure. C: Comparison of NSE levels between the two groups after treatment. Note: NSE, neuron-specific enolase; SFS, Simple febrile seizure; CFS, Complex febrile seizure.
Figure 2.
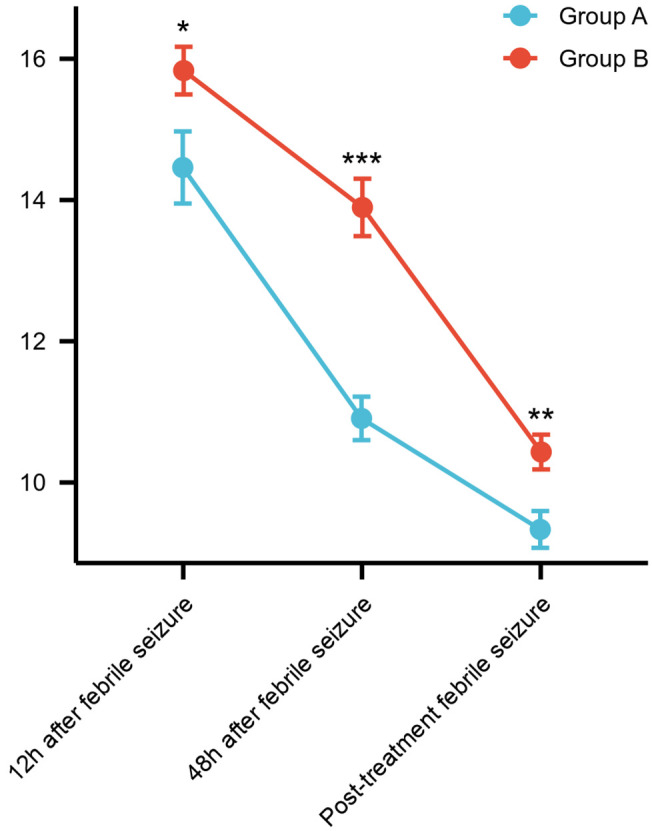
Comparison of the trends in NSE levels between the two groups. Note: NSE, neuron-specific enolase.
Diagnostic value of EEG combined with NSE in differentiating children with febrile seizures
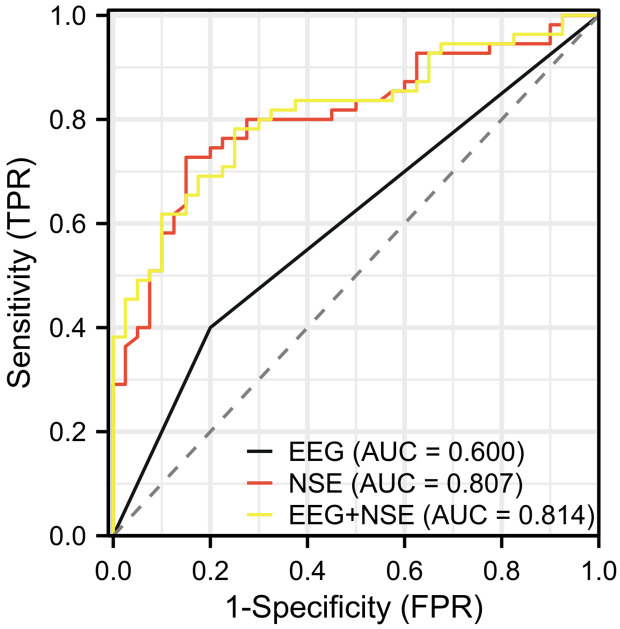
ROC curve was utilized to assess the clinical diagnostic value of EEG combined with NSE in differentiating children with febrile seizures. The results indicated that the areas under the curve (AUCs) for EEG, NSE, and their combined detection in differentiating febrile seizures were 0.600, 0.807, and 0.814, respectively. The specificity for these methods was 80.00%, 85.00%, and 75.00%, while the sensitivity was 40.00%, 72.73%, and 78.18%, respectively. Detailed results are shown in Table 5 and Figure 3.
Table 5.
Diagnostic value of EEG combined with NSE in differentiating children with febrile seizures
| Area under the curve (AUC) | Confidence interval (CI) | Cut-off | Sensitivity | Specificity | Youden index | |
|---|---|---|---|---|---|---|
| EEG | 0.600 | 0.509-0.691 | 0.500 | 40.00% | 80.00% | 20.00% |
| NSE | 0.807 | 0.719-0.895 | 12.425 | 72.73% | 85.00% | 57.73% |
| EEG+NSE | 0.814 | 0.728-0.899 | 0.542 | 78.18% | 75.00% | 53.18% |
Note: EEG, electroencephalogram; NSE, neuron-specific enolase.
Figure 3.
ROC analysis of EEG, NSE and their combined detection for the differential diagnosis of febrile seizures. Note: ROC, Receiver operator characteristic; EEG, electroencephalogram; NSE, neuron-specific enolase; SFS, Simple febrile seizure; CFS, Complex febrile seizure.
Predictive value of EEG combined with NSE for brain function prognosis in children with febrile seizures
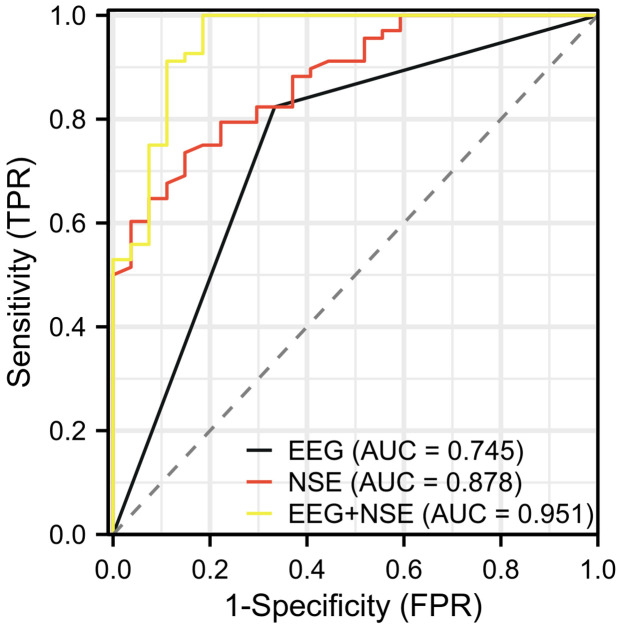
The 95 patients were further divided into a favorable outcome group (n=68) and an unfavorable outcome group (n=27) for subsequent study (Table 6). ROC curve analysis was utilized to assess the predictive value of EEG combined with NSE for brain function prognosis in children with febrile seizures. The results showed that the AUCs for predicting the prognosis of children with febrile seizures was 0.745 for EEG, 0.878 for NSE, and 0.951 for their combined detection. The specificity for these methods was 66.67%, 58.72%, and 81.48%, respectively, while the sensitivity was 82.35%, 73.53%, and 100.00%, respectively. The detailed results are shown in Table 7 and Figure 4.
Table 6.
Distribution of patients with different prognostic outcomes
| n=95 | Favorable outcome group (n=68) | Unfavorable outcome group (n=27) | |
|---|---|---|---|
| EEG | |||
| Normal | 65/68.42 | 56/82.35 | 9/33.33 |
| Abnormal | 30/31.58 | 12/17.65 | 18/66.67 |
| Febrile seizure | |||
| SFS | 40/42.11 | 36/52.94 | 4/14.81 |
| CFS | 55/57.89 | 32/40.06 | 23/85.19 |
Note: EEG, electroencephalogram; SFS, Simple febrile seizure; CFS, Complex febrile seizure.
Table 7.
Predictive value of EEG combined with NSE for brain function prognosis in children with febrile seizures
| Area under the curve (AUC) | Confidence interval (CI) | Cut-off | Sensitivity | Specificity | Youden index | |
|---|---|---|---|---|---|---|
| EEG | 0.745 | 0.644-0.847 | 0.5 | 82.35% | 66.67% | 49.02% |
| NSE | 0.878 | 0.809-0.947 | 12.695 | 73.53% | 85.19% | 58.72% |
| EEG+NSE | 0.951 | 0.901-1.000 | 0.60881 | 100.00% | 81.48% | 81.48% |
Note: EEG, electroencephalogram; NSE, neuron-specific enolase.
Figure 4.
ROC analysis of EEG, NSE and their combined detection in predicting the prognosis in children with febrile seizure. Note: ROC, Receiver operator characteristic; EEG, electroencephalogram; NSE, neuron-specific enolase.
Identification of risk factors for post-seizure sequelae in pediatric patients with febrile seizures
Results from the univariate analysis indicated that EEG findings, febrile seizure type, perinatal abnormalities, the number of first seizure, and NSE levels were associated with post-seizure sequelae in pediatric patients with febrile seizure (Table 8). These variables with statistical significance were further assigned with values for subsequent multivariate analysis (Table 9). Logistic multivariate analysis revealed that EEG findings, febrile seizure type, perinatal abnormalities, and NSE levels were all independent risk factors affecting post-seizure sequelae in pediatric patients with febrile seizures (Table 10).
Table 8.
Univariate analysis of factors affecting patient prognosis
| Factors | Unfavorable outcome group (n=27) | Favorable outcome group (n=68) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 0.029 | 0.865 | ||
| ≤4 | 16 | 39 | ||
| >4 | 11 | 29 | ||
| Sex | 1.295 | 0.255 | ||
| Male | 12 | 39 | ||
| Female | 15 | 29 | ||
| EEG | 21.49 | <0.001 | ||
| Normal | 9 | 56 | ||
| Abnormality | 18 | 12 | ||
| Febrile seizure | 11.52 | 0.001 | ||
| SFS | 4 | 36 | ||
| CFS | 23 | 32 | ||
| Perinatal abnormalities | 12.69 | <0.001 | ||
| Yes | 14 | 11 | ||
| No | 13 | 57 | ||
| Number of first seizures | 8.609 | 0.003 | ||
| ≤5 | 5 | 35 | ||
| >5 | 22 | 33 | ||
| NSE | 15.55±2.84 | 11.47±2.16 | 7.537 | <0.001 |
Note: EEG, electroencephalogram; NSE, neuron-specific enolase; SFS, Simple febrile seizure; CFS, Complex febrile seizure.
Table 9.
Assignment table
| Factors | Assignment |
|---|---|
| EEG | Normal =0, Abnormal =1 |
| Febrile seizure | Simple febrile seizure (SFS) =0, Complex febrile seizure (CFS) =1 |
| Perinatal abnormalities | Yes =1, No =0 |
| Number of first seizures | ≤5=0, >5=1 |
| NSE | Original data input |
| Prognosis | Favorable outcomes =0, Adverse outcomes =1 |
Note: EEG, electroencephalogram; NSE, neuron-specific enolase; SFS, Simple febrile seizure; CFS, Complex febrile seizure.
Table 10.
Multivariate analysis of factors affecting patient prognosis
| B | S.E. | Wals | Sig. | Exp (B) | 95% C.I. of the EXP (B) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower limit | Upper limit | ||||||
| EEG | 2.323 | 0.969 | 5.746 | 0.017 | 10.207 | 1.527 | 68.211 |
| Febrile seizure | 3.847 | 1.717 | 5.017 | 0.025 | 46.838 | 1.617 | 1356.403 |
| Perinatal abnormalities | 3.124 | 1.118 | 7.803 | 0.005 | 22.744 | 2.54 | 203.666 |
| Number of first seizures | 1.815 | 1.067 | 2.894 | 0.089 | 6.143 | 0.759 | 49.742 |
| NSE | 1.174 | 0.36 | 10.618 | 0.001 | 3.236 | 1.597 | 6.56 |
Note: EEG, electroencephalogram; NSE, neuron-specific enolase.
Discussion
Febrile seizure is a common type of epileptic seizure seen in children, typically occurring between the ages of 6 months and 5 years, with the highest frequency observed between 18 months and 3 years [14,15]. Febrile seizures are usually triggered by high fever and often associated with infectious diseases such as upper respiratory tract infections, acute tonsillitis, and other related conditions [16]. Febrile seizures can be categorized into two types: simple febrile seizures (SFS) [17] and complex febrile seizures (CFS) [18]. SFS is defined as a seizure lasting less than 15 minutes without recurrence [19], while CFS is characterized by seizures lasting longer than 15 minutes, occurring multiple times within 24 hours, involving asymmetric patterns, or presenting with neurological abnormalities [20]. While most febrile seizures do not result in long-term brain damage, CFS may increase the risk of future epilepsy in affected children. Therefore, accurate diagnosis and reliable prediction of brain function prognosis in children with febrile seizures holds significant clinical significance.
EEG is a technique used to record the brain’s electrical activity by placing electrodes on the scalp. It detects and measures the electrical signals generated by neurons [21]. In the study of CFS, researchers have identified various types of abnormal EEG discharge in affected children, including sharp slow waves and spike-slow wave complexes [22]. This study compared EEG findings between children with CFS and SFS and revealed that the incidence of EEG abnormalities was significantly higher in children with CFS compared to those with SFS, indicating that CFS is associated with a greater prevalence of EEG abnormalities. Additionally, this study revealed that NSE levels in children with CFS were notably higher than those in children with SFS at 12 and 48 hours after the onset of febrile seizures. NSE is a marker released into the blood during neuronal injury or death, and its level can reflect the extent of neuronal damage [23]. The results of this study suggest that CFS may be associated with more severe neuronal dysfunction and potential neuronal damage.
In this study, the diagnostic value of EEG and NSE for differentiating simple and complex febrile seizures was also evaluated. The results indicated that the AUCs for EEG, NSE, and their combined detection in differentiating febrile seizures were 0.600, 0.807, and 0.814, respectively. The specificity for these methods was 80.00%, 85.00%, and 75.00%, while the sensitivity was 40.00%, 72.73%, and 78.18%, respectively. Based on the results, we can observe that the combined detection has a higher AUC and sensitivity, and a relatively lower specificity, indicating that the EEG combined with NSE offers a better overall performance in differentiating SFS and CFS. The high sensitivity of the combined detection can assist in accurately identifying children with complex febrile seizures. However, the decrease in specificity may result in a certain level of misdiagnosis.
In a study by Chen et al. [24], the ROC curve analysis revealed an AUC of 0.806 for NSE in distinguishing between mild gastroenteritis and mild gastroenteritis with febrile seizures. Similarly, our study also demonstrated that the AUC for NSE in differentiating febrile seizures in children was above 0.8, indicating comparable findings. These results suggest that NSE is a valuable biomarker for the differential diagnosis of febrile seizures, aligning with the findings of Chen et al.’s research.
In terms of predicting brain function prognosis, the study revealed that the AUC of EEG, NSE and their combined detection were 0.745 (specificity: 66.67%, sensitivity: 82.35%), 0.878 (specificity: 58.72%, sensitivity: 73.53%) and 0.951 (specificity: 81.48%, sensitivity: 100.00%), respectively. These results indicate that the combined detection has a high AUC value and excellent sensitivity for predicting brain function prognosis in children with febrile seizures, although its specificity is relatively lower. This means that the combination of EEG and NSE detection can be an effective tool for predicting the brain function prognosis in children with febrile seizure. However, improving specificity is necessary to reduce the rate of misdiagnosis. In addition, Logistic multivariate analysis identified EEG findings, febrile seizure type, perinatal abnormalities, and NSE levels as independent risk factors influencing post-seizure sequelae in pediatric patients with febrile seizures.
Based on the above results, it can be concluded that EEG combined with NSE detection has a certain value in the differential diagnosis and prognosis prediction of brain function for children with febrile seizures. The combination of EEG and NSE demonstrates a relatively high sensitivity, which can assist in accurately identifying children with febrile seizures. However, the relatively low specificity of the combined detection may result in a certain rate of misdiagnosis. In practical applications, it is important to interpret the combined detection results alongside clinical history and other auxiliary examinations to ensure a comprehensive and accurate assessment.
The results of this study are of great significance for improving the diagnosis and prognosis of febrile seizures. Common diagnostic methods for febrile seizures include clinical evaluation, medical history, temperature measurement, neurological examination, blood tests, lumbar puncture, EEG, and occasionally neuroimaging. While EEG is a simple, non-invasive method that provides information on the electrical activity of the brain, its diagnostic value is relatively low in the differential diagnosis of febrile seizures. As a biomarker, NSE can reflect the extent of brain tissue injury and has high sensitivity and specificity for the diagnosis of complex febrile seizures (CFS). Therefore, the combined application of EEG and NSE can strengthen the advantages of both detection methods, thereby enhancing the accuracy and reliability of the diagnosis.
There are still some limitations to this study. Firstly, the retrospective nature of the analysis introduces inherent limitations, including potential incomplete information retrieval and data bias. Secondly, the sample size is relatively small, which may affect the stability and generalizability of the results. To address these issues, future studies should aim to expand the sample size and conduct multi-center prospective studies. Such improvements would enhance the robustness and applicability of the findings, ultimately leading to better diagnostic and prognostic strategies for classifying febrile seizures.
Conclusions
In conclusion, the combination of EEG and NSE demonstrates certain value in the differential diagnosis and prediction of brain function prognosis in children with febrile seizures. The combined use of these two methods can improve diagnostic accuracy and holds significant practical value.
Disclosure of conflict of interest
None.
References
- 1.Smith DK, Sadler KP, Benedum M. Febrile seizures: risks, evaluation, and prognosis. Am Fam Physician. 2019;99:445–450. [PubMed] [Google Scholar]
- 2.Hashimoto R, Suto M, Tsuji M, Sasaki H, Takehara K, Ishiguro A, Kubota M. Use of antipyretics for preventing febrile seizure recurrence in children: a systematic review and meta-analysis. Eur J Pediatr. 2021;180:987–997. doi: 10.1007/s00431-020-03845-8. [DOI] [PubMed] [Google Scholar]
- 3.Paul SP, Rogers E, Wilkinson R, Paul B. Management of febrile convulsion in children. Emerg Nurse. 2015;23:18–25. doi: 10.7748/en.23.2.18.e1431. [DOI] [PubMed] [Google Scholar]
- 4.Mewasingh LD, Chin RFM, Scott RC. Current understanding of febrile seizures and their long-term outcomes. Dev Med Child Neurol. 2020;62:1245–1249. doi: 10.1111/dmcn.14642. [DOI] [PubMed] [Google Scholar]
- 5.Patel AD, Vidaurre J. Complex febrile seizures: a practical guide to evaluation and treatment. J Child Neurol. 2013;28:762–767. doi: 10.1177/0883073813483569. [DOI] [PubMed] [Google Scholar]
- 6.Gould L, Delavale V, Plovnick C, Wisniewski T, Devinsky O. Are brief febrile seizures benign? A systematic review and narrative synthesis. Epilepsia. 2023;64:2539–2549. doi: 10.1111/epi.17720. [DOI] [PubMed] [Google Scholar]
- 7.Kaminska A, Eisermann M, Plouin P. Child EEG (and maturation) Handb Clin Neurol. 2019;160:125–142. doi: 10.1016/B978-0-444-64032-1.00008-4. [DOI] [PubMed] [Google Scholar]
- 8.Muller-Putz GR. Electroencephalography. Handb Clin Neurol. 2020;168:249–262. doi: 10.1016/B978-0-444-63934-9.00018-4. [DOI] [PubMed] [Google Scholar]
- 9.Shah PB, James S, Elayaraja S. EEG for children with complex febrile seizures. Cochrane Database Syst Rev. 2017;10:CD009196. doi: 10.1002/14651858.CD009196.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu MY, Liu WJ, Wang H, Wang WD, Liu NW, Lu Y. NSE from diffuse large B-cell lymphoma cells regulates macrophage polarization. Cancer Manag Res. 2019;11:4577–4595. doi: 10.2147/CMAR.S203010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu L, Zha Z, Zhang P, Li D, Liu G. NSE, positively regulated by LINC00657-miR-93-5p axis, promotes small cell lung cancer (SCLC) invasion and epithelial-mesenchymal transition (EMT) process. Int J Med Sci. 2021;18:3768–3779. doi: 10.7150/ijms.58415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang LT, Xu X, Han H, Cao SM, Li LL, Lv J, Zhang LR, Li JG. The value of NSE to predict ICU mortality in patients with septic shock: a prospective observational study. Medicine (Baltimore) 2022;101:e30941. doi: 10.1097/MD.0000000000030941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson JL, Carapetian SA, Hageman JR, Kelley KR. Febrile seizures. Pediatr Ann. 2013;42:249–254. doi: 10.3928/00904481-20131122-09. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A. Febrile seizures. Continuum (Minneap Minn) 2016;22:51–59. doi: 10.1212/CON.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 15.Cerisola A, Chaibun E, Rosas M, Cibils L. Febrile seizures: questions and answers. Medicina (B Aires) 2018;78(Suppl 2):18–24. [PubMed] [Google Scholar]
- 16.Wu G, Hu J, Zhu H, Wu S, Huang S, Liu Z. Treatment with melatonin ameliorates febrile convulsion via modulating the MEG3/miR‑223/PTEN/AKT signaling pathway. Int J Mol Med. 2021;48:154. doi: 10.3892/ijmm.2021.4987. [DOI] [PubMed] [Google Scholar]
- 17.Falsaperla R, Marino S, Vitaliti G, Bonadies A, Marino SD, Pavone P, Romano C, Savoia F, Cali C, Ruggieri M, Lubrano R, Tipo V. Simple febrile seizures: new cut off for the duration of the crises. Acta Neurol Belg. 2023;123:1339–1344. doi: 10.1007/s13760-023-02211-3. [DOI] [PubMed] [Google Scholar]
- 18.Whelan H, Harmelink M, Chou E, Sallowm D, Khan N, Patil R, Sannagowdara K, Kim JH, Chen WL, Khalil S, Bajic I, Keval A, Greydanus D. Complex febrile seizures-A systematic review. Dis Mon. 2017;63:5–23. doi: 10.1016/j.disamonth.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Kumari PL, Rajamohanan K, Krishnan ASA. Risk factors of first episode simple febrile seizures in children aged 6 month to 5 year: a case control study. Indian Pediatr. 2022;59:871–874. [PubMed] [Google Scholar]
- 20.Friese G, Collopy KT. Febrile seizures. Learn to distinguish simple febrile seizures from complex seizures. EMS Mag. 2010;39:52–57. [PubMed] [Google Scholar]
- 21.Rubinos C, Alkhachroum A, Der-Nigoghossian C, Claassen J. Electroencephalogram monitoring in critical care. Semin Neurol. 2020;40:675–680. doi: 10.1055/s-0040-1719073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah PB, James S, Elayaraja S. EEG for children with complex febrile seizures. Cochrane Database Syst Rev. 2015:CD009196. doi: 10.1002/14651858.CD009196.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Lascarrou JB, Miailhe AF, le Gouge A, Cariou A, Dequin PF, Reignier J, Coupez E, Quenot JP, Legriel S, Pichon N, Thevenin D, Boulain T, Frat JP, Vimeux S, Colin G, Desroys du Roure F. NSE as a predictor of death or poor neurological outcome after non-shockable cardiac arrest due to any cause: ancillary study of HYPERION trial data. Resuscitation. 2021;158:193–200. doi: 10.1016/j.resuscitation.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Chen Y, Zhong JM. Detection and diagnostic value of serum NSE and S100B protein levels in patients with seizures associated with mild gastroenteritis: a retrospective observational study. Medicine (Baltimore) 2020;99:e23439. doi: 10.1097/MD.0000000000023439. [DOI] [PMC free article] [PubMed] [Google Scholar]