Abstract
Objectives: This meta-analysis aimed to evaluate the combined effectiveness of Magnetic Resonance Imaging (MRI) and mammography in detecting breast cancer in women with dense breasts. Methods: A comprehensive search was conducted across PubMed, Web of Science, and EMBASE databases up to December 31, 2023, to identify relevant studies. Studies focusing on breast cancer detection in women with dense breast tissue and providing data on the sensitivity, specificity, or positive predictive value of combined MRI and mammography screening, or the use of MRI following a negative mammogram, were included. The meta-analysis was conducted using Stata 15.0, and study quality was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. Results: Ten studies, involving 51,602 participants, were included in the meta-analysis. The combined use of MRI and mammography for breast cancer detection in women with dense breasts yielded a pooled sensitivity of 0.87 (95% CI: 0.79-0.92), specificity of 0.95 (95% CI: 0.89-0.97), positive likelihood ratio of 2.55 (95% CI: 1.45-4.46), negative likelihood ratio of 0.11 (95% CI: 0.07-0.17), diagnostic score of 3.18 (95% CI: 2.35-4.02), and diagnostic ratio of 24.14 (95% CI: 10.44-55.81), and an area under the Summary Receiver Operating Characteristic curve of 0.97 (95% CI: 0.95-0.98). Conclusion: This meta-analysis demonstrated that the combination of MRI and mammography enhanced breast cancer detection in women with dense breasts. This synergistic approach significantly improves detection sensitivity in this high-risk group.
Keywords: Breast cancer, dense breast tissue, Magnetic Resonance Imaging (MRI), mammography, meta-analysis
Introduction
Breast cancer is a widespread and complex disease that remains a significant health concern for women worldwide. It involves the uncontrolled growth of cells within the breast tissue, which, if left undetected and untreated, can metastasize to other parts of the body [1,2]. Early detection through screening is essential for improving patient outcomes and survival rates, as it provides more treatment options and a higher chance of successful intervention [3].
Mammography, a form of low-dose X-ray imaging, has long been the primary method for breast cancer screening due to its ability to detect abnormalities such as tumors and calcifications [4]. However, the efficacy of mammography is greatly influenced by breast tissue density, which varies significantly among individuals. Breast tissue density refers to the ratio of glandular and connective tissue to fatty tissue [5]. Women with dense breast tissue, especially those with heterogeneous or extremely dense tissue, are at an elevated risk for breast cancer and may have reduced mammographic sensitivity [6,7]. As a result, mammograms may fail to detect cancer in these women, leading to delayed diagnosis and treatment.
Magnetic Resonance Imaging (MRI) is an advanced imaging technique that uses a strong magnetic field and radio waves to generate detailed images of the body’s internal structures [8,9]. MRI is known for its superior soft tissue contrast, allowing for clear differentiation between various tissue types and the detection of abnormalities not visible on mammograms [10]. MRI is particularly useful in evaluating the extent of breast cancer, since it can identify smaller lesions and is more sensitive in detecting cancer in women with dense breast tissue [11]. Although MRI has the potential to complement mammography, studies on the combined use of both methods for breast cancer detection have yielded mixed results. Some research suggests that adding MRI to mammography significantly improves detection rates, especially in high-risk populations and those with dense breasts [12]. However, concerns about the cost-effectiveness of this approach, the possibility of increased false positives, and the invasiveness of MRI have been raised. This meta-analysis aims to thoroughly evaluate the sensitivity, specificity, and positive predictive value of MRI when used alongside mammography, while also addressing the benefits and challenges of this integrated imaging strategy.
Materials and methods
This meta-analysis was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13], and the standards outlined in the Cochrane Handbook [14]. This study was registered on the INPLASY platform with registration number INPLASY2024100028.
Literature search
We conducted a search for studies examining the predictive value of MRI when used in conjunction with mammography for diagnosing breast cancer in women with dense breasts. The search was performed across the PubMed, Web of Science, and EMBASE databases, covering the period from the inception of each database through December 31, 2023. The search strategy included the following terms: “Magnetic Resonance Imaging” or “MRI Scans” or “Magnetic Resonance Images”, and “Mammography” or “X-ray Breast Tomosynthesis”, “Digital Breast Tomosynthesis”, and “Breast Cancer” or “Breast Neoplasms” or “Breast Tumor” or “Breast Carcinoma”, and “Dense Breasts”. Two experienced researchers independently conducted the search, and any discrepancies were resolved through consultation or by involving a third researcher.
Literature screening
Inclusion criteria were: (1) Studies focused on breast cancer detection in women with dense breast tissue; (2) English-language publications; (3) Studies reporting sensitivity, specificity, predictive diagnostic score, diagnostic ratio, positive likelihood ratio, negative likelihood ratio for combined mammography and MRI screening, or MRI follow-up after a negative mammogram.
Exclusion criteria included: (1) Reviews, conference abstracts, commentaries, or editorials; (2) Studies without full-text availability; (3) Studies lacking accuracy-related indicators or positive predictive values without underlying data.
Following deduplication, two researchers (YFL and DQL) independently screened the literature by reviewing titles, abstracts, and full texts when necessary, applying the predefined inclusion and exclusion criteria.
Data extraction
Two independent researchers (YFL and DQL) extracted the following data from the included studies: (1) General information: title, publication year, and authors; (2) Study characteristics: design type, setting; (3) Screening methodology: combined mammography and MRI or MRI following a negative mammogram; (4) Diagnostic reference standard; (5) Outcome data: true positives, false positives, true negatives, false negatives.
Discrepancies were resolved through discussion with the senior author (HW).
Quality assessment
The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool was used to evaluate the quality of the included studies, focusing on two dimensions: risk of bias and clinical applicability. The bias assessment included four domains: case selection (three questions), index test (two questions), reference standard (two questions), and flow and timing (four questions). Clinical applicability was evaluated separately for case selection, index test, and reference standard. A domain was considered to have poor clinical applicability if the actual situation poorly matched the research question, good applicability if it closely matched, and unclear applicability if it was difficult to assess due to incomplete information. Two researchers (YFL and DQL) independently extracted data and assessed study quality, with discrepancies resolved through discussion and, if needed, by involving a third researcher.
Statistical analysis
Statistical analyses were performed using Stata 15.0 (Stata Corp LLC). Forest plots were generated to summarize the pooled sensitivity, specificity, predictive diagnostic score, diagnostic ratio, positive likelihood ratio, and negative likelihood ratio of the studies. A summary receiver operating characteristic (SROC) curve was constructed to assess overall accuracy. Heterogeneity in sensitivity and specificity was evaluated using the I2 statistic alongside the Q-test. An I2 value ≤ 25% indicated low heterogeneity, an I2 value between 25% and 75% was considered moderate, and I2 ≥ 75% indicated high heterogeneity. Due to heterogeneity between studies and the negative correlation between sensitivity and specificity, a bivariate random-effects model was used to pool sensitivity and specificity. A sensitivity analysis was conducted to assess the robustness of the results. The Deeks test was applied to detect publication bias, with a P-value < 0.05 indicating evidence of bias. Subgroup analyses were conducted to explore the influence of different study types, types of dense breast tissue, and detection methods on diagnostic accuracy. Studies were stratified into subgroups based on these characteristics. Pooled sensitivity, specificity, Diagnostic Odds Ratio, and Area Under the Curve (AUC) were calculated for each subgroup using random-effects models. Heterogeneity within each subgroup was also assessed using the I2 statistic and Q-test. Statistical significance was set at P < 0.05.
Results
Study selection
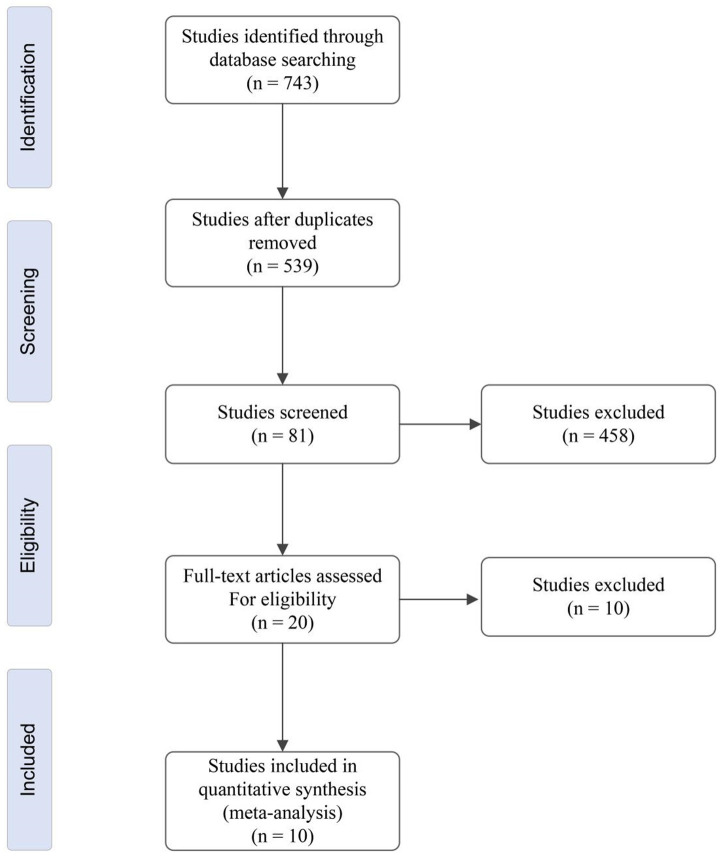
Figure 1 illustrates the study selection process. A total of 743 studies were initially identified. After removing duplicates, 539 studies remained. Of these, 458 studies were excluded for not meeting the inclusion criteria. Of the remaining 81 studies, 61 were excluded due to repetitive or insufficient data. Subsequently, 20 studies underwent full-text review to assess eligibility. Ultimately, 10 original research articles were included in this meta-analysis.
Figure 1.
Selection flow chart for identifying studies eligible for inclusion in the meta-analysis.
Characteristics of included studies
The main characteristics of the 10 studies included in the meta-analysis are summarized in Table 1. The studies were published between 2000 and 2021, with sample sizes ranging from 30 to 40,373 participants, totaling 51,602 individuals. Eight studies involved combined MRI and mammography (MRI+M) screenings for all participants. Two studies first performed mammography screening, followed by MRI for participants with negative mammography results, and biopsy for those with positive mammograms.
Table 1.
Main features of the studies involved in the meta-analysis
| Author | Year | Sample size | Study type | Age (years) | Detection method | Reference standard | Breast density type |
|---|---|---|---|---|---|---|---|
| Tilanus et al. [25] | 2000 | 109 | Observational study | 41.5 | M (-): MRI M (+): biopsy | Pathology + 12-month follow-up | Dense |
| Bakker et al. [26] | 2019 | 40,373 | RCT | 50-70 | M (-): MRI M (+): biopsy | Pathology + 24-month follow-up | Extremely dense |
| Berg et al. [27] | 2012 | 2809 | RCT | 56.8 | MRI+M | Pathology + 12-month follow-up | Dense |
| Chen et al. [28] | 2017 | 356 | Prospective study | 48.2 | M (-): MRI M (+): biopsy | Pathology + 12-month follow-up | Dense |
| Kriege et al. [29] | 2006 | 1952 | Screening study | 25-70 | MRI+M | Pathology + 36-month follow-up | Dense |
| Rubinstein et al. [30] | 2006 | 30 | Prospective study | 41.4 | MRI+M | Pathology + 6-month follow-up | Dense |
| Saadatmand et al. [31] | 2019 | 1355 | RCT | 44.7 | MRI+M | Pathology + 12-month follow-up | Dense |
| Strahle et al. [32] | 2017 | 671 | Prospective study | 55.7 | MRI+M | Pathology | Dense |
| Veenhuizen et al. [33] | 2021 | 3436 | Prospective multicenter trial | 54 | MRI+M | Pathology + 24-month follow-up | Extremely dense |
| Weinstein et al. [34] | 2020 | 511 | Retrospective study | 58 | MRI+M | Pathology + 6-month follow-up | Dense |
RCT: randomized controlled trial; M: mammography; MRI: magnetic resonance imaging; M (-): MRI: MRI imaging was conducted for individuals with negative mammography results.
Quality evaluation
As depicted in Table 2, one study exhibited a high-risk bias concerning the flow and timing of data collection, while two studies showed high-risk bias in patient selection. Overall, the QUADAS-2 assessment indicates that most studies had a low risk of bias and minimal concerns regarding applicability.
Table 2.
Quality assessment of the ten included studies
| Included study | Risk of bias | Clinical applicability | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Patient selection | Index text | Reference standard | Flow and timing | Patient selection | Index text | Reference standard | |
| Tilanus et al. [25] | Low risk | Low risk | Low risk | High-risk | Low risk | Low risk | Low risk |
| Bakker et al. [26] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Berg et al. [27] | Low risk | Low risk | Unclear | Low risk | Low risk | Unclear | Low risk |
| Chen et al. [28] | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear | Low risk |
| Kriege et al. [29] | High risk | Low risk | Unclear | Low risk | Low risk | Low risk | Low risk |
| Rubinstein et al. [30] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Saadatmand et al. [31] | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear | Low risk |
| Strahle et al. [32] | High risk | Low risk | Unclear | Low risk | Unclear | Low risk | Low risk |
| Veenhuizen et al. [33] | Low risk | Low risk | Low risk | Unclear | Low risk | Low risk | Low risk |
| Weinstein et al. [34] | Low risk | Low risk | Unclear | Low risk | Low risk | Low risk | Low risk |
Meta-analysis of combined effects
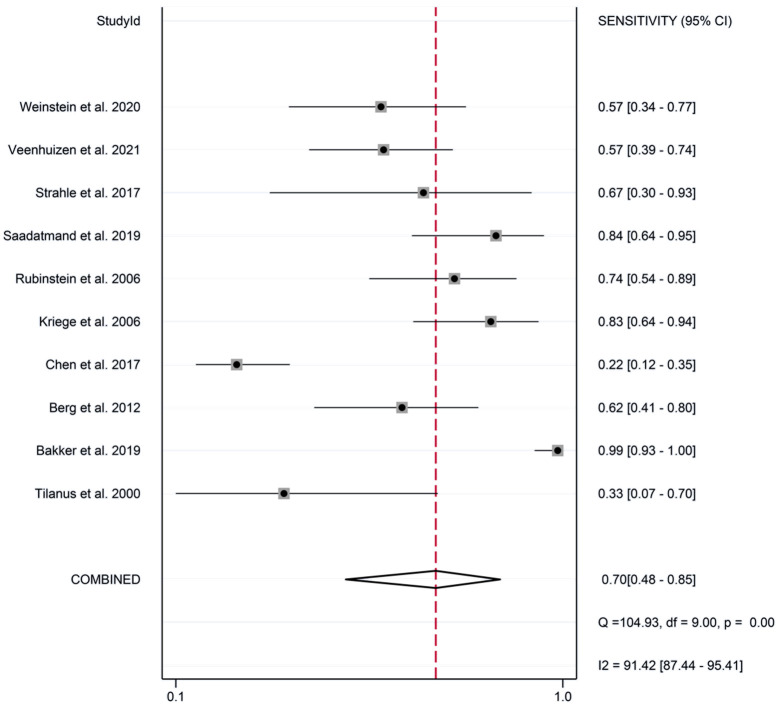
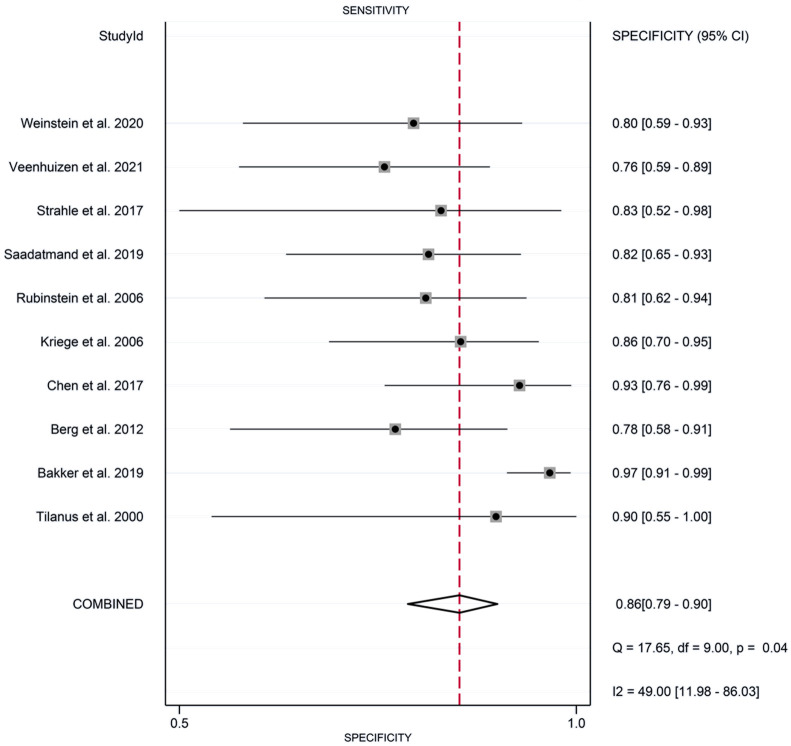
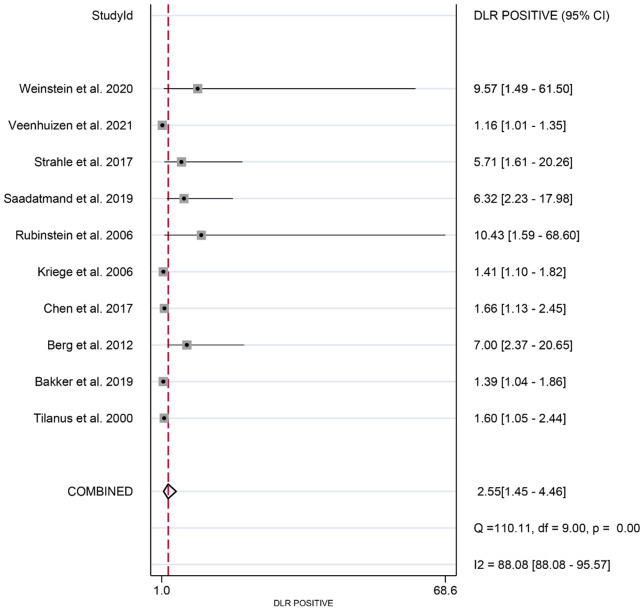
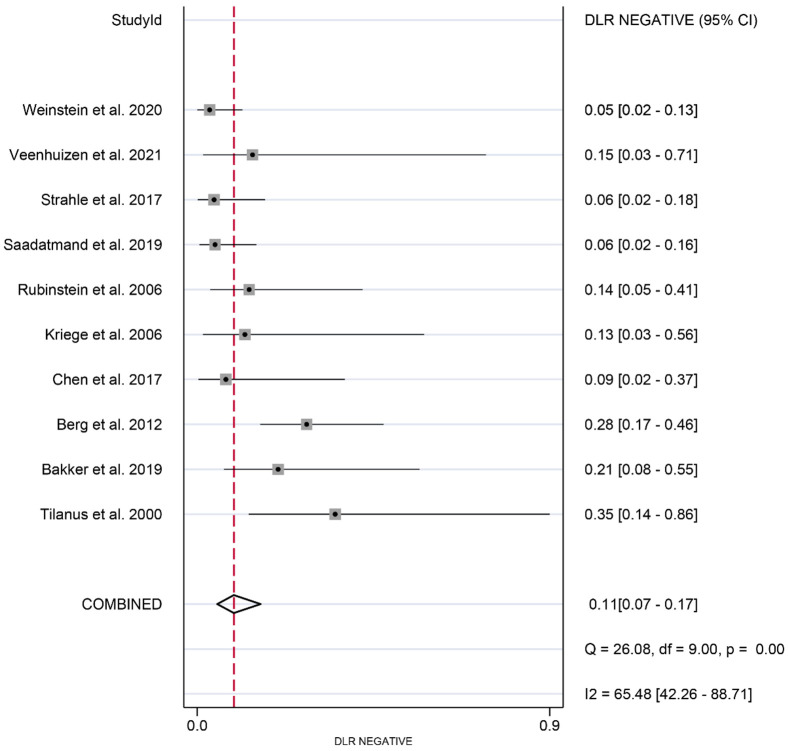
The meta-analysis revealed the following pooled results: sensitivity of 0.87 (95% CI: 0.79-0.92) (Figure 2), specificity of 0.86 (95% CI: 0.79-0.90) (Figure 3), positive likelihood ratio of 2.55 (95% CI: 1.45-4.46) (Figure 4), and negative likelihood ratio of 0.11 (95% CI: 0.07-0.17) (Figure 5). Additionally, the diagnostic score was 3.18 (95% CI: 2.35-4.02) (Figure 6), diagnostic ratio was 24.14 (95% CI: 10.44-55.81) (Figure 7), and the area under the SROC curve was 0.97 (95% CI: 0.95-0.98) (Figure 8).
Figure 2.
Forest plots of pooled sensitivity of synergistic effect of magnetic resonance imaging and mammography in the detection of breast cancer in women with dense breasts.
Figure 3.
Forest plots of pooled specificity of synergistic effect of magnetic resonance imaging and mammography in the detection of breast cancer in women with dense breasts.
Figure 4.
Forest plots of pooled positive likelihood ratio of synergistic effect of magnetic resonance imaging and mammography in the detection of breast cancer in women with dense breasts.
Figure 5.
Forest plots of pooled negative likelihood ratio of synergistic effect of magnetic resonance imaging and mammography in the detection of breast cancer in women with dense breasts.
Figure 6.
Forest plots of diagnostic score of synergistic effect of magnetic resonance imaging and mammography in the detection of breast cancer in women with dense breasts.
Figure 7.
Summary receiver operating characteristic curve of the accuracy of synergistic effect of magnetic resonance imaging and mammography in the detection of breast cancer in women with dense breasts.
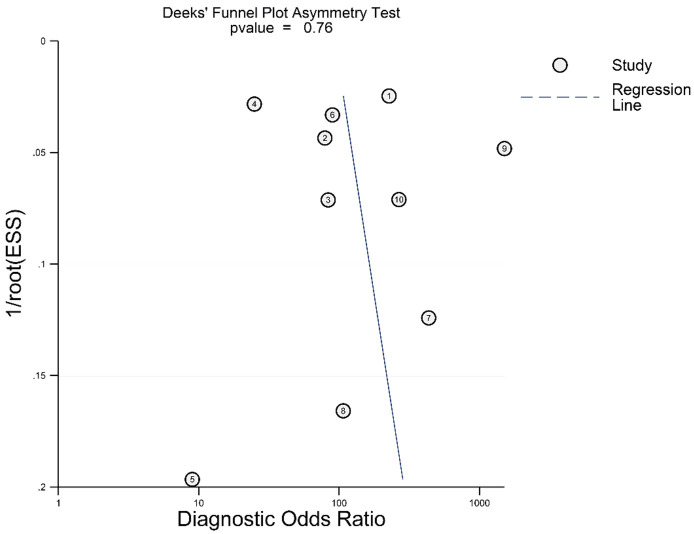
Figure 8.
Deek’s funnel plot of the accuracy of synergistic effect of magnetic resonance imaging and mammography in the detection of breast cancer in women with dense breasts.
Sensitivity analysis
The sensitivity analysis indicated that excluding any single study, studies with ≥ 2 outcomes or overlapping participants, or studies with high or unknown risk in certain quality assessment aspects did not significantly affect the pooled sensitivity and specificity. This suggested that the results were robust (Table 3).
Table 3.
Sensitivity analysis of synergistic impact of magnetic resonance imaging and mammography in the detection of breast cancer in women with dense breasts
| Included study | Sensitivity | Specificity |
|---|---|---|
| Tilanus et al. [25] | 0.93 (0.86-0.95) | 0.85 (0.83-0.90) |
| Bakker et al. [26] | 0.94 (0.87-0.96) | 0.86 (0.83-0.92) |
| Berg et al. [27] | 0.94 (0.83-0.95) | 0.84 (0.81-0.89) |
| Chen et al. [28] | 0.92 (0.83-0.93) | 0.87 (0.83-0.92) |
| Kriege et al. [29] | 0.93 (0.82-0.94) | 0.84 (0.81-0.91) |
| Rubinstein et al. [30] | 0.94 (0.82-0.95) | 0.85 (0.83-0.92) |
| Saadatmand et al. [31] | 0.92 (0.84-0.96) | 0.87 (0.83-0.92) |
| Strahle et al. [32] | 0.93 (0.83-0.94) | 0.86 (0.81-0.90) |
| Veenhuizen et al. [33] | 0.94 (0.83-0.96) | 0.85 (0.80-0.92) |
| Weinstein et al. [34] | 0.95 (0.88-0.96) | 0.86 (0.83-0.92) |
Publication bias
The funnel plot generated by Deeks’ test did not show significant asymmetry (Figure 5), indicating a low risk of publication bias, with a P-value of 0.61.
Subgroup analysis
Statistically significant differences in diagnostic accuracy were observed across subgroups categorized by study type, breast tissue density, and detection method. The subgroup analysis in Table 4 indicates that combining MRI with mammography yielded high diagnostic performance across all subgroups, particularly in women with dense breasts. The analysis demonstrated consistent sensitivity and specificity across the categories, highlighting the complementary role of MRI in enhancing breast cancer detection. The high AUC values further confirm the reliability of this combined imaging approach.
Table 4.
Subgroup analysis of magnetic resonance imaging and mammography in diagnosis of breast cancer in women with dense breasts
| Classification | Studies | Sensitivity (95% CI) | Specificity (95% CI) | DOR (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|
| Study type | |||||
| RCT | 3 | 0.88 (0.80-0.93) | 0.94 (0.90-0.96) | 130.9 (72.5-237.0) | 0.97 (0.95-0.98) |
| Observational study | 1 | 0.85 (0.77-0.91) | 0.92 (0.89-0.94) | 69.3 (44.8-107.5) | 0.96 (0.94-0.97) |
| Prospective study | 4 | 0.89 (0.82-0.95) | 0.96 (0.91-0.98) | 135.2 (79.3-249.0) | 0.98 (0.95-0.99) |
| Screening study | 1 | 0.86 (0.81-0.94) | 0.95 (0.92-0.98) | 139.6 (83.6-258.3) | 0.95 (0.92-0.97) |
| Retrospective study | 1 | 0.87 (0.81-0.95) | 0.93 (0.87-0.96) | 137.5 (81.9-251.6) | 0.97 (0.94-0.98) |
| Dense type of breast tissue | |||||
| Extremely dense | 3 | 0.90 (0.82-0.94) | 0.93 (0.89-0.95) | 144.8 (84.9-246.0) | 0.97 (0.95-0.98) |
| Heterogeneous dense | 4 | 0.87 (0.79-0.92) | 0.95 (0.92-0.97) | 221.1 (137.8-353.7) | 0.96 (0.94-0.97) |
| Homogeneous dense | 3 | 0.86 (0.78-0.91) | 0.91 (0.88-0.93) | 81.2 (56.5-116.1) | 0.95 (0.93-0.97) |
| Detection method | |||||
| MRI + Mammography | 7 | 0.88 (0.80-0.93) | 0.93 (0.90-0.95) | 100.7 (70.4-144.3) | 0.97 (0.95-0.98) |
| Mammography negative followed by MRI | 3 | 0.87 (0.79-0.92) | 0.91 (0.88-0.93) | 78.2 (54.1-113.0) | 0.96 (0.94-0.97) |
RCT: randomized controlled trial; CI: Confidence Interval; DOR: Diagnostic Odds Ratio; AUC: Area Under the Curve.
Discussion
Breast cancer remains a significant global health issue, and early detection is critical for improving treatment outcomes and survival rates. While mammography has long been regarded as the gold standard for breast cancer screening, its effectiveness varies because of the differences in breast tissue density among individuals [15,16]. Women with denser breast tissue, particularly those with heterogeneous or extremely dense tissue types, face a higher risk of breast cancer and may experience reduced mammographic sensitivity [17]. Notably, a substantial proportion of Asian women - more than half - have dense breast tissue. As dense breast tissue is an established independent risk factor for breast cancer, there is growing interest in the benefit of combining MRI with mammography for enhanced detection [18]. MRI is valued for its superior soft tissue contrast resolution, allowing it to identify lesions that may be missed on mammography. However, primary studies on the efficacy of this combined approach have yielded inconsistent results [19].
The meta-analysis demonstrated that the combined use of MRI and mammography resulted in a pooled sensitivity of 0.87 (95% CI: 0.79-0.92) and a pooled specificity of 0.95 (95% CI: 0.89-0.97) for detecting breast cancer in women with dense breast tissue. These findings imply that adding MRI to mammography significantly enhances sensitivity for breast cancer detection in this population. The high specificity further indicates that this dual-modality approach is reliable for excluding cancer, thus reducing the likelihood of false positives and unnecessary biopsies. The increased sensitivity of MRI in breast cancer detection, as evidenced in this meta-analysis, can be attributed to its ability to visualize smaller lesions and its superior contrast resolution, which allows better differentiation between tissue types. This is especially important for women with dense breast tissue, where mammography alone may be less effective since dense parenchyma can mask abnormalities. These results align with the findings of Comstock et al. [20], who reported improved sensitivity in breast cancer detection when MRI was added, although with a slight decrease in specificity.
Another key advantage of MRI is its ability to assess tumor vascularity and metabolic activity. Techniques such as dynamic contrast-enhanced MRI (DCE-MRI) provide insights into tumor perfusion and permeability, both crucial factors for tumor growth and progression [21]. This ability helps explain why MRI can detect cancers that are not visible on mammography. Additionally, Alaref et al. [22] demonstrated the potential of diffusion-weighted imaging (DWI)-based MRI in detecting breast cancer in women with dense breasts, suggesting that MRI’s multifaceted approach - evaluating lesions from multiple perspectives - was superior to mammography, which primarily relies on calcifications and morphologic features.
This meta-analysis provided further insight into the diagnostic utility of combining MRI and mammography for breast cancer detection. The positive likelihood ratio of 2.55 (95% CI: 1.45 to 4.46) indicated a significant increase in the odds of breast cancer when both MRI and mammography are positive, compared to when only mammography is positive. This suggests that a positive result is more likely to reflect true breast cancer, thus supporting more confident diagnosis and timely intervention. Conversely, the negative likelihood ratio of 0.11 (95% CI: 0.07 to 0.17) shows that a negative result from the combined screening substantially reduces the probability of breast cancer. This is crucial for reducing patient anxiety and avoiding unnecessary tests or invasive procedures, such as biopsies. The diagnostic score of 3.18 (95% CI: 2.35 to 4.02) and diagnostic ratio of 24.14 (95% CI: 10.44 to 55.81) further substantiate the robustness of the combined screening approach. These metrics suggest that the combined method is more likely to correctly identify breast cancer, and the high diagnostic ratio suggests that the screening effectively identifies true cases while minimizing false positives. These findings reinforce the idea that MRI supplementation to mammography significantly enhances breast cancer screening performance.
The issue of false positives is particularly significant, as it can lead to increased anxiety, additional testing, and unnecessary biopsies for patients. It is essential to balance the benefits of early cancer detection with the psychological and financial burdens caused by false positives. The results of this meta-analysis call for further investigation into optimizing the use of MRI alongside mammography, including the development of better criteria to identify patients who would benefit the most from MRI screening.
While this meta-analysis suggests a promising role for the combination of MRI and mammography in breast cancer detection, the clinical applicability of this approach must be evaluated in light of practical challenges. MRI is more expensive than mammography, and there is potential for increased false positives and the invasiveness of the procedure. Early diagnosis and treatment are crucial for improving breast cancer prognosis and reducing disease burden. Mammography, ultrasound, and MRI are widely used in breast cancer screening, with each offering significant diagnostic value. Mammography, in particular, is recommended globally as a routine breast cancer screening method, contributing to a substantial reduction in breast cancer mortality.
Previous meta-analyses have revealed that mammography alone has a sensitivity of 0.74 and specificity of 0.93 in detecting breast cancer in women with dense breasts [23]. This study found that MRI-assisted mammography for this population results in a slight decrease in specificity but a notable increase in sensitivity. Another study compared the effectiveness of mammography alone to mammography combined with other imaging techniques [24]. The findings revealed that combining mammography with MRI significantly improves breast cancer detection rates compared to mammography alone. Specifically, the weighted average sensitivity and specificity were 0.92 and 0.91 for women with non-dense breasts, and 0.82 and 0.80 for women with dense breasts, respectively.
Despite the strengths of this meta-analysis, several limitations exist. First, the included studies varied in sample size, design, and reference standards for breast cancer diagnosis, which may have affected the reliability of the combined results. Additionally, the meta-analysis did not account for factors such as patient age, menopausal status, and hormonal factors, which may have influenced the diagnostic accuracy of combined MRI and mammography. These factors should be considered in future research.
In conclusion, this meta-analysis underscored the synergistic effect of MRI and mammography in detecting breast cancer in women with dense breasts. The findings suggest that combined imaging modalities significantly improve sensitivity in this high-risk group. However, the clinical applicability of this approach must be carefully weighed against challenges such as cost and the risk of false positives.
Disclosure of conflict of interest
None.
References
- 1.Tzeng YT, Hsiao JH, Tseng LM, Hou MF, Li CJ. Breast cancer organoids derived from patients: a platform for tailored drug screening. Biochem Pharmacol. 2023;217:115803. doi: 10.1016/j.bcp.2023.115803. [DOI] [PubMed] [Google Scholar]
- 2.Upadhyay N, Wolska J. Imaging the dense breast. J Surg Oncol. 2024;130:29–35. doi: 10.1002/jso.27661. [DOI] [PubMed] [Google Scholar]
- 3.Sardanelli F, Magni V, Rossini G, Kilburn-Toppin F, Healy NA, Gilbert FJ. The paradox of MRI for breast cancer screening: high-risk and dense breasts-available evidence and current practice. Insights Imaging. 2024;15:96. doi: 10.1186/s13244-024-01653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farkas AH, Nattinger AB. Breast cancer screening and prevention. Ann Intern Med. 2023;176:ITC161–ITC176. doi: 10.7326/AITC202311210. [DOI] [PubMed] [Google Scholar]
- 5.Kwon MR, Chang Y, Ham SY, Cho Y, Kim EY, Kang J, Park EK, Kim KH, Kim M, Kim TS, Lee H, Kwon R, Lim GY, Choi HR, Choi J, Kook SH, Ryu S. Screening mammography performance according to breast density: a comparison between radiologists versus standalone intelligence detection. Breast Cancer Res. 2024;26:68. doi: 10.1186/s13058-024-01821-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrielson M, Hammarström M, Bergqvist J, Lång K, Rosendahl AH, Borgquist S, Hellgren R, Czene K, Hall P. Baseline breast tissue characteristics determine the effect of tamoxifen on mammographic density change. Int J Cancer. 2024;155:339–351. doi: 10.1002/ijc.34939. [DOI] [PubMed] [Google Scholar]
- 7.Winkelman AJ, Tulenko K, Epstein SH, Nguyen JV, Ford C, Miller MM. Breast cancer screening with automated breast US and mammography vs handheld US and mammography in women with dense breasts in a real-world clinical setting. J Breast Imaging. 2024;6:493–501. doi: 10.1093/jbi/wbae039. [DOI] [PubMed] [Google Scholar]
- 8.Udayakumar D, Madhuranthakam AJ, Doğan BE. Magnetic resonance perfusion imaging for breast cancer. Magn Reson Imaging Clin N Am. 2024;32:135–150. doi: 10.1016/j.mric.2023.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Cozzi A, Schiaffino S. Preoperative breast MRI in women with dense breasts: can we keep up with a rapidly changing scenario? Eur Radiol. 2023;33:8077–8079. doi: 10.1007/s00330-023-10075-7. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka M, Iima M, Miyake KK, Honda M. Multiparametric approach to breast cancer with emphasis on magnetic resonance imaging in the Era of personalized breast cancer treatment. Invest Radiol. 2024;59:26–37. doi: 10.1097/RLI.0000000000001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisen A, Fletcher GG, Fienberg S, George R, Holloway C, Kulkarni S, Seely JM, Muradali D. Breast magnetic resonance imaging for preoperative evaluation of breast cancer: a systematic review and meta-analysis. Can Assoc Radiol J. 2024;75:118–135. doi: 10.1177/08465371231184769. [DOI] [PubMed] [Google Scholar]
- 12.Stout NK, Miglioretti DL, Su YR, Lee CI, Abraham L, Alagoz O, de Koning HJ, Hampton JM, Henderson L, Lowry KP, Mandelblatt JS, Onega T, Schechter CB, Sprague BL, Stein S, Trentham-Dietz A, van Ravesteyn NT, Wernli KJ, Kerlikowske K, Tosteson ANA. Breast cancer screening using mammography, digital breast tomosynthesis, and magnetic resonance imaging by breast density. JAMA Intern Med. 2024;184:1222–1231. doi: 10.1001/jamainternmed.2024.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lapin B, Boehnke JR. Introduction to “PRISMA-COSMIN for outcome measurement instruments 2024”. Qual Life Res. 2024;33:2025–2027. doi: 10.1007/s11136-024-03731-y. [DOI] [PubMed] [Google Scholar]
- 14.Langaliya A, Alam MK, Hegde U, Panakaje MS, Cervino G, Minervini G. Occurrence of temporomandibular disorders among patients undergoing treatment for obstructive sleep apnoea syndrome (OSAS) using mandibular advancement device (MAD): a systematic review conducted according to PRISMA guidelines and the cochrane handbook for systematic reviews of interventions. J Oral Rehabil. 2023;50:1554–1563. doi: 10.1111/joor.13574. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Wu J, Zhou XS, Shi F, Shen D. Recent advancements in artificial intelligence for breast cancer: Image augmentation, segmentation, diagnosis, and prognosis approaches. Semin Cancer Biol. 2023;96:11–25. doi: 10.1016/j.semcancer.2023.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Kerlikowske K, Zhu W, Su YR, Sprague BL, Stout NK, Onega T, O’Meara ES, Henderson LM, Tosteson ANA, Wernli K, Miglioretti DL. Supplemental magnetic resonance imaging plus mammography compared with magnetic resonance imaging or mammography by extent of breast density. J Natl Cancer Inst. 2024;116:249–257. doi: 10.1093/jnci/djad201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong W, Zhu J, Hong C, Liu X, Li S, Chen Y, Zhang B, Li X. Diagnostic accuracy of cone-beam breast computed tomography and head-to-head comparison of digital mammography, magnetic resonance imaging and cone-beam breast computed tomography for breast cancer: a systematic review and meta-analysis. Gland Surg. 2023;12:1360–1374. doi: 10.21037/gs-23-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obermann M, Nohava L, Frass-Kriegl R, Soanca O, Ginefri JC, Felblinger J, Clauser P, Baltzer PAT, Laistler E. Panoramic magnetic resonance imaging of the breast with a wearable coil vest. Invest Radiol. 2023;58:799–810. doi: 10.1097/RLI.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramli Hamid MT, Ab Mumin N, Abdul Hamid S, Ahmad Saman MS, Rahmat K. Abbreviated breast magnetic resonance imaging (MRI) or digital breast tomosynthesis for breast cancer detection in dense breasts? A retrospective preliminary study with comparable results. Clin Radiol. 2024;79:e524–e531. doi: 10.1016/j.crad.2023.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Comstock CE, Gatsonis C, Newstead GM, Snyder BS, Gareen IF, Bergin JT, Rahbar H, Sung JS, Jacobs C, Harvey JA, Nicholson MH, Ward RC, Holt J, Prather A, Miller KD, Schnall MD, Kuhl CK. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746–756. doi: 10.1001/jama.2020.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai WC, Chang KM, Kao KJ. Dynamic contrast enhanced MRI and intravoxel incoherent motion to identify molecular subtypes of breast cancer with different vascular normalization gene expression. Korean J Radiol. 2021;22:1021–1033. doi: 10.3348/kjr.2020.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alaref A, Hassan A, Sharma Kandel R, Mishra R, Gautam J, Jahan N. Magnetic resonance imaging features in different types of invasive breast cancer: a systematic review of the literature. Cureus. 2021;13:e13854. doi: 10.7759/cureus.13854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ontario Health (Quality) Supplemental screening as an adjunct to mammography for breast cancer screening in people with dense breasts: a health technology assessment. Ont Health Technol Assess Ser. 2023;23:1–293. [PMC free article] [PubMed] [Google Scholar]
- 24.Duijm LEM. Dense breasts at breast cancer screening: can DWI-based breast MRI without contrast help us in the pursuit of personalized screening? Eur Radiol. 2024;34:4727–4729. doi: 10.1007/s00330-023-10323-w. [DOI] [PubMed] [Google Scholar]
- 25.Tilanus-Linthorst MM, Obdeijn IM, Bartels KC, de Koning HJ, Oudkerk M. First experiences in screening women at high risk for breast cancer with MR imaging. Breast Cancer Res Treat. 2000;63:53–60. doi: 10.1023/a:1006480106487. [DOI] [PubMed] [Google Scholar]
- 26.Bakker MF, de Lange SV, Pijnappel RM, Mann RM, Peeters PHM, Monninkhof EM, Emaus MJ, Loo CE, Bisschops RHC, Lobbes MBI, de Jong MDF, Duvivier KM, Veltman J, Karssemeijer N, de Koning HJ, van Diest PJ, Mali WPTM, van den Bosch MAAJ, Veldhuis WB, van Gils CH DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091–2102. doi: 10.1056/NEJMoa1903986. [DOI] [PubMed] [Google Scholar]
- 27.Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, Böhm-Vélez M, Mahoney MC, Evans WP 3rd, Larsen LH, Morton MJ, Mendelson EB, Farria DM, Cormack JB, Marques HS, Adams A, Yeh NM, Gabrielli G ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394–1404. doi: 10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen SQ, Huang M, Shen YY, Liu CL, Xu CX. Abbreviated MRI protocols for detecting breast cancer in women with dense breasts. Korean J Radiol. 2017;18:470–475. doi: 10.3348/kjr.2017.18.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kriege M, Brekelmans CT, Obdeijn IM, Boetes C, Zonderland HM, Muller SH, Kok T, Manoliu RA, Besnard AP, Tilanus-Linthorst MM, Seynaeve C, Bartels CC, Kaas R, Meijer S, Oosterwijk JC, Hoogerbrugge N, Tollenaar RA, Rutgers EJ, de Koning HJ, Klijn JG. Factors affecting sensitivity and specificity of screening mammography and MRI in women with an inherited risk for breast cancer. Breast Cancer Res Treat. 2006;100:109–119. doi: 10.1007/s10549-006-9230-z. [DOI] [PubMed] [Google Scholar]
- 30.Rubinstein WS, Latimer JJ, Sumkin JH, Huerbin M, Grant SG, Vogel VG. Prospective screening study of 0.5 Tesla dedicated magnetic resonance imaging for the detection of breast cancer in young, high-risk women. BMC Womens Health. 2006;6:10. doi: 10.1186/1472-6874-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saadatmand S, Geuzinge HA, Rutgers EJT, Mann RM, de Roy van Zuidewijn DBW, Zonderland HM, Tollenaar RAEM, Lobbes MBI, Ausems MGEM, van’t Riet M, Hooning MJ, Mares-Engelberts I, Luiten EJT, Heijnsdijk EAM, Verhoef C, Karssemeijer N, Oosterwijk JC, Obdeijn IM, de Koning HJ, Tilanus-Linthorst MMA FaMRIsc study group. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): a multicentre, randomised, controlled trial. Lancet Oncol. 2019;20:1136–1147. doi: 10.1016/S1470-2045(19)30275-X. [DOI] [PubMed] [Google Scholar]
- 32.Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162:283–295. doi: 10.1007/s10549-017-4112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veenhuizen SGA, de Lange SV, Bakker MF, Pijnappel RM, Mann RM, Monninkhof EM, Emaus MJ, de Koekkoek-Doll PK, Bisschops RHC, Lobbes MBI, de Jong MDF, Duvivier KM, Veltman J, Karssemeijer N, de Koning HJ, van Diest PJ, Mali WPTM, van den Bosch MAAJ, van Gils CH, Veldhuis WB DENSE Trial Study Group. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278–286. doi: 10.1148/radiol.2021203633. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein SP, Korhonen K, Cirelli C, Schnall MD, McDonald ES, Pantel AR, Zuckerman S, Borthakur A, Conant EF. Abbreviated breast magnetic resonance imaging for supplemental screening of women with dense breasts and average risk. J. Clin. Oncol. 2020;38:3874–3882. doi: 10.1200/JCO.19.02198. [DOI] [PubMed] [Google Scholar]