Abstract
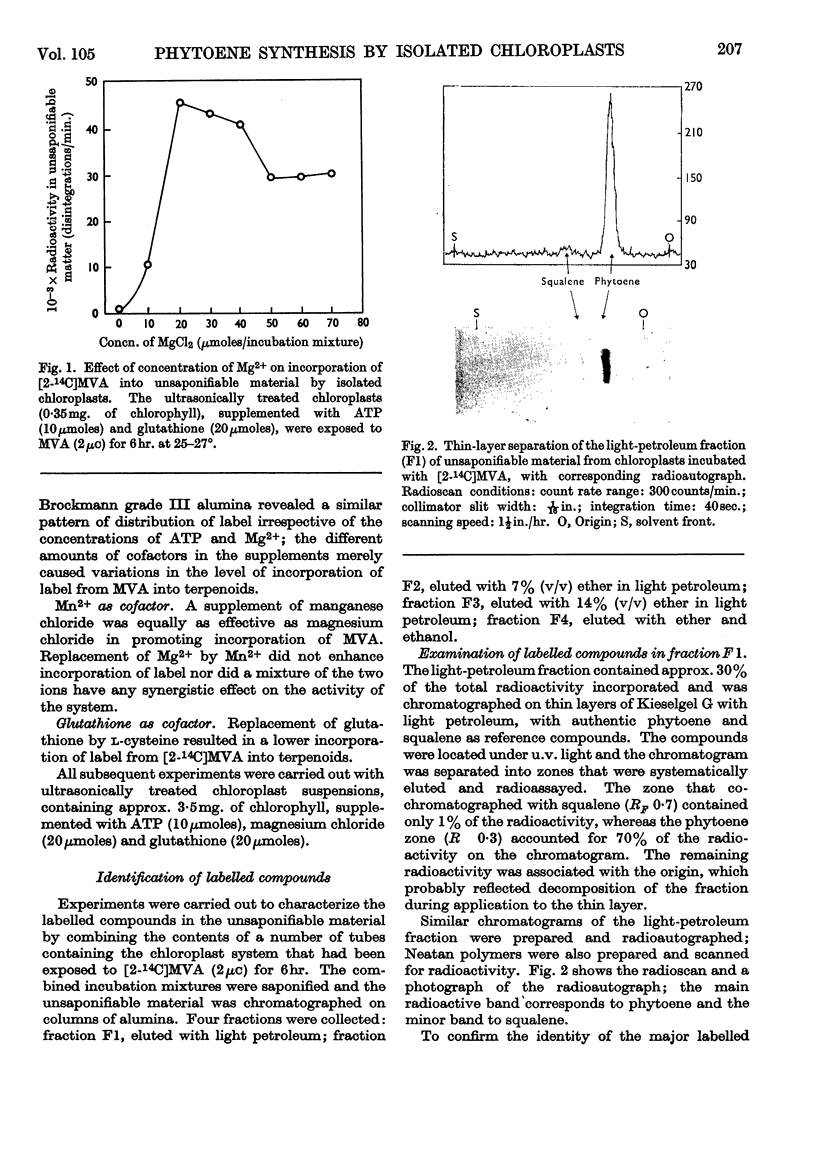
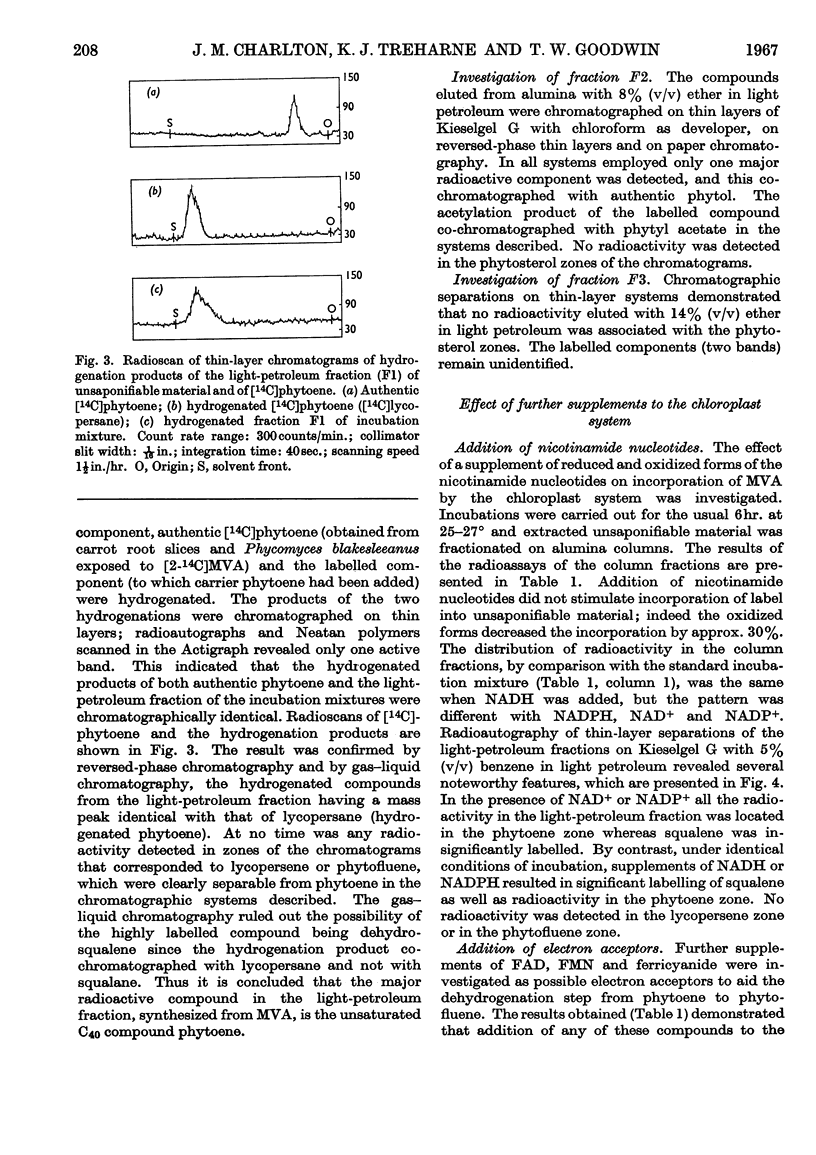
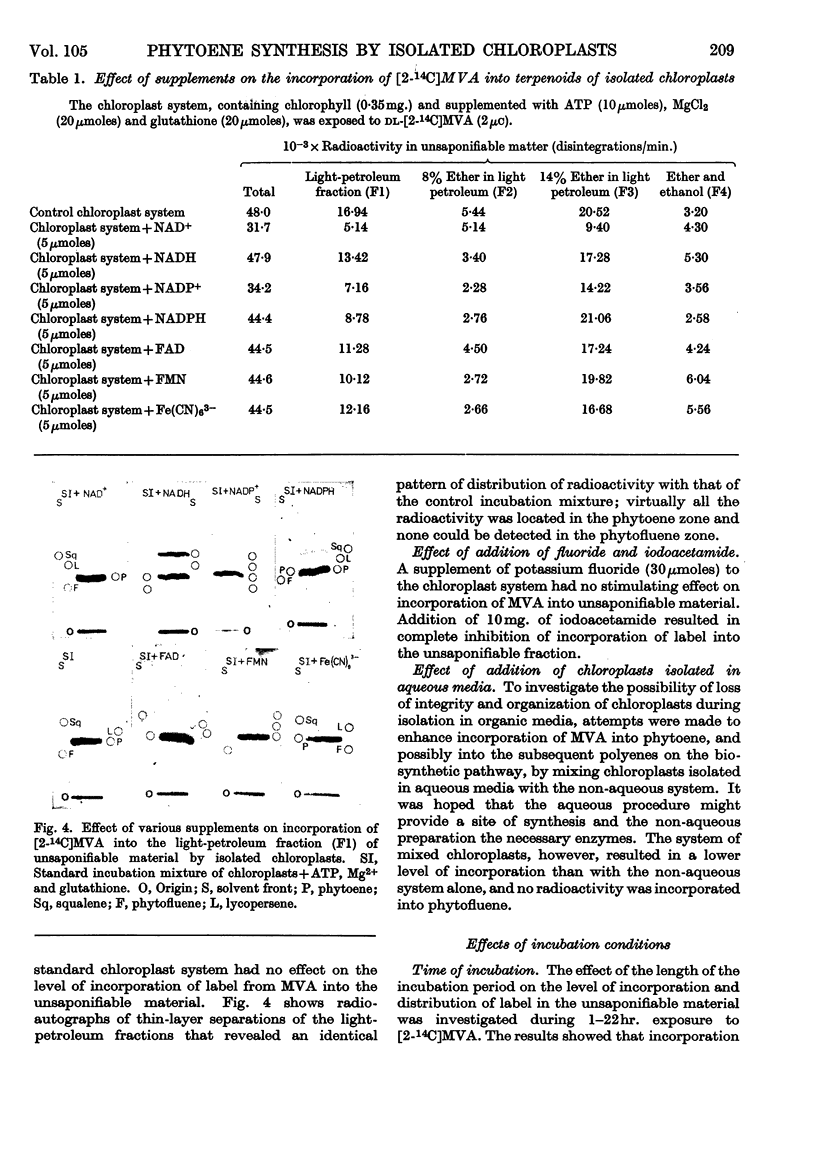
1. Chloroplasts prepared by the non-aqueous technique will, after fragmentation by ultrasonic treatment, incorporate [2-14C]mevalonic acid into phytoene, the first C40 compound formed in the biosynthetic sequence to coloured carotenoids. 2. With suspensions containing 3·5mg. of chlorophyll, the optimum amounts of cofactor required were ATP (10μmoles), magnesium chloride (20μmoles) and glutathione (20μmoles); neither NAD+ nor NADP+ was required. 3. Very small amounts of squalene are also formed and synthesis is stimulated by addition of NADH or NADPH. Phytoene synthesis was not affected by the presence of these cofactors and no lycopersene (the C40 homologue of squalene) was detected. 4. The phytol side chain of chlorophyll is also labelled under these conditions. 5. Preparations of developing chloroplasts are more active than preparations of mature chloroplasts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON D. G., PORTER J. W. The biosynthesis of phytoene and other carotenes by enzymes of isolated higher plant plastids. Arch Biochem Biophys. 1962 Jun;97:509–519. doi: 10.1016/0003-9861(62)90115-7. [DOI] [PubMed] [Google Scholar]
- Dennis D. T., Coultate T. P. Phosphofructokinase, a regulatory enzyme in plants. Biochem Biophys Res Commun. 1966 Oct 20;25(2):187–191. doi: 10.1016/0006-291x(66)90578-x. [DOI] [PubMed] [Google Scholar]
- GOODMAN D. S., POPJAK G. Studies on the biosynthesis of cholesterol. XII. Synthesis of allyl pyrophosphates from mevalonate and their conversion into squalene with liver enzymes. J Lipid Res. 1960 Jul;1:286–300. [PubMed] [Google Scholar]
- GOODWIN T. W. Studies in carotenogenesis. 25. The incorporation of 14CO2, [2-14C] acetate and [2-14C]mevalonate into beta-carotene by illuminated etiolated maize seedings. Biochem J. 1958 Dec;70(4):612–617. doi: 10.1042/bj0700612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEBER U. [Comparative studies on chloroplasts obtained by isolation operations in non-aqueous and in aqueous milieu. I. A study of the relationships of chloroplast protein to total protein in the leaf cell]. Z Naturforsch B. 1960 Feb;15B:95–99. [PubMed] [Google Scholar]
- HEBER U. [Comparative studies on chloroplasts obtained by isolation operations in non-aqueous and in aqueous milieu. II. A criticism of the purity and enzyme localizations in chloroplasts]. Z Naturforsch B. 1960 Feb;15B:100–109. [PubMed] [Google Scholar]
- KAKUTANI Y., SUZUE G., TANAKA S. THE ENZYMATIC CONVERSION OF PHYTOENE TO BETA-CAROTENE. J Biochem. 1964 Aug;56:195–196. doi: 10.1093/oxfordjournals.jbchem.a127979. [DOI] [PubMed] [Google Scholar]
- LEECH R. M. THE ISOLATION OF STRUCTURALLY INTACT CHLOROPLASTS. Biochim Biophys Acta. 1964 May 25;79:637–639. doi: 10.1016/0926-6577(64)90235-9. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Passonneau J. V. Kinetic evidence for multiple binding sites on phosphofructokinase. J Biol Chem. 1966 May 25;241(10):2268–2279. [PubMed] [Google Scholar]
- POPJAK G., GOODMAN W. S., CORNFORTH J. W., CORNFORTH R. H., RYHAGE R. Studies on the biosynthesis of cholesterol. XV. Mechanism of squalene biosynthesis from farnesyl pyrophosphate and from mevalonate. J Biol Chem. 1961 Jul;236:1934–1947. [PubMed] [Google Scholar]
- PORTER J. W., ANDERSON D. G. The biosynthesis of carotenes. Arch Biochem Biophys. 1962 Jun;97:520–528. doi: 10.1016/0003-9861(62)90116-9. [DOI] [PubMed] [Google Scholar]
- Rogers L. J., Shah S. P., Goodwin T. W. Intracellular localization of mevalonate-activating enzymes in plant cells. Biochem J. 1966 May;99(2):381–388. doi: 10.1042/bj0990381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treharne K. J., Mercer E. I., Goodwin T. W. Incorporation of [14C] carbon dioxide and [2-14C] mevalonic acid into terpenoids of higher plants during chloroplast development. Biochem J. 1966 Apr;99(1):239–245. doi: 10.1042/bj0990239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K., Racker E. Regulatory mechanisms in carbohydrate metabolism. VII. Hexokinase and phosphofructokinase. J Biol Chem. 1965 Dec;240(12):4682–4688. [PubMed] [Google Scholar]