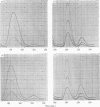
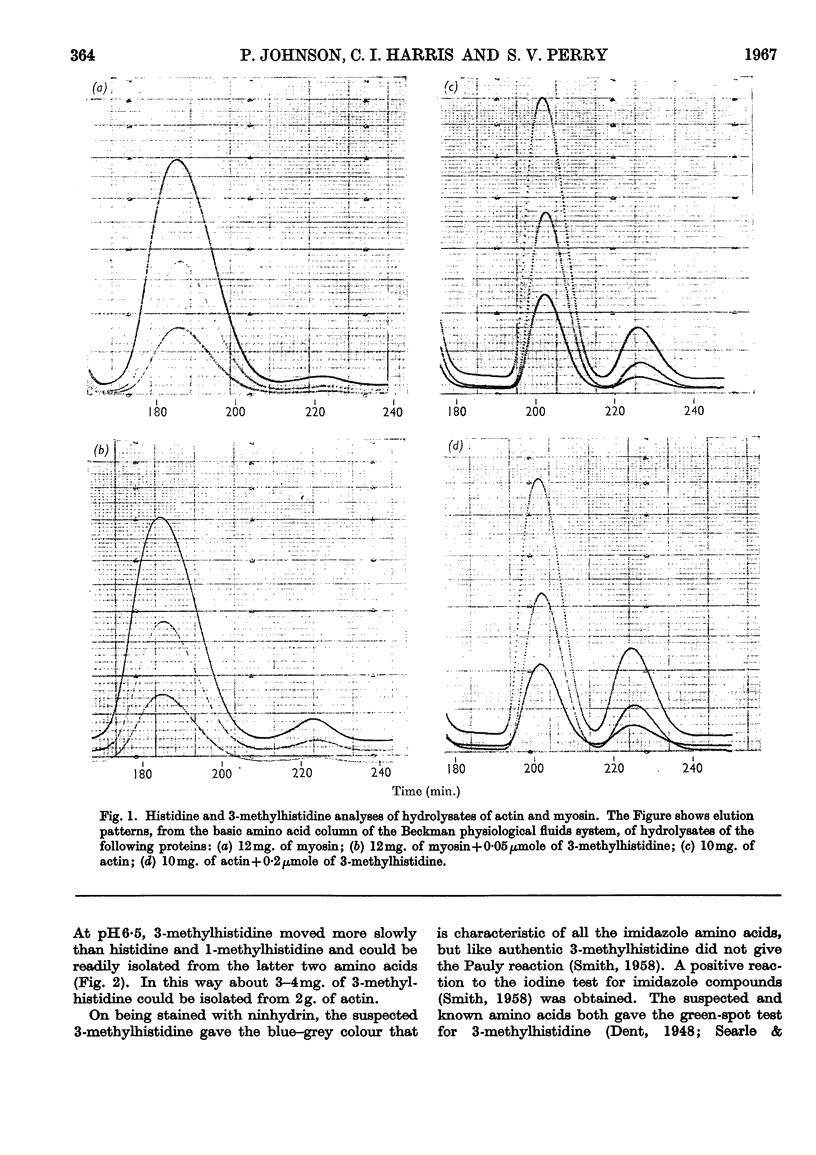
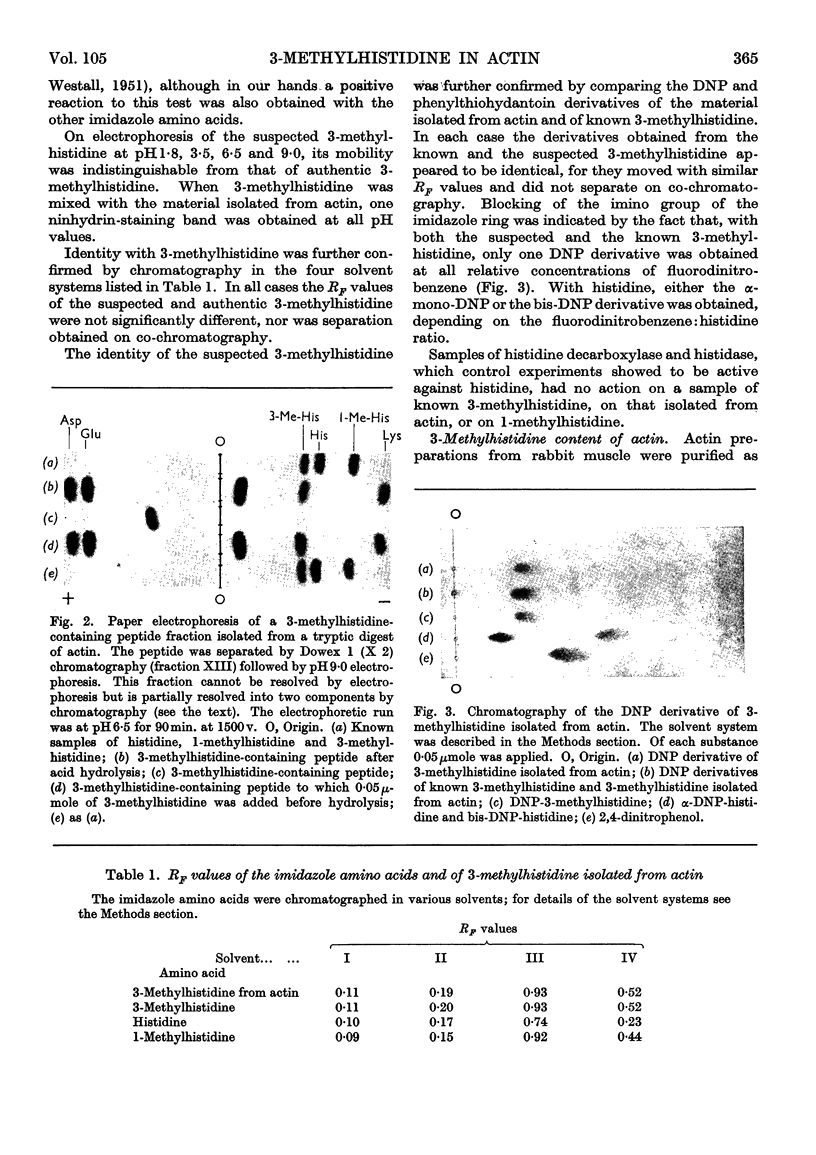
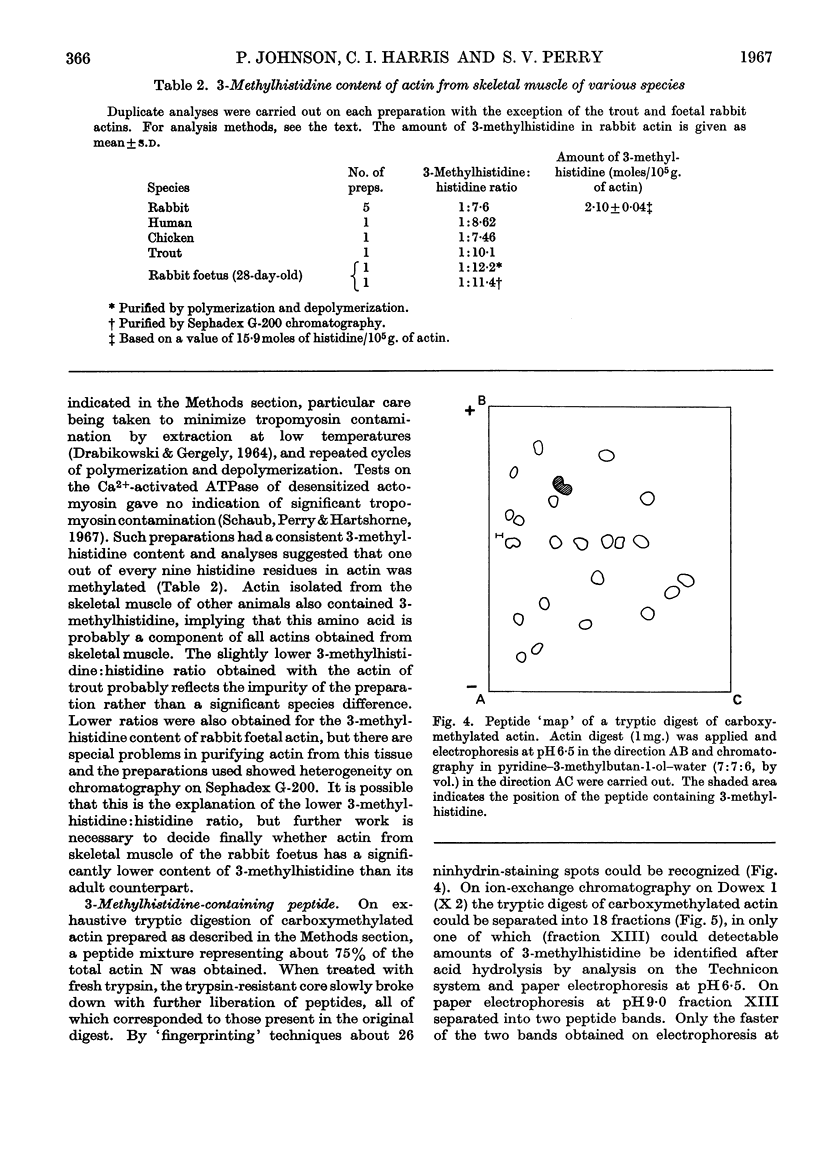
Abstract
1. By the use of the extended elution system for basic amino acid analysis, 3-methylhistidine has been detected in hydrolysates of actin isolated from mammalian, fish and bird skeletal muscle. 2. Evidence is presented to indicate that 3-methylhistidine forms part of the primary structure and that in rabbit actin this residue is restricted to one peptide fraction obtained from the tryptic digest. 3. Rabbit skeletal-muscle actin has a 3-methylhistidine:histidine ratio 1:7·6, indicating a minimum molecular weight of 47600. 4. Adult rabbit myosin contains approximately 2 3-methylhistidine residues/mol. These residues are localized in the heavy meromyosin part of the molecule, and are restricted to the major component obtained after succinylation.
Full text
PDF


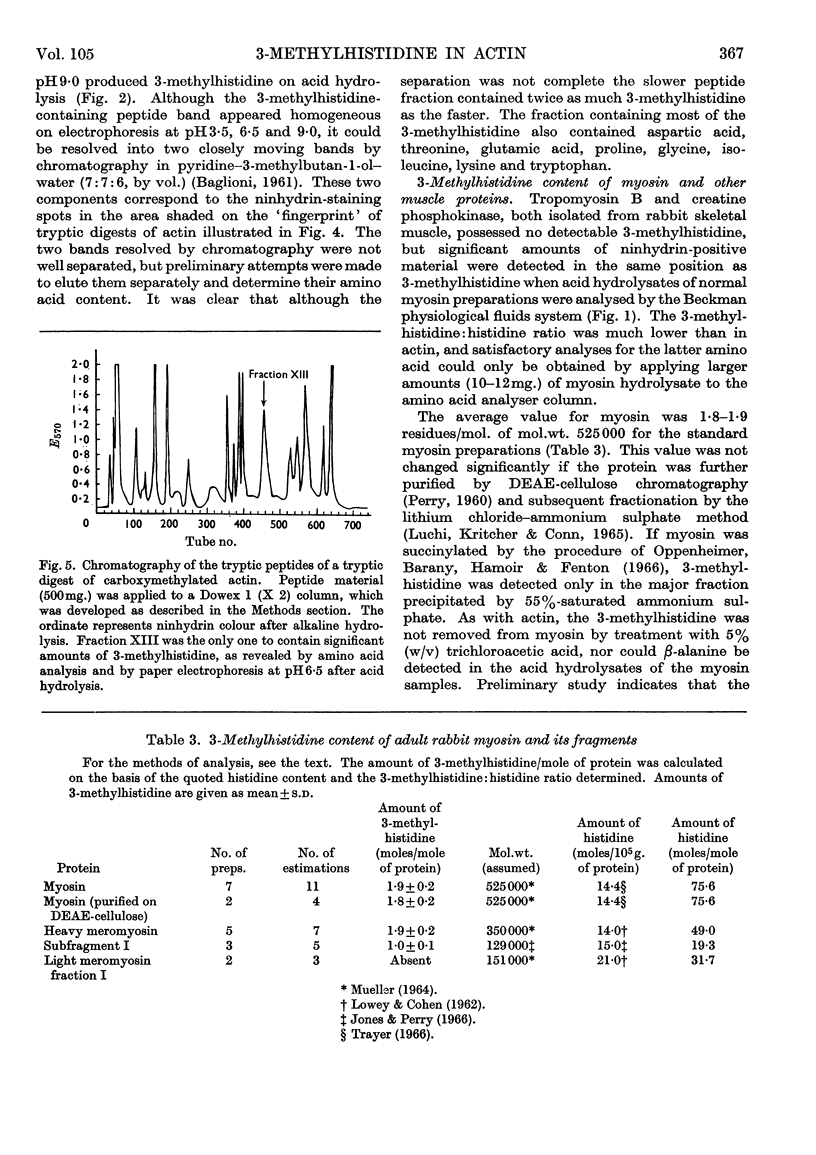



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P., REES M. W. Epsilon-N-Methyl-lysine in bacterial flagellar protein. Nature. 1959 Jul 4;184:56–57. doi: 10.1038/184056b0. [DOI] [PubMed] [Google Scholar]
- Asatoor A. M., Armstrong M. D. 3-methylhistidine, a component of actin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):168–174. doi: 10.1016/0006-291x(67)90229-x. [DOI] [PubMed] [Google Scholar]
- BAGLIONI C. An improved method for the fingerprinting of human hemoglobin. Biochim Biophys Acta. 1961 Apr 1;48:392–396. doi: 10.1016/0006-3002(61)90490-5. [DOI] [PubMed] [Google Scholar]
- BAILEY K. End-group assay in some proteins of the keratin-myosin group. Biochem J. 1951 Jun;49(1):23–27. doi: 10.1042/bj0490023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARSTEN M. E., MOMMAERTS W. F. A study of actin by means of starch gel electrophoresis. Biochemistry. 1963 Jan-Feb;2:28–32. doi: 10.1021/bi00901a006. [DOI] [PubMed] [Google Scholar]
- COCKS D. H., DENNIS P. O., NELSON T. H. ISOLATION OF 3-METHYL HISTIDINE FROM WHALEMEAT EXTRACT AND THE PREPARATION OF SOME DERIVATIVES. Nature. 1964 Apr 11;202:184–185. doi: 10.1038/202184a0. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Dent C. E. A study of the behaviour of some sixty amino-acids and other ninhydrin-reacting substances on phenol-;collidine' filter-paper chromatograms, with notes as to the occurrence of some of them in biological fluids. Biochem J. 1948;43(2):169–180. [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H., MOORE S., STEIN W. H. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [PubMed] [Google Scholar]
- Johnson P., Harris C. I., Perry S. V. 3-methylhistidine in actin and other muscle proteins. Biochem J. 1967 Jun;103(3):79P–79P. [PMC free article] [PubMed] [Google Scholar]
- Jones J. M., Perry S. V. The biological activity of subfragment 1 prepared from heavy meromyosin. Biochem J. 1966 Jul;100(1):120–129. doi: 10.1042/bj1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASAI M., NAKANO E., OOSAWA F. POLYMERIZATION OF ACTIN FREE FROM NUCLEOTIDES AND DIVALENT CATIONS. Biochim Biophys Acta. 1965 Mar 29;94:494–503. doi: 10.1016/0926-6585(65)90058-0. [DOI] [PubMed] [Google Scholar]
- KRANS H. M., VAN EIJKH, WESTENBRINK H. G. A STUDY OF G-ACTIN. Biochim Biophys Acta. 1965 Apr 12;100:193–201. doi: 10.1016/0304-4165(65)90441-1. [DOI] [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. I. Isolation of the crystalline enzyme from rabbit muscle. J Biol Chem. 1954 Jul;209(1):191–201. [PubMed] [Google Scholar]
- LAKI K., MARUYAMA K., KOMINZ D. R. Evidence for the interaction between tropomyosin and actin. Arch Biochem Biophys. 1962 Aug;98:323–330. doi: 10.1016/0003-9861(62)90190-x. [DOI] [PubMed] [Google Scholar]
- LOWEY S., COHEN C. Studies on the structure of myosin. J Mol Biol. 1962 Apr;4:293–308. doi: 10.1016/s0022-2836(62)80007-2. [DOI] [PubMed] [Google Scholar]
- MARTONOSI A., GOUVEA M. A., GERGELY J. Studies on actin. IV. Actin as a component of myosin-B. J Biol Chem. 1960 Nov;235:3169–3173. [PubMed] [Google Scholar]
- MIHASHI K. MOLECULAR CHARACTERISTICS OF G-ADP ACTIN. Arch Biochem Biophys. 1964 Sep;107:441–448. doi: 10.1016/0003-9861(64)90300-5. [DOI] [PubMed] [Google Scholar]
- MUELLER H. MOLECULAR WEIGHT OF MYOSIN AND MEROMYOSINS BY ARCHIBALD EXPERIMENTS PERFORMED WITH INCREASING SPEED OF ROTATIONS. J Biol Chem. 1964 Mar;239:797–804. [PubMed] [Google Scholar]
- Mueller H. Characterization of the molecular region containing the active sites of myosin. J Biol Chem. 1965 Oct;240(10):3816–3828. [PubMed] [Google Scholar]
- Oppenheimer H., Bárány K., Hamoir G., Fenton J. Polydispersity of succinylated myosin. Arch Biochem Biophys. 1966 Jul;115(1):233–234. doi: 10.1016/s0003-9861(66)81064-0. [DOI] [PubMed] [Google Scholar]
- PERRY S. V., CORSI A. Extraction of proteins other than myosin from the isolated rabbit myofibril. Biochem J. 1958 Jan;68(1):5–12. doi: 10.1042/bj0680005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V. The chromatography of L-myosin on diethylaminoethylcellulose. Biochem J. 1960 Jan;74:94–101. doi: 10.1042/bj0740094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V., ZYDOWO M. The nature of the extra protein fraction from myofibrils of striated muscle. Biochem J. 1959 Feb;71(2):220–228. doi: 10.1042/bj0710220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAFTERY M. A., COLE R. D. Tryptic cleavage at cysteinyl peptide bonds. Biochem Biophys Res Commun. 1963 Mar 25;10:467–472. doi: 10.1016/0006-291x(63)90381-4. [DOI] [PubMed] [Google Scholar]
- RYLE A. P., SANGER F., SMITH L. F., KITAI R. The disulphide bonds of insulin. Biochem J. 1955 Aug;60(4):541–556. doi: 10.1042/bj0600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARLE J. M., WESTALL R. G. The occurrence of free methylhistidine in urine. Biochem J. 1951 Apr;48(4):1–1. [PubMed] [Google Scholar]
- SJOQUIST J. Determination of amino acids as phenyl thiohydantoin derivatives. III. Quantitative determination of 3-phenyl-2-thiohydantoins from paper chromatograms. Biochim Biophys Acta. 1960 Jun 17;41:20–30. doi: 10.1016/0006-3002(60)90364-4. [DOI] [PubMed] [Google Scholar]
- TALLAN H. H., STEIN W. H., MOORE S. 3-Methylhistidine, a new amino acid from human urine. J Biol Chem. 1954 Feb;206(2):825–834. [PubMed] [Google Scholar]
- WITTMANN H. G., BRAUNITZER G. Isolation and composition of all tryptic peptides of TMV. Virology. 1959 Dec;9:726–728. doi: 10.1016/0042-6822(59)90170-9. [DOI] [PubMed] [Google Scholar]