Abstract

The injection of CO2 into shale gas reservoirs can not only enhance shale gas recovery (ESGR), but also realize CO2 geological storage (CGS). In this study, the competitive adsorption behaviors of CO2 and CH4 in shale were systematically reviewed, and the implication for shale gas recovery efficiency and CO2 storage potential were discussed. The adsorption advantage of shale for CO2 compared to CH4 provides a guarantee of the feasibility of supercritical CO2 (ScCO2) enhanced shale gas exploitation technology. The selective adsorption coefficient of CO2 and CH4 by shale (SCO2/CH4) is an important parameter in evaluating the competitive adsorption behavior of CO2/CH4 in shale gas reservoirs, which is closely related to the mineral composition, reservoir temperature, pressure conditions, water content, and mixed gas composition ratio. In addition, the injection type, injection mode, and injection rate of gases also exhibit different effects on CO2/CH4 competitive adsorption. Furthermore, the interaction between ScCO2 and the water–rock system will change the mineral composition and microstructure of shale, which will lead to changes in the adsorption behavior of shale on CO2 and CH4, so its influence on the competitive adsorption of CO2/CH4 cannot be ignored. Future research should integrate different research methods and combine with practical engineering to reveal the competitive adsorption mechanism of CO2/CH4 in shale reservoirs from both micro and macro aspects. This study can provide support for the integration technology of ScCO2 enhanced shale gas exploitation and its geological storage.
1. Introduction
Shale gas is a kind of unconventional natural gas, which mainly exists in adsorbed and free states in organic matter, clay minerals, and pore and fracture structures of shale, with the adsorbed gas accounting for 20–85% of the total gas content.1 Hydraulic fracturing is one of the main technical means of shale gas exploitation, but the recovery rate of shale gas is generally low (<30%).2 The typical curve of shale gas production over time is represented in Figure 1, and the gas production process of shale reservoirs can be divided into three stages, namely, the rapid decay stage (<400 days), the slow decrease stage (400–1000 days), and the stable development stage (>1000 days). A widely accepted explanation is that the free gas is mainly recovered in the early stage of shale gas production and the adsorbed gas is predominantly recovered in the later stage.3,4 In view of the fact that adsorbed gas mainly exists in the nanoscale pores of shale, its recovery requires a greater driving force, which is one of the predominant reasons for the low gas production in the later stage of shale gas development.
Figure 1.
Actual production amount of shale gas wells.5 (Photograph courtesy of Yang, R., copyright 2022, and the images in the figure are free domain.)
Numerous studies6−9 have indicated that the adsorption capacity of shale for CO2 is significantly stronger than that of CH4, thus the CO2 huff-puff technology was suggested to be adopted in the later stage of shale gas development according to the shale oil reservoir development method. After injecting CO2 into the shale gas reservoir, the CO2 molecules will gradually enter the microscale or nanoscale pore structure along the fractured crack network and then exchange gas molecules with the residual adsorbed CH4 in the form of competitive adsorption, which can promote the conversion of CH4 from an adsorbed state to a free state, thereby improving the efficiency of shale gas recovery. Simultaneously, part of injected CO2 will be captured by shale reservoirs to achieve CO2 geological sequestration (Figure 2). Accordingly, the competitive adsorption behavior of CO2/CH4 after injecting CO2 into the shale gas reservoir is a key factor affecting shale gas recovery and CO2 storage.9,10
Figure 2.

Schematic diagram of the integration of shale gas recovery and CO2 geological storage.
In order to realize the efficient development of shale gas and CO2 geological storage, a large number of scholars have conducted a series of studies and achieved significant results. This study systematically reviews and summarizes the previous research results, aiming to provide theoretical guidance for ScCO2 enhanced shale gas exploitation technology. The targets and scope of this study include the following two parts:
-
(1)
The effects of different factors on the competitive adsorption behavior of CO2 and CH4 in shale are reviewed and summarized, including the content and type of organic matter, mineral composition, reservoir temperature and pressure conditions, water content, mixed gas composition ratio, injection type, injection mode, injection rate, and ScCO2–water–rock interaction.
-
(2)
Based on the published relevant research, the shortcomings are pointed out, and then the directions of future attention and research are proposed.
2. CO2/CH4 Competitive Adsorption Behaviors in Shale
After CO2 is injected into shale gas reservoirs, CO2 and CH4 actually exist in the form of mixed gas. In view of the remarkable differences in the adsorption behavior of CO2 and CH4 in shale, the two kinds of gas molecules will compete for adsorption sites in the micro- or nanoscale pores of shale in the form of competitive adsorption, which will generate a certain impact on shale gas recovery and CO2 geological storage. A large number of pure component gas adsorption experiments manifest that the thermodynamic parameters such as adsorption heat, Gibbs free energy, and entropy loss of CO2 in shale are higher than those of CH4; moreover, the adsorption capacity of CO2 in shale is about 2–10 times that of CH4.11−13 It can be concluded that a greater adsorption advantage for CO2 existed in shale rather than CH4.7,13,14 Accordingly, after CO2 injection into the shale reservoir, CO2 molecules will compete with adsorbed CH4 molecules for adsorption sites due to the difference in the adsorption of CO2 and CH4 in shale, which indirectly promotes the desorption of CH4 and improves the recovery rate of shale gas. Simultaneously, part of the injected CO2 will be captured and stored in shale reservoirs. Currently, laboratory experiments, molecular simulations, and numerical analyses were principally used to investigate the competitive adsorption behavior of CO2/CH4 in shale.7,15,16
2.1. Methods
2.1.1. Laboratory Experiments
The mixed gas adsorption test is the most direct and effective means to characterize the competitive adsorption characteristics of different gases in porous materials, while it is different from the pure component gas adsorption test due to the relatively complex experimental system, resulting in increased difficulty in the experiment. Figure 3 illustrates the typical mixed gas adsorption device.15 It can be seen that the test device mainly consists of five parts: a gas mixing system, pressurization system, adsorption system, gas component calibration system, and computer control system. Compared with the pure component gas adsorption test, the main difficulties in the mixed gas adsorption test are as follows: 1) accurately configuring the composition ratio of the mixed gas; 2) accurately calculating the mixed gas adsorption capacity; and 3) obtaining kinetic adsorption data of mixed gases.
Figure 3.
Mixed gas adsorption system.15 (Photograph courtesy of Qin, C., copyright 2021, and the images in the figure are free domain.)
2.1.2. Molecular Dynamics Simulation
The difficulty with laboratory experiments is mainly reflected in the high accuracy requirements of experimental instruments, the complexity of the adsorption capacity calculation, and the challenge of analyzing various potential influencing factors. Thus, molecular simulation methods have gradually attracted the attention of scholars. Currently, density functional theory (DFT) based on quantum mechanics, the grand canonical Monte Carlo (GCMC) method based on statistical mechanics, and the molecular dynamics simulation (MD) based on Brownian motion are widely used.17 According to the types of adsorbents, the corresponding inorganic matter models and organic matter models along with inorganic and organic compound models have been established.18,19 Among them, the commonly used inorganic models mainly include quartz and clay minerals (montmorillonite, illite) models,20 the organic models mainly include graphene nanomodels and kerogen models (mainly containing three types of kerogen: types I–III),12,21,22 and the composite models use multilayer graphene structures.23 Generally, the molecular simulation method can be used to conduct targeted research on different variables so as to obtain the influence of different potential factors (organic matter type, mineral composition, pore structure, temperature, pressure, water content, gas component ratio, etc.) on the competitive adsorption of multicomponent gases. Figure 4 shows the construction method of a composite heterogeneous nanopore model containing organic and inorganic matter.
Figure 4.
Construction method of a composite heterogeneous nanopore model containing organic matter and inorganic matter.24 (Photograph courtesy of Ma, J., copyright 2022, and the images in the figure are free domain.)
2.1.3. Numerical Analysis
Currently, the numerical simulation software applied to the competitive adsorption of multicomponent gases includes GEM, COMET, GCOMP, SIMED, and TOUGH2.25−29 Taking GEM software as an example, by determining the mass equation, the relative permeability equation, and the adsorption equation, setting reservoir-related parameters (mineral composition, porosity, permeability, etc.) and initial conditions (initial pressure, temperature, water saturation, etc.), the migration process of CO2 and CH4 in the target geological body can be simulated to obtain the final CH4 yield and CO2 storage capacity so as to analyze the adsorption and displacement mechanism of CO2 replacement for CH4 in shale (Figure 5).
Figure 5.
Schematic diagram of the double-porosity and double-permeability homogeneous model.30
2.2. Influence Factors
A large number of competitive adsorption experiments of two-component and three-component gases on a coal matrix have been carried out and achieved remarkable results,31,32 which have laid a theoretical foundation for studying the competitive adsorption characteristics of CO2/CH4 in shale. However, the adsorption capacity of shale for CO2 and CH4 is relatively weak compared to that of the coal matrix, and there are inevitable test errors in the mixed gas adsorption test, which makes it more difficult to conduct the multicomponent gas adsorption experiment in shale. According to the related report,33 it can be found that the adsorption capacity of CO2/CH4 mixed gas in shale is between the values for pure CH4 and pure CO2 and displays an increasing trend with the increase in CO2 concentration in the mixed gas. Based on the change in the molar fraction of each component in the free phase and the adsorbed phase of the mixed gas before and after adsorption, the selective adsorption coefficient can be used to evaluate the selective adsorption behavior of CO2 and CH4 in shale.15 The calculation procedure is shown in Figure 6
| 1 |
where SCO2/CH4 is the selective adsorption coefficient of CO2 and CH4 in shale (dimensionless), which is closely related to the properties of shale samples, water content, system temperature, and pressure; xCO2 and xCH4 represent the molar fractions of CO2 and CH4 in the adsorption phase (dimensionless); yCO2 and yCH4 represent the mole fractions of CO2 and CH4 in the free phase (dimensionless). Some scholars use local density to indicate the difference in the adsorption capacity of gases, and the local density represents the number of gas molecules in a specific region or location. Due to its large molecular size and strong polarity, CO2 interacts more strongly and generally binds to the surface of porous materials (such as shale and coal) more readily than CH4. As a result, CO2 exhibits a larger local density and exhibits an adsorption capacity different from that of CH4 molecules. Qi34 uses the simplified local density theory (SLD) of multicomponent gases to calculate the actual local densities of different gases in the pores. The competitive adsorption capacity of CH4 and CO2 gases on the pore surface was obtained by comparing the local densities. The CO2/CH4 competitive adsorption experiments conducted in shale are represented in Table 1, which mainly involve the effects of the shale physical structure, reservoir conditions, and gas component ratio on SCO2/CH4, and the following conclusions can be drawn.
Figure 6.
Calculation procedure of SCO2/CH4.
Table 1. CO2/CH4 Competitive Adsorption Experiments of Different Shale Samples.
| References | Samples | Conditions | Main findings |
|---|---|---|---|
| Xie et al.35 | Longmaxi | 0–20 MPa 288–328 K | SCO2/CH4 decreases with the increases in CO2 molar fraction, temperature, and pressure but is in direct proportion to TOC and clay mineral content. |
| Gu et al.36,37 | Longmaxi Wufeng | 0–2 MPa 278–318 K | SCO2/CH4 decreases with the increases in TOC, microporous content, and temperature but is inversely proportional to clay mineral content. |
| Qin et al.15 | Yanchang | 0–6 MPa 313 K | The molar fraction of CO2 has no obvious effect on SCO2/CH4. SCO2/CH4 gradually decreases with growing pressure; the ScCO2–shale interaction resultes in a slight decline in SCO2/CH4. |
| Liu et al.9 | Longmaxi | 0–10 MPa 303 K | SCO2/CH4 is proportional to the molar fraction of CO2 and inversely proportional to the pressure. |
| Du et al.38 | Wufeng | 0–2 MPa 278–318 K | SCO2/CH4 enhances with the decreases in molar fraction of CO2 and pressure, but the effect of temperature is not obvious. |
| Ortiz et al.39 | Outcropping shale in the Iberian Range (Spain) | 0–2 MPa -- | SCO2/CH4 increases first and then decreases as the pressure grows. |
| Qi et al.40 | Wufeng–Longmaxi | 0–20 MPa 303 K/353 K | SCO2/CH4 is proportional to the molar fraction of CO2 and temperature. |
| Liao et al.6 | Wufen Longmaxi Yanchang | 0–10 MPa 323 K | SCO2/CH4 increases first and then decreases with the molar fraction of CO2; the variation with pressure is affected by the CO2/CH4 mixing ratio. |
| Ma et al.41 | Longmaxi | 0–15 MPa 323–363 K | The molar fraction of CO2 in the mixed gas affects the trend of SCO2/CH4 with pressure. |
2.2.1. Effect of Mineral Compositions
SCO2/CH4 is generally proportional to the content of TOC and inversely proportional to the clay mineral content. The effect of TOC on SCO2/CH4 is mainly related to the large number of micropore structures developed in shale organic matter.42 An acceptable explanation is that the micropores belong to high-energy adsorption sites in shale, and the adsorption advantage of shale for CO2 is particularly prominent in high-energy adsorption sites.43 In addition, relevant scholars44−47 have investigated the effect of organic matter type on the competitive adsorption behavior of CO2 and CH4 in shale, and the results indicated that the adsorption capacity and SCO2/CH4 of different kerogens are listed as follows: IA < IID < IIC < IIB < IIA < IIIA. (IA is typical of hydrogen-rich kerogen, II is deposited in marine environments, and IIIA is deposited in deltaic environments. The chemical formulas of kerogen types of II-A, II-B, II-C, and II-D are C252H294O24N6S3, C234H263O14N5S2, C242H219O13N5S2, and C175H102O9N4S2, respectively) (Figure 7 (b)). In addition, they found that the N groups, S groups, and O groups in kerogen exhibit a stronger adsorption capacity for CO2. A similar conclusion obtained by Huang et al.46 showed that CO2 gas was preferentially adsorbed in the S groups in the kerogen of IA and IIA models and the N groups in the kerogen of IIIA models. The influence of clay minerals on SCO2/CH4 is also closely related to their mineral types, and the orders of SCO2/CH4 for the clay minerals are montmorillonite > illite > kaolinite.48 Specially, CO2 molecules tend to occupy higher energy adsorption sites in montmorillonite and illite due to the cation exchange at low pressure, while a mixture of CO2 and CH4 begins to occupy the middle of the pore when the adsorption layer reaches saturation at higher pressures46 (Figure 7 (a)).
Figure 7.
Effect of the types of clay minerals and organic matter on SCO2/CH4. (a) Clay minerals;48 (b) organic matter.47 (Photograph courtesy of Hu, X., copyright 2019, and Sui, H., copyright 2020, and the images in the figure are free domain.).
It is worth noting that although SCO2/CH4 is negatively correlated with the content of clay minerals, it exhibits a positive correlation with the content of clay minerals after standardizing clay minerals with TOC (Figure 8), indicating that the influence of TOC on SCO2/CH4 may weaken the effects of clay minerals. The competitive adsorption behavior of CO2 and CH4 in clay minerals is not only related to its internal pore structure but also affected by its hydrophilic properties.49 Water molecules will occupy part of the adsorption sites in clay minerals, thus affecting the adsorption behavior of CO2 and CH4. Therefore, it is necessary to consider the potential influence of hydrophilic properties on the competitive adsorption behavior of CO2 and CH4 in clay minerals. Through molecular simulation studies, Hu et al.48 found that although cation exchange sites are mostly in clay minerals, the 1:1 layer structure of kaolinite lacks the interlayer space necessary for cation accommodation and exchange, coupled with the strong internal bonding and lack of charge-balancing requirements, so it does not have cation exchange. Cations may alter the adsorption sites’ energy landscape and significantly improve SCO2/CH4; therefore, the selective adsorption capacity of kaolinite is not as good as that of montmorillonite and illite. Additionally, they consider that the charge effect may be the main factor that leads to a stronger adsorption capacity of CO2 than CH4 in clay minerals. Specifically, the presence of exchangeable cations will affect the ionic strength of shale pore water and the charge distribution on the shale surface, enhancing the solubility of CO2 in the water phase. In addition, due to the different polarization properties of CO2 and CH4, the electrostatic interaction between CO2 molecules and the shale surface is stronger than that of CH4, so it is easier to adsorb.
Figure 8.
Relationship of SCO2/CH4 versus TOC and clay content.35 (Photograph courtesy of Xie, W., copyright 2022, and the images in the figure are free domain.)
2.2.2. Effects of Pore Size and Content
Aiming at the influence of micropores, Duan et al.36 and Xie et al.35 denoted that SCO2/CH4 was directly proportional to the micropore content through experiments, while an unobvious correlation between micropore content and SCO2/CH4 was represented in the study of Gu et al.37 A possible interpretation is that the micropores obtained in the study of Gu et al.37 are not the real micropore content of shale (the low-temperature N2 adsorption method was used to measure the pore structure of shale in their study, while this method can test pores only above 1.5 nm). In addition, molecular simulation has been used to study the effect of different pore size ranges on the competitive adsorption of CO2 and CH4. Zhou et al.50 indicated that SCO2/CH4 decrease with the increase in pore size based on the model of kaolinite clay minerals, while a different result was obtained according to a graphene slit model of organic nanopores in shale.51 They51 discovered that SCO2/CH4 first increased and then decreased in the pore size range of 1.0–4.0 nm, and there was a maximum value of around 1.5–2.0 nm (Figure 9). Furthermore, Qi et al.52 denoted that 0.42 nm is the critical size that determines which gas in CO2 and CH4 is preferentially adsorbed, and CH4 will be preferentially adsorbed when the pore size is less than 0.42 nm; otherwise, CO2 will be preferentially adsorbed. The possible explanations for this phenomenon are as follows: The pore wall exhibits a repulsive effect on gas molecules, and the repulsive effect on CO2 in smaller pores (≤0.42 nm) is greater than that of CH4, resulting in a weakened adsorption capacity for CO2. The CO2 molecule (carbon dioxide) is smaller than the CH4 molecule (methane) and has a linear structure, whereas the CH4 molecule is spherical. Thus, smaller CO2 molecules are more likely to enter smaller nanopores. In addition, due to the interaction of the pore wall, the adsorption capacity of the gas molecules will be enhanced, and smaller CO2 molecules can adapt to the pore geometry more easily and form stronger adsorption on the pore wall. In nanopores, the van der Waals forces between the gas molecules and the pore wall have a significant effect on the adsorption. Finally, because CO2 molecules have stronger polarity and higher dipole moments, the van der Waals forces between CO2 molecules and the pore wall are stronger than those for CH4 molecules. In smaller pores, the diffusion rate of gas molecules is limited, while smaller CO2 molecules diffuse at a faster rate than CH4 molecules, which makes it easier for them to diffuse through pores and occupy adsorption sites.52
Figure 9.
Effect of pore structure on SCO2/CH4. (a) Shale samples,37 (b) kaolinite,50 and (c) organic matter.51 (Photograph courtesy of Gu, M., copyright 2017, Zhou, W., copyright 2019, and Zhou, W., copyright 2018. The images in the figure are free domain.)
It is worth mentioning that the variations of pore size can affect the adsorption behavior of CO2 and CH4 in shale. Related studies53−55 have demonstrated that CO2 and CH4 are mainly adsorbed in the form of micropore filling in micropores, while they are mainly adsorbed in the form of monolayers in mesopores and macropores. This is a topic of constant debate regarding whether the gas adsorption mechanism in shale is monolayer adsorption or micropore filling. Zhou et al.50 pointed out that the strong adsorption layer is mainly due to the monolayer adsorption mechanism, while the weak adsorption layer is caused by micropore filling. Their research shows that in micropores, gas molecules first form a single layer of adsorption (strong adsorption layer) on the pore wall, and as the pressure increases or the pore diameter decreases, gas molecules begin to fill in the pores to form weak adsorption layers. The change in pore size (1–6 nm) has no obvious effect on monolayer adsorption but has a great effect on micropore filling adsorption. With the increase in pore size, the effect of micropore filling adsorption gradually weakens, and when the pore size is larger than 2 nm, the effect can be ignored (Figure 10). This may be related to factors such as gas pressure, temperature, fluid density, and pore size. Under high-pressure conditions, gas molecules are more inclined to form multilayer adsorption in the pores and the increase in temperature will reduce the density of gas molecules in the adsorption layer, so low temperature is conducive to gas adsorption. In addition, the increase in pore size will weaken the adsorption affinity of the pore structure to gas molecules, thus reducing the adsorption capacity of gas molecules. A similar conclusion was obtained by Zeng et al.,56 and they manifested that the increase in pore size would weaken the adsorption affinity of the pore structure to gas molecules, thus reducing the number of adsorbed gas molecules. It should be noted that the development of pore fracture structure in shale is closely correlated with the diversity of mineral species, but the effect of the pore structure of different minerals on the SCO2/CH4 has received little attention.
Figure 10.

Selectivities in different adsorption layers.50 (Photograph courtesy of Zhou, W., copyright 2019, and the image in the figure is free domain.)
2.2.3. Effect of Reservoir Temperature and Pressure Conditions
SCO2/CH4 is inversely proportional to the system temperature, but the influence of the system pressure remains to be further studied. Regarding the influence of temperature, although the adsorption capacity of shale for CO2 and CH4 decreases with increasing temperature,57 the degree of decrease in CO2 adsorption is higher than that of CH4 due to the critical temperature of CO2 being higher than that of CH4, resulting in a decreasing trend of SCO2/CH4 with increasing temperature. The conjecture can be verified by the research results in Figure 11. And Bai58 studied the adsorption behavior of CO2 molecules under subcritical and supercritical temperatures and found that the change law of gas adsorption-phase properties was determined by the special properties of near-critical fluid and the pore structure of adsorbent. Below the critical temperature, because the adsorption energy of gas molecules is usually lower than the thermal energy in the body phase, they are inclined to be adsorbed. However, as the temperature approaches or exceeds the critical temperature, the thermal energy increases, the interaction of gas molecules is weakened, and the active sites on the surface of the adsorbent are saturated faster, which may lead to the desorption of gas and reduce the adsorption amount. In addition, when the critical temperature is exceeded, the pores may expand or contract, affecting the accessibility of the pores and the availability of adsorption sites. Due to the high critical temperature of CO2, there are differences between CO2 and CH4 in the adsorption process in terms of intermolecular force and adsorption heat, so its adsorption behavior is more sensitive when it is close to the critical temperature. The adsorption heat of CO2 is usually higher than that of CH4, so more heat is released during the adsorption process. At higher temperatures, the thermal movement of the CO2 molecules is intensified, so they will be easier to desorb from the adsorbent surface.
Figure 11.
Effect of pressure (MPa) and temperature (K for (a) and (b),°C for (c) and (d)) on SCO2/CH4. (a) Shale organic nanopores,51 (b) shale of the Longmaxi Formation,59 (c) shale of the Longmaxi Formation,54 and (d) shale of the Longmaxi Formation.54 (Photograph courtesy of Zhou, W., copyright 2018, Lu, T., copyright 2022, and Xie, W., copyright 2021. The images in the figure are free domain.)
In view of the influence of pressure, relevant scholars7,50 found that SCO2/CH4 decreased with increasing adsorption pressure through laboratory tests, and they indicated that the gas molecules will be preferentially adsorbed at the high-energy adsorption site of kerogen under low-pressure conditions. In this case, the interactions between kerogen and gas molecules is dominant, and the adsorption advantage of CO2 is reflected.60 As the pressure increases, the high-energy adsorption sites are gradually occupied, and the gas molecules can compete for only the adsorption sites on the low-energy adsorption sites. In this case, the interaction force of the gas molecules is dominant, thus resulting in the weakening of the adsorption advantage of CO2, and SCO2/CH4 gradually tends to be balanced.47 However, different results were discovered by Liao et al.6 and Ortiz et al.39 They discovered that the SCO2/CH4 of some shale samples increased first and then decreased with increasing pressure (Figure 12). A possible explanation is that the TOC and clay mineral (illite) content of the tested samples are relatively high, resulting in the adsorption capacity of the high-energy adsorption site in shale not reaching the saturation state under low-pressure conditions. In addition, the gas injection volume will increase with increasing pressure, while the adsorption capacity of CO2/CH4 in shale in the whole adsorption system is limited, which can lead to the increase of residual gas. Therefore, the influence of the free phase ratio on SCO2/CH4 will be weakened according to eq 1; namely, the yCO2/yCH4 will gradually approach the original gas injection ratio with the increase in gas injection, thus resulting in the decrease in SCO2/CH4.
Figure 12.

Change in SCO2/CH4 with adsorption pressure in shale.6 (Photograph courtesy of Liao, Q., copyright 2023, and the image in the figure is free domain.)
2.2.4. Effect of Water Content
The variation of SCO2/CH4 with water content is closely related to the type of organic matter in shale. Huang et al.61 indicated that the SCO2/CH4 of the kerogen of IIA, IIC, and IID increased with increasing water content while that of kerogen with IIB decreased with increasing water content when the pressure is >3 MPa (Figure 13). In addition, the distribution of water molecules in kerogen varies significantly under different water content conditions. Relevant scholars46,47,62 denoted that the water molecules will be irregularly dispersed in kerogen pores and mainly exist on the pore surface in the form of water film under the condition of low water content while most of the water molecules will form clusters and distribute in the pore center under the condition of high water content, which will lead to the pore space of kerogen being occupied by a large number of water molecules, thus reducing the adsorption capacity of kerogen for CO2 and CH4. On this basis, Zhou et al.50 indicated that the polarity of water molecules is stronger than that of CO2 and CH4 molecules, which is the main reason that water molecules are more likely to occupy the adsorption site of kerogen. Simultaneously, the water molecules also tend to adsorb to each other and form clusters on hydrophilic clay minerals, which will further interfere with the adsorption of the CO2 and CH4 molecules. Furthermore, it is worth emphasizing that the molar ratio of mixed gas will change the variation of SCO2/CH4 with the water content. Zhang et al.63 discovered that the SCO2/CH4 in kerogen decreased sharply with the increase in water content when the molar fraction of CO2 is high while it increased with increasing water content when the molar fraction of CH4 is high.
Figure 13.
Effect of the water content on SCO2/CH4. (a) Different kerogen models;61 (b) type II-D kerogens (10 water, 50 water, and 100 water mean 10, 50, and 100 water molecules).47 (Photograph courtesy of Huang, L., copyright 2018, and Sui, H., copyright 2020, and the images in the figure are free domain.).
2.2.5. Effect of the Proportion of Each Component in the Mixed Gas
The effect of the variation of the CO2 molar fraction on SCO2/CH4 remains to be further studied. With the increase in the CO2 molar fraction in the CO2/CH4 gas mixture, SCO2/CH4 exhibited a decreasing trend reported by Xie et al.35 while it displayed a slightly increasing trend published by Qin et al.15 In addition, Liao et al.6 discovered that the SCO2/CH4 obtained by the mixing ratio of 50%:50% was significantly higher than that of other mixing ratios (Figure 14). It is pointed out that the variation of SCO2/CH4 with the proportion of mixed gas components may be related to the difference in molecular properties of different gases and the adsorption affinity of different adsorption sites in shale for gas molecules.50,64 However, this inference is only given a qualitative description and lacks a sufficient explanation at present. Consequently, it is necessary to explore the interaction relationship of different gas molecules in shale from the molecular level, thereby providing a theoretical reference for the influence mechanism of the gas mixing ratio.
Figure 14.
Effect of the mixing ratio of CO2/CH4 on SCO2/CH4.6,15,35 (Photograph courtesy of Liao, Q., copyright 2023, Qin, C., copyright 2021, and Xie, W., copyright 2022, and the images in the figure are free domain.)
2.2.6. Effect of the Injection Gas
In addition to injecting CO2 gas into shale reservoirs, the injection of N2 or a mixture of N2 and CO2 can also improve the recovery rate of shale gas to a certain extent, while significant differences existed in the replacement mechanism of the two injected gases. Related studies through experiments and simulations65−68 have indicated that both CO2 and N2 injection can improve the recovery rate of shale gas to a certain extent (2–80%), but the injection effects of the two schemes are significantly different (Figure 15). They demonstrated that the injection effect of N2 for shale gas recovery is obviously better than that of CO2, which is mainly due to the fact that the injection of N2 can promote the desorption of CH4 by reducing the partial pressure of CH4, and this effect is stronger than the influence of CO2/CH4 competitive adsorption on shale gas recovery. Accordingly, it is recommended to inject a mixed gas of CO2 and N2 into the shale reservoir in practical engineering projects, which can not only improve shale gas recovery but also achieve CO2 geological storage. However, how to choose the appropriate injection ratio of CO2 and N2 is a problem worthy of attention, which may be related to the geological conditions, physical parameters, and construction technology of shale reservoirs and should be paid attention to in future practical projects.
Figure 15.
Effects of different CO2/N2 injection ratio on recovery efficiency of shale gas (solid lines mean <4 MPa overpressure injection and dashed lines mean <8 MPa overpressure).65 (Photograph courtesy of Li, Z., copyright 2019, and the image in the figure is free domain.)
2.2.7. Effect of the Injection Mode and Injection Rate
Li et al.69 used a reservoir simulation model of multicomponent transport in dual porosity sorbing and swelling media to compare different injection modes, and they found that compared with the continuous injection of CO2 into shale reservoirs for improving shale gas recovery, the effect of huff and puff injection is more significant. In addition, the effectiveness of ESGR and CGS can be affected by the injection rate, and the increase of the gas injection rate is beneficial to ESGR and CGS when the injection rate is low while an opposite effect existed when the injection rate exceeds a certain threshold (Figure 16). Consequently, a reasonable gas injection rate should be selected according to the actual shale reservoir conditions.
Figure 16.
Effects of changes in CO2 and N2 injection rates on gas recovery and CO2 storage.67 (Photograph courtesy of Ma, H., copyright 2022, and the image in the figure is free domain.)
As can be seen from Figure 16, the increase in the injection rate can improve the recovery rate of shale gas before fracture breakthrough, and the main reason is that the permeability of the fracture cannot be effectively improved when the gas injection rate is low, thus leading to CH4 molecules that cannot flow out. However, there is a threshold for the injection rate, and the shale gas recovery rate will decrease as the injection rate increases when the injection rate exceeds this threshold (Figure 16 (a) dark blue curve, 0.2 MMSCF/day, MMSCF is a measure of the natural gas volume and stands for million standard cubic feet). A possible interpretation is that the fracture breakthrough time may be shortened after exceeding the injection rate threshold and it is difficult to extract natural gas because the fracture permeability is not effectively improved. In addition, it can be seen from Figure 16 (b) that the injected CO2 can be successfully sealed as long as the breakthrough point is not reached. Although more CH4 can be recovered with the increase in the gas injection rate, the utilization efficiency of CO2 is not improved as depicted in Figure 16 (c).
2.2.8. Effect of ScCO2 Immersion
Simulation studies indicate that the effect of ScCO2 immersion on ESGR and CO2 sequestration is negligible,70 but different conclusions have been obtained in laboratory experiments.15,68,71 It can be clearly observed that all values of SCO2/CH4 decreased to varying degrees after ScCO2 immersion (Figure 17 (a)), signifying that ScCO2 weakened the selective adsorption advantage of shale to CO2 to a certain extent, and they indicated that the decrease in SCO2/CH4 is mainly related to the change in the pore structure of shale. As described in Figure 17 (b), it can be seen that some micropores and mesopores in shale were converted to macropores after ScCO2 immersion.72−75 Theoretically, CO2 molecules can enter smaller pore structures compared with CH4 molecules because the molecular diameter of CO2 is smaller than that of CH4. Therefore, the expansion of the pore structure in shale after ScCO2 immersion will reduce the adsorption advantage of shale for CO2, thus leading to a decrease in SCO2/CH4.
Figure 17.
Effect of ScCO2 immersion on SCO2/CH4 and pore structure of shale.15,74 (Photograph courtesy of Qin, C., copyright 2021, and Zhou, J., copyright 2021, and the images in the figure are free domain.)
In addition, the influence of changes in organic matter and the mineral composition of shale after ScCO2 treatment on SCO2/CH4 cannot be ignored. Relevant studies76,77 indicated that after CO2 is dissolved in water, water molecules will react to form carbonic acid, reducing the pH value of the solution, extracting organic matter from shale, dissolving some carbonates (calcite, dolomite) and clay minerals (illite, kaolinite, montmorillonite, etc.) in the shale, thus producing some secondary minerals (such as quartz, kaolinite, etc.), and finally resulting in an increase in the percentage content of quartz in shale (Figure 18). According to Figure 8 and Figure 18, it can be inferred that the decrease in the TOC content of shale after ScCO2 treatment may result in a decrease in SCO2/CH4.
Figure 18.
Variations in shale mineral composition before and after ScCO2 exposure.76
3. Implications for Shale Gas Recovery and CO2 Geological Storage
After CO2 injection into shale reservoirs, the competitive adsorption of CO2/CH4 will affect the efficiency of shale gas recovery and the potential for CO2 storage, and relevant on-site experiments have been conducted in recent years.10,78 For example, the first field test of CO2 injection in the United States was conducted in the Chattanooga shale reservoir in Morgan County, Tennessee10 (Figure 19). About 500 tons of CO2 was injected into the shale reservoir by engineering in 2 weeks, the well was sealed and soaked for four months. It is found that the shale gas recovery rate was improved, the recovery rate of shale gas after CO2 injection was about 8 times higher than before injection, and the production life of the gas well was extended by 15 months.
Figure 19.
CO2-ESGR project in the Chachattooga shale reservoir.10 (Copyright 2017 and the image in the figure is free domain.)
In practical engineering, in order to maximize shale gas recovery and CO2 storage, the appropriate injection gas and injection scheme should be selected according to the geological conditions of the shale reservoir. In addition, the interactions between ScCO2 and water–rock systems will change the mineral composition and pore structure of shale, which will lead to changes in the adsorption behavior of shale on CO2 and CH4, thus affecting the adsorption and replacement effect of CO2 on CH4 (Figure 20). Therefore, after CO2 is injected into shale reservoirs, the appropriate soaking time is also an important factor to be considered, which can be determined through a combination of experimental research and numerical simulation.
Figure 20.
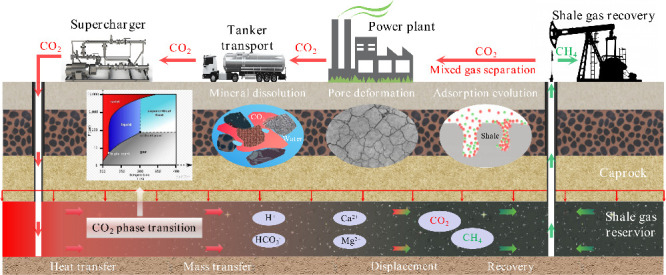
Schematic diagram of ScCO2 enhanced shale gas exploitation and its geological storage integration.
In addition, the assessment of the CO2 storage potential after the depletion of shale gas reservoirs is also an important issue that needs to be addressed. Currently, scholars79−82 have carried out a large number of studies on deep saline aquifers, depleted oil and gas fields, deep unminable coal beds, and shallow seas as CO2 storage geological bodies and have denoted that the CO2 storage potential of geological bodies is not only affected by geological conditions such as scale, burial depth, porosity, permeability but also closely related to technology, economy, policy measures, and other factors. The calculation of the storage capacity is a key task for evaluating the CO2 storage potential of geologic bodies. According to the differences in the purpose of evaluation and the understanding levels of geologic bodies, a technology–economic resource pyramid model was proposed by relevant scholars83 to characterize the potential of CO2 geological storage (Figure 21). This model divides the potential of CO2 storage into four levels—theoretical storage capacity (TSC), effective storage capacity (ESC), actual storage capacity (ASC), and matching storage capacity (MSC)—and suggests that the initial assessment of the geological CO2 storage potential should focus on TSC and ESC.
Figure 21.
Pyramid model of the CO2 geological storage capacity.
Previous studies80,82,84,85 have denoted that there are great differences in the CO2 sequestration mechanism among different sequestration geological bodies, and the evaluation methods of the CSLF (Carbon Sequestration Leaders Forum), US-DOE (United States Department of Energy), UE (European Union), RIPED&CUP evaluation method, novel evaluation method, and three parameters method synchronous storage calculation method were established (Table 2). Among them, the current mainstream methods mainly include the US-DOE and USGS methods based on volume balance theory,84,86 the CSLF method based on material balance theory,82 and the RIPED&CUP method based on the dissolution trapping mechanism.87 In addition, it should be noted that the CO2 storage capacity of the geological body is closely related to its TOC and micropore content. When the geological body has the characteristics of a shallow burial depth, abnormal pressure, and high temperature, it is difficult to reach the supercritical state after CO2 injection, so it cannot be used as an ideal CO2 storage site.88 In general, there are significant differences in the CO2 storage capacity calculated by different calculation models. Therefore, the combination of experimental and theoretical research methods should be used in practical engineering to comprehensively consider the potential impact of various factors.
Table 2. Calculation Methods for CO2 Storage Capacity.
| Measuring Methods | Mathematical model | Representative researchers | |
|---|---|---|---|
| US-DOE-NETL method | GCO2 = VEV[ρCO2ϕEϕ + ρSCO2(1 – ϕ)Es(d) × Es(m)]M = ρr(CO2)·Ahφ(1 – Swi)·BE | Ma et al.83 | |
| CSLF | Mt(CO2) = ρr(CO2)·[RfAhφ(1 – Swi) – Viw+Vpw]Me(CO2) = CeMt(CO2) | Bachu et al.82 | |
| USGS | TASR = (RSV + BSV)ρr(CO2) | Brennan et al.89 | |
| RIPED&CUP |
|
Shen et al.87 | |
| Novel evaluation methods | Rezk et al.90 | ||
| Three-parameter calculation method of CSSP | Wang et al.91 |
4. Challenges and Perspectives
After CO2 is injected into shale gas reservoirs, the composition and microstructure of shale are altered in the process of the ScCO2–water–rock interaction, leading to variations in the adsorption behavior of CO2, CH4, and their mixed gases in shale, which affects the adsorption and displacement effect of CO2 on residual CH4. Currently, a large amount of research15,92−94 has been carried out on the mineral composition, microstructure, and adsorption characteristics of shale under ScCO2–water–rock action, but the following problems still need to be studied further.
CO2/CH4 competitive adsorption is a key factor affecting shale gas recovery and CO2 geological storage.9,46 However, there are currently difficulties in conducting mixed gas experiments and complex calculation processes for adsorption capacity, which leads to relatively enormous errors in the current test results and poor consistency among numerous research conclusions. Accordingly, it is necessary to improve the mixed gas adsorption test equipment and optimize the calculation method of the adsorption amount, thus systematically carrying out an in-depth analysis of the influence mechanism of different factors on the competitive adsorption of mixed gas via mixed gas adsorption tests combined with molecular simulation technology, finally revealing the competitive adsorption mechanism of CO2/CH4 in shale reservoirs.
Both CO2 and N2 injection can improve shale gas recovery to a certain extent,67,95 but how to reasonably select the CO2/N2 mixed injection ratio from the perspectives of safety and economy is an important issue that needs to be addressed in future research. There is a threshold for the influence of the gas injection rate on ESGR and CGS, which may be closely related to shale reservoir geological conditions, gas injection modes (continuous injection, segmented injection, or pulsed injection), injected gas properties, etc. Future studies should pay attention to the influence of these potential factors. The effects of ScCO2 injection on the composition and microstructure of shale cannot be ignored, thus the potential influences of ScCO2–water–rock interaction on ESGR and CGS need to be considered in the future simulation process by combining with laboratory test results. In addition, there are significant differences in the CO2 storage capacity calculated by different CO2 storage calculation models, hence various factors should be considered comprehensively and the calculation method of the CO2 storage capacity should be carefully selected in practical engineering.
5. Conclusions
This study systematically expounds on the competitive adsorption process of CO2 and CH4 in shale, analyzes the influence mechanism of different factors on SCO2/CH4, and discusses the potential influence of CO2/CH4 competitive adsorption on shale gas recovery and CO2 geological storage. The main conclusions are as follows:
-
(1)
SCO2/CH4 is generally proportional to TOC and inversely proportional to clay mineral content, which can be interpreted by the abundant high-energy adsorption sites in organic matter and the hydrophobicity of clay minerals. SCO2/CH4 decreases with increasing temperature while the effects of pressure and the proportion of mixed gas components on SCO2/CH4 are relatively complex, which are mainly related to the changes in the adsorption site of gas molecules and the interactions among gas molecules in shale. In addition, the variation of SCO2/CH4 with water content is controlled by the type of organic matter in shale and the molar ratio of mixed gas.
-
(2)
The injection type (CO2 or N2), injection mode (continuous injection or pulse injection), and injection rate of gases exhibit significant effects on shale gas recovery and CO2 geological storage, and the CO2/N2 mixed gas injection scheme is recommended in actual engineering projects.
-
(3)
The ScCO2–water–rock interaction can dissolve organic matter and minerals of shale, change the microscopic pore structure of shale, lead to changes in the adsorption behavior of CO2 and CH4 in shale, and affect the adsorption and replacement effects of CO2 on CH4 in shale during the soaking process. Therefore, the potential impact of ScCO2 soaking on CO2/CH4 competitive adsorption in practical engineering cannot be ignored.
-
(4)
Both CO2 and N2 injection can improve the recovery rate of shale gas to a certain extent, but the injection effects of the two schemes are significantly different. It is recommended to use a CO2/N2 mixed gas injection scheme in practical engineering. The effect of CO2 pulse injection is obviously better than that of continuous injection, and a reasonable gas injection rate should be selected according to actual shale reservoir conditions.
-
(5)
Significant differences existed in the CO2 storage mechanism for different geological bodies, and the CO2 storage capacity of a geological body is closely related to TOC and the micropore content while the geological body with a shallow burial depth, abnormal pressure, and high temperature is not an ideal place for CO2 sequestration.
Acknowledgments
The research presented here was supported by Guizhou Provincial Basic Research Program (Natural Science) (ZK[2022]099 and ZK[2024]026); Young Talent Introduction Program of GZU (no. (2021) 63); Basic Research Project of GZU (no. 2023)53); and Key Technologies and Engineering Tests of Shale Gas Benefit Development in Guizhou Province ([2022]ZD005).
The authors declare no competing financial interest.
References
- Zhang C.-Y.; Chai X.-S.; Xiao X.-M. A simple method for correcting for the presence of minor gases when determining the adsorbed methane content in shale. Fuel 2015, 150, 334–338. 10.1016/j.fuel.2015.02.050. [DOI] [Google Scholar]
- Zhang F.; Damjanac B.; Maxwell S. Investigating Hydraulic Fracturing Complexity in Naturally Fractured Rock Masses Using Fully Coupled Multiscale Numerical Modeling. Rock Mechanics and Rock Engineering 2019, 52 (12), 5137–5160. 10.1007/s00603-019-01851-3. [DOI] [Google Scholar]
- Zhang X.; Lu Y.; Tang J.; Zhou Z.; Liao Y. Experimental study on fracture initiation and propagation in shale using supercritical carbon dioxide fracturing. Fuel 2017, 190, 370–378. 10.1016/j.fuel.2016.10.120. [DOI] [Google Scholar]
- Middleton R. S.; Gupta R.; Hyman J. D.; Viswanathan H. S. The shale gas revolution: Barriers, sustainability, and emerging opportunities. Applied Energy 2017, 199, 88–95. 10.1016/j.apenergy.2017.04.034. [DOI] [Google Scholar]
- Yang R.; Liu X.; Yu R.; Hu Z.; Duan X. Long short-term memory suggests a model for predicting shale gas production. Applied Energy 2022, 322, 119415. 10.1016/j.apenergy.2022.119415. [DOI] [Google Scholar]
- Liao Q.; Zhou J.; Xian X.; Yang K.; Zhang C.; Dong Z.; Yin H. Competition adsorption of CO2/CH4 in shale: Implications for CO2 sequestration with enhanced gas recovery. Fuel 2023, 339, 127400. 10.1016/j.fuel.2023.127400. [DOI] [Google Scholar]
- Liu B.; Qi C.; Mai T.; Zhang J.; Zhan K.; Zhang Z.; He J. Competitive adsorption and diffusion of CH4/CO2 binary mixture within shale organic nanochannels. Journal of Natural Gas Science and Engineering 2018, 53, 329–336. 10.1016/j.jngse.2018.02.034. [DOI] [Google Scholar]
- Wang S.; Zhou S.; Pan Z.; Elsworth D.; Yan D.; Wang H.; Liu D.; Hu Z. Response of pore network fractal dimensions and gas adsorption capacities of shales exposed to supercritical CO2: Implications for CH4 recovery and carbon sequestration. Energy Reports 2023, 9, 6461–6485. 10.1016/j.egyr.2023.05.266. [DOI] [Google Scholar]
- Liu J.; Xie L.; Elsworth D.; Gan Q. CO2/CH4 Competitive Adsorption in Shale: Implications for Enhancement in Gas Production and Reduction in Carbon Emissions. Environ. Sci. Technol. 2019, 53 (15), 9328–9336. 10.1021/acs.est.9b02432. [DOI] [PubMed] [Google Scholar]
- Louk K.; Ripepi N.; Luxbacher K.; Gilliland E.; Tang X.; Keles C.; Schlosser C.; Diminick E.; Keim S.; Amante J.; et al. Monitoring CO2 storage and enhanced gas recovery in unconventional shale reservoirs: Results from the Morgan County, Tennessee injection test. Journal of Natural Gas Science and Engineering 2017, 45, 11–25. 10.1016/j.jngse.2017.03.025. [DOI] [Google Scholar]
- Klewiah I.; Berawala D. S.; Alexander Walker H. C.; Andersen P. Ø.; Nadeau P. H. Review of experimental sorption studies of CO2 and CH4 in shales. Journal of Natural Gas Science and Engineering 2020, 73, 103045. 10.1016/j.jngse.2019.103045. [DOI] [Google Scholar]
- Rani S.; Padmanabhan E.; Prusty B. K. Review of gas adsorption in shales for enhanced methane recovery and CO2 storage. J. Pet. Sci. Eng. 2019, 175, 634–643. 10.1016/j.petrol.2018.12.081. [DOI] [Google Scholar]
- Zhou J.; Liu M.; Xian X.; Jiang Y.; Liu Q.; Wang X. Measurements and modelling of CH4 and CO2 adsorption behaviors on shales: Implication for CO2 enhanced shale gas recovery. Fuel 2019, 251, 293–306. 10.1016/j.fuel.2019.04.041. [DOI] [Google Scholar]
- Ke H.; Jiren T.; Helmut M. Investigation of supercritical shale gas adsorption in shale based on the Ono-Kondo lattice model. Journal of China Coal Society 2021, 46 (8), 2479–2487. 10.13487/j.cnki.imce.021550. [DOI] [Google Scholar]
- Qin C.; Jiang Y.; Zhou J.; Song X.; Liu Z.; Li D.; Zhou F.; Xie Y.; Xie C. Effect of supercritical CO2 extraction on CO2/CH4 competitive adsorption in Yanchang shale. Chemical Engineering Journal 2021, 412, 128701. 10.1016/j.cej.2021.128701. [DOI] [Google Scholar]
- Wang T.; Tian S.; Li G.; Sheng M.; Ren W.; Liu Q.; Zhang S. Molecular simulation of CO2/CH4 competitive adsorption on shale kerogen for CO2 sequestration and enhanced gas recovery. J. Phys. Chem. C 2018, 122 (30), 17009–17018. 10.1021/acs.jpcc.8b02061. [DOI] [Google Scholar]
- Jin D.; Lu X.; Zhang M.; Wei S.; Zhu Q.; Shi X.; Shao Y.; Wang W.; Guo W. The adsorption behaviour of CH4 on microporous carbons: effects of surface heterogeneity. Phys. Chem. Chem. Phys. 2014, 16 (22), 11037. 10.1039/c3cp55107e. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Cao D. Molecular simulation of displacement of shale gas by carbon dioxide at different geological depths. Chem. Eng. Sci. 2016, 156, 121–127. 10.1016/j.ces.2016.09.002. [DOI] [Google Scholar]
- Lee T.; Bocquet L.; Coasne B.. Activated desorption at heterogeneous interfaces and long-time kinetics of hydrocarbon recovery from nanoporous media. Nat. Commun. 2016, 7 ( (1), ), 10.1038/ncomms11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adewumi Babatunde K.; Mamo Negash B.; Rashik Mojid M.; Ahmed T. Y.; Regassa Jufar S. Molecular simulation study of CO2/CH4 adsorption on realistic heterogeneous shale surfaces. Appl. Surf. Sci. 2021, 543, 148789. 10.1016/j.apsusc.2020.148789. [DOI] [Google Scholar]
- Liu Z.; Bai B.; Wang Y.; Ding Z.; Li J.; Qu H.; Jia Z. Experimental study of friction reducer effect on dynamic and isotherm of methane desorption on Longmaxi shale. Fuel 2021, 288, 119733. 10.1016/j.fuel.2020.119733. [DOI] [Google Scholar]
- Lim Y.-I.; Bhatia S. K. Simulation of methane permeability in carbon slit pores. J. Membr. Sci. 2011, 369 (1–2), 319–328. 10.1016/j.memsci.2010.12.009. [DOI] [Google Scholar]
- Cheng H.; Lei G. Multilayer graphene nanostructure separate CO2/CH4 mixture: Combining molecular simulations with ideal adsorbed solution theory. Chem. Phys. Lett. 2016, 661, 31–35. 10.1016/j.cplett.2016.08.061. [DOI] [Google Scholar]
- Ma J.; Yao M.; Yang Y.; Zhang X. Comprehensive review on stability and demulsification of unconventional heavy oil-water emulsions. J. Mol. Liq. 2022, 350, 118510. 10.1016/j.molliq.2022.118510. [DOI] [Google Scholar]
- Zhang S. H.; Tang S. H.; Li Z. C.; Pan Z. J.; Liu B. Competitive sorption and diffusion of methane and carbon dioxide mixture in Carboniferous-Permian anthracite of south Qinshui Basin, China. Arabian Journal of Geosciences 2020, 13 (24), Article. 10.1007/s12517-020-06303-9. [DOI] [Google Scholar]
- Pan Z.; Connell L. D. Comparison of adsorption models in reservoir simulation of enhanced coalbed methane recovery and CO2 sequestration in coal. International Journal of Greenhouse Gas Control 2009, 3 (1), 77–89. 10.1016/j.ijggc.2008.05.004. [DOI] [Google Scholar]
- Zhan J.; Niu Z.; Li M.; Zhang Y.; Ma X.; Fan C.; Wang R.; Zendehboudi S. Numerical Simulation and Modeling on CO2 Sequestration Coupled with Enhanced Gas Recovery in Shale Gas Reservoirs. Geofluids 2021, 2021, 1–15. 10.1155/2021/9975296. [DOI] [Google Scholar]
- Siyu L.; Guodong Y.; Mian H.; Shuguo Y.; Xin M.; Qi B. Effects of artificial fracture parameters on CO2 sequestration and CH4 production in CO2-ESGR. Chemical Engineering of Oil &Gas 2024, 53 (2), 94–100. 10.3969/j.issn.1007-3426.2024.02.014. [DOI] [Google Scholar]
- Shabani B.; Vilcáez J. A fast and robust TOUGH2 module to simulate geological CO2 storage in saline aquifers. Computers & Geosciences 2018, 111, 58–66. 10.1016/j.cageo.2017.10.012. [DOI] [Google Scholar]
- Shuguo Y.; Guodong Y.; Tao F.; Xin M.; Wei C.; Mian H.; Tianqing G. Effects of Physical Parameters of Shale on CO2 Storage Capacity with Different Mechanisms. Geological Journal of China Universities 2023, 29 (1), 37–46. 10.16108/j.issn1006-7493.2022071. [DOI] [Google Scholar]
- Lee H.-H.; Kim H.-J.; Shi Y.; Keffer D.; Lee C.-H. Competitive adsorption of CO2/CH4 mixture on dry and wet coal from subcritical to supercritical conditions. Chemical Engineering Journal 2013, 230, 93–101. 10.1016/j.cej.2013.06.036. [DOI] [Google Scholar]
- Songhang Z.; Shouren Z.; Shuheng T.; Di X.; Bing L. Adsorption and transport of methane and carbon dioxide mixture in anthracite. Journal of China Coal Society 2021, 46 (2), 544–555. 10.13225/j.cnki.jccs.XR20.1746. [DOI] [Google Scholar]
- Luo X.; Wang S.; Wang Z.; Jing Z.; Lv M.; Zhai Z.; Han T. Adsorption of methane, carbon dioxide and their binary mixtures on Jurassic shale from the Qaidam Basin in China. International Journal of Coal Geology 2015, 150–151, 210–223. 10.1016/j.coal.2015.09.004. [DOI] [Google Scholar]
- Rongrong Q.Study on Multi-Component Competitive Adsorption Mechanism of Shale Gas; China University of Petroleum, Beijing, 2019. [Google Scholar]
- Xie W.; Wang M.; Chen S.; Vandeginste V.; Yu Z.; Wang H. Effects of gas components, reservoir property and pore structure of shale gas reservoir on the competitive adsorption behavior of CO2 and CH4. Energy 2022, 254, 124242. 10.1016/j.energy.2022.124242. [DOI] [Google Scholar]
- Duan S.; Gu M.; Du X.; Xian X. Adsorption Equilibrium of CO2 and CH4 and Their Mixture on Sichuan Basin Shale. Energy Fuels 2016, 30 (3), 2248–2256. 10.1021/acs.energyfuels.5b02088. [DOI] [Google Scholar]
- Gu M.; Xian X.; Duan S.; Du X. Influences of the composition and pore structure of a shale on its selective adsorption of CO2 over CH4. Journal of Natural Gas Science and Engineering 2017, 46, 296–306. 10.1016/j.jngse.2017.07.011. [DOI] [Google Scholar]
- Du X.; Cheng Y.; Liu Z.; Hou Z.; Wu T.; Lei R.; Shu C. Study on the adsorption of CH4, CO2 and various CH4/CO2 mixture gases on shale. Alexandria Engineering Journal 2020, 59 (6), 5165–5178. 10.1016/j.aej.2020.09.046. [DOI] [Google Scholar]
- Ortiz Cancino O. P.; Pino Pérez D.; Pozo M.; Bessieres D. Adsorption of pure CO2 and a CO2/CH4 mixture on a black shale sample: Manometry and microcalorimetry measurements. J. Pet. Sci. Eng. 2017, 159, 307–313. 10.1016/j.petrol.2017.09.038. [DOI] [Google Scholar]
- Qi R.; Ning Z.; Wang Q.; Zeng Y.; Huang L.; Zhang S.; Du H. Sorption of Methane, Carbon Dioxide, and Their Mixtures on Shales from Sichuan Basin, China. Energy Fuels 2018, 32 (3), 2926–2940. 10.1021/acs.energyfuels.7b03429. [DOI] [Google Scholar]
- Ma Y.; Yue C.; Li S.; Xu X.; Niu Y. Study of CH4 and CO2 competitive adsorption on shale in Yibin, Sichuan Province of China. Carbon Resources Conversion 2019, 2 (1), 35–42. 10.1016/j.crcon.2018.11.005. [DOI] [Google Scholar]
- Ma X.; Guo S. Comparative Study on Shale Characteristics of Different Sedimentary Microfacies of Late Permian Longtan Formation in Southwestern Guizhou, China. Minerals 2019, 9 (1), 20. 10.3390/min9010020. [DOI] [Google Scholar]
- Wang Q.; Huang L. Molecular insight into competitive adsorption of methane and carbon dioxide in montmorillonite: Effect of clay structure and water content. Fuel 2019, 239, 32–43. 10.1016/j.fuel.2018.10.149. [DOI] [Google Scholar]
- Rezlerová E.; Brennan J. K.; Lísal M.. Methane and carbon dioxide in dual-porosity organic matter: Molecular simulations of adsorption and diffusion. AIChE J. 2021, 67 ( (3), ), 10.1002/aic.16655. [DOI] [Google Scholar]
- Tesson S.; Firoozabadi A. Methane Adsorption and Self-Diffusion in Shale Kerogen and Slit Nanopores by Molecular Simulations. J. Phys. Chem. C 2018, 122 (41), 23528–23542. 10.1021/acs.jpcc.8b07123. [DOI] [Google Scholar]
- Huang L.; Ning Z.; Wang Q.; Zhang W.; Cheng Z.; Wu X.; Qin H. Effect of organic type and moisture on CO2/CH4 competitive adsorption in kerogen with implications for CO2 sequestration and enhanced CH4 recovery. Applied Energy 2018, 210, 28–43. 10.1016/j.apenergy.2017.10.122. [DOI] [Google Scholar]
- Sui H.; Zhang F.; Wang Z.; Wang D.; Wang Y. Effect of Kerogen Maturity, Water Content for Carbon Dioxide, Methane, and Their Mixture Adsorption and Diffusion in Kerogen: A Computational Investigation. Langmuir 2020, 36 (33), 9756–9769. 10.1021/acs.langmuir.0c01191. [DOI] [PubMed] [Google Scholar]
- Hu X.; Deng H.; Lu C.; Tian Y.; Jin Z. Characterization of CO2/CH4 Competitive Adsorption in Various Clay Minerals in Relation to Shale Gas Recovery from Molecular Simulation. Energy Fuels 2019, 33 (9), 8202–8214. 10.1021/acs.energyfuels.9b01610. [DOI] [Google Scholar]
- Pan B.; Gong C.; Wang X.; Li Y.; Iglauer S. The interfacial properties of clay-coated quartz at reservoir conditions. Fuel 2020, 262, 116461. 10.1016/j.fuel.2019.116461. [DOI] [Google Scholar]
- Zhou W.; Wang H.; Yan Y.; Liu X. Adsorption mechanism of CO2/CH4 in kaolinite clay: Insight from molecular simulation. Energy Fuels 2019, 33 (7), 6542–6551. 10.1021/acs.energyfuels.9b00539. [DOI] [Google Scholar]
- Zhou W.; Zhang Z.; Wang H.; Yan Y.; Liu X. Molecular insights into competitive adsorption of CO2/CH4 mixture in shale nanopores. RSC Adv. 2018, 8 (59), 33939–33946. 10.1039/C8RA07486K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi R.; Ning Z.; Wang Q.; Huang L.; Wu X.; Cheng Z.; Zhang W. Measurements and modeling of high-pressure adsorption of CH4 and CO2 on shales. Fuel 2019, 242, 728–743. 10.1016/j.fuel.2018.12.086. [DOI] [Google Scholar]
- Zhou W.; Wang H.; Yang X.; Liu X.; Yan Y. Confinement effects and CO2/CH4 competitive adsorption in realistic shale kerogen nanopores. Ind. Eng. Chem. Res. 2020, 59 (14), 6696–6706. 10.1021/acs.iecr.9b06549. [DOI] [Google Scholar]
- Xie W.; Wang M.; Wang H. Adsorption Characteristics of CH4 and CO2 in Shale at High Pressure and Temperature. ACS Omega 2021, 6 (28), 18527–18536. 10.1021/acsomega.1c02921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Javadpour F.; Feng Q. Fast mass transport of oil and supercritical carbon dioxide through organic nanopores in shale. Fuel 2016, 181, 741–758. 10.1016/j.fuel.2016.05.057. [DOI] [Google Scholar]
- Zeng K.; Jiang P.; Lun Z.; Xu R. Molecular Simulation of Carbon Dioxide and Methane Adsorption in Shale Organic Nanopores. Energy Fuels 2019, 33 (3), 1785–1796. 10.1021/acs.energyfuels.8b02851. [DOI] [Google Scholar]
- Pan Z.; Ye J.; Zhou F.; Tan Y.; Connell L. D.; Fan J. CO2 storage in coal to enhance coalbed methane recovery: a review of field experiments in China. International Geology Review 2018, 60 (5–6), 754–776. 10.1080/00206814.2017.1373607. [DOI] [Google Scholar]
- Bai P. S.Studies on Adsorption Behavior of CO2 on Porous Solids near the Critical Temperature. Tianjin University, 2003. [Google Scholar]
- Lu T.; Zeng K.; Jiang P.; Zhou B.; Xu R. Competitive adsorption in CO2 enhancing shale gas: Low-field NMR measurement combined with molecular simulation for selectivity and displacement efficiency model. Chemical Engineering Journal 2022, 440, 135865. 10.1016/j.cej.2022.135865. [DOI] [Google Scholar]
- Sun H.; Zhao H.; Qi N.; Li Y. Molecular insights into the enhanced shale gas recovery by Carbon Dioxide in Kerogen Slit-Nanopores. J. Phys. Chem. C 2017, 121, 10233. 10.1021/acs.jpcc.7b02618. [DOI] [Google Scholar]
- Huang L.; Ning Z.; Wang Q.; Qi R.; Zeng Y.; Qin H.; Ye H.; Zhang W. Molecular simulation of adsorption behaviors of methane, carbon dioxide and their mixtures on kerogen: Effect of kerogen maturity and moisture content. Fuel 2018, 211, 159–172. 10.1016/j.fuel.2017.09.060. [DOI] [Google Scholar]
- Jin Z.; Firoozabadi A. Effect of water on methane and carbon dioxide sorption in clay minerals by Monte Carlo simulations. Fluid Phase Equilib. 2014, 382, 10–20. 10.1016/j.fluid.2014.07.035. [DOI] [Google Scholar]
- Zhang K.; Jiang H.; Qin G. Utilization of zeolite as a potential multi-functional proppant for CO2 enhanced shale gas recovery and CO2 sequestration: A molecular simulation study of the impact of water on adsorption in zeolite and organic matter. Fuel 2021, 292, 120312. 10.1016/j.fuel.2021.120312. [DOI] [Google Scholar]
- Sun J.; Chen C.; Hu W.; Cui J.; Jiang L.; Liu Y.; Zhao Y.; Li W.; Song Y. Asymmetric competitive adsorption of CO2/CH4 binary mixture in shale matrix with heterogeneous surfaces. Chemical Engineering Journal 2021, 422, 130025. 10.1016/j.cej.2021.130025. [DOI] [Google Scholar]
- Li Z.; Elsworth D. Controls of CO2–N2 gas flood ratios on enhanced shale gas recovery and ultimate CO2 sequestration. J. Pet. Sci. Eng. 2019, 179, 1037–1045. 10.1016/j.petrol.2019.04.098. [DOI] [Google Scholar]
- Li L.; Huang Z.; Duan X.; Niu H.; Zhan S.; Yang Z.; Fan Z.; Zhang T. Insights into Enhancing Shale Oil Recovery Postfracturing with CO2 Huff and Puff and Elastic Depletion Development Strategies. Energy Fuels 2024, 38 (5), 3997–4009. 10.1021/acs.energyfuels.3c04549. [DOI] [Google Scholar]
- Ma H.; Yang Y.; Zhang Y.; Li Z.; Zhang K.; Xue Z.; Zhan J.; Chen Z. Optimized schemes of enhanced shale gas recovery by CO2-N2 mixtures associated with CO2 sequestration. Energy Conversion and Management 2022, 268, 116062. 10.1016/j.enconman.2022.116062. [DOI] [Google Scholar]
- Li L.; Su Y.; Hao Y.; Zhan S.; Lv Y.; Zhao Q.; Wang H. A comparative study of CO2 and N2 huff-n-puff EOR performance in shale oil production. J. Pet. Sci. Eng. 2019, 181, 106174. 10.1016/j.petrol.2019.06.038. [DOI] [Google Scholar]
- Li X.; Elsworth D. Geomechanics of CO2 enhanced shale gas recovery. Journal of Natural Gas Science and Engineering 2015, 26, 1607–1619. 10.1016/j.jngse.2014.08.010. [DOI] [Google Scholar]
- Lan Y.; Yang Z.; Wang P.; Yan Y.; Zhang L.; Ran J. A review of microscopic seepage mechanism for shale gas extracted by supercritical CO2 flooding. Fuel 2019, 238, 412–424. 10.1016/j.fuel.2018.10.130. [DOI] [Google Scholar]
- Yin H.; Zhou J.; Jiang Y.; Xian X.; Liu Q. Physical and structural changes in shale associated with supercritical CO2 exposure. Fuel 2016, 184, 289–303. 10.1016/j.fuel.2016.07.028. [DOI] [Google Scholar]
- Meng S.; Jin X.; Tao J.; Wang X.; Zhang C. Evolution Characteristics of Mechanical Properties under Supercritical Carbon Dioxide Treatment in Shale Reservoirs. ACS Omega 2021, 6 (4), 2813–2823. 10.1021/acsomega.0c05136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A.; Sanguinito S.; Tkach M.; Natesakhawat S.; Kutchko B.; Fazio J.; Cvetic P. Investigating the role of water on CO2-Utica Shale interactions for carbon storage and shale gas extraction activities – Evidence for pore scale alterations. Fuel 2019, 242, 744–755. 10.1016/j.fuel.2019.01.091. [DOI] [Google Scholar]
- Zhou J.; Yang K.; Zhou L.; Jiang Y.; Xian X.; Zhang C.; Tian S.; Fan M.; Lu Z.. Microstructure and mechanical properties alterations in shale treated via CO2/CO2-water exposure. J. Pet. Sci. Eng. 2021, 196, 108088. 10.1016/j.petrol.2020.108088. [DOI] [Google Scholar]
- Zhou D.; Zhang G.; Huang Z.; Zhao J.; Wang L.; Qiu R. Changes in microstructure and mechanical properties of shales exposed to supercritical CO2 and brine. International Journal of Rock Mechanics and Mining Sciences 2022, 160, 105228. 10.1016/j.ijrmms.2022.105228. [DOI] [Google Scholar]
- Qin C.; Jiang Y.; Luo Y.; Zhou J.; Liu H.; Song X.; Li D.; Zhou F.; Xie Y. Effect of supercritical CO2 saturation pressures and temperatures on the methane adsorption behaviours of Longmaxi shale. Energy 2020, 206, 118150. 10.1016/j.energy.2020.118150. [DOI] [Google Scholar]
- Jiang Y.; Luo Y.; Lu Y.; Qin C.; Liu H. Effects of supercritical CO2 treatment time, pressure, and temperature on microstructure of shale. Energy 2016, 97, 173–181. 10.1016/j.energy.2015.12.124. [DOI] [Google Scholar]
- Du X.-D.; Gu M.; Duan S.; Xian X.-F.. Investigation of CO2–CH4 Displacement and Transport in Shale for Enhanced Shale Gas Recovery and CO2 Sequestration. J. Energy Resour. Technol. 2017, 139 ( (1), ), 10.1115/1.4035148. [DOI] [Google Scholar]
- Bachu S.; Bonijoly D.; Bradshaw J.; Burruss R.; Holloway S.; Christensen N. P.; Mathiassen O. M. CO2 storage capacity estimation: Methodology and gaps. International Journal of Greenhouse Gas Control 2007, 1 (4), 430–443. 10.1016/S1750-5836(07)00086-2. [DOI] [Google Scholar]
- Hang Y.; Qi L.; Bo P. Research progress in evaluation of carbon storage potential based on CO2 flooding technology. Clean Coal Technology 2021, 27 (2), 107–116. 10.13226/j.issn.1006-6772.CCUS20100905. [DOI] [Google Scholar]
- Zhao D. F.; Liao X. W.; Yin D. D. Evaluation of CO2 enhanced oil recovery and sequestration potential in low permeability reservoirs, Yanchang Oilfield, China. Journal of the Energy Institute 2014, 87 (4), 306–313. 10.1016/j.joei.2014.03.031. [DOI] [Google Scholar]
- Bachu S.Comparison Between Methodologies Recommended for Estimation of CO2 Storage Capacity in Geological Media. Carbon Sequestration Leadership Forum 2008. [Google Scholar]
- Ma L.; Fauchille A.-L.; Ansari H.; Chandler M.; Ashby P.; Taylor K.; Pini R.; Lee P. D. Linking multi-scale 3D microstructure to potential enhanced natural gas recovery and subsurface CO2 storage for Bowland shale, UK. Energy Environ. Sci. 2021, 14 (8), 4481–4498. 10.1039/D0EE03651J. [DOI] [Google Scholar]
- Ting L.; Xin M.; Yujie D.; Xiaolin J.; Jie F.; Chenglong Z. Research status of CO2 geological storage potential evaluation methods at home and abroad. Geological Survey Of China 2021, 8 (4), 101–108. 10.19388/j.zgdzdc.2021.04.11. [DOI] [Google Scholar]
- Jessen K.; Kovscek A. R.; Orr F. M. Increasing CO2 storage in oil recovery. Energy Conversion and Management 2005, 46 (2), 293–311. 10.1016/j.enconman.2004.02.019. [DOI] [Google Scholar]
- Huang L.; Zhou W.; Xu H.; Wang L.; Zou J.; Zhou Q. Dynamic fluid states in organic-inorganic nanocomposite: Implications for shale gas recovery and CO2 sequestration. Chemical Engineering Journal 2021, 411, 128423. 10.1016/j.cej.2021.128423. [DOI] [Google Scholar]
- Pingping S.; Xinwei L.; Qingjie L. Methodology for estimation of CO2 storage capacity in reservoirs. Petroleum Exploration and Development 2009, 36, 216–220. 10.1016/S1876-3804(09)60121-X. [DOI] [Google Scholar]
- Strąpoć D.; Mastalerz M.; Schimmelmann A.; Drobniak A.; Hasenmueller N. R. Geochemical constraints on the origin and volume of gas in the New Albany Shale (Devonian–Mississippian), eastern Illinois Basin. AAPG Bulletin 2010, 94 (11), 1713–1740. 10.1306/06301009197. [DOI] [Google Scholar]
- Brennan B. S. T.; Burruss R. C.; Merrill M. D.; Freeman P. A.; Ruppert L. F.. A probabilistic assessment methodology for the evaluation of geologic carbon dioxide storage. USGS; 2010.
- Rezk M. G.; Foroozesh J.; Zivar D.; Mumtaz M. CO2 storage potential during CO2 enhanced oil recovery in sandstone reservoirs. Journal of Natural Gas Science and Engineering 2019, 66, 233–243. 10.1016/j.jngse.2019.04.002. [DOI] [Google Scholar]
- Gaofeng W.; Shunxi Q.; Chunxia H.; Xiangyu C.. Calculation of Carbon Dioxide Simultaneous Sequestration Potential in Low Permeable Reservoirs. Science Technology and Engineering 2019, 19( (27), ), 148–154 [Google Scholar]
- Fatah A.; Ben Mahmud H.; Bennour Z.; Gholami R.; Hossain M. The impact of supercritical CO2 on the pore structure and storage capacity of shales. Journal of Natural Gas Science and Engineering 2022, 98, 104394. 10.1016/j.jngse.2021.104394. [DOI] [Google Scholar]
- Ao X.; Lu Y.; Tang J.; Chen Y.; Li H. Investigation on the physics structure and chemical properties of the shale treated by supercritical CO2. Journal of CO2 Utilization 2017, 20, 274–281. 10.1016/j.jcou.2017.05.028. [DOI] [Google Scholar]
- Bai B.; Ni H.-j.; Shi X.; Guo X.; Ding L. The experimental investigation of effect of supercritical CO2 immersion on mechanical properties and pore structure of shale. Energy 2021, 228, 120663. 10.1016/j.energy.2021.120663. [DOI] [Google Scholar]
- Du X.; Gu M.; Liu Z.; Zhao Y.; Sun F.; Wu T. Enhanced Shale Gas Recovery by the Injections of CO2, N2, and CO2/N2 Mixture Gases. Energy Fuels 2019, 33 (6), 5091–5101. 10.1021/acs.energyfuels.9b00822. [DOI] [Google Scholar]