Abstract
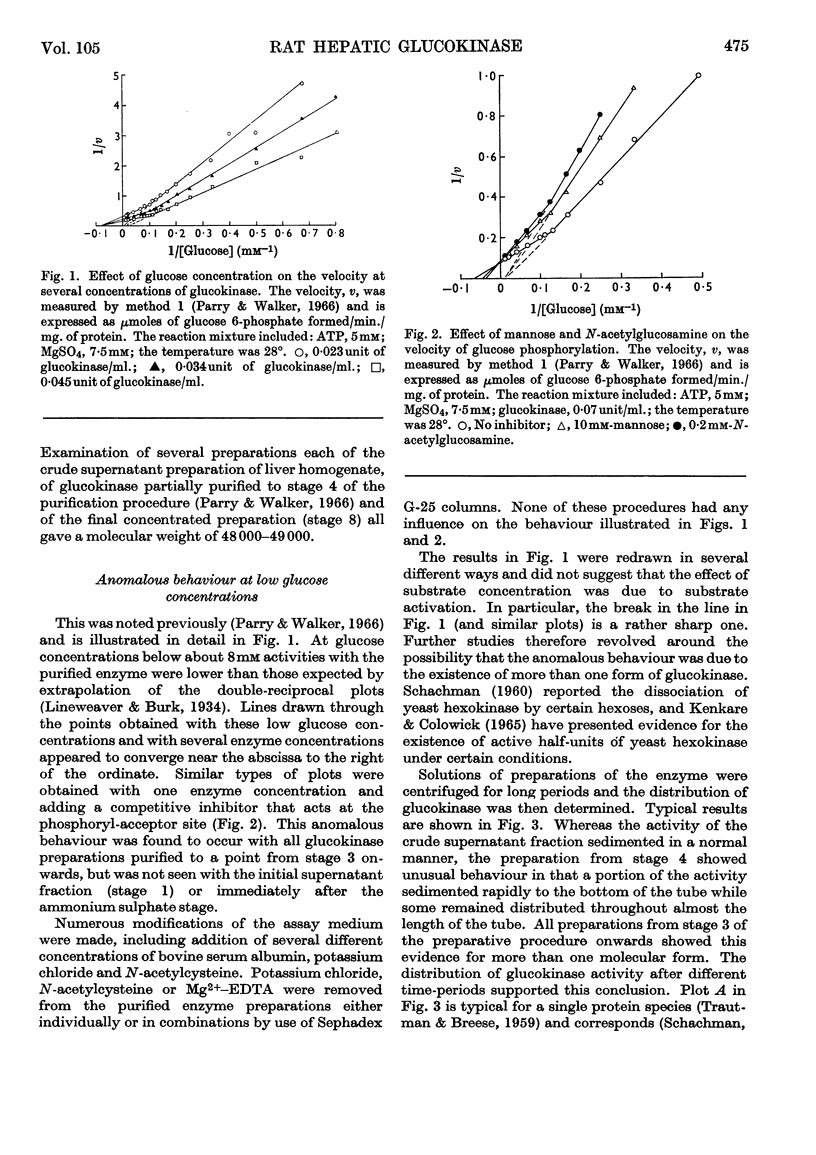
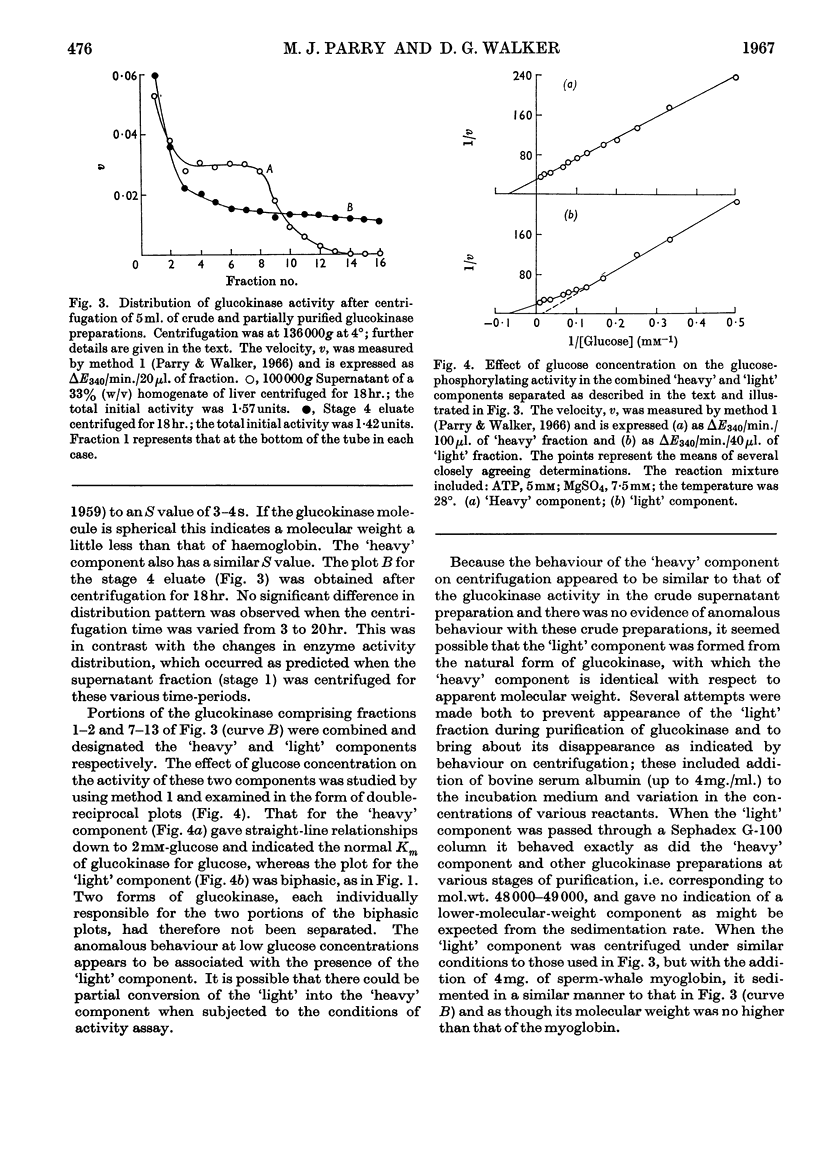
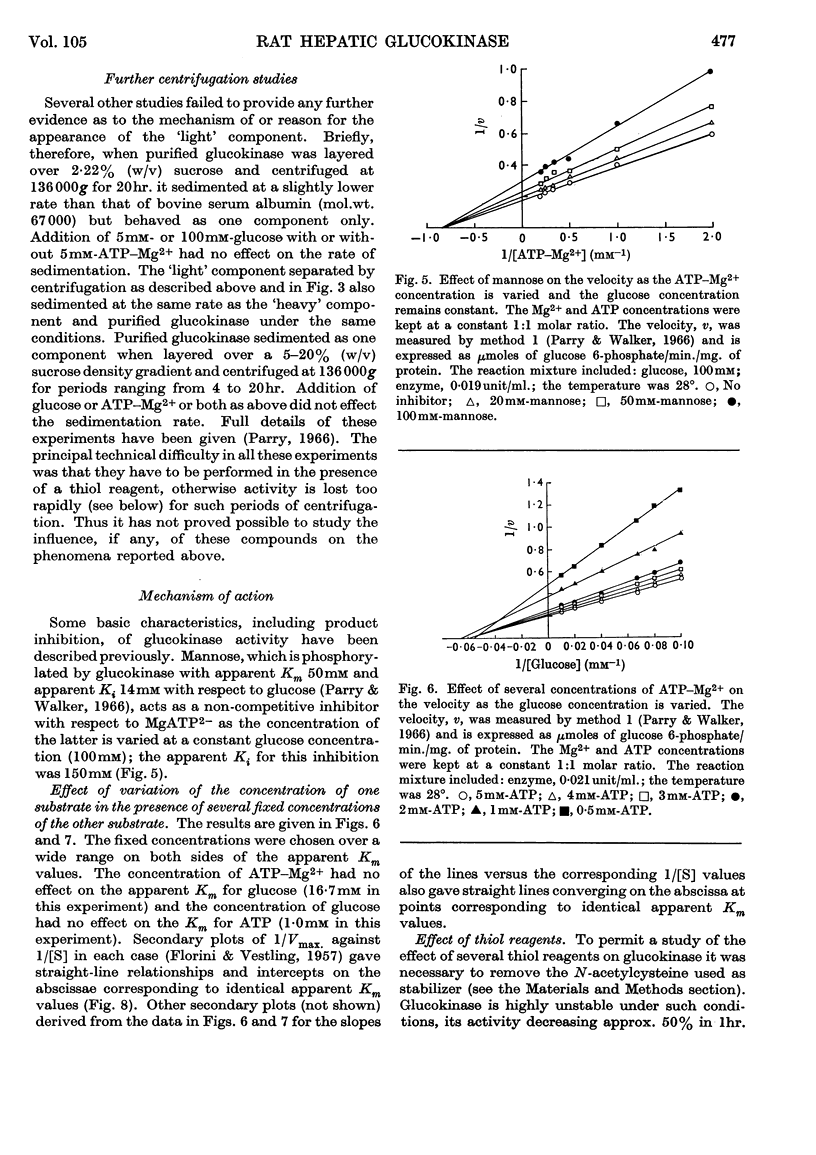
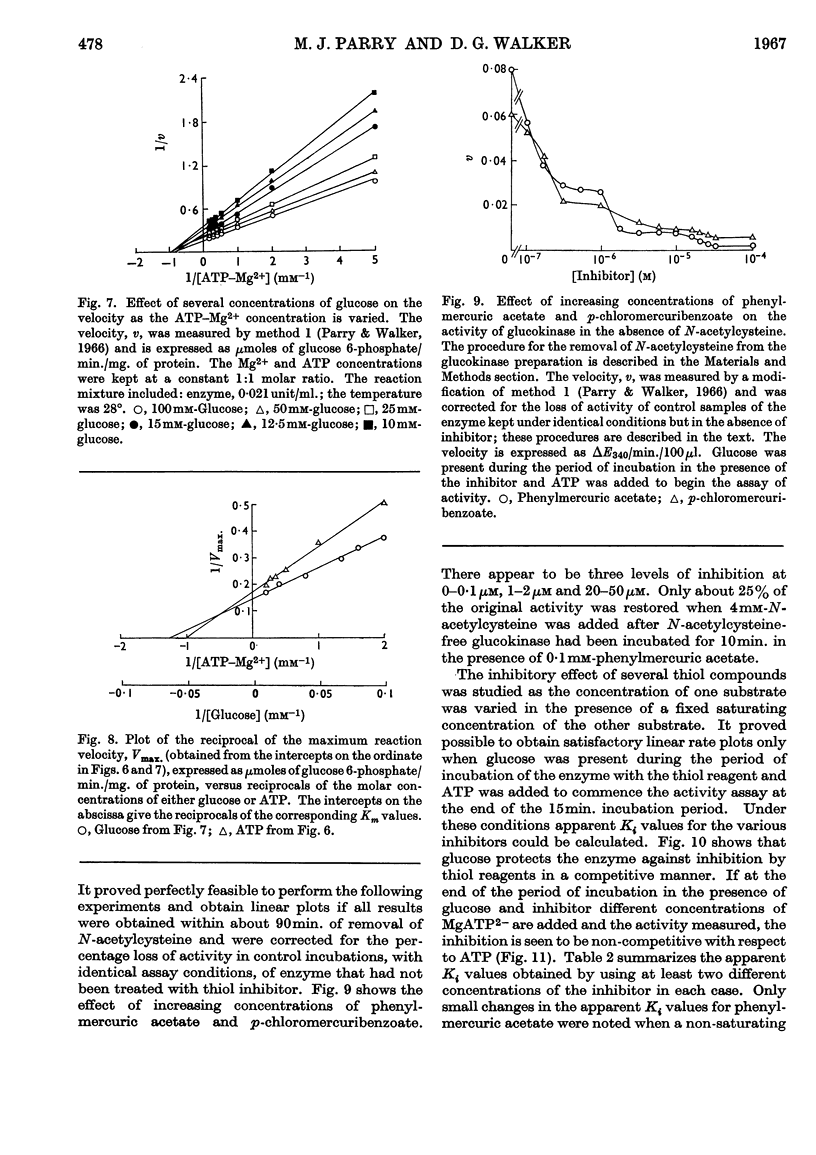
1. Magnesium ions are the most effective bivalent ions in the glucokinase reaction. 2. The molecular weight of rat hepatic glucokinase is 48000–49000 as assessed by gel filtration on Sephadex G-100. 3. Anomalous kinetic behaviour at low glucose concentrations appears to be due to the formation during the purification procedure of fragments possessing modified catalytic properties, but is unlikely to be of physiological significance. 4. Extension of previous studies (Parry & Walker, 1966) suggests that glucokinase catalyses a reaction of the random Bi Bi type similar to that of yeast hexokinase. 5. The inhibitory effects of various thiol reagents suggest that a thiol group may be involved at or near the binding site of the acceptor molecule.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALLAGHAN O. H., WEBER G. Kinetic studies on rabbitmuscle myokinase. Biochem J. 1959 Nov;73:473–485. doi: 10.1042/bj0730473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- FASELLA P., HAMMES G. G. Studies of the enzyme hexokinase. IV. The role of sulfhydryl groups. Arch Biochem Biophys. 1963 Feb;100:295–297. doi: 10.1016/0003-9861(63)90075-4. [DOI] [PubMed] [Google Scholar]
- FLORINI J. R., VESTLING C. S. Graphical determination of the dissociation constants for two-substrate enzyme systems. Biochim Biophys Acta. 1957 Sep;25(3):575–578. doi: 10.1016/0006-3002(57)90529-2. [DOI] [PubMed] [Google Scholar]
- FROMM H. J., SILVERSTEIN E., BOYER P. D. EQUILIBRIUM AND NET REACTION RATES IN RELATION TO THE MECHANISM OF YEAST HEXOKINASE. J Biol Chem. 1964 Nov;239:3645–3652. [PubMed] [Google Scholar]
- FROMM H. J., ZEWE V. Kinetic studies of the brain hexokinase reaction. J Biol Chem. 1962 May;237:1661–1667. [PubMed] [Google Scholar]
- FROMM H. J., ZEWE V. Kinetic studies of yeast hexokinase. J Biol Chem. 1962 Oct;237:3027–3032. [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- HASS L. F., BOYER P. D., REYNARD A. M. Studies on possible phosphoryl enzyme formation in catalysis by hexokinase, pyruvate kinase, and glucose 6-phosphatase. J Biol Chem. 1961 Aug;236:2284–2291. [PubMed] [Google Scholar]
- Hanson T. L., Fromm H. J. Rat skeletal muscle hexokinase. I. Kinetics and reaction mechanism. J Biol Chem. 1965 Nov;240(11):4133–4139. [PubMed] [Google Scholar]
- Kaji A., Colowick S. P. Adenosine triphosphatase activity of yeast hexokinase and its relation to the mechanism of the hexokinase reaction. J Biol Chem. 1965 Nov;240(11):4454–4462. [PubMed] [Google Scholar]
- Kaji A. The reaction of yeast hexokinase with sulfhydryl reagents. Biochim Biophys Acta. 1966 Jul 6;122(1):43–56. doi: 10.1016/0926-6593(66)90090-7. [DOI] [PubMed] [Google Scholar]
- Kenkare U. W., Colowick S. P. Reversible inactivation and dissociation of yeast hexokinase. J Biol Chem. 1965 Dec;240(12):4570–4584. [PubMed] [Google Scholar]
- McLean P., Brown J. Activities of some enzymes concerned with citrate and glucose metabolism in transplanted rat hepatomas. Biochem J. 1966 Mar;98(3):874–882. doi: 10.1042/bj0980874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. F., Cleland W. W. Isotope exchange studies of the mechanism of the reaction catalyzed by adenosine triphosphate: creatine phosphotransferase. J Biol Chem. 1966 Feb 10;241(3):673–683. [PubMed] [Google Scholar]
- Morrison J. F., James E. The mechanism of the reaction catalysed by adenosine triphosphate-creatine phosphotransferase. Biochem J. 1965 Oct;97(1):37–52. doi: 10.1042/bj0970037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry M. J., Walker D. G. Purification and properties of adenosine 5'-triphospae-D-glucose 6-phosphotransferase from rat liver. Biochem J. 1966 May;99(2):266–274. doi: 10.1042/bj0990266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALAS J., SALAS M., VINUELA E., SOLS A. GLUCOKINASE OF RABBIT LIVER. J Biol Chem. 1965 Mar;240:1014–1018. [PubMed] [Google Scholar]
- Toews C. J. Kinetic studies with skeletal-muscle hexokinase. Biochem J. 1966 Sep;100(3):739–744. doi: 10.1042/bj1000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virden R., Watts D. C. The role of thiol groups in the structure and mechanism of action of arginine kinase. Biochem J. 1966 Apr;99(1):162–172. doi: 10.1042/bj0990162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. G. ON THE PRESENCE OF TWO SOLUBLE GLUCOSE-PHOSPHORYLATING ENZYMES IN ADULT LIVER AND THE DEVELOPMENT OF ONE OF THESE AFTER BIRTH. Biochim Biophys Acta. 1963 Oct 1;77:209–226. doi: 10.1016/0006-3002(63)90494-3. [DOI] [PubMed] [Google Scholar]
- WATTS D. C., RABIN B. R. A study of the 'reactive' sulphydryl groups of adenosine 5'-triphosphate-creatine phosphotransferase. Biochem J. 1962 Dec;85:507–516. doi: 10.1042/bj0850507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. G., Khan H. H., Eaton S. W. Enzymes catalysing the phosphorylation of hexoses in neonatal animals. Biol Neonat. 1965;9(1):224–239. [PubMed] [Google Scholar]


