Abstract
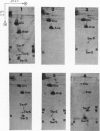
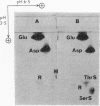
1. Partial acid hydrolysates of histones from various origins and of protamine were analysed by a two-dimensional ionophoretic procedure to reveal strongly acidic ninhydrin-positive components. 2. Histone fractions prepared by extraction with sulphuric acid gave rise to spots identified as serine O-sulphate and threonine O-sulphate. These two compounds, which were not found in hydrolysates of corresponding fractions prepared by extraction with hydrochloric acid, were artifacts. 3. Hydrolysis of proteins in the presence of traces of sulphate can lead to the formation of the O-sulphates of serine and threonine. This can cause errors, which may sometimes be serious, in amino acid analyses of proteins. 4. O-Phosphoserine was obtained in small amounts from some histone fractions and from protamine, but was undetectable in other histone fractions, notably those of lower lysine content.
Full text
PDF
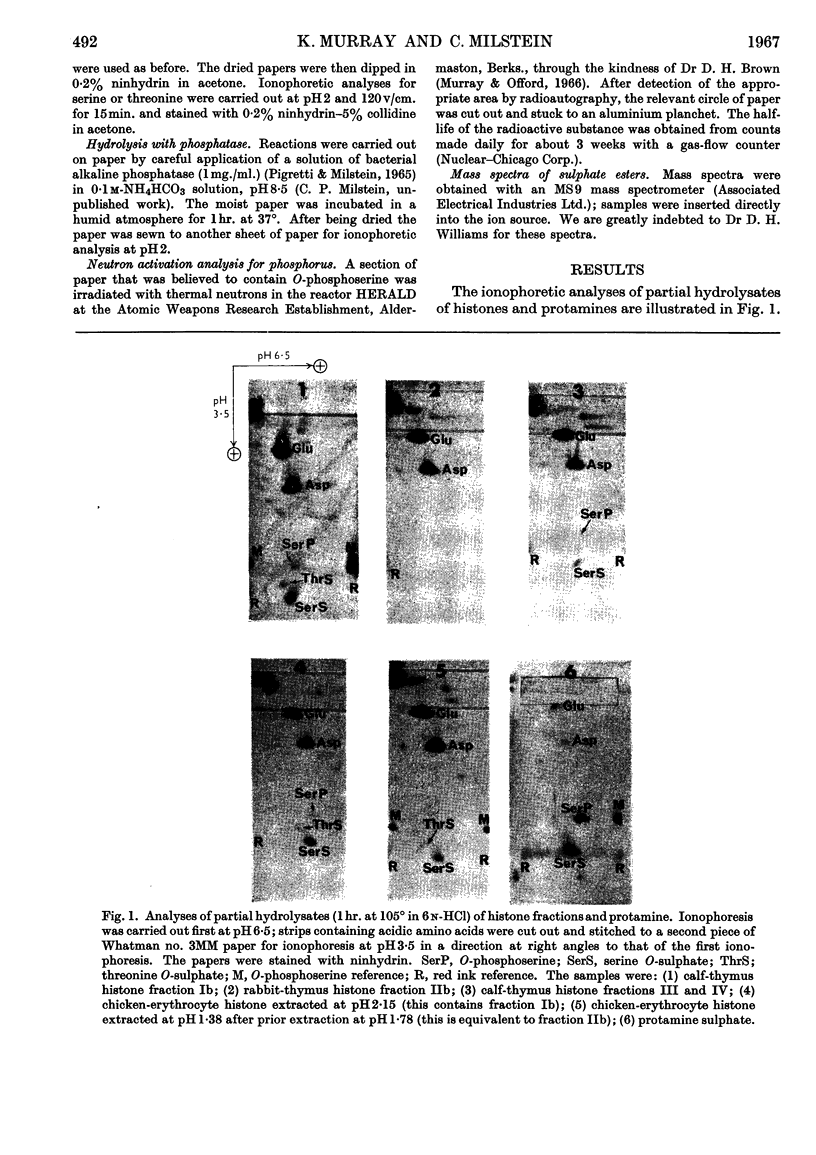
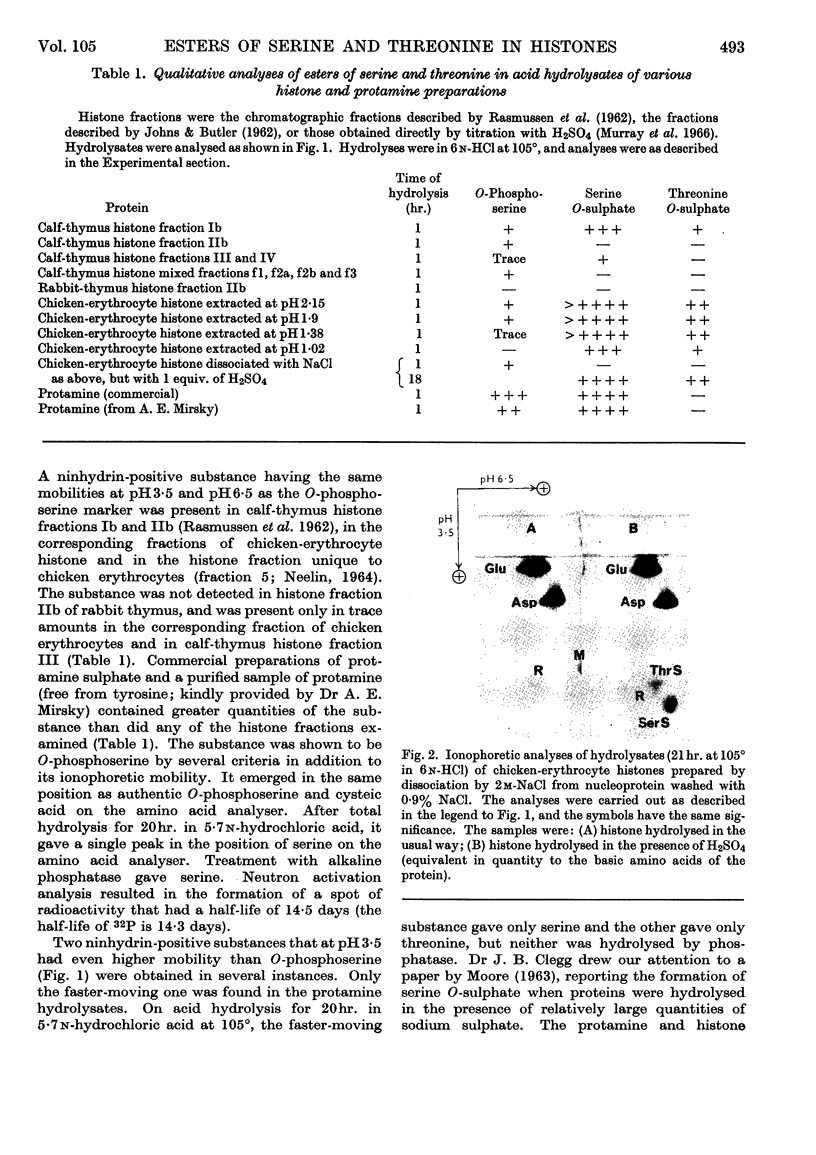


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IKAWA M., SNELL E. E. Artifact production through esterification of glutamic acid during analytical procedures. J Biol Chem. 1961 Jul;236:1955–1959. [PubMed] [Google Scholar]
- JOHNS E. W., BUTLER J. A. Further fractionations of histones from calf thymus. Biochem J. 1962 Jan;82:15–18. doi: 10.1042/bj0820015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILSTEIN C., SANGER F. An amino acid sequence in the active centre of phosphoglucomutase. Biochem J. 1961 Jun;79:456–469. doi: 10.1042/bj0790456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C. The amino acid sequence around the reactive serine residue in alkaline phosphatase from Escherichia coli. Biochem J. 1964 Aug;92(2):410–421. doi: 10.1042/bj0920410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Offord R. E. Use of neutron activation in the characterization of small quantities of nucleic acids. Nature. 1966 Jul 23;211(5047):376–378. doi: 10.1038/211376a0. [DOI] [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Metabolic properties of histones from rat liver and thymus gland. Biochem J. 1966 Mar;98(3):888–897. doi: 10.1042/bj0980888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIGRETTI M. M., MILSTEIN C. ACID INACTIVATION OF AND INCORPORATION OF PHOSPHATE INTO ALKALINE PHOSPHATASE FROM ESCHERICHIA COLI. Biochem J. 1965 Jan;94:106–113. doi: 10.1042/bj0940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RASMUSSEN P. S., MURRAY K., LUCK J. M. On the complexity of calf thymus histone. Biochemistry. 1962 Jan;1:79–89. doi: 10.1021/bi00907a013. [DOI] [PubMed] [Google Scholar]