Abstract
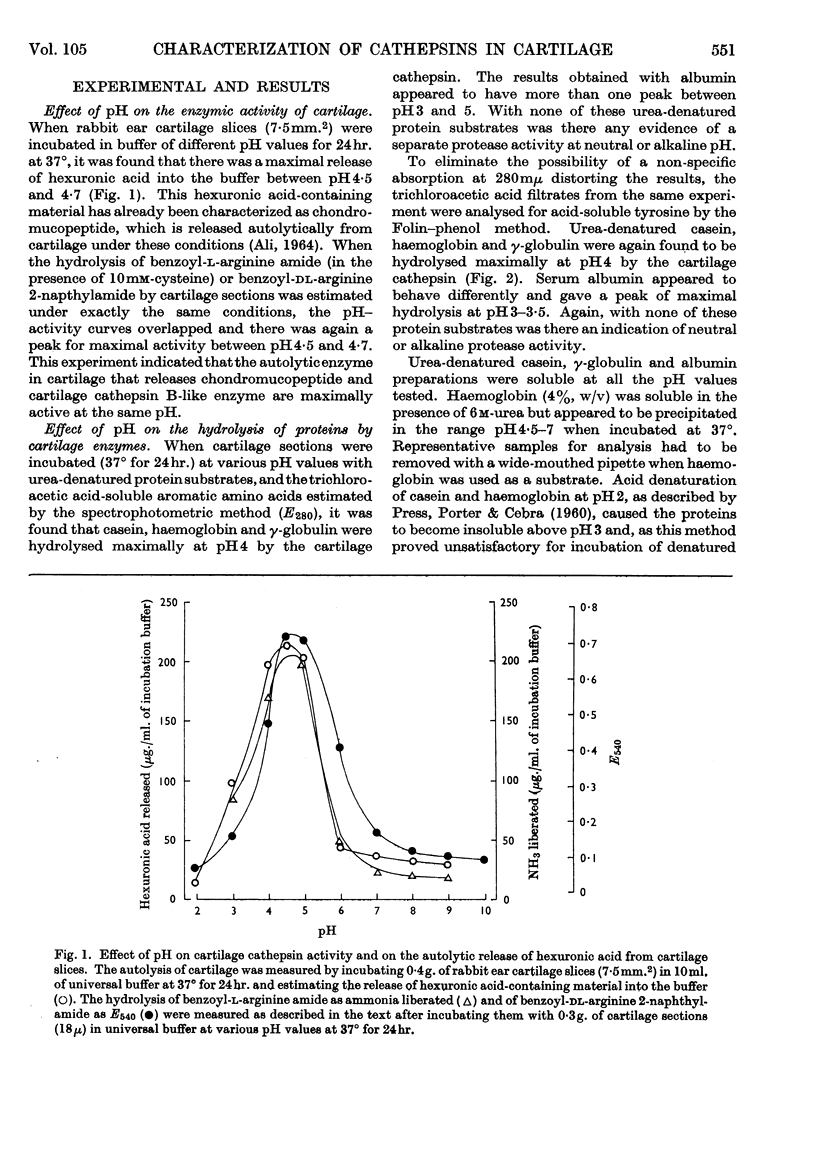
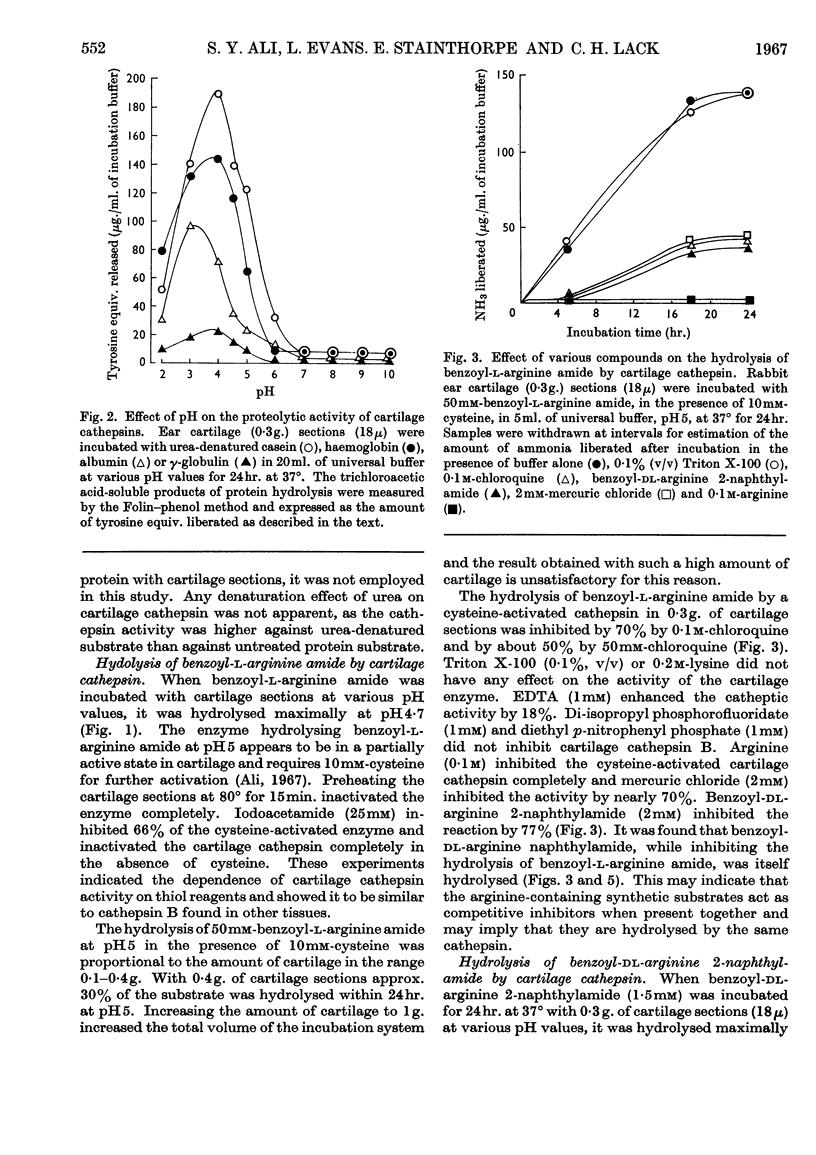
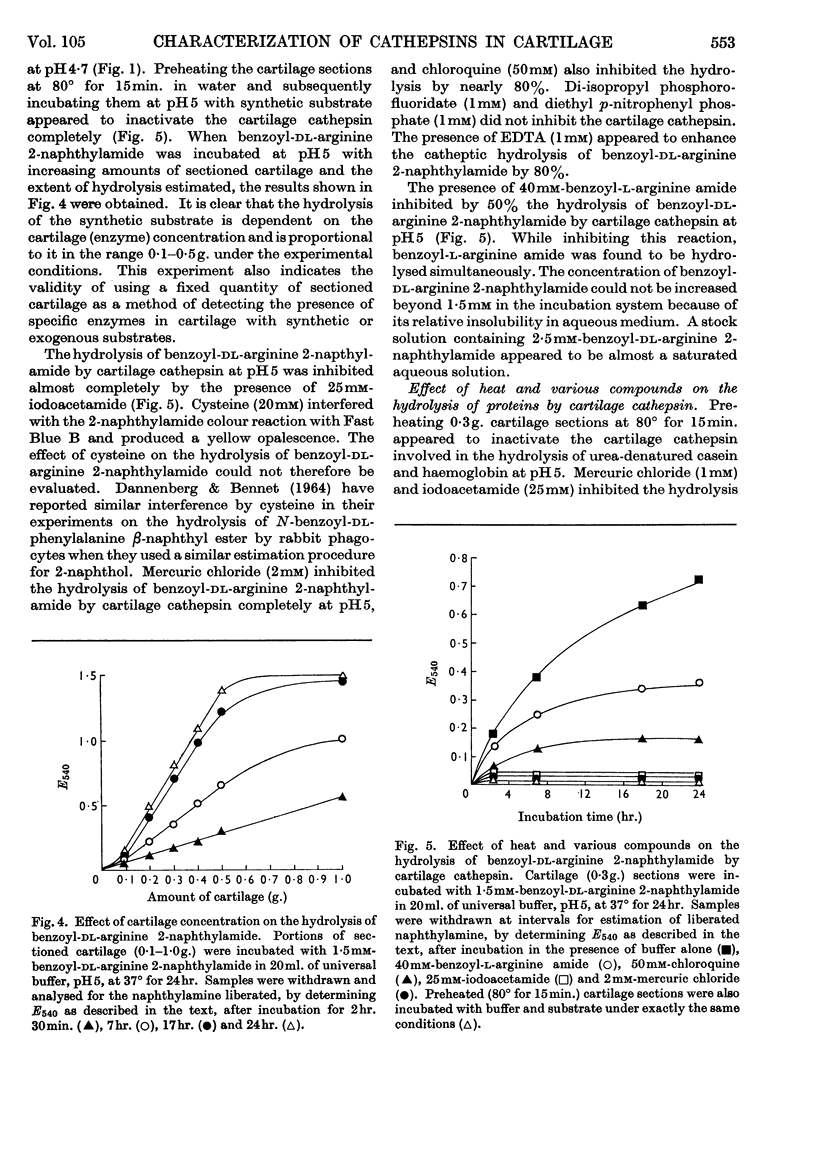
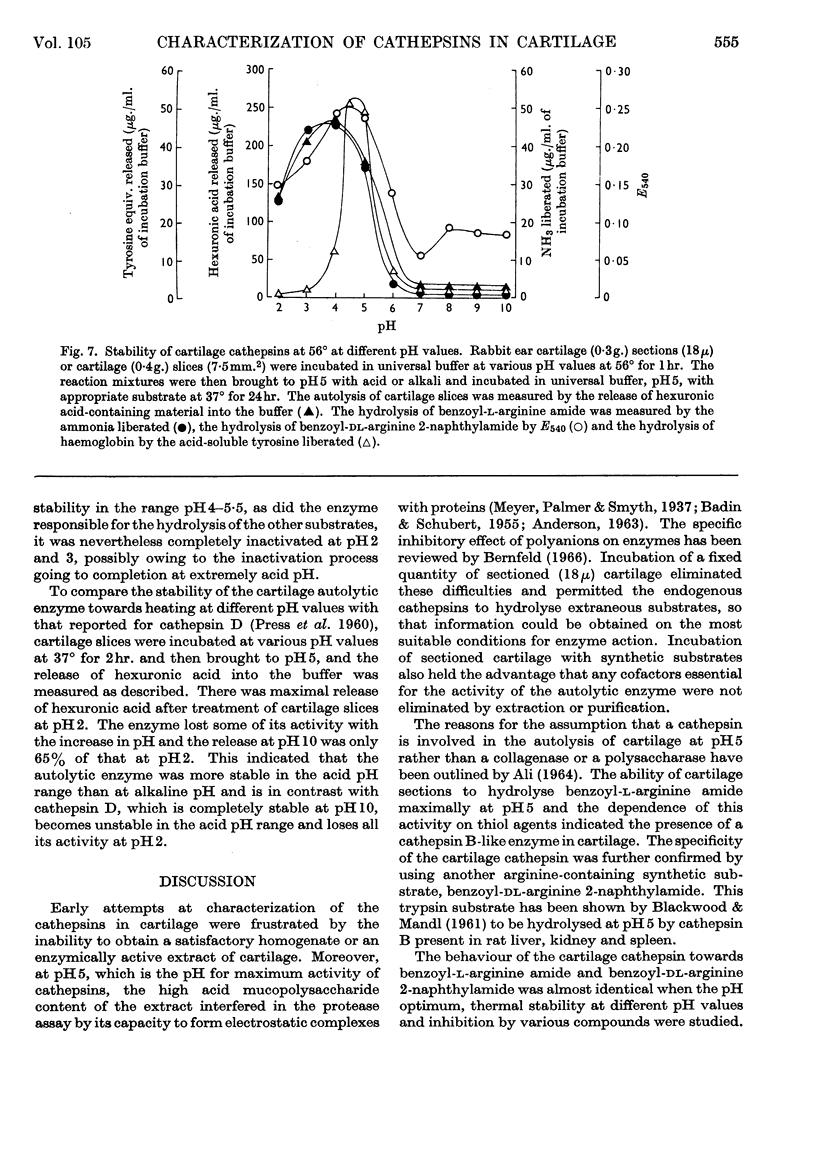
The presence of a cathepsin B-like enzyme in rabbit ear cartilage was established by the use of the synthetic substrates benzoyl-l-arginine amide and benzoyl-dl-arginine 2-naphthylamide. This was facilitated by using a technique that permits the incubation of a fixed weight of thin (18μ) cartilage sections with an appropriate exogenous substrate. The enzymic properties of cathepsin B in cartilage have been compared with an endogenous enzyme that liberates chondromucopeptide by degrading the cartilage matrix autocatalytically at pH5. Besides being maximally active at pH4·7, these cartilage enzymes are enhanced in activity by cysteine and inhibited by arginine analogues, iodoacetamide, chloroquine and mercuric chloride. They are not inhibited by EDTA, di-isopropyl phosphorofluoridate and diethyl p-nitrophenyl phosphate. When inhibiting the release of chondromucopeptide from cartilage at pH5, the arginine-containing synthetic substrates are hydrolysed simultaneously. These enzymes also share the same heat-inactivation characteristics at various pH values, being stable at acid pH and unstable at neutral and alkaline pH. The experimental evidence indicates that a cathepsin B-like enzyme may be partly responsible for the autolytic degradation of cartilage matrix at pH5.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALI S. Y., LACK C. H. STUDIES ON THE TISSUE ACTIVATOR OF PLASMINOGEN. DISTRIBUTION OF ACTIVATOR AND PROTEOLYTIC ACTIVITY IN THE SUBCELLULAR FRACTIONS OF RABBIT KIDNEY. Biochem J. 1965 Jul;96:63–74. doi: 10.1042/bj0960063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON A. J. THE FORMATION OF CHONDROMUCOPROTEIN-FIBRINOGEN AND CHONDROMUCOPROTEIN-BETA-LIPOPROTEIN COMPLEXES. Biochem J. 1963 Sep;88:460–469. doi: 10.1042/bj0880460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. Y. The degradation of cartilage matrix by an intracellular protease. Biochem J. 1964 Dec;93(3):611–618. doi: 10.1042/bj0930611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BADIN J., SCHUBERT M. Conditions of formation of euglobulin-like precipitates from serum proteins and chondroitin sulfate. J Clin Invest. 1955 Aug;34(8):1312–1316. doi: 10.1172/JCI103177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAUFAY H., DE DUVE C. Tissue fractionation studies. 9. Enzymic release of bound hydrolases. Biochem J. 1959 Dec;73:604–609. doi: 10.1042/bj0730604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLLET A. J., HANDY J. R., STURGILL B. C. Chondroitin sulfate concentration and protein-polysaccharide composition of articular cartilage in osteoarthritis. J Clin Invest. 1963 Jun;42:853–859. doi: 10.1172/JCI104777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Chondromucoprotein-degrading enzymes. Nature. 1966 Sep 10;211(5054):1188–1189. doi: 10.1038/2111188a0. [DOI] [PubMed] [Google Scholar]
- Bouma J. M., Gruber M. Intracellular distribution of cathepsin B and cathepsin C in rat liver. Biochim Biophys Acta. 1966 Feb 14;113(2):350–358. doi: 10.1016/s0926-6593(66)80074-7. [DOI] [PubMed] [Google Scholar]
- COLLINS D. H., McELLIGOTT T. F. Sulphate (35SO4) uptake by chondrocytes in relation to histological changes in osteoarthritic human articular cartilage. Ann Rheum Dis. 1960 Dec;19:318–330. doi: 10.1136/ard.19.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. An inhibitor of liver alcohol dehydrogenase in preparations of reduced diphosphopyridine nucleotide. Nature. 1961 Sep 9;191:1098–1099. doi: 10.1038/1911098a0. [DOI] [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, BENNETT W. E. HYDROLYTIC ENZYMES OF RABBIT MONONUCLEAR EXUDATE CELLS. I. QUANTITATIVE ASSAY AND PROPERTIES OF CERTAIN PROTEASES, NON-SPECIFIC ESTERASES, AND LIPASES OF MONONUCLEAR AND POLYMORPHONUCLEAR CELLS AND ERYTHROCYTES. J Cell Biol. 1964 Apr;21:1–13. doi: 10.1083/jcb.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R., BAUDHUIN P. Distribution of enzymes between subcellular fractions in animal tissues. Adv Enzymol Relat Subj Biochem. 1962;24:291–358. doi: 10.1002/9780470124888.ch6. [DOI] [PubMed] [Google Scholar]
- DINGLE J. T., LUCY J. A., FELL H. B. Studies on the mode of action of excess of vitamin A. 1. Effect of excess of vitamin A on the metabolism and composition of embryonic chick-limb cartilage grown in organ culture. Biochem J. 1961 Jun;79:497–500. doi: 10.1042/bj0790497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGLE J. T. Studies on the mode of action of excess of vitamin A. 3. Release of a bound protease by the action of vitamin A. Biochem J. 1961 Jun;79:509–512. doi: 10.1042/bj0790509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELL H. B., DINGLE J. T. Studies on the mode of action of excess of vitamin A. 6. Lysosomal protease and the degradation of cartilage matrix. Biochem J. 1963 May;87:403–408. doi: 10.1042/bj0870403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FESSEL J. M., CHRISMAN O. D. ENZYMATIC DEGRADATION OF CHONDROMUCOPROTEIN BY CELL-FREE EXTRACTS OF HUMAN CARTILAGE. Arthritis Rheum. 1964 Aug;7:398–405. doi: 10.1002/art.1780070406. [DOI] [PubMed] [Google Scholar]
- GERBER D. A. EFFECT OF CHLOROQUINE ON THE SULFHYDRYL GROUP AND THE DENATURATION OF BOVINE SERUM ALBUMIN. Arthritis Rheum. 1964 Jun;7:193–200. doi: 10.1002/art.1780070302. [DOI] [PubMed] [Google Scholar]
- MEGO J. L., MCQUEEN J. D. THE UPTAKE AND DEGRADATION OF INJECTED LABELED PROTEINS BY MOUSE-LIVER PARTICLES. Biochim Biophys Acta. 1965 Apr 12;100:136–143. doi: 10.1016/0304-4165(65)90436-8. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., PLAPINGER R. E., SELIGMAN A. M. ROLE OF SOME STRUCTURAL FEATURES OF SUBTRATES ON TRYPSIN ACTIVITY. Arch Biochem Biophys. 1964 Nov;108:266–274. doi: 10.1016/0003-9861(64)90386-8. [DOI] [PubMed] [Google Scholar]
- PRESS E. M., PORTER R. R., CEBRA J. The isolation and properties of a proteolytic enzyme, cathepsin D, from bovine spleen. Biochem J. 1960 Mar;74:501–514. doi: 10.1042/bj0740501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS L. THE EFFECTS OF PAPAIN, VITAMIN A, AND CORTISONE ON CARTILAGE MATRIX IN VIVO. Biophys J. 1964 Jan;4:SUPPL207–SUPPL213. doi: 10.1016/s0006-3495(64)86939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


