Abstract
Nonalcoholic fatty liver disease (NAFLD) encompasses a spectrum of chronic liver conditions, ranging from simple steatosis to nonalcoholic steatohepatitis, which may progress to fibrosis/cirrhosis. Here, the GSE163211 data set was analyzed, and Asah1 (encoding acid ceramidase) was identified as a crucial lysosomal gene that positively correlated with NAFLD stages in obese patients. To evaluate the role of Asah1 in the progression of NAFLD, Asah1fl/fl/Albcre mice (hepatocyte-specific deletion of Asah1) and Asah1 floxed (Asah1fl/fl/wild-type) mice were fed with either a normal diet or a high-fat, high-cholesterol paigen diet (PD) for 20 weeks. Hepatocyte-specific Asah1 ablation markedly aggravated PD-induced hepatic steatosis, hepatitis, and apoptosis, and resulted in marked fibrotic changes. In addition, Asah1 gene ablation exacerbated PD-induced portal venous hemodynamic abnormality. In cultured hepatocytes, Asah1 gene knockdown resulted in increased ceramide and cholesterol levels but did not affect triglyceride level. Knocking down Asah1 gene also exhibited broad impacts on lipid homeostasis pathways, including lipogenesis, fatty acid uptake, fatty acid oxidation, and lipid transport. Furthermore, Asah1 knockdown resulted in increased endoplasmic reticulum stress and lipid droplet biogenesis. Finally, Asah1 gene knockdown impaired chaperone-mediated autophagy. These results suggest that Asah1 functions as an important regulator of hepatic lipid homeostasis, and its deficiency exacerbates hepatocyte lipotoxicity and injury, and promotes the development of fibrotic nonalcoholic steatohepatitis.
Nonalcoholic fatty liver disease (NAFLD) is the most common form of liver condition in the world, with a global prevalence of 32%.1 NAFLD is closely associated with the incidence of multiple metabolic diseases, such as central obesity, dyslipidemia, hypertension, and type 2 diabetes.2 Furthermore, NAFLD increases the risk of cardiovascular diseases, such as myocardial infarction and stroke.3,4 The hallmark of NAFLD is hepatosteatosis, the deposition of hepatocellular neutral lipids resulting from disrupted hepatic lipid homeostasis.5 Approximately 25% of patients with NAFLD progress to nonalcoholic steatohepatitis (NASH), an advanced NAFLD form manifested by hepatocyte ballooning degeneration and hepatitis.6 Persistent chronic liver inflammation and liver injury result in liver fibrosis, which may further progress to cirrhosis, and lead to the development of severe liver complications, such as portal hypertension and liver carcinoma.7, 8, 9 A cohort study reported that the presence of NASH does not increase liver-related morbidity or overall mortality, whereas the fibrosis stage is related with severe liver diseases and predicts mortality.10 Similarly, another study showed that fibrosis severity serves as a determinant of mortality in patients with NASH.11 Therefore, understanding the underlying mechanism of the development of fibrotic NASH is important for developing therapies and improving the outcomes of patients with NAFLD.
As the central organ mediating lipid metabolism, the liver is responsible for orchestrating the balance of lipid synthesis, uptake, storage, and disposal. Under normal circumstances, hepatocellular lipid acquisition [eg, de novo lipogenesis and fatty acid (FA) uptake] is balanced with lipid storage and lipid consumption [eg, FA oxidation and very-low-density lipoprotein (VLDL) export].12 However, during the development of NAFLD, one or more of these pathways may be perturbed, leading to lipid accumulation within hepatocytes.13 Excessive accumulation of lipotoxic lipids, including oxysterols, sphingolipids (most notably ceramide), and saturated FAs, induces hepatocellular damage and apoptosis, leading to the release of damage-associated molecular patterns or mediators, including high mobility group box 1 (HMGB1), reactive oxygen species, oxysterols, oxidized phospholipids, apoptotic bodies, and extracellular vesicles.14,15 These hepatocyte-released damage-associated molecular patterns or mediators can directly activate hepatic stellate cells, leading to their myofibroblast transformation and extracellular matrix deposition.6 Moreover, these damage-associated molecular patterns or mediators trigger innate immune responses, resulting in monocyte recruitment and the release of profibrotic mediators, including transforming growth factor-β.6 Owing to these pathologic mechanisms, the disruption of lipid homeostasis may be an early event in liver fibrosis, thus playing a critical role in the progression of fibrotic NASH.
Lipid droplets (LDs) are the major lipid storage organelles in hepatocytes and influence a wide range of physiological processes. The abundance of LDs, due to imbalance in biogenesis and catabolism, is intimately linked to the metabolic state of the liver and correlates with the severity of NAFLD.16 The biogenesis of LDs takes place in the cytosolic leaflet in the endoplasmic reticulum (ER), where the enzymes catalyzing the synthesis of neutral lipids are located. Imbalanced lipid homeostasis and lipid overload induce ER stress and promote LD biogenesis, whereas impaired LD catabolism and lipolysis exacerbate ER stress.17 LD catabolism begins with the degradation of LD membrane-coating proteins, especially the perilipin family member perilipin 2 (PLIN2), a substrate of chaperone-mediated autophagy (CMA).12,18 During CMA, the Lys-Phe-Glu-Arg-Gln (KFERQ) domain of PLIN2 is recognized by a heat shock cognate protein called heat shock cognate 71-kDa protein (HSC70), which delivers PLIN2 to lysosomal lumen via lysosome-associated membrane protein 2A (LAMP2A). This allows cytosolic lipases to enter the LD core and facilitates the lipolysis process. Alternatively, LDs can be degraded via macroautophagy, during which autophagosomes sequester LDs and fuse with lysosomes, followed by the catabolism of LD cargos via lysosomal acid lipase.5,19
Acid ceramidase (encoded by the Asah1 gene) is a lysosomal hydrolase that converts ceramide into sphingosine and FA. Ceramide is an important lipotoxic inducer implicated in insulin resistance, oxidative stress, apoptosis, inflammation, and fibrosis pathways.20,21 Several clinical studies have shown that hepatic ceramide levels increase with the progression of NAFLD.22 Genetic mutations in Asah1 cause Farber disease, a rare lysosomal storage disorder characterized by hepatomegaly, ascites, liver fibrosis, and even liver failure.23 In addition, Asah1P361R/P361R mice with loss-of-function mutations in the Asah1 gene exhibit liver injury, leukocyte infiltration, and progressive tissue fibrosis.24 Hepatocyte-specific Asah1 gene deficiency increases ceramide accumulation and aggravates hepatic steatosis.25 However, the role and mechanism of Asah1 in hepatic lipid homeostasis and fibrotic NASH have not been investigated. To address these questions, in this study, a clinical data set was analyzed, which showed a positive correlation between Asah1 expression with NAFLD stages. Next, whether hepatic Asah1 deficiency exacerbates NAFLD-related phenotypes and promotes liver fibrosis was investigated. Finally, whether Asah1 regulates lipid homeostasis and controls LD biogenesis and/or catabolism in cultured hepatocytes was investigated.
Materials and Methods
Differentially Expressed Gene Analysis and Data Visualization
Hepatic gene expression profile GSE163211 was obtained from the Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo, last accessed October 14, 2024). GSE163211 was conducted on the Nanostring nCounter platform (Seattle, WA), containing 795 genes and 5 housekeeping genes (CLTC, GUSB, PGK1, SDHA, and TUBB).26 Differentially expressed gene (DEG) analysis was conducted by using limma R package version 3.58.1 on GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r, last accessed October 14, 2024). Gene Ontology enrichment was performed by using clusterProfiler R package version 4.10.0 (https://bioconductor.org/packages/release/bioc/html/clusterProfiler.html). Circos plot was generated using the enrichplot package version 1.22.0 (https://bioconductor.org/packages/release/bioc/html/enrichplot.html), whereas heat map plots were generated using the pheatmap package version 1.0.12 (https://cran.r-project.org/web/packages/pheatmap/index.html).
Animal Experiments
All animal studies were conducted under the NIH Guide for the Care and Use of Laboratory Animals.27 All the animal experiments were approved by the Institutional Animal Care and Use Committee of University of Houston (Houston, TX). Mice were housed in a temperature- and humidity-controlled room, provided with rodent chow and water ad libitum with a 12-hour light/12-hour dark cycle. Hepatocyte-specific Asah1 knockout mouse line (Asah1fl/fl/AlbCre) was generated by crossing Asah1 floxed mice [Asah1fl/fl/wild type (WT)] with Alb-Cre mice, and Asah1 floxed mice were used as control. Male Asah1fl/fl/WT mice (C57BL/6 J background; aged 8 to 24 weeks) and Asah1fl/fl/AlbCre mice were randomly divided into four groups, fed with either normal diet (ND) or high-fat, high-cholesterol Paigen diet (PD; Research Diets D12336; 35% kcal fat, 1.25% cholesterol) for 20 weeks. The mice were sacrificed after 20 weeks, and livers were isolated for various analyses described later, including biochemical assays, histologic staining, immunofluorescence staining, and real-time quantitative PCR analysis. All mice were fasted overnight (at least 12 hours) before sacrifice. All mice used in the in vivo studies were genotyped for Asah1fl/fl/AlbCre and Cre recombinase gene to confirm liver-specific gene deletion of acid ceramidase α subunit, genotyped as previously described.25 The primers for Asah1fl/fl/AlbCre mice were as follows: 5′-ACAACTGTGTAGGATTCACGCATTCTCC-3′ (forward) and 5′-TCGATCTATGAAATGTCGCTGTCGG-3′ (reverse). The primers for internal control (AC exon) were as follows: 5′-CTAGGCCACAGAATTGAAAGATCT-3′ (forward) and 5′-GTAGGTGGAAATTCTAGCATCATCC-3′ (reverse). The primers for Cre recombinase gene were as follows: 5′-CTAGGCCACAGAATTGAAAGATCT-3′ (forward) and 5′-GTAGGTGGAAATTCTAGCATCATCC-3′ (reverse). The PCR products were separated by gel electrophoresis on 3% agarose gels and visualized by ethidium bromide fluorescence, as previously described.25
Cell Line and Cell Treatment
Human hepatocellular carcinoma cell line HepG2 was purchased from ATCC (Manassas, VA) and maintained in Eagle's minimum essential medium with 10% fetal bovine serum, cultured in a humidified incubator with 5% CO2 at 37°C. Palmitic acid, 7-ketocholesterol (7K), and chloroquine were purchased from Sigma-Aldrich (St. Louis, MO). Oleic acid was purchased from Cayman (Ann Arbor, MI). Palmitic acid was dissolved in 0.1 mol/L NaOH at 75°C and diluted in fatty acid–free bovine serum albumin solution. Oleic acid and 7K were dissolved in 100% ethanol. Chloroquine was dissolved in water. For gene silencing, cells were transfected with Asah1 siRNA or negative control siRNA (Santa Cruz Biotechnology, Dallas, TX) using a silentFect Lipid Reagent for RNAi (Bio-Rad, Hercules, CA), according to the manufacturer's protocol.
Measurement of Aminotransferase Activities and Lipid Concentration
Aminotransferase activities, total cholesterol (TC), and triglyceride (TG) concentration in mouse plasma, liver tissue, or HepG2 cells were determined according to the manufacturer's protocols (BioAssay Systems, Hayward, CA). Nonesterified fatty acid concentration in mouse plasma was determined according to the manufacturer's protocol (Elabscience, Houston, TX).
Histologic and Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling Staining
Liver paraffin sections (4 μm thick) were used to perform hematoxylin and eosin staining (Teomics, Houston, TX), Masson staining (Sigma-Aldrich), Sirius red staining (Sigma-Aldrich), and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining (Roche, Basel, Switzerland) were performed according to the manufacturer's protocols.
Immunofluorescence Staining
Liver and cell immunofluorescence staining were performed as previously described.4,28 Briefly, for liver immunofluorescence staining, frozen liver sections (8 μm thick) were fixed with acetone. For cell immunofluorescence staining, HepG2 cells were fixed by 4% paraformaldehyde. Then, liver sections or cells were washed with phosphate-buffered saline (PBS), blocked with 10% donkey serum for 1 hour, and then incubated with primary antibodies at room temperature for 1 hour and overnight at 4°C. Next, liver sections or cells were washed with PBS, followed by incubation with Alexa Fluor 488– and/or Alexa Fluor 555–labeled secondary antibodies (Thermo Fisher, Waltham, MA) for 1 hour at room temperature. Finally, liver sections or cells were mounted with Vectashield PLUS antifade mounting medium with DAPI (Vector, Newark, CA), and imaged using Leica SP8 STED Super Resolution Confocal Microscopy (Wetzlar, Germany). CD45, collagen I, and LAMP2A primary antibodies were purchased from Abcam (Cambridge, MA). Cleaved caspase-3 primary antibody was purchased from Cell Signaling Technology (Danvers, MA). HSC70 primary antibody was purchased from Santa Cruz Biotechnology. Ceramide primary antibody was purchased from Enzo Biochem (Farmingdale, NY). For quantification, the mean fluorescence intensity was analyzed using Image-Pro Plus software version 6.0 (Media Cybernetics, Rockville, MD).
Doppler Ultrasound Imaging
Mouse portal venous hemodynamics were determined using a Vevo 3100 Imaging system (VisualSonics, Toronto, ON, Canada), as previously described.29 Briefly, mice were anesthetized with 1.5% isoflurane and placed on the detection platform in the supine position. Mouse body temperature was maintained at 37°C, while the electrocardiogram and respiration were monitored during the whole procedure. Mouse portal venous hemodynamics were analyzed by using B-mode, color Doppler mode, and pulsed wave Doppler mode. Maximum velocity (Vmax), minimum velocity (Vmin), and mean velocity (Vmean) were used to calculate pulsatility index (PI) with the equation: PI = (Vmax – Vmin)/Vmax.30
Real-Time Quantitative PCR
Cell total RNA was extracted by TRIzol reagent and reverse transcribed by iScript Reverse Transcription Supermix (Bio-Rad), according to the manufacturer's protocol. Gene expression was determined by measuring mRNA levels using iTaq Universal SYBR Green Supermix (Bio-Rad), according to the manufacturer's protocol. Primers were purchased from Eurofins Scientific (Luxembourg City, Luxembourg), and the sequences are listed in Table 1.
Table 1.
Primer Sequences for Quantitative RT-PCR Analysis
| Gene symbol | Species | Forward primer | Reverse primer |
|---|---|---|---|
| Adgre1 | Mouse | 5′-TGACTCACCTTGTGGTCCTAA-3′ | 5′-CTTCCCAGAATCCAGTCTTTCC-3′ |
| Nlrp3 | Mouse | 5′-GGAGAGACCTTTATGAGAAAGCAA-3′ | 5′-GCTGTCTTCCTGGCATATCACA-3′ |
| Casp1 | Mouse | 5′-AACACTTTGAAGTGCCCAAGC-3′ | 5′-CACTCCTTGTTTCTCTCCACG-3′ |
| Il18 | Mouse | 5′-CCTTTGAGGCATCCAGGACAA-3′ | 5′-CGGGGCCTGAGGATTATAGC-3′ |
| Tnf | Mouse | 5′-ATGGCCTCCCTCTCATCAGT-3′ | 5′-TTTGCTACGACGTGGGCTAC-3′ |
| Ccl2 | Mouse | 5′-CAGGTCCCTGTCATGCTTCT-3′ | 5′-GTGGGGCGTTAACTGCATCT-3′ |
| Ppia | Mouse | 5′-CCCACCGTGTTCTTCGACAT-3′ | 5′-CCAGTGCTCAGAGCTCGAAA-3′ |
| Tgfb1 | Mouse | 5′-TTGCTTCAGCTCCACAGAGA-3′ | 5′-TGGTTGTAGAGGGCAAGGAC-3′ |
| Col1a1 | Mouse | 5′-GGGGCAAGACAGTCATCGAA-3′ | 5′-GAGGGAACCAGATTGGGGTG-3′ |
| Col2a1 | Mouse | 5′-ACTTGCCAAGACCTGAAACTCTG-3′ | 5′-AAACTTTCATGGCGTCCAAGG-3′ |
| Mmp2 | Mouse | 5′-GCCCCCATGAAGCCTTGTTT-3′ | 5′-GGTCATAGTCCTCGGTGGTG-3′ |
| Mmp9 | Mouse | 5′-CAGCCGACTTTTGTGGTCTTC-3′ | 5′-CGGTACAAGTATGCCTCTGCCA-3′ |
| Apoa1 | Mouse | 5′-GGCAGAGACTATGTGTCCCAGT-3′ | 5′-GCTGACTAACGGTTGAACCCAG-3′ |
| Abca1 | Mouse | 5′-GGAGCCTTTGTGGAACTCTTCC-3′ | 5′-CGCTCTCTTCAGCCACTTTGAG-3′ |
| Abcg1 | Mouse | 5′-GACACCGATGTGAACCCGTTTC-3′ | 5′-GCATGATGCTGAGGAAGGTCCT-3′ |
| Nceh1 | Mouse | 5′-CGGTATTTCTGGAGACAGTGCTG-3′ | 5′-GGTGTGTTGAAGTCCAAAGCCTG-3′ |
| Cyp7a1 | Mouse | 5′-CACCATTCCTGCAACCTTCTGG-3′ | 5′-ATGGCATTCCCTCCAGAGCTGA-3′ |
| Cyp8b1 | Mouse | 5′-GGTACGCTTCCTCTATCGCC-3′ | 5′-GAGGGATGGCGTCTTATGGG-3′ |
| Cyp2c70 | Mouse | 5′-AAGCTCTGATTGACCAGGGAG-3′ | 5′-GCCGGGTTTGTTTCCATGTT-3′ |
| Abcb11 | Mouse | 5′-CCTTGGTAGAGAAGAGGCGACA-3′ | 5′-ATGGCTACCCTTTGCTTCTGCC-3′ |
| Srebf1 | Mouse | 5′-CGACTACATCCGCTTCTTGCAG-3′ | 5′-CCTCCATAGACACATCTGTGCC-3′ |
| Scd1 | Mouse | 5′-GCAAGCTCTACACCTGCCTCTT-3′ | 5′-CGTGCCTTGTAAGTTCTGTGGC-3′ |
| Fasn1 | Mouse | 5′-CACAGTGCTCAAAGGACATGCC-3′ | 5′-CACCAGGTGTAGTGCCTTCCTC-3′ |
| Fads2 | Mouse | 5′-TTCCTGGAGAGCCACTGGTTTG-3′ | 5′-GAAGAAGGACTGCTCCACATTGC-3′ |
| Srebf2 | Mouse | 5′-AGAAAGAGCGGTGGAGTCCTTG-3′ | 5′-GAACTGCTGGAGAATGGTGAGG-3′ |
| Hmgcr | Mouse | 5′-GTGCGTAAGCGCAGTTCCTT-3′ | 5′-CACAGTCCTTGGATCCTCCG-3′ |
| Ldlr | Mouse | 5′-CCAATCGACTCACGGGTTCA-3′ | 5′-CAACCACCATTGGGGAGGAG-3′ |
| Pparg1 | Mouse | 5′-GAAAGACAACGGCAAATCACC-3′ | 5′-GGGGGTGATATGTTTGAACCTG-3′ |
| Pparg2 | Mouse | 5′-TGCTGTTATGGGTGAAACTCTG-3′ | 5′-CTGTGTCAACCATGGTAAATTTCTT-3′ |
| Cd36 | Mouse | 5′-GATGAATGGTTGAGACCCCGT-3′ | 5′-ATTTCAGAAGGCAGCAACTTC-3′ |
| Lpl | Mouse | 5′-GCGTAGCAGGAAGTCTGACCAA-3′ | 5′-AGCGTCATCAGGAGAAAGGCGA-3′ |
| Pnpla2 | Mouse | 5′-ACCTTCGCAATCTCTACCGC-3′ | 5′-TGGGTTGGTTCAGTAGGCCA-3′ |
| Hprt1 | Mouse | 5′-GTTGGGCTTACCTCACTGCT-3′ | 5′-TAATCACGACGCTGGGACTG-3′ |
| SREBF2 | Human | 5′-CTCCATTGACTCTGAGCCAGGA-3′ | 5′-GAATCCGTGAGCGGTCTACCAT-3′ |
| HMGCR | Human | 5′-TGATTGACCTTTCCAGAGCAAG-3′ | 5′-CTAAAATTGCCATTCCACGAGC-3′ |
| PPARG1 | Human | 5′-GACAGGAAAGACAACAGACAAATC-3′ | 5′-GGGGTGATGTGTTTGAACCTG-3′ |
| PPARG2 | Human | 5′-TCCATGCTGTTATGGGTGAA-3′ | 5′-TGTGTCAACCATGGTATTTTC-3′ |
| CD36 | Human | 5′-GGCTGTGACCGGAACTGTG-3′ | 5′-AGGTCTCCAACTGGCATTAGAA-3′ |
| FATP5 | Human | 5′-TGGAGGAGATCCTTCCCAAGC-3′ | 5′-TGGTCCCCGAGGTATAGATGAA-3′ |
| FGF21 | Human | 5′-CTGTGGGTTTCTGTGCTGG-3′ | 5′-CCGGCTTCAAGGCTTTCAG-3′ |
| ABCA1 | Human | 5′-ACCCACCCTATGAACAACATGA-3′ | 5′-GAGTCGGGTAACGGAAACAGG-3′ |
| APOB | Human | 5′-TGCTCCACTCACTTTACCGTC-3′ | 5′-TAGCGTCCAGTGTGTACTGAC-3′ |
| APOE | Human | 5′-GTTGCTGGTCACATTCCTGG-3′ | 5′-GCAGGTAATCCCAAAAGCGAC-3′ |
| TM6SF2 | Human | 5′-GCATTGATGAGCGCCCTAATC-3′ | 5′-AGTGGGTCATAGGAGACCTCG-3′ |
| HPRT1 | Human | 5′-CCTGGCGTCGTGATTAGTGA-3′ | 5′-CGAGCAAGACGTTCAGTCCT-3′ |
| FITM2 | Human | 5′-GTCTGTGCTGCATGAGGTGAA-3′ | 5′-CCAGATGAAAGTCAGAATGCCC-3′ |
| BECN1 | Human | 5′-GAAGACGTGGAAAAGAACCGC-3′ | 5′-CAGCCTGAAGTTATTGATTGTGC-3′ |
| ATG3 | Human | 5′-GCCGTTAAAGAGATCACACTGG-3′ | 5′-CATAGCCAAACAACCATAATCGTGG-3′ |
| ATG5 | Human | 5′-CAGCTCTTCCTTGGAACATC-3′ | 5′-GGCTGTGGGATGATACTAATATG-3′ |
| ATG12 | Human | 5′-CTGCTGGCGACACCAAGAAA-3′ | 5′-CGTGTTCGCTCTACTGCCC-3′ |
| LC3 | Human | 5′-AAGGCGCTTACAGCTCAATG-3′ | 5′-CTGGGAGGCATAGACCATGT-3′ |
| SQSTM1 | Human | 5′-ATCGGAGGATCCGAGTGT-3′ | 5′-TGGCTGTGAGCTGCTCTT-3′ |
Western Blot Analysis
Western blot analysis was performed as previously described.31,32 Briefly, HepG2 cells were boiled at 95°C for 5 minutes, separated on 10% to 12% SDS-polyacrylamide gels, and transferred to 0.22-μm polyvinylidene difluoride membranes. Next, the polyvinylidene difluoride membrane was blocked by 5% bovine serum albumin at room temperature for 2 hours and incubated with primary antibodies for 1 hour at room temperature and 4°C overnight. Following, bands were incubated with corresponding IRDye 800CW purified immunoglobulin (LI-COR, Lincoln, NE) or horseradish peroxidase–conjugated secondary antibodies (Thermo Fisher). Finally, bands were detected by a LI-COR Western Blot Imaging system and analyzed by ImageJ software version 1.50i (NIH, Bethesda, MD; https://imagej.net/ij). Acid ceramidase, sterol O-acyltransferase 1 (SOAT1), SOAT2, and HSC70 primary antibodies were purchased from Santa Cruz Biotechnology. Sterol regulatory element–binding transcription factor 1 (SREBF1), diacylglycerol O-acyltransferase 1 (DGAT1), DGAT2, carnitine palmitoyltransferase 1A (CPT1A), glyceraldehyde-3-phosphate dehydrogenase, LAMP1, and LAMP2A primary antibodies were purchased from Abcam. Adipose triglyceride lipase (ATGL), β-actin, binding Ig protein (BIP), C/EBP homologous protein (CHOP), calnexin, protein disulfide isomerase (PDI), microtubule-associated proteins 1A/1B light chain 3C (LC3), and sequestosome-1 (SQSTM1) primary antibodies were purchased from Cell Signaling Technology. Low-density lipoprotein receptor (LDLR) primary antibody was purchased from Biovision Technologies (Exton, PA). Peroxisome proliferator-activated receptor α (PPARα) primary antibody was purchased from Invitrogen Thermo Fisher (Waltham, MA). PLIN2 primary antibody was purchased from Proteintech (Rosemont, IL).
Cell Oil Red O Staining
HepG2 cells were treated with FA plus 7K for 24 hours. Twenty-four hours later, cell culture medium was removed, and cells were washed three times with PBS, followed by fixation in 4% paraformaldehyde for 15 minutes, and then three PBS washes. Next, cells were stained with 60% oil red O for 30 minutes at room temperature. Subsequently, oil red O was removed, washed by PBS for three times, 200 μL isopropanol was added to each well, and the plates were incubated for 10 minutes on a shaker. Following that, 100 μL isopropanol was transferred to a 96-well plate, and the absorbance was determined at 510 nm.
Fluorescence and Flow Cytometry Analysis of Cell Staining
Bodipy (493/503) was purchased from Invitrogen Thermo Fisher. Filipin was purchased from Cayman. HepG2 cells were stained with Bodipy for 1 hour at a 1:100 dilution or Filipin for 2 hours at a working solution of 0.05 mg/mL. For fluorescence imaging analysis, Bodipy was removed an hour later, washed with PBS, and then fixed with 4% paraformaldehyde for 15 minutes. Subsequently, cells were washed with PBS three times, mounted with vectashield PLUS antifade mounting medium with DAPI (Vector), and then imaged by Leica SP8 STED Super Resolution Confocal Microscopy. For flow cytometry analysis, cells were harvested by trypsin and fixed in 4% paraformaldehyde. Next, cells were washed three times with PBS, and analyzed by an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA). Unstained HepG2 cells were used as negative control to set the gate for positive cells.
Statistical Analysis
Results were presented as means ± SEMs and analyzed using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA). P values were calculated by using analysis of variances. P < 0.05 was considered as statistically significant, while P < 0.01 was considered as highly significant.
Results
Hepatic Asah1 Gene Expression Increases with Progressive NAFLD Stages in Obese Patients
To explore the most influential genes associated with NAFLD progression, the data set GSE163211 from the Gene Expression Omnibus database was analyzed. Liver tissue samples were collected by liver biopsy. Gene expression analysis was conducted among obese patients with four different NAFLD stages: normal liver biopsy (n = 76), steatosis (n = 88), NASH without fibrosis (n = 72), and NASH with fibrosis (n = 82).26 Multigroup analysis was performed to identify the top 50 DEGs. Next, Gene Ontology was examined, and functional enrichment analysis was performed on the basis of Biological Process, Cellular Component, and Molecular Function. The top 10 terms of Gene Ontology enrichment are shown in Figure 1A, and the genes categorized by Cellular Component category are shown in a circus plot (Figure 1B). One of the key sites relevant to NAFLD progression was the lysosome, which is known as a key organelle involved in lipid degradation. The five DEGs categorized to lysosomal lumen include IFI30, CTSK, LUM, PDGFRB, and Asah1. The heat map of lysosomal DEGs is displayed in Figure 1C, and the normalized gene counts are presented in Figure 1D. All lysosomal lumen genes increased with the progression of NAFLD stages (Figure 1, C and D). Of these five genes, further studies were focused on Asah1 because it is responsible for lysosomal degradation of ceramide, a key lipotoxic lipid.33 Asah1 expression was significantly increased in patients with NASH with fibrosis compared with normal, steatosis, and NASH without fibrosis groups (Figure 1D), indicating that Asah1 gene expression is closely associated with NAFLD progression, especially the onset of liver fibrosis.
Figure 1.
Hepatic Asah1 gene expression increases with progressive nonalcoholic fatty liver disease (NAFLD) stages in obese patients. A: Gene Ontology (GO) enrichment analysis of differentially expressed genes (DEGs) from the GSE163211 data set. Circle size represents child GO term number, whereas circle color represents the significance of enrichment. B: Circos plot of the top 10 terms on Cellular Component (CC) category. C: Heat map of DEGs categorized to the lysosomal lumen. D: Violin plots of DEGs enriched in lysosomal lumen category. Gene counts were normalized to housekeeping genes. n = 76 normal liver biopsy group; n = 88 steatosis group; n = 72 nonalcoholic steatohepatitis (NASH) without fibrosis (NASH F0) group; n = 82 NASH with fibrosis (NASH F1-F4) group. ∗∗P < 0.01. BP, Biological Process; CTSK, cathepsin K; IFI30, interferon-γ–inducible protein 30 preproprotein; LUM, lumican; MF, Molecular Function; PDGFRB, platelet-derived growth factor receptor-β.
Hepatocyte-Specific Asah1 Gene Deletion Aggravates PD-Induced Steatosis and Injury in the Liver of Mice
To understand the role of Asah1 in NAFLD development, hepatocyte-specific Asah1 gene knockout (Asah1fl/fl/AlbCre) mice and Asah1fl/fl/WT controls were treated with ND or PD for 20 weeks. PD had no significant effect on the body weight of mice (Figure 2A). However, it led to a significant increase in liver weight, liver index, hepatomegaly, and yellow coloration in Asah1fl/fl/WT mice, with these changes being more pronounced in Asah1fl/fl/AlbCre mice (Figure 2, B–D). Then, liver dysfunction was analyzed by evaluating plasma alanine aminotransferase and aspartate aminotransferase levels. PD increased alanine aminotransferase but not aspartate aminotransferase in Asah1fl/fl/WT mice, whereas both alanine aminotransferase and aspartate aminotransferase in Asah1fl/fl/AlbCre mice increased further with PD (Figure 2, E and F). Additionally, PD increased TC and nonesterified fatty acid levels in the plasma of Asah1fl/fl/WT, whereas TC but not nonesterified fatty acid level was further elevated in Asah1fl/fl/AlbCre mice (Figure 2, G and H). In contrast, neither PD nor Asah1 gene deficiency had a significant effect on plasma TG level (Figure 2I). PD significantly increased both TC and TG in the liver of Asah1fl/fl/WT mice, whereas TC level was further increased in Asah1fl/fl/AlbCre mice (Figure 2, J and K). As shown in Figure 2L, PD increased steatosis and hepatocyte ballooning (hematoxylin and eosin staining) in the liver of Asah1fl/fl/WT mice, and apoptotic cells were also observed via terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling and cleaved caspase 3 staining. These effects were more pronounced in Asah1fl/fl/AlbCre mice.
Figure 2.
Hepatocyte-specific Asah1 gene deletion aggravates Paigen diet (PD)–induced steatosis and injury in mice liver. A–C: Mouse body weight, liver weight, and liver index changes. Liver index was calculated as liver weight/body weight × 100. D: Representative images of mouse liver. E and F: Mouse plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) level. G–I: Mouse plasma total cholesterol (TC), nonesterified fatty acid (NEFA), and triglyceride (TG) levels. J and K: Mouse liver TC and TG level. L: Representative images of hematoxylin and eosin (H&E) staining, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining, and cleaved caspase 3 (cle-CASP3) immunofluorescence (IF) staining. Data are represented as means ± SEM (A–C and E–K). ∗P < 0.05, ∗∗P < 0.01. Scale bars: 50 μm (L, H&E staining); 20 μm (L, TUNEL and cleaved-CASP3 IF staining). ND, normal diet; WT, wild type.
Hepatocyte-Specific Asah1 Gene Deletion Aggravates Liver Inflammation and Induces Fibrosis in the Liver of Mice Fed PD
The effects of hepatocyte-specific Asah1 gene ablation on hepatitis and fibrosis were further examined. Immunofluorescence staining revealed that PD significantly induced infiltration of CD45+ immune cells in Asah1fl/fl/WT mice, which was more severe in Asah1fl/fl/AlbCre mice (Figure 3A). The effect of PD on the hepatic mRNA levels of various inflammatory markers or mediators was also investigated, including adhesion G-protein–coupled receptor E1 (ADGRE1; a hepatic monocyte marker), NLR family pyrin domain containing 3 (NLRP3) inflammasome components (NLRP3 and caspase 1), IL-18, IL-1β, tumor necrosis factor (TNF)-α, and chemokine (C-C motif) ligand (CCL) 2. The expression of IL-1β level was low (data not shown). As shown in Figure 3B, PD significantly increased the hepatic mRNA levels of ADGRE1 and IL-18 in Asah1fl/fl/WT mice, but had no significant effect on NLRP3, caspase 1, TNF-α, and CCL2. In contrast, PD induced a higher increase in the hepatic mRNA levels of all these genes in Asah1fl/fl/AlbCre mice compared with Asah1fl/fl/WT mice.
Figure 3.
Hepatocyte-specific Asah1 gene deletion aggravates liver inflammation and induces fibrosis in the liver of mice fed Paigen diet (PD). A: Representative immunofluorescence images of CD45 staining. B: Real-time quantitative PCR (qPCR) results of hepatic mRNA abundance, normalized to peptidylprolyl isomerase A (PPIA). C: Representative images of Masson staining, Sirius red staining, and collagen I immunofluorescence staining. D: qPCR results of hepatic mRNA abundance, normalized to PPIA. Data are represented as means ± SEM (B and D). n = 5 to 6 per group (A–D). ∗P < 0.05, ∗∗P < 0.01. Scale bars: 50 μm (A and C, Sirius red and collagen I immunofluorescence staining); 100 μm (C, Masson staining). ADGRE1, adhesion G-protein–coupled receptor E1; CASP1, caspase 1; CCL2, C-C motif chemokine ligand 2; COL1A1, collagen type I α 1 chain; COL2A1, collagen type II α 1 chain; MMP, matrix metallopeptidase; ND, normal diet; NLRP3, NLR family pyrin domain containing 3; TGF-β, transforming growth factor-β; TNF, tumor necrosis factor; WT, wild type.
Next, to examine liver fibrosis, collagen was detected with the use of Masson staining, Sirus red staining, and immunofluorescence staining. As shown in Figure 3C, there was no significant difference in the expression of collagen in the liver of Asah1fl/fl/WT mice between PD and ND groups, indicating that PD alone was not sufficient to induce significant liver fibrosis. In contrast, PD remarkably induced fibrotic changes in Asah1fl/fl/AlbCre mice with the accumulation of hepatic collagen. This is supported by the widespread distribution of blue collagen (Masson staining), conspicuous increase in Sirius red staining, and marked increase in collagen I immunofluorescence staining. Consistent with the increase in collagen staining, PD significantly increased the hepatic mRNA levels of transforming growth factor-β, collagen type I α 1 chain, collagen type II α 1 chain, matrix metallopeptidase-2, and matrix metallopeptidase-9 in Asah1fl/fl/AlbCre mice compared with Asah1fl/fl/WT mice (Figure 3D).
Hepatocyte-Specific Asah1 Gene Deletion Exacerbates PD-Induced Hemodynamical Abnormality in Mouse Portal Vein
Using Doppler ultrasound imaging, whether the increased severity of NAFLD in Asah1fl/fl/AlbCre mice was associated with exacerbated portal vein hemodynamical abnormalities was investigated. Representative Doppler ultrasound images are shown in Figure 4A. The summarized data in Figure 4, B–D, demonstrate that PD significantly reduced portal venous Vmax, Vmin, and Vmean in Asah1fl/fl/WT mice. Compared with Asah1fl/fl/WT mice, Asah1fl/fl/AlbCre mice had similar portal blood flow velocity under the basal condition with ND, but exhibited much lower velocity under PD. In addition, the portal vein PI remained unchanged between all mouse groups (Figure 4E). These results suggest that PD leads to a decrease in portal venous velocity, and hepatic Asah1 gene deficiency further exacerbates this phenomenon.
Figure 4.
Hepatocyte-specific Asah1 gene deletion exacerbates Paigen diet (PD)–induced hemodynamical abnormality in mouse portal vein. A: Representative ultrasound Doppler images of the portal venous wave. B: Maximum velocity (Vmax). C: Minimum velocity (Vmin). D: Mean velocity. E: Pulsatility index. Pulsatility index was calculated by (Vmax – Vmin)/Vmax. Data are represented as means ± SEM (B–E). ∗P < 0.05, ∗∗P < 0.01. ND, normal diet; WT, wild type.
Asah1 Deficiency Increases Hepatocellular Lipid Accumulation
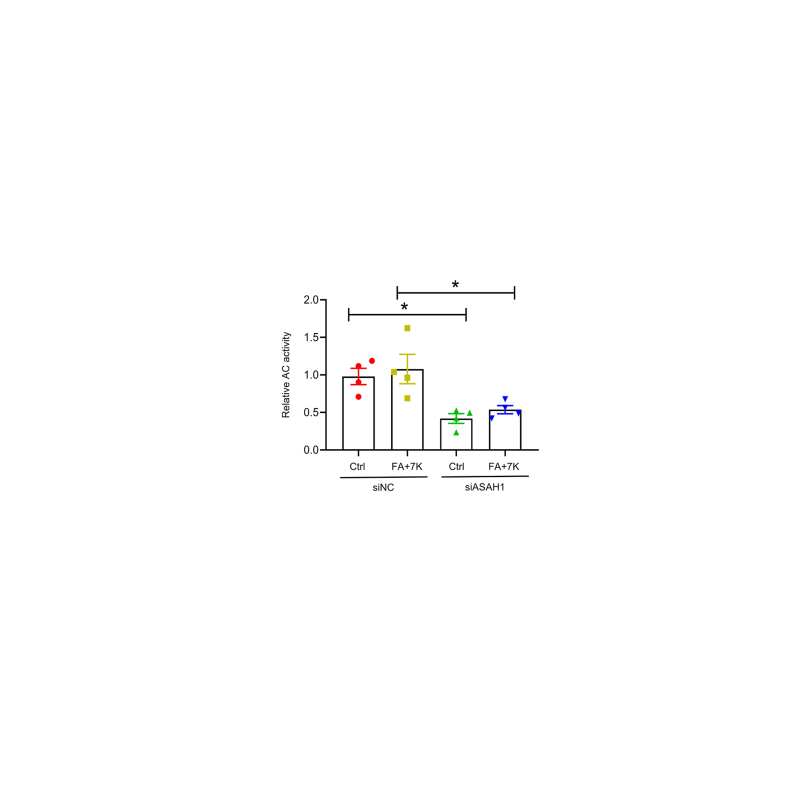
Next, the effect of Asah1 deficiency on the enhanced lipid accumulation was investigated. The Asah1 gene was silenced in cultured HepG2 hepatocytes, followed by treatment with a lipid mixture consisting of free FAs (300 μmol/L; oleic acid:palmitic acid = 2:1) and 7K (40 μmol/L) for 24 hours. Asah1 mRNA level, acid ceramidase protein expression, and acid ceramidase activity in HepG2 cells were significantly reduced by Asah1 gene silencing (Figure 5, A and B, and Supplemental Figure S1). Immunofluorescence staining further demonstrated that FA + 7K treatment increased ceramide levels in HepG2 cells, which were enhanced by Asah1 gene silencing (Figure 5C). Intracellular neutral lipid [majorly TG and cholesterol ester (CE)] accumulation was evaluated by oil red O staining. FA + 7K enhanced intracellular oil red O content, and when Asah1 was silenced, oil red O staining was further enhanced (Figure 5D). As shown in Figure 5, E and F, quantification of neutral lipids revealed that FA + 7K treatment alone increased TG level, whereas the TC level only showed an upward trend. Interestingly, Asah1 gene silencing did not affect TG level (Figure 5E), but significantly increased TC levels (Figure 5F). Considering that TG level remained unchanged, elevated oil red O content is more likely induced by increased CE level. Free cholesterol level was determined by Filipin staining, which showed that Asah1 gene silencing markedly enhanced intracellular free cholesterol content (Figure 5G). These data suggest that Asah1 deficiency enhances the accumulation of lipids in hepatocytes by increasing intracellular cholesterol level.
Figure 5.
Asah1 deficiency increases hepatocellular lipid accumulation. A: Relative Asah1 mRNA abundance determined by real-time quantitative PCR (qPCR). B: Representative Western blot images and summarized data of acid ceramidase (AC). C: Representative immunofluorescence images of ceramide staining and the quantitative image. D: Relative cell oil red O content. E: Cell triglyceride (TG) level. F: Cell total cholesterol (TC) level. G: Representative images of Filipin staining. H and I: Representative Western blot images and summarized data. J: Relative cell mRNA abundance determined by qPCR. K: Relative hepatic mRNA abundance determined by qPCR. Data are represented as means ± SEM (A–F and I–K). n = 4 per group (A–C and G); n = 3 to 4 per group (H–J); n = 5 to 6 per group (K). ∗P < 0.05, ∗∗P < 0.01. Scale bar = 10 μm (C and G). ABCA1, ATP-binding cassette subfamily A member 1; ABCB11, ATP-binding cassette subfamily B member 11; ABCG1, ATP-binding cassette subfamily G member 1; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoE, apolipoprotein E; ATGL, adipose triglyceride lipase; CPT1A, carnitine palmitoyltransferase 1A; CYP2C70, cytochrome P450 family 7 subfamily C polypeptide 70; CYP7A1, cytochrome P450 family 7 subfamily A member 1; CYP8B1, cytochrome P450 family 8 subfamily B member 1; DGAT, diacylglycerol O-acyltransferase; FADS2, fatty acid desaturase 2; FASN, fatty acid synthase; FATP5, fatty acid transport protein-5; FGF21, fibroblast growth factor 21; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; HPRT1, hypoxanthine phosphoribosyltransferase 1; LDLR, low-density lipoprotein receptor; LPL, lipoprotein lipase; nCEH, neutral cholesterol ester hydrolase; PPARα, peroxisome proliferator-activated receptor-α; PPARG, peroxisome proliferator-activated receptor-γ; PPIA, peptidylprolyl isomerase A; SCD, stearoyl-CoA desaturase; SOAT, sterol O-acyltransferase; SREBF, sterol regulatory element–binding transcription factor; TM6SF2, transmembrane 6 superfamily member 2.
To understand how Asah1 deficiency leads to lipid retention, changes in genes related to lipid homeostasis were evaluated. DEGs associated with lipid homeostasis during NAFLD progression were identified by analyzing the GSE163211 data set, which are shown in Supplemental Figure S2A. The gene expression of FGF21, PLIN2, LDLR, CD36, APOE, and TM6SF2 were positively correlated with NAFLD progression in obese patients, whereas APOB, PPARG, and PPARA are down-regulated (Supplemental Figure S2B). Additionally, SREBF1 expression was increased in patients with NASH without fibrosis but decreased with the occurrence of fibrosis. Next, the influence of Asah1 gene deficiency on these DEGs and their related signaling pathways were analyzed on human HepG2 hepatocytes using Western blot analysis or real-time PCR. As shown in Figure 5, H and I, Asah1 silencing significantly increased lipogenesis-related genes, including SREBF1 and SOAT1/2, but not DGAT1/2. SREBF2 and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), which are involved in cholesterol biosynthesis, are reduced by Asah1 gene deficiency (Figure 5J). In addition, Asah1 silencing significantly increased lipid uptake/transport–related genes, including CD36, fatty acid transport protein-5 (FATP5), and LDLR, in both the basal condition and FA + 7K treatment groups (Figure 5, H–J). In contrast, Asah1 silencing had no effect on the triglyceride hydrolase ATGL, a key player in the lipolysis pathway (Figure 5, H and I). The expression of fatty acid oxidation-related genes, including PPARα, PPARG1, CPT1A, and FGF21, was increased by Asah1 silencing under the basal condition or after FA + 7K treatment (Figure 5, H and I). Notably, although PPARG1 was induced by Asah1 gene deficiency, PPARG2 remained unchanged (Figure 5J). Furthermore, Asah1 silencing significantly decreased the mRNA levels of cholesterol efflux transporter ATP-binding cassette subfamily A member 1 (ABCA1) and lipoprotein biogenesis/secretion–related genes, including apolipoprotein B, apolipoprotein E, and TM6SF2 (Figure 5J).
The expression of cholesterol efflux-related genes apolipoprotein A1, ABCA1, and neutral cholesterol ester hydrolase in the liver of Asah1fl/fl/AlbCre mice was also reduced compared with controls, whereas the expression of ATP-binding cassette subfamily G member 1 (ABCG1) was increased (Figure 5K). The mRNA levels of bile acid synthesis-related genes cytochrome P450 family 7 subfamily A member 1 (CYP7A1), cytochrome P450 family 7 subfamily C polypeptide 70 (CYP2C70), and ATP-binding cassette subfamily B member 11 (ABCB11) in the liver were markedly decreased by Asah1 gene deficiency, whereas cytochrome P450 family 8 subfamily B member 1 (CYP8B1) remained unchanged (Figure 5K). The mRNA levels of SREBF1 and fatty acid saturation-related gene fatty acid synthase 1 (FASN1) in the liver were also significantly increased by Asah1 gene deficiency, whereas the expression of stearoyl-CoA desaturase (SCD) and fatty acid desaturase (FADS) exhibited an increasing trend, but was not statistically significant (Figure 5K). The expression of lipid uptake/transport–related genes CD36 and LDLR was increased by Asah1 gene deficiency, whereas cholesterol de novo synthesis enzyme HMGCR was decreased (Figure 5K). Additionally, TG hydrolase lipoprotein lipase (LPL) and ATGL, SREBF2, and PPARG1 were not affected by Asah1 gene deficiency (Figure 5K). PPARG2 was not detected.
Asah1 Gene Deficiency Enhances LD Formation and ER Stress in Vitro
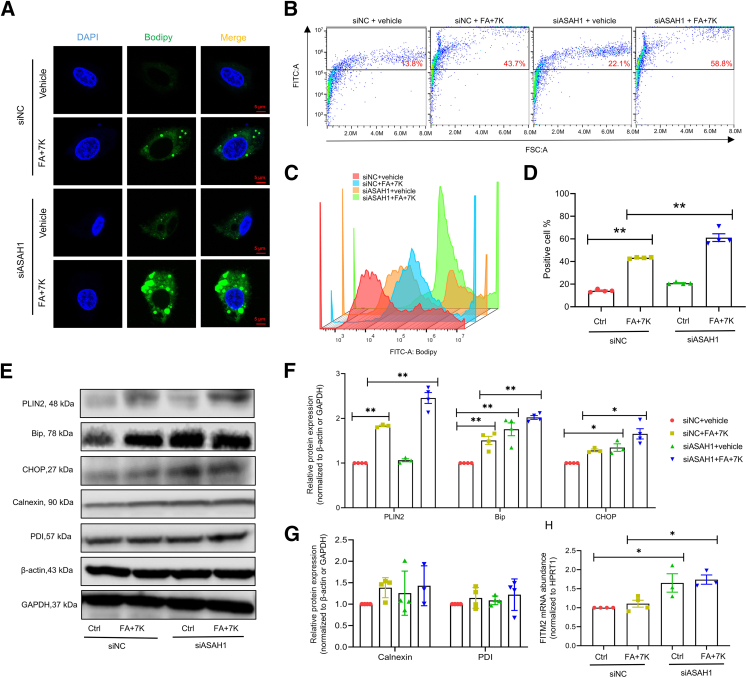
To further investigate whether increased lipid accumulation in hepatocytes is associated with enhanced formation of LDs, the major lipid storage organelle, Bodipy staining was performed to visualize LDs and analyze their size and number. As shown in Figure 6A, Asah1 silencing increased the number and size of LDs induced by FA + 7K treatment. As shown in Figure 6, B–D, flow cytometry analysis confirmed that Asah1 silencing significantly increased the percentage of Bodipy-stained cells induced by FA + 7K treatment. Consistently, Asah1 silencing enhanced the expression of LD coating protein PLIN2 induced by FA + 7K (Figure 6, E and F).
Figure 6.
Asah1 deficiency enhances lipid droplet formation and endoplasmic reticulum stress in vitro. A: Representative Bodipy staining images. B–D: Representative flow cytometry analysis of Bodipy staining images, shown by a scatterplot, histogram plot, and the summarized data. E–G: Representative Western blot images and summarized data. H: Relative fat storage–inducing transmembrane protein 2 mRNA abundance determined by real-time quantitative PCR. Data are represented as means ± SEM (D and F–H). n = 4 per group (A–D); n = 3 to 4 per group (E–H). ∗P < 0.05, ∗∗P < 0.01. Scale bar = 5 μm (A). Bip, binding Ig protein; CHOP, C/EBP homologous protein; Ctrl, control; FA, fatty acid; FITC, fluorescein isothiocyanate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; 7K, 7-ketocholesterol; PDI, protein disulfide isomerase; PLIN2, perilipin 2; siASAH1, ASAH1 siRNA; siNC, negative control siRNA.
LD biogenesis takes place in the ER membrane. Next, whether Asah1 gene deficiency enhances ER stress was investigated by analyzing the protein expression of the unfolded protein response signaling regulators, including glucose-regulated protein 78 (GRP78)/Bip and CHOP. As shown in Figure 6, E and F, lipid loading of HepG2 cells with FA + 7K significantly increased the expression of Bip, but not CHOP. Furthermore, Asah1 silencing led to the increased expression of both Bip and CHOP, which were further enhanced in the presence of FA + 7K (Figure 6, E and F). In contrast, the expression of ER-resident proteins calnexin and PDI did not change significantly in all groups (Figure 6, E and G). Interestingly, Asah1 silencing increased the mRNA level of fat storage inducing transmembrane protein 2 (FITM2), a crucial ER protein involved in LD biogenesis (Figure 6H).
Asah1 Deficiency Impairs CMA but Enhances Macroautophagy in Hepatocytes
LAMP2A-mediated CMA and macroautophagy are two crucial pathways mediating LD catabolism and lipolysis. Asah1 silencing significantly reduced the protein expression of LAMP2A in HepG2 cells and aggravated the decrease of LAMP2A induced by FA + 7K (Figure 7, A and B). In contrast, the expression of lysosomal membrane protein LAMP1 and molecular chaperone HSC70 remained unchanged in HepG2 cells under all conditions (Figure 7, A and B). Next, confocal microscopy was used to observe the interaction between LAMP2A and HSC70 in HepG2 cells. As shown in Figure 7C, Asah1 silencing reduced the yellow colocalization puncta under basal conditions, whereas in the presence of FA + 7K, the yellow puncta were almost diminished. These results suggest that Asah1 deficiency down-regulates LAMP2A, thereby weakening its interaction with HSC70. The expression of LAMP2A and its interaction with HSC70 were decreased in Asah1fl/fl/AlbCre mice under ND or PD conditions compared with Asah1fl/fl/WT mice (Figure 7D).
Figure 7.
Asah1 deficiency impairs chaperone-mediated autophagy in hepatocytes. A and B: Representative Western blot images and summarized data. C: Representative immunofluorescence images of lysosome-associated membrane glycoprotein 2A (LAMP2A) and heat shock cognate 71-kDa protein (HSC70) in HepG2 cells. The yellow boxed areas represent the areas of interest. D: Representative immunofluorescence images of LAMP2A and HSC70 of liver sections. E: Relative mRNA abundance determined by real-time quantitative PCR. F–H: Representative Western blot images and summarized data. Data are represented as means ± SEM (B, E, G, and H). n = 4 per group (A–C and F–H); n = 5 to 6 per group (D); n = 3 to 4 per group (E). ∗P < 0.05, ∗∗P < 0.01. Scale bars: 2.5 μm (C); 50 μm (D). ATG, autophagy-related protein; BECN1, beclin-1; Ctrl, control; FA, fatty acid; 7K, 7-ketocholesterol; LC3, microtubule-associated proteins 1A/1B light chain 3C; ND, normal diet; PD, Paigen diet; siASAH1, ASAH1 siRNA; siNC, negative control siRNA; SQSTM1, sequestosome-1.
To examine the effect of Asah1 deficiency on the macroautophagy pathway, the protein and mRNA expression of macroautophagy-related genes were analyzed, including beclin-1 (BECN1), autophagy-related protein 3 (ATG3), ATG5, ATG12, LC3, and SQSTM1/p62, in HepG2 cells. FA + 7K alone did not significantly alter the mRNA levels of these macroautophagy-related genes despite an observed downward trend (Figure 7E). However, Asah1 deficiency significantly up-regulated the mRNA levels of autophagosome biogenesis genes, including BECN1, ATG3, ATG5, ATG12, and LC3, under both the basal condition or on FA + 7K treatment (Figure 7E). In contrast, no change was observed in the mRNA of SQSTM1/p62, which encodes an autophagy receptor. Consistently, FA + 7K alone did not affect the protein expressions of LC3-II or SQSTM1/p62, whereas Asah1 silencing increased LC3-II and reduced SQSTM1/p62 (Figure 7, F–H). In addition, when the autophagic flux was blocked by the lysosome inhibitor chloroquine, LC3-II and SQSTM1 were accumulated (Figure 7, F–H). In the presence of chloroquine (5 μmol/L), Asah1 silencing further increased the accumulation of LC3-II (Figure 7, F–H). These results suggest that Asah1 deficiency increases macroautophagy induction in hepatocytes rather than decreasing autophagic flux.
Discussion
The current study investigated the role of acid ceramidase in lipid homeostasis and the progression of NAFLD to fibrotic NASH. Hepatic steatosis, inflammation, and cell death were significantly enhanced in Asah1fl/fl/AlbCre mice treated with PD compared with Asah1fl/fl/WT mice. These exacerbated pathologic changes in the liver were accompanied by a transition from mild nonfibrotic NASH to severe fibrotic NASH, characterized by extensive extracellular matrix deposition and increased intrahepatic resistance in Asah1fl/fl/AlbCre mice treated with PD. Furthermore, the down-regulation of acid ceramidase activity by Asah1 gene silencing perturbed lipid homeostasis, especially lipophagy, by impairing LAMP2A-dependent chaperone-mediated autophagy. These findings provide new insights into the critical role of Asah1 gene in preventing hepatic lipotoxicity and progression to fibrotic NASH.
Genetic mutations in Asah1 gene cause Farber disease, a rare lysosomal storage disorder characterized by hepatomegaly, ascites, liver inflammation and fibrosis, or liver failure.23,24,34 In addition, Asah1P361R/P361R mice, which carry loss-of-function mutations in the Asah1 gene, exhibit liver injury, leukocyte infiltration, apoptosis, and progressive tissue fibrosis.24 Although these studies have established a clear link between the Asah1 gene and liver disease, the cell-specific role of Asah1 in hepatic lipotoxicity, liver damage, and progression of NAFLD remains largely unknown. To address this question, we recently crossed Asah1 floxed mice with albumin-Cre mice to generate hepatocyte-specific knockout mice (Asah1fl/fl/AlbCre).25 Characterization of these Asah1fl/fl/AlbCre mice revealed that the absence of acid ceramidase leads to the accumulation of lysosomal ceramides and increased steatosis in the liver.25 In the present study, these mice were used and treated with PD to induce a murine NAFLD model, with the aim of investigating whether hepatocyte-specific deletion of Asah1 promotes the progression of NAFLD to fibrotic NASH. Liver pathologies of PD-treated Asah1fl/fl/AlbCre and Asah1fl/fl/WT mice were characterized and compared, including liver index, transaminase activities, lipid contents, inflammation, and fibrosis. As expected, PD treatment significantly increased liver index, transaminase activity, lipid content, and apoptosis in the liver of Asah1fl/fl/WT mice, indicating liver enlargement and injury, which were further aggravated in Asah1fl/fl/AlbCre mice. Furthermore, Asah1fl/fl/WT mice on PD had increased CD45+ immune cell infiltration and expression of inflammatory genes, including ADGRE1 (F4/80) and IL-18, but not TNF-α or CCL2, whereas Asah1fl/fl/AlbCre mice had increased CD45+ cells in the liver and increased all these inflammatory genes. Importantly, Asah1fl/fl/WT mice did not show a significant increase in extracellular matrix protein (collagen I) deposition or related gene expression on PD, whereas Asah1fl/fl/AlbCre mice showed the opposite effect. Therefore, these results indicate that PD induced steatosis with mild inflammation in Asah1fl/fl/WT mice, whereas PD induced severe liver pathology, particularly with aggravated inflammation and the induction of fibrosis, in Asah1fl/fl/AlbCre mice.
Previous studies have demonstrated that hepatitis and cell death are highly correlated with NAFLD progression. For example, CD45+ immune cell infiltration is clinically associated with NAFLD severity.35 Infiltration of F4/80 (encoded by ADGRE1)–positive monocytes differentiates into macrophages and fosters progression from NAFLD to fibrotic NASH.36 In recent years, the NLRP3 inflammasome has emerged as a crucial caspase-1 activation platform, highly expressed in macrophages, hepatocytes, and hepatic stellate cells, and participate in liver injury, hepatitis, and fibrosis by mediating the secretion of proinflammatory cytokines.37 It has been reported that NLRP3 inflammasome-dependent IL-18, but not IL-1β maturation, serves as a potential modulator of early liver injury of NAFLD.38 Consistently, IL-18 expression was markedly induced in the liver of Asah1fl/fl/AlbCre mice, whereas the expression of IL-1β was low. During NASH progression, the NLRP3 inflammasome is a major cause of multiple types of cell death, such as pyroptosis, apoptosis, and necroptosis.37 TNF-α is another important cytokine secreted by activated liver-infiltrating immune cells and lipotoxic hepatocytes. TNF-α triggers an intense immune response by mediating the release of various proinflammatory mediators, such as IL-18, IL-6, CCL2, and CCL5. Additionally, it promotes the migration of monocytes, neutrophils, and lymphocytes to the liver by inducing intercellular adhesion molecule 1 expression.39,40 Enhanced hepatic TNF-α level also promotes apoptosis via the TNF receptor 1 signaling pathway.39 TNF-α provokes transforming growth factor-β production and thereby stimulates collagen production.39 In addition, TNF-α promotes collagen deposition and extracellular matrix stabilization by enhancing periostin and tissue inhibitor of metalloproteinase 1 (TIMP-1) expression, respectively. In this study, Asah1fl/fl/AlbCre mice exhibited characteristics of more advanced NAFLD or fibrotic NASH compared with mild nonfibrotic NASH in Asah1fl/fl/WT mice. Hepatocyte ballooning occurs due to a specific form of apoptosis that distinguishes NASH from simple steatosis.41 Herein, Asah1fl/fl/AlbCre mice had exacerbated hepatocyte ballooning compared with Asah1fl/fl/WT mice. Furthermore, Asah1fl/fl/AlbCre mice had increased expression of NLRP3 inflammasome (NLRP3 and casapase-1), TNF-α, or CCL2, which are implicated in fibrotic NASH but were not observed in Asah1fl/fl/WT mice. Therefore, these findings support the view that hepatocyte-specific deletion of Asah1 promotes inflammatory cell death and fibrosis in the liver, leading to the transition from mild, nonfibrotic NASH to fibrotic NASH under lipotoxic conditions.
During the progression of NAFLD, portal blood flow may be obstructed by increased intrahepatic resistance, which could be multifactorial, depending on the stage of NAFLD.42,43 In simple NAFLD (steatosis) or nonfibrotic NASH, lipid accumulation results in hepatocyte swelling, and endothelial dysfunction leads to sinusoidal contraction, both contributing to increased intrahepatic resistance. In advanced NAFLD, diffuse destruction and regeneration of liver parenchyma lead to fibrosis and distortion of liver architecture, which may obstruct and further increase resistance to portal blood flow. Previous studies have shown that portal venous velocity is inversely correlated with steatosis and fibrosis severity in patients with NAFLD.42,43 Early-stage liver fibrosis in patients with chronic hepatitis can be predicted by abnormal portal venous Vmax.44 Consistently, the present study, for the first time, demonstrated that PD induced a significant decrease in portal venous Vmax, Vmin, and Vmean in Asah1fl/fl/WT mice, whereas these hemodynamic abnormalities were exacerbated in Asah1fl/fl/AlbCre mice. Portal venous PI has also been reported as a predictor for fibrosis in patients with NAFLD.45 However, no significant difference in PI between all groups was observed. Nonetheless, the present data suggest that hepatocyte-specific Asah1 deficiency promotes liver fibrosis and thereby exacerbates portal blood flow reduction.
Ceramide accumulation contributes to both NAFLD and alcoholic liver disease in various models.46,47 Ceramide levels are elevated in the liver tissue from patients with Farber disease,24 loss-of-function mutant Asah1P361R/P361R mice,24 or Asah1fl/fl/AlbCre mice.25 These previous studies by others and this study suggest that the absence of acid ceramidase in hepatocytes leads to lysosomal ceramide accumulation and, in turn, to lipid accumulation and NAFLD in the mouse liver. However, the role of Asah1 in various pathways related to lipid homeostasis remains undefined. In the present study, results showed that Asah1fl/fl/AlbCre mice had higher steatosis than Asah1fl/fl/WT mice, which was associated with enhanced TC accumulation, but TG and nonesterified fatty acid levels remained unaffected. This phenomenon was further confirmed in cultured HepG2 hepatocytes by showing that Asah1 silencing enhanced ceramide production, accumulation of neutral lipids, and TC and free cholesterol levels, but not TG levels. Therefore, these results indicate that Asah1 deficiency enhances the accumulation of neutral lipids in hepatocytes by increasing intracellular cholesterol levels.
The next aim was to gain insights in understanding how Asah1 deficiency leads to enhanced lipid retention (cholesterol or cholesterol esters) in hepatocytes. The present study showed that Asah1 silencing had broad effects on gene expression related to lipid homeostasis in human HepG2 hepatocytes, with significant up-regulation of genes involved in lipogenesis (SREBF1, SOAT1/2), lipid uptake/transport (CD36, FATP5, and LDLR), and fatty acid oxidation (PPARα, PPARG1, CPT1A, and FGF21), and down-regulation of genes involved in cholesterol synthesis (SREBF2, HMGCR), cholesterol efflux (ABCA1), and lipoprotein biogenesis/secretion (apolipoprotein B, apolipoprotein E, and TM6SF2). Among them, SREBF1 is a crucial transcription factor that mediates de novo fatty acid synthesis. CD36 and FATP5 are two pivotal fatty acid transporters, and LDLR is the major receptor for cholesterol uptake. SOAT1/2 are two acyltransferases that convert cholesterol into CEs. ABCA1 is a key cholesterol transporter that mediates the efflux of unesterified cholesterol. Up-regulation of these genes by Asah1 silencing suggests that Asah1 deficiency enhances cholesterol uptake and retention, favoring the synthesis of CEs. Increased intracellular cholesterol levels inhibit SREBF2 signaling and cholesterol de novo biosynthesis via HMGCR.48 Therefore, the observed reductions in SREBF2 or HMGCR may be a consequence of enhanced cholesterol retention. Notably, the expression of genes for TG synthesis (DGAT1/2) and hydrolysis (ATGL) was not changed in HepG2 cells. These data are consistent with the finding that Asah1 deficiency had no effect on TGs in cultured HepG2 hepatocytes and in the liver of Asah1fl/fl/AlbCre mice. The up-regulation of fatty acid oxidation genes suggests that Asah1 deficiency increases fatty acid consumption in hepatocytes, which is likely an adaptive response to excessive fatty acid accumulation. In hepatocytes, TGs and CEs are packaged into VLDL with apolipoproteins (apolipoprotein B, apolipoprotein E) and released from liver.49 TM6SF2 plays a critical role in VLDL lipidation and VLDL secretion in the ER, and its deficiency promotes hepatic steatosis and fibrosis.50 Therefore, the down-regulation of these genes suggests that Asah1 deficiency impairs the assembly and release of VLDL, thereby favoring retention of lipids in cytoplasmic LDs. The mechanism of how acid ceramidase and the ceramide pathway specifically regulate cholesterol-related lipid homeostasis pathways remains unclear and deserves further investigation in future studies.
Gene expression data in the liver of Asah1fl/fl/AlbCre mice further support the view that Asah1 gene deficiency increases cholesterol retention and CE accumulation in hepatocytes in vivo. First, Asah1 gene deficiency decreases cholesterol efflux-related genes, including apolipoprotein A1, ABCA1, and neutral cholesterol ester hydrolase. ABCA1 and ABCG1 are mutually complementary, and the deficiency of ABCA1 can induce compensatory induction of ABCG1,51 so the increase in ABCG1 may be attributed to the compensatory up-regulation caused by reduced ABCA1. Second, Asah1 gene deficiency decreases hepatic bile acid synthesis-related genes, including CYP7A1, CYP2C70, and ABCB11, indicating that reduced hepatic bile acid synthesis leads to hepatic cholesterol retention. Third, Asah1 gene deficiency does not increase SREBF2 or HMGCR in mouse livers, indicating that cholesterol de novo biosynthesis does not contribute to increased intracellular cholesterol levels. Fourth, Asah1 gene deficiency increases SREBF1, LDLR, CD36, and FASN1 levels in mouse livers, indicating that fatty acid synthesis and uptake are up-regulated in favor of CE synthesis. Finally, Asah1 gene deficiency showed no effects on LPL and ATGL in mouse livers, suggesting that TG metabolism is not affected.
The major lipid storage organelle in hepatocytes is the cytosolic LD, where lipids (including FA, cholesterol, and ceramide) are stored in the form of TG, CE, and acylceramide, respectively. Excessive LD deposition is associated with increased lipid accumulation and NAFLD progression.52 In hepatocytes, LD accumulation is determined by the dynamic balance between LD biogenesis and its catabolism. PLIN2 is an LD coating protein, and increased PLIN2 expression has been reported to promote LD accumulation.53 FITM2 is a crucial ER membrane protein that does not regulate the synthesis of lipids but rather segregates them into cytosolic LDs, so that increased FITM2 levels favor the formation of the cytosolic LD rather than VLDLs within the ER lumen.54 The role of Asah1 in LD biogenesis is unclear. ER stress triggers LD biogenesis under lipotoxic conditions to maintain cellular lipid homeostasis.17 Because ceramide and free cholesterol have been shown to be inducers of ER stress, increased LD formation is often accompanied by elevated ER stress.55,56 Furthermore, ER stress has been implicated in systemic inflammation and hepatic fibrosis during the progression of NAFLD.57 In the present study, Asah1 silencing resulted in increased LD size and number, PLIN2 and FITM2 expression, and ER stress in HepG2 cells. Therefore, these results suggest that Asah1 deficiency leads to aggravated lipotoxicity and elevated ceramide and cholesterol levels, triggering ER stress and LD formation in hepatocytes.
The present study further revealed that Asah1 deficiency also affects CMA and macroautophagy, two important pathways for LD catabolism and lysosomal lipolysis (also known as lipophagy). LD catabolism or lipophagy is initiated with the degradation of LD membrane-coating proteins, such as PLIN2, a substrate of CMA.12,18 The molecular chaperone protein HSC70 binds to PLIN2 and delivers it to lysosomes for degradation via LAMP2A. When LDs lose PLIN2, cytoplasmic lipase, including ATGL, can access the lipid core for lipolysis, leading to LD shrinkage. On the contrary, defective LAMP2A expression inhibits the degradation of PLIN2, thereby inhibiting lipolysis and causing LD accumulation.58,59 In addition to CMA, macroautophagy sequesters small- or medium-sized LDs into autophagosomes, which then fuse with lysosomes and are degraded by lysosomal acid lipase.5,19 Macroautophagy regulates LD degradation under both basal conditions and lipogenic stimulation, and pharmacologic inhibition of macroautophagy is associated with LD accumulation.60 In the present study, Asah1 deficiency down-regulated the expression of LAMP2A and decreased its interaction with HSC70, but increased genes related to macroautophagy. These results indicate that Asah1 deficiency impairs CMA but enhances macroautophagy. Consistent with the present findings, a previous study demonstrated that in CMA-defective cells induced by LAMP2A blockade, macroautophagy was up-regulated, a phenomenon attributed to compensation for CMA function.61 Lipids, such as cholesterol and ceramide, as well as ER stress are known to directly induce macroautophagy.62,63 Therefore, Asah1 deficiency may down-regulate LAMP2A-dependent CMA and impairs lipophagy, a major factor in lipid accumulation, whereas lipotoxicity up-regulates macroautophagy and LD biogenesis as secondary effects. The precise mechanism of how LAMP2A is regulated by acid ceramidase or ceramide is unclear and warrants further investigations.
In summary, the present study demonstrated that hepatocyte-specific Asah1 gene ablation significantly exacerbates PD-induced NASH and leads to severe liver fibrosis. Importantly, it revealed that Asah1 deficiency specifically regulates cholesterol levels and LAMP2A-dependent CMA in hepatocytes. These results provide novel insights into understanding the key role of acid ceramidase in lipid homeostasis and progression of NAFLD to fibrotic NASH in metabolic disorders.
Disclosure Statement
None declared.
Footnotes
Supported by NIH grants R01HL122937 (Y.Z.) and R01HL150007 (X.L.).
Supplemental material for this article can be found at http://doi.org/10.1016/j.ajpath.2024.11.003.
Supplemental Data
Supplemental Figure S1.
Relative acid ceramidase (AC) activity. AC activity in HepG2 cells was determined by measuring the fluorescence change of Bodipy ceramide. n = 4. ∗P < 0.05. Ctrl, control; FA, fatty acid; 7K, 7-ketocholesterol; siASAH1, ASAH1 siRNA; siNC, negative control siRNA.
Supplemental Figure S2.
Differentially expressed genes (DEGs) related to lipid metabolism among different nonalcoholic fatty liver disease (NAFLD) stages in obese patients. A: Heat map of DEGs related to lipid metabolism from the GSE163211 data set (https://www.ncbi.nlm.nih.gov/geo). B: Violin plots of DEGs. Gene counts were normalized to housekeeping genes. n = 76 normal liver biopsy group; n = 88 steatosis group; n = 72 nonalcoholic steatohepatitis (NASH) without fibrosis (NASH F0) group; n = 82 NASH with fibrosis (NASH F1-F4) group. ∗P < 0.05, ∗∗P < 0.01. ApoB, apolipoprotein B; ApoE, apolipoprotein E; FGF21, fibroblast growth factor 21; LDLR, low-density lipoprotein receptor; PLIN2, perilipin 2; PPARA, peroxisome proliferator-activated receptor α; PPARG, peroxisome proliferator-activated receptor γ; SREBF1, sterol regulatory element–binding transcription factor 1; TM6SF2, transmembrane 6 superfamily member 2.
References
- 1.Teng M.L., Ng C.H., Huang D.Q., Chan K.E., Tan D.J., Lim W.H., Yang J.D., Tan E., Muthiah M.D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2023;29:S32–S42. doi: 10.3350/cmh.2022.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pouwels S., Sakran N., Graham Y., Leal A., Pintar T., Yang W., Kassir R., Singhal R., Mahawar K., Ramnarain D. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. 2022;22:63. doi: 10.1186/s12902-022-00980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim K.S., Hong S., Han K., Park C.Y. Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study. BMJ. 2024;384 doi: 10.1136/bmj-2023-076388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuo R., Ye L.F., Huang Y., Song Z.Q., Wang L., Zhi H., Zhang M.Y., Li J.Y., Zhu L., Xiao W.J., Shang H.C., Zhang Y., He R.R., Chen Y. Hepatic small extracellular vesicles promote microvascular endothelial hyperpermeability during NAFLD via novel-miRNA-7. J Nanobiotechnol. 2021;19:396. doi: 10.1186/s12951-021-01137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mashek D.G. Hepatic lipid droplets: a balancing act between energy storage and metabolic dysfunction in NAFLD. Mol Metabol. 2021;50 doi: 10.1016/j.molmet.2020.101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwabe R.F., Tabas I., Pajvani U.B. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology. 2020;158:1913–1928. doi: 10.1053/j.gastro.2019.11.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bataller R., Brenner D.A. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng C.H., Lim W.H., Hui Lim G.E., Hao Tan D.J., Syn N., Muthiah M.D., Huang D.Q., Loomba R. Mortality outcomes by fibrosis stage in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023;21:931–939.e5. doi: 10.1016/j.cgh.2022.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryou M., Stylopoulos N., Baffy G. Nonalcoholic fatty liver disease and portal hypertension. Explor Med. 2020;1:149–169. doi: 10.37349/emed.2020.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagström H., Nasr P., Ekstedt M., Hammar U., Stål P., Hultcrantz R., Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Vilar-Gomez E., Calzadilla-Bertot L., Wai-Sun Wong V., Castellanos M., Aller-de la Fuente R., Metwally M., Eslam M., Gonzalez-Fabian L., Alvarez-Quiñones Sanz M., Conde-Martin A.F., De Boer B., McLeod D., Hung Chan A.W., Chalasani N., George J., Adams L.A., Romero-Gomez M. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155:443–457.e17. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 12.Seebacher F., Zeigerer A., Kory N., Krahmer N. Hepatic lipid droplet homeostasis and fatty liver disease. Semin Cell Dev Biol. 2020;108:72–81. doi: 10.1016/j.semcdb.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Ipsen D.H., Lykkesfeldt J., Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75:3313–3327. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng Y., Faber K.N., de Meijer V.E., Blokzijl H., Moshage H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol Int. 2021;15:21–35. doi: 10.1007/s12072-020-10121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loomba R., Friedman S.L., Shulman G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–2564. doi: 10.1016/j.cell.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulze R.J., McNiven M.A. Lipid droplet formation and lipophagy in fatty liver disease. Semin Liver Dis. 2019;39:283–290. doi: 10.1055/s-0039-1685524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarc E., Petan T. Lipid droplets and the management of cellular stress. Yale J Biol Med. 2019;92:435–452. [PMC free article] [PubMed] [Google Scholar]
- 18.Fader Kaiser C.M., Romano P.S., Vanrell M.C., Pocognoni C.A., Jacob J., Caruso B., Delgui L.R. Biogenesis and breakdown of lipid droplets in pathological conditions. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.826248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu C., Fan J. Links between autophagy and lipid droplet dynamics. J Exp Bot. 2022;73:2848–2858. doi: 10.1093/jxb/erac003. [DOI] [PubMed] [Google Scholar]
- 20.Pagadala M., Kasumov T., McCullough A.J., Zein N.N., Kirwan J.P. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol Metabol. 2012;23:365–371. doi: 10.1016/j.tem.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y., Li X., Becker K.A., Gulbins E. Ceramide-enriched membrane domains--structure and function. Biochim Biophys Acta. 2009;1788:178–183. doi: 10.1016/j.bbamem.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Denimal D., Béland-Bonenfant S., Pais-de-Barros J.P., Rouland A., Bouillet B., Duvillard L., Vergès B., Petit J.M. Plasma ceramides are associated with MRI-based liver fat content but not with noninvasive scores of liver fibrosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2023;22:310. doi: 10.1186/s12933-023-02049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Y., McCorvy J.D., Harpsøe K., Lansu K., Yuan S., Popov P., Qu L., Pu M., Che T., Nikolajsen L.F., Huang X.P., Wu Y., Shen L., Bjørn-Yoshimoto W.E., Ding K., Wacker D., Han G.W., Cheng J., Katritch V., Jensen A.A., Hanson M.A., Zhao S., Gloriam D.E., Roth B.L., Stevens R.C., Liu Z.J. 5-HT(2C) receptor structures reveal the structural basis of GPCR polypharmacology. Cell. 2018;172:719–730.e14. doi: 10.1016/j.cell.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu F.P.S., Molino S., Sikora J., Rasmussen S., Rybova J., Tate E., Geurts A.M., Turner P.V., McKillop W.M., Medin J.A. Hepatic pathology and altered gene transcription in a murine model of acid ceramidase deficiency. Lab Invest. 2019;99:1572–1592. doi: 10.1038/s41374-019-0271-4. [DOI] [PubMed] [Google Scholar]
- 25.Yuan X., Bhat O.M., Zou Y., Zhang Y., Li P.L. Contribution of hepatic steatosis-intensified extracellular vesicle release to aggravated inflammatory endothelial injury in liver-specific Asah1 gene knockout mice. Am J Pathol. 2023;193:493–508. doi: 10.1016/j.ajpath.2022.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subudhi S., Drescher H., Dichtel L., Bartsch L., Chung R., Hutter M., Gee D., Meireles O., Witkowski E., Gelrud L., Masia R., Osganian S., Gustafson J., Rwema S., Bredella M., Bhatia S., Warren A., Miller K., Lauer G., Corey K. Distinct hepatic gene-expression patterns of NAFLD in patients with obesity. Hepatol Commun. 2022;6:77–89. doi: 10.1002/hep4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Committee for the Update of the Guide for the Care and Use of Laboratory Animals. National Research Council . National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals: Eighth Edition. [Google Scholar]
- 28.Wang Y.T., Li X., Chen J., McConnell B.K., Chen L., Li P.L., Chen Y., Zhang Y. Activation of TFEB ameliorates dedifferentiation of arterial smooth muscle cells and neointima formation in mice with high-fat diet. Cell Death Dis. 2019;10:676. doi: 10.1038/s41419-019-1931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolachala V.L., Jiang R., Abramowsky C.R., Gupta N.A. Contrast-based real-time assessment of microcirculatory changes in a fatty liver after ischemia reperfusion injury. J Pediatr Gastroenterol Nutr. 2016;62:429–436. doi: 10.1097/MPG.0000000000001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baikpour M., Ozturk A., Dhyani M., Mercaldo N.D., Pierce T.T., Grajo J.R., Samir A.E. Portal venous pulsatility index: a novel biomarker for diagnosis of high-risk nonalcoholic fatty liver disease. Am J Roentgenol. 2020;214:786–791. doi: 10.2214/AJR.19.21963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Q., Zuo R., Wang K., Nong F.F., Fu Y.J., Huang S.W., Pan Z.F., Zhang Y., Luo X., Deng X.L., Zhang X.X., Zhou L., Chen Y. Oroxindin inhibits macrophage NLRP3 inflammasome activation in DSS-induced ulcerative colitis in mice via suppressing TXNIP-dependent NF-κB pathway. Acta Pharmacol Sin. 2020;41:771–781. doi: 10.1038/s41401-019-0335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y.T., Chen J., Li X., Umetani M., Chen Y., Li P.L., Zhang Y. Contribution of transcription factor EB to adipoRon-induced inhibition of arterial smooth muscle cell proliferation and migration. Am J Physiol Cell Physiol. 2019;317:C1034–C1047. doi: 10.1152/ajpcell.00294.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hajduch E., Lachkar F., Ferré P., Foufelle F. Roles of ceramides in non-alcoholic fatty liver disease. J Clin Med. 2021;10:792. doi: 10.3390/jcm10040792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehlert K., Frosch M., Fehse N., Zander A., Roth J., Vormoor J. Farber disease: clinical presentation, pathogenesis and a new approach to treatment. Pediatr Rheumatol Online J. 2007;5:15. doi: 10.1186/1546-0096-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwenger K.J.P., Chen L., Chelliah A., Da Silva H.E., Teterina A., Comelli E.M., Taibi A., Arendt B.M., Fischer S., Allard J.P. Markers of activated inflammatory cells are associated with disease severity and intestinal microbiota in adults with non-alcoholic fatty liver disease. Int J Mol Med. 2018;42:2229–2237. doi: 10.3892/ijmm.2018.3800. [DOI] [PubMed] [Google Scholar]
- 36.Alabdulaali B., Al-Rashed F., Al-Onaizi M., Kandari A., Razafiarison J., Tonui D., Williams M.R., Blériot C., Ahmad R., Alzaid F. Macrophages and the development and progression of non-alcoholic fatty liver disease. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1195699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufmann B., Kui L., Reca A., Leszczynska A., Kim A.D., Booshehri L.M., Wree A., Friess H., Hartmann D., Broderick L., Hoffman H.M., Feldstein A.E. Cell-specific deletion of NLRP3 inflammasome identifies myeloid cells as key drivers of liver inflammation and fibrosis in murine steatohepatitis. Cell Mol Gastroenterol Hepatol. 2022;14:751–767. doi: 10.1016/j.jcmgh.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hohenester S., Kanitz V., Schiergens T., Einer C., Nagel J., Wimmer R., Reiter F.P., Gerbes A.L., De Toni E.N., Bauer C., Holdt L., Mayr D., Rust C., Schnurr M., Zischka H., Geier A., Denk G. IL-18 but not IL-1 signaling is pivotal for the initiation of liver injury in murine non-alcoholic fatty liver disease. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21228602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vachliotis I.D., Polyzos S.A. The role of tumor necrosis factor-alpha in the pathogenesis and treatment of nonalcoholic fatty liver disease. Curr Obes Rep. 2023;12:191–206. doi: 10.1007/s13679-023-00519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagata N., Chen G., Xu L., Ando H. An update on the chemokine system in the development of NAFLD. Medicina. 2022;58:761. doi: 10.3390/medicina58060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singla T., Muneshwar K.N., Pathade A.G., Yelne S. Hepatocytic ballooning in non-alcoholic steatohepatitis: bridging the knowledge gap and charting future avenues. Cureus. 2023;15 doi: 10.7759/cureus.45884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulusan S., Yakar T., Koc Z. Evaluation of portal venous velocity with Doppler ultrasound in patients with nonalcoholic fatty liver disease. Korean J Radiol. 2011;12:450–455. doi: 10.3348/kjr.2011.12.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nababan S.H.H., Lesmana C.R.A. Portal hypertension in nonalcoholic fatty liver disease: from pathogenesis to clinical practice. J Clin Translat Hepatol. 2022;10:979–985. doi: 10.14218/JCTH.2021.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamaguchi K., Seko Y., Sakai T., Kitano S., Okabe H., Kataoka S., Moriguchi M., Umemura A., Itoh Y. Comparison of portal vein hemodynamics with ultrasound-based elastography for the prediction of liver fibrosis in patients with chronic liver disease. Sci Rep. 2023;13:3425. doi: 10.1038/s41598-023-30279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu S., Archard R., McLeod L., Banh A., Con D., Ardalan Z., Kutaiba N. Portal venous pulsatility index as a predictor of fibrosis in patients with non-alcoholic fatty liver disease. Australas J Ultrasound Med. 2022;25:36–41. doi: 10.1002/ajum.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kasumov T., Li L., Li M., Gulshan K., Kirwan J.P., Liu X., Previs S., Willard B., Smith J.D., McCullough A. Ceramide as a mediator of non-alcoholic fatty liver disease and associated atherosclerosis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Correnti J.M., Juskeviciute E., Swarup A., Hoek J.B. Pharmacological ceramide reduction alleviates alcohol-induced steatosis and hepatomegaly in adiponectin knockout mice. Am J Physiol Gastrointest Liver Physiol. 2014;306:G959–G973. doi: 10.1152/ajpgi.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L., Ma M.Y., Sun M., Jiang L.Y., Zhao X.T., Fang X.X., Man Lam S., Shui G.H., Luo J., Shi X.J., Song B.L. Endogenous sterol intermediates of the mevalonate pathway regulate HMGCR degradation and SREBP-2 processing. J Lipid Res. 2019;60:1765–1775. doi: 10.1194/jlr.RA119000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venugopal S.K., Anoruo M., Jialal I. In StatPearls [Internet] StatPearls Publishing; Treasure Island, FL: 2024. Biochemistry, Low Density Lipoprotein.https://www.ncbi.nlm.nih.gov/books/NBK500010 Available at: (last updated April 17, 2023) [Google Scholar]
- 50.Newberry E.P., Hall Z., Xie Y., Molitor E.A., Bayguinov P.O., Strout G.W., Fitzpatrick J.A.J., Brunt E.M., Griffin J.L., Davidson N.O. Liver-specific deletion of mouse Tm6sf2 promotes steatosis, fibrosis, and hepatocellular cancer. Hepatology. 2021;74:1203–1219. doi: 10.1002/hep.31771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yvan-Charvet L., Ranalletta M., Wang N., Han S., Terasaka N., Li R., Welch C., Tall A.R. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X., Zhang K. Endoplasmic reticulum stress-associated lipid droplet formation and type II diabetes. Biochem Res Int. 2012;2012 doi: 10.1155/2012/247275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Itabe H., Yamaguchi T., Nimura S., Sasabe N. Perilipins: a diversity of intracellular lipid droplet proteins. Lipids Health Dis. 2017;16:83. doi: 10.1186/s12944-017-0473-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kadereit B., Kumar P., Wang W.J., Miranda D., Snapp E.L., Severina N., Torregroza I., Evans T., Silver D.L. Evolutionarily conserved gene family important for fat storage. Proc Natl Acad Sci U S A. 2008;105:94–99. doi: 10.1073/pnas.0708579105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de la Monte S.M. Triangulated mal-signaling in Alzheimer's disease: roles of neurotoxic ceramides, ER stress, and insulin resistance reviewed. J Alzheim Dis. 2012;30 Suppl 2:S231–S249. doi: 10.3233/JAD-2012-111727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sozen E., Ozer N.K. Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: an updated mini-review. Redox Biol. 2017;12:456–461. doi: 10.1016/j.redox.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maiers J.L., Malhi H. Endoplasmic reticulum stress in metabolic liver diseases and hepatic fibrosis. Semin Liver Dis. 2019;39:235–248. doi: 10.1055/s-0039-1681032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiao L., Wang H.F., Xiang L., Ma J., Zhu Q., Xu D., Zheng H., Peng J.Q., Zhang S., Lu H.X., Chen W.Q., Zhang Y. Deficient chaperone-mediated autophagy promotes lipid accumulation in macrophage. J Cardiovasc Translat Res. 2021;14:661–669. doi: 10.1007/s12265-020-09986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma S.Y., Sun K.S., Zhang M., Zhou X., Zheng X.H., Tian S.Y., Liu Y.S., Chen L., Gao X., Ye J., Zhou X.M., Wang J.B., Han Y. Disruption of Plin5 degradation by CMA causes lipid homeostasis imbalance in NAFLD. Liver Int. 2020;40:2427–2438. doi: 10.1111/liv.14492. [DOI] [PubMed] [Google Scholar]
- 60.Singh R. Autophagy and regulation of lipid metabolism. Results Probl Cell Differ. 2010;52:35–46. doi: 10.1007/978-3-642-14426-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Massey A.C., Kaushik S., Sovak G., Kiffin R., Cuervo A.M. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci U S A. 2006;103:5805–5810. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li K., Deng Y., Deng G., Chen P., Wang Y., Wu H., Ji Z., Yao Z., Zhang X., Yu B., Zhang K. High cholesterol induces apoptosis and autophagy through the ROS-activated AKT/FOXO1 pathway in tendon-derived stem cells. Stem Cell Res Ther. 2020;11:131. doi: 10.1186/s13287-020-01643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pattingre S., Bauvy C., Levade T., Levine B., Codogno P. Ceramide-induced autophagy: to junk or to protect cells? Autophagy. 2009;5:558–560. doi: 10.4161/auto.5.4.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]