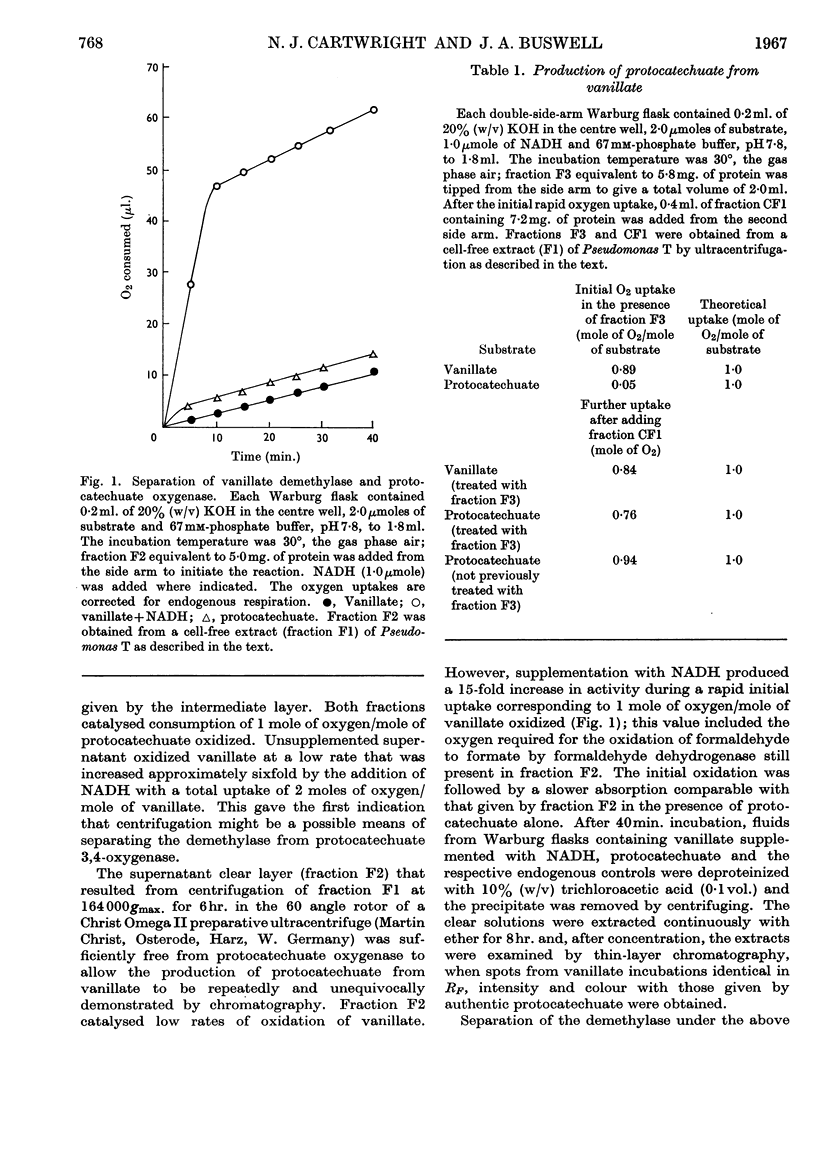
Abstract
1. Protocatechuate 3,4-oxygenase in the soluble part of a cell-free extract of Pseudomonas fluorescens (strain T) sedimented more rapidly than vanillate O-demethylase under specified conditions in a preparative ultracentrifuge. 2. The supernatant from this process contained vanillate O-demethylase and formaldehyde dehydrogenase, and when supplemented with NADH oxidized vanillate with an uptake of 1 mole of oxygen/mole of substrate and accumulation of protocatechuate. 3. This uptake was decreased to 0·5mole/mole of substrate in the presence of semicarbazide as trapping agent for formaldehyde. 4. Reasons are presented for the process of methyl group removal from vanillate being oxidative demethylation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cartwright N. J., Smith A. R. Bacterial attack on phenolic ethers: An enzyme system demethylating vanillic acid. Biochem J. 1967 Mar;102(3):826–841. doi: 10.1042/bj1020826. [DOI] [PMC free article] [PubMed] [Google Scholar]


