Abstract
Background
Obesity is a global health issue which has been widely accepted as an aging related pathogenesis. α-Klotho is a protein involved in aging process, mineral metabolism, insulin sensitivity, and the pathogenesis of various age-related diseases. Adiposity correlates with lower soluble α-Klotho, but the role of fat distribution and inflammation remains unclear. The body roundness index (BRI) refines central adiposity assessment beyond BMI. Herein, We aimed to investigate the relationship of BRI, inflammation and serum level of soluble α-Klotho.
Methods
We conducted a cross-sectional analysis of 9,958 U.S. adults (40–79 years) from the 2007–2016 NHANES. We examined association between BRI and serum α-Klotho (SαKl) levels, controlling for demographic, socioeconomic, lifestyle, and clinical factors. We also assessed whether inflammatory markers mediated the BRI–SαKl relationship.
Results
BRI was inversely associated with SαKl levels (P < 0.05). A significant sex interaction was found (P < 0.001), while BRI was positively correlated with multiple proinflammatory markers, which were all inversely related to SαKl levels. Mediation analyses showed inflammatory markers accounted for 20.5% (WBC), 18.0% (neutrophils), and 12.3% (platelets) of the BRI–SαKl association.
Conclusion
More severe central adiposity measured by BRI was related to lower SαKl, which may partly be attributed to inflammation. These findings underscore the importance of fat distribution and inflammation in obesity-related aging and may guide interventions to preserve SαKl levels. Longitudinal studies are needed to confirm causality and inform future strategies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-025-02541-6.
Keywords: α−Klotho, Aging, Obesity, Body roundness index, NHANES
Introduction
Obesity is a global health concern linked to a myriad of chronic conditions, including cardiovascular disease (CVD), diabetes, chronic low-grade inflammation, and accelerated aging [1]. Traditional measures of adiposity, such as Body Mass Index (BMI), often fail to capture body fat distribution and overall metabolic risk [2]. The Body Roundness Index (BRI), developed to incorporate waist circumference into an anthropometric assessment of body shape, provides a more refined estimation of central adiposity and better reflects metabolic risk than BMI alone [3–5]. Moreover, growing evidence suggests a strong association between BRI and inflammatory markers, including C-reactive protein and interleukin-6, implying that greater body roundness may drive systemic inflammation [6].
α-Klotho (αKl), a protein involved in aging process and aging-associated diseases. It is predominantly expressed in the kidneys and brain. It plays a pivotal role in mineral metabolism and insulin sensitivity [7], thereby positioning itself as an important biomarker for aging-related health issues. Reduced levels of soluble αKl are associated with several aging-related diseases, such as chronic kidney disease (CKD) [8], osteoporosis [9], atherosclerosis [10], and cognitive decline [11], highlighting its broad clinical relevance. Moreover, αKl is integral to energy balance and glucose homeostasis, influencing processes such as insulin secretion, β-cell health, lipid oxidation in adipose tissue, and hepatic gluconeogenesis [12]. The protein's ability to modulate these processes underscores its potential as a biomarker for metabolic health and its utility in clinical settings. Inflammatory conditions, which downregulate αKl expression, further complicate the pathophysiology of age-related disorders [13], with elevated levels of inflammatory cytokines, like interleukin-6 and TNF-α (tumor necrosis factor-alpha) being inversely related to soluble αKl levels [14, 15]. Notably, central adiposity—a marker of metabolic dysfunction, inflammation, and oxidative stress— has been found to suppressed αKl expression [16, 17].
Given αKl extensive involvement in both aging and metabolic regulation, αKl serves not only as a key indicator of biological aging but also as a potential therapeutic target for managing age-related health conditions, making it a critical focus for public health and clinical research. This broader understanding of αKl 's roles enriches our investigation into body composition metrics beyond the conventional use of BMI. While previous research has predominantly utilized BMI, it may not adequately capture the nuances of fat distribution, inflammation, and metabolic regulation. Our study introduces the BRI as a more precise and accessible metric for assessing central adiposity. Despite the recognized importance of both BRI and αKl in health and disease, whether inflammatory pathways play a vital role in their association remains insufficiently understood. We hypothesize that systemic inflammation may mediate the relationship between BRI and αKl levels. This hypothesis is based on the well-established role of inflammation in linking adiposity to aging-related outcomes [18], as well as the known suppressive effects of pro-inflammatory cytokines on αKl expression [19]. Clarifying the relationship between BRI and αKl may offer deeper insights into the mechanisms underlying obesity-related aging and inform more precise intervention strategies.
In this cross-sectional study, we analyzed data from the National Health and Nutrition Examination Survey (NHANES) to evaluate the relationship between BRI and serum αKl (SαKl) levels. We also assessed the mediating role of inflammatory markers in this association. Our findings aim to elucidate the potential impact of body roundness on aging biomarkers, thereby contributing to a broader understanding of metabolic health and longevity.
Methods
This cross-sectional study used data from the NHANES, a program conducted by the U.S. Centers for Disease Control and Prevention (CDC) that employs a stratified, multistage probability sampling design to obtain a representative sample of the U.S. civilian, non-institutionalized population. This NHANES-based research was approved by the National Center for Health Statistics ethics review board, with all participants providing written informed consent. The study adhered to the Declaration of Helsinki and complied with STROBE guidelines for observational studies.
Study participants
Data from five consecutive NHANES cycles (2007–2016) were included, as SαKl measurements were available exclusively for participants aged 40–79 years during this period. From an initial pool of 50,588 participants, individuals not meeting eligibility criteria were sequentially removed. Overall, 31,244 did not fall within the 40–79-year age bracket; 5,580 had missing SαKl data; 587 were missing BRI measurements; 8 were pregnant; 1,530 had a history of cancer; 54 lacked data on inflammatory markers; and 1,627 had missing information for other key variables. Ultimately, 9,958 participants remained in the final sample (Fig. S1).
BRI definition
BRI was calculated according to the formula developed by Thomas et al. [3], which incorporates waist circumference and height to better estimate body shape and visceral fat distribution:
Waist circumference and height were measured by trained examiners at mobile examination centers following standardized NHANES protocols. Standing height was measured using a calibrated stadiometer, and waist circumference was measured at the level of the iliac crest using a flexible tape measure.
SαKl level
SαKl levels were measured in serum samples collected from participants aged 40–79 years at mobile examination centers, following standardized protocols. Samples were processed, stored at −80 °C, and subsequently shipped to the Northwest Lipid Metabolism and Diabetes Research Laboratories (University of Washington) for analysis between 2019 and 2020, adhering to strict quality control measures [20].
SαKl concentrations were determined using a commercial enzyme-linked immunosorbent assay kit (IBL International, Japan), with all procedures adhering to the manufacturer's instructions. Each sample was analyzed in duplicate to ensure reliability. The average value was used for the final analysis.
Inflammatory markers
Inflammatory markers were derived from complete blood count data obtained during NHANES physical examinations. Trained phlebotomists drew blood samples using standardized protocols, which were then stored and analyzed in CDC-certified laboratories to ensure accuracy and reliability.
The inflammatory markers included in our study—neutrophil count, lymphocyte count, platelet count, monocyte count, white blood cell (WBC) count, systemic immune inflammation index (SII), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) —were selected based on their established roles in assessing systemic inflammation. These markers have been widely used in previous research to evaluate immune responses in chronic diseases, and aging [21–23]. SII was calculated as (platelet count × neutrophil count) / lymphocyte count. It provides a comprehensive measure of systemic inflammation by reflecting the balance between pro-inflammatory immune responses (neutrophils and platelets) and anti-inflammatory (lymphocytes) responses. NLR was computed as the ratio of the absolute neutrophil count to the absolute lymphocyte count, PLR as the ratio of the absolute platelet count to the absolute lymphocyte count, and LMR as the ratio of the absolute lymphocyte count to the absolute monocyte count.
Covariates
Potential confounders included age; race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and Others); marital status (married/living with a partner or living alone), poverty income ratio (PIR) (< 1.30, 1.30 − 2.99, and ≥ 3.00); education level (less than high school, high school/ GED, or above high school); and physical activity level (inactive, moderate, or vigorous). Alcohol intake was categorized as never, former, light-to-moderate, or heavy drinker according to established criteria [24], and smoking status was classified as never, former, or current smoker according to published guidelines [25].
Comorbidities, including diabetes, hypertension, CKD, and CVD, were also taken into consideration. Diabetes and hypertension were identified according to established definitions [26], while CKD and CVD were determined based on self-reported physician diagnoses.
Statistical analysis
This study utilized 10 years (2007–2016) of NHANES data, with survey weights applied according to NHANES guidelines to ensure national representativeness. Baseline characteristics were summarized using weighted means ± standard errors (SE) for continuous variables and weighted percentages (95% confidence intervals [CI]) for categorical variables. Group differences were assessed using weighted linear regression for continuous variables and the chi-square test for categorical variables. Multivariate linear regression examined the relationship between BRI, inflammatory markers, and SαKl levels.
Three multivariate models were developed. Model 1 was unadjusted. Model 2 adjusted for age, gender, and race/ethnicity. Model 3 included additional adjustments for marital status, PIR, education level, smoking status, alcohol consumption status, physical activity, diabetes, hypertension, CKD, and CVD. BRI was analyzed both as a continuous and categorical variable, with BRI values divided into quartiles (Q1 to Q4) based on population distribution to capture both linear and non-linear associations with SαKl levels. Stratified analyses were also performed to explore the relationship between the BRI and SαKl in subgroups defined by age (< / ≥ 60 years), gender, and comorbidities.
A mediation analysis was conducted to investigate whether inflammatory markers mediated the association between BRI and SαKl level using Model 3. The mediation effect was quantified as the proportion of the indirect effect (path a*b) to the total effect, and its significance was assessed using bootstrapping (5,000 iterations) to obtain the bias-corrected 95% CIs. The mediation was considered significant if the 95% CI did not include zero.
Sensitivity analyses were conducted to ensure the robustness and validity of our findings. To assess potential selection bias, we summarized the characteristics of participants excluded due to missing data and compared them with those included in the study. Furthermore, we standardized the BRI using Z-score transformation and subjected the data to three multivariate models to confirm the stability and reliability. Additionally, we excluded participants with an estimated glomerular filtration rate (eGFR) less than 60 mL/min, calculated using the CKD-EPI formula. This exclusion was based on the premise that severe kidney impairment might confound the relationship between BRI and SαKl levels.
A two-tailed P < 0.05 was considered statistically significant. All analyses were performed using R (version 4.3.2) and Free Statistics software (version 1.9). Survey weighting and multivariate regression were operated with the “survey” package, mediation analysis was operated with the “mediation” package.
Results
Characteristics
The study included 9,958 participants aged 40–79 years, representing an estimated 82,122,431 individuals from the 2007–2016 cycle (weighted proportions: 51.22% female; mean age: 55.1 years). Table 1 summarizes the weighted population characteristics across BRI quartiles (Q1: 3.43 ± 0.02; Q2: 4.88 ± 0.01; Q3: 6.17 ± 0.01; Q4: 8.89 ± 0.05). Among all participants, the mean BRI was 5.69 ± 0.04, and the mean SαKl level was 848.98 ± 5.41 pg/mL. The racial/ethnic distribution was 6.89% Mexican American, 4.60% other Hispanic, 72.85% Non-Hispanic White, 9.41% Non-Hispanic Black, and 6.25% from other racial individuals.
Table 1.
Baseline characteristics of the participants in NHANES, 2007 to 2016
| Characteristic | Total (n = 9958) | Q1 (n = 2490) | Q2 (n = 2489) | Q3 (n = 2489) | Q4 (n = 2490) | P |
|---|---|---|---|---|---|---|
| α-klotho (pg/mL) | 848.98 ± 5.41 | 870.68 ± 8.97 | 851.03 ± 10.00 | 831.46 ± 7.52 | 838.09 ± 7.47 | 0.003 |
| Age (years) | 55.07 ± 0.16 | 52.55 ± 0.27 | 55.03 ± 0.25 | 56.45 ± 0.29 | 56.79 ± 0.26 | < 0.001 |
| BRI | 5.69 ± 0.04 | 3.43 ± 0.02 | 4.88 ± 0.01 | 6.17 ± 0.01 | 8.89 ± 0.05 | < 0.001 |
| Gender (%) | < 0.001 | |||||
| Female | 51.22 (47.28,55.17) | 51.85 (49.18,54.51) | 44.51 (41.75,47.28) | 48.12 (45.91,50.32) | 61.39 (58.74,64.04) | |
| Male | 48.78 (44.98,52.57) | 48.15 (45.49,50.82) | 55.49 (52.72,58.25) | 51.88 (49.68,54.09) | 38.61 (35.96,41.26) | |
| Race/ethnicity (%) | < 0.001 | |||||
| Mexican American | 6.89 (5.47, 8.32) | 3.36 (2.58, 4.13) | 6.75 (5.08, 8.42) | 9.63 (7.49,11.76) | 8.57 (6.11,11.03) | |
| Other Hispanic | 4.60 (3.59, 5.62) | 3.53 (2.60,4.46) | 4.45 (3.23,5.66) | 5.60 (4.11,7.09) | 5.06 (3.68,6.44) | |
| Non-Hispanic White | 72.85 (64.80,80.91) | 74.72 (71.96,77.48) | 73.57 (70.10,77.05) | 71.12 (67.10,75.15) | 71.52 (67.37,75.67) | |
| Non-Hispanic Black | 9.41 (8.24,10.57) | 9.37 (7.91,10.83) | 8.25 (6.75, 9.76) | 8.98 (7.20,10.75) | 11.22 (8.86,13.58) | |
| Others | 6.25 (5.41, 7.08) | 9.02 (7.54,10.49) | 6.98 (5.40, 8.55) | 4.67 (3.55, 5.80) | 3.63 (2.39, 4.88) | |
| Marital status (%) | < 0.001 | |||||
| Married/living with partner | 70.81 (64.84,76.77) | 73.08 (70.79,75.37) | 74.02 (71.48,76.56) | 71.90 (69.44,74.36) | 63.22 (60.13,66.30) | |
| Living alone | 29.19 (27.06,31.32) | 26.92 (24.63,29.21) | 25.98 (23.44,28.52) | 28.10 (25.64,30.56) | 36.78 (33.70,39.87) | |
| PIR (%) | < 0.001 | |||||
| Low | 17.42 (15.65,19.20) | 14.00 (12.04,15.96) | 16.09 (13.99,18.19) | 18.83 (16.27,21.40) | 21.69 (19.24,24.14) | |
| Middle | 26.27 (23.75,28.80) | 22.28 (19.73,24.84) | 25.03 (22.47,27.59) | 26.83 (24.04,29.62) | 31.99 (29.53,34.44) | |
| High | 56.31 (50.73,61.88) | 63.72 (60.32,67.12) | 58.88 (55.18,62.59) | 54.34 (50.86,57.81) | 46.32 (43.12,49.53) | |
| Education level (%) | < 0.001 | |||||
| Less than high school | 16.29 (14.58,17.99) | 12.41 (10.17,14.64) | 16.05 (14.08,18.02) | 18.64 (16.16,21.12) | 18.88 (16.49,21.27) | |
| High school or GED | 22.79 (20.31,25.27) | 18.87 (16.49,21.25) | 22.97 (20.50,25.44) | 24.45 (21.76,27.15) | 25.65 (23.11,28.19) | |
| Above high school | 60.92 (55.45,66.40) | 68.72 (65.26,72.18) | 60.98 (57.58,64.37) | 56.91 (53.53,60.28) | 55.47 (52.55,58.40) | |
| Smoking status (%) | < 0.001 | |||||
| Never | 52.49 (48.56,56.42) | 56.00 (53.05,58.94) | 51.17 (48.19,54.16) | 51.53 (48.55,54.52) | 50.74 (48.27,53.20) | |
| Former | 28.81 (25.83,31.79) | 21.44 (19.35,23.54) | 29.69 (26.84,32.54) | 32.36 (29.43,35.28) | 33.12 (30.26,35.97) | |
| Current | 18.70 (16.97,20.43) | 22.56 (19.92,25.20) | 19.14 (16.97,21.31) | 16.11 (14.67,17.54) | 16.15 (13.97,18.32) | |
| Alcohol consumption (%) | < 0.001 | |||||
| Never | 10.34 (9.18,11.50) | 8.26 (6.81, 9.72) | 8.56 (7.32, 9.81) | 11.87 (10.12,13.62) | 13.36 (11.42,15.30) | |
| Former | 17.54 (16.03,19.05) | 12.35 (10.27,14.44) | 14.54 (12.88,16.21) | 20.66 (18.30,23.01) | 24.13 (21.73,26.52) | |
| Light-to-moderate | 55.80 (50.73,60.87) | 62.69 (58.84,66.54) | 59.81 (57.05,62.58) | 50.77 (47.74,53.79) | 47.90 (44.64,51.17) | |
| Heavy | 16.32 (14.80,17.85) | 16.69 (14.37,19.01) | 17.08 (15.18,18.99) | 16.71 (14.66,18.76) | 14.61 (12.60,16.61) | |
| Physical activity (%) | < 0.001 | |||||
| Inactive | 49.00 (44.89,53.11) | 35.95 (32.34,39.57) | 44.79 (41.54,48.04) | 54.30 (51.92,56.69) | 64.33 (61.42,67.24) | |
| Moderate | 31.16 (27.90,34.42) | 30.68 (27.31,34.05) | 33.56 (30.64,36.48) | 30.50 (28.11,32.89) | 29.64 (26.98,32.30) | |
| Vigorous | 19.84 (17.35,22.33) | 33.37 (29.52,37.21) | 21.65 (18.86,24.44) | 15.19 (13.06,17.33) | 6.03 (4.92, 7.13) | |
| Diabetes (%) | < 0.001 | |||||
| No | 81.41 (74.84,87.97) | 94.17 (93.11,95.22) | 87.62 (85.96,89.28) | 78.81 (76.37,81.24) | 61.37 (58.82,63.92) | |
| Yes | 18.59 (17.20,19.99) | 5.83 (4.78, 6.89) | 12.38 (10.72,14.04) | 21.19 (18.76,23.63) | 38.63 (36.08,41.18) | |
| Hypertension (%) | < 0.001 | |||||
| No | 53.31 (48.97,57.65) | 71.04 (68.52,73.56) | 56.66 (53.74,59.59) | 48.74 (46.28,51.20) | 32.53 (30.13,34.93) | |
| Yes | 46.69 (43.14,50.24) | 28.96 (26.44,31.48) | 43.34 (40.41,46.26) | 51.26 (48.80,53.72) | 67.47 (65.07,69.87) | |
| CKD (%) | < 0.001 | |||||
| No | 84.91 (78.33,91.50) | 89.52 (88.48,90.56) | 88.15 (86.09,90.21) | 84.85 (83.08,86.61) | 75.65 (73.59,77.70) | |
| Yes | 15.09 (13.78,16.39) | 10.48 (9.44,11.52) | 11.85 (9.79,13.91) | 15.15 (13.39,16.92) | 24.35 (22.30,26.41) | |
| CVD (%) | < 0.001 | |||||
| No | 90.07 (83.25,96.88) | 94.52 (93.44,95.60) | 92.09 (90.67,93.51) | 89.22 (87.43,91.01) | 83.18 (81.38,84.98) | |
| Yes | 9.93 (8.98,10.88) | 5.48 (4.40, 6.56) | 7.91 (6.49, 9.33) | 10.78 (8.99,12.57) | 16.82 (15.02,18.62) | |
| Neutrophil (1000 cell/ul) | 4.25 ± 0.03 | 3.90 ± 0.05 | 4.15 ± 0.06 | 4.30 ± 0.04 | 4.74 ± 0.05 | < 0.001 |
| Lymphocyte (1000 cell/ul) | 2.06 ± 0.01 | 1.92 ± 0.02 | 2.02 ± 0.02 | 2.13 ± 0.02 | 2.22 ± 0.02 | < 0.001 |
| Platelet (1000 cell/ul) | 241.78 ± 1.10 | 237.55 ± 1.96 | 240.43 ± 1.82 | 241.86 ± 1.87 | 248.38 ± 1.46 | < 0.001 |
| Monocyte (1000 cell/ul) | 0.56 ± 0.00 | 0.52 ± 0.01 | 0.56 ± 0.01 | 0.57 ± 0.01 | 0.60 ± 0.01 | < 0.001 |
| WBC (1000 cell/ul) | 7.12 ± 0.04 | 6.57 ± 0.06 | 6.97 ± 0.07 | 7.26 ± 0.05 | 7.84 ± 0.06 | < 0.001 |
| SII | 541.19 ± 5.47 | 522.74 ± 9.27 | 540.90 ± 10.00 | 526.74 ± 9.07 | 578.64 ± 8.47 | < 0.001 |
| NLR | 2.23 ± 0.02 | 2.18 ± 0.03 | 2.23 ± 0.04 | 2.19 ± 0.03 | 2.32 ± 0.03 | 0.001 |
| PLR | 127.87 ± 0.95 | 134.10 ± 1.52 | 130.34 ± 1.63 | 123.46 ± 1.47 | 121.92 ± 1.23 | < 0.001 |
| LMR | 3.97 ± 0.03 | 3.97 ± 0.05 | 3.88 ± 0.04 | 4.01 ± 0.04 | 4.03 ± 0.05 | 0.08 |
BRI Body Roundness Index, PIR Poverty income ratio, CKD Chronic kidney disease, CVD Cardiovascular disease, WBC White blood cell, SII Systemic immune-inflammatory, NLR Neutrophil-to-lymphocyte ratio, PLR Platelet-to-lymphocyte ratio, LMR Lymphocyte-to-monocyte ratio
Participants with higher BRI were generally older, predominantly female, and more likely to be Mexican American or Non-Hispanic Black, as well as living alone. They also exhibited lower PIR, lower education levels, reduced physical activity, and fewer current smokers. Additionally, they reported lower alcohol consumption and had a higher prevalence of comorbid conditions and elevated inflammation indicators.
Univariate analysis of SαKl levels
A univariate analysis (Table S1) identified age, BRI, gender, race/ethnicity, smoking status, alcohol consumption, hypertension, CKD, CVD, and inflammatory markers as significantly associated with SαKl levels.
BRI and SαKl levels
Three models were employed to explore the association between BRI and SαKl levels (Table 2). Across all models, higher BRI was inversely associated with SαKl levels. After fully adjustment, individuals in the highest BRI quartile (Q4) had significantly lower SαKl levels (−38.46 pg/ml) compared to those in the lowest quartile (Q1) (P = 0.002).
Table 2.
Associations between BRI and serum α − klotho levels by multivariate linear regression
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| BRI, continuous | −5.76 (−9.05, −2.47) | < 0.001 | −5.94 (−9.32, −2.56) | < 0.001 | −7.53 (−11.10, −3.96) | < 0.001 |
| BRI, categories | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | −19.64 (−44.23, 4.94) | 0.122 | −12.45 (−37.31, 12.41) | 0.329 | −12.94 (−37.99, 12.11) | 0.316 |
| Q3 | −39.21 (−59.27, −19.16) | < 0.001 | −32.32 (−52.43, −12.21) | 0.002 | −36.65 (−56.91, −16.39) | < 0.001 |
| Q4 | −32.59 (−53.82, −11.36) | 0.004 | −31.68 (−53.84, −9.51) | 0.007 | −38.46 (−61.15, −15.78) | 0.002 |
| P for trend | < 0.001 | 0.002 | < 0.001 | |||
| BRI, per SD | −13.05 (−20.51, −5.60) | < 0.001 | −13.46 (−21.12, −5.80) | < 0.001 | −17.06 (−25.15, −8.97) | < 0.001 |
Model 1: Adjusted for none
Model 2: Adjusted for age, gender, race/ethnicity
Model 3: Adjusted for age, gender, race/ethnicity, marital status, PIR, education level, smoking status, alcohol consumption status, physical activity, diabetes, hypertension, CKD, and CVD
Abbreviations: CI Confidence interval, BRI body roundness index, PIR poverty income ratio, CKD chronic kidney disease, CVD cardiovascular disease
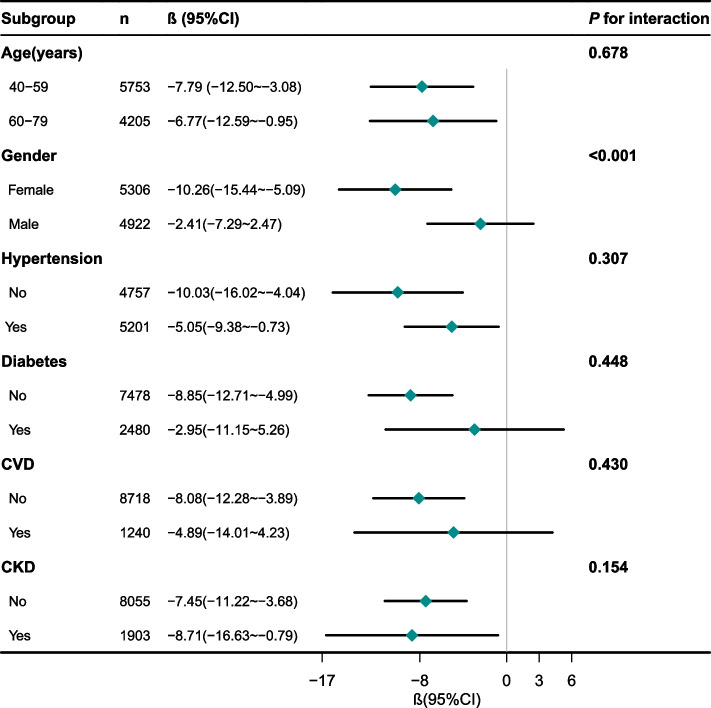
Stratified analyses demonstrated that the inverse association between BRI and SαKl remained robust across several subgroups, including individuals aged 40–59 years, 60–79 years, females, and those with or without hypertension or CKD (Fig. 1). In contrast, this association was not statistically significant among males or individuals with diabetes or CVD. A significant interaction by gender (P for interaction < 0.001) suggests that the influence of BRI on SαKl may differ from males to females.
Fig. 1.
Subgroup analyses of the association between BRI and serum α−Klotho levels
Each stratification adjusted for all factors (age, gender, race/ethnicity, marital status, PIR, education level, smoking status, alcohol consumption status, physical activity, diabetes, hypertension, CKD, and CVD) except the stratification factor itself. Abbreviations: PIR, Poverty income ratio; CKD, chronic kidney disease; CVD, cardiovascular disease
BRI, inflammatory markers and SαKl levels
We utilized three models to examine the relationships between BRI and inflammatory markers (Table 3). In summary, BRI was significantly positively associated with multiple inflammatory markers, including neutrophil count (β = 0.14), lymphocyte count (β = 0.05), platelet count (β = 2.01), monocyte count (β = 0.01), WBC count (β = 0.21), and SII (β = 7.20). In contrast, PLR was negatively associated with BRI (β = −2.25).
Table 3.
Associations between BRI and inflammation related indicators
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Neutrophil | 0.15 (0.13, 0.17) | < 0.001 | 0.17 (0.15, 0.19) | < 0.001 | 0.14 (0.12, 0.16) | < 0.001 |
| Lymphocyte | 0.05 (0.04, 0.06) | < 0.001 | 0.05 (0.04, 0.06) | < 0.001 | 0.05 (0.04, 0.06) | < 0.001 |
| Platelet | 2.07 (1.32, 2.83) | < 0.001 | 2.10 (1.36, 2.85) | < 0.001 | 2.01 (1.18, 2.84) | < 0.001 |
| Monocyte | 0.01 (0.01, 0.02) | < 0.001 | 0.01 (0.01, 0.02) | < 0.001 | 0.01 (0.01, 0.02) | < 0.001 |
| WBC | 0.22 (0.20, 0.25) | < 0.001 | 0.24 (0.22, 0.27) | < 0.001 | 0.21 (0.18, 0.23) | < 0.001 |
| SII | 10.85 (7.02, 14.67) | < 0.001 | 11.60 (7.72, 15.48) | < 0.001 | 7.20 (2.79, 11.61) | 0.002 |
| NLR | 0.03 (0.02, 0.04) | < 0.001 | 0.03 (0.02, 0.04) | < 0.001 | 0.01 (−0.002, 0.03) | 0.091 |
| PLR | −1.94 (−2.57, −1.30) | < 0.001 | −2.17 (−2.82, −1.53) | < 0.001 | −2.25 (−2.91, −1.60) | < 0.001 |
| LMR | 0.01 (−0.01, 0.03) | 0.443 | 0.004 (−0.02, 0.02) | 0.716 | 0.01 (−0.01, 0.03) | 0.228 |
Model 1: Adjusted for none
Model 2: Adjusted for age, gender, race/ethnicity
Model 3: Adjusted for age, gender, race/ethnicity, marital status, PIR, education level, smoking status, alcohol consumption status, physical activity, diabetes, hypertension, CKD, and CVD
Abbreviations: CI Confidence interval, WBC White blood cell, SII Systemic immune-inflammatory, NLR Neutrophil-to-lymphocyte ratio, PLR Platelet-to-lymphocyte ratio, LMR Lymphocyte-to-monocyte ratio, PIR Poverty income ratio, CKD Chronic kidney disease, CVD Cardiovascular disease
Similarly, three models evaluated the relationships between inflammatory markers and SαKl level (Table 4). Inflammatory markers, including neutrophil count (β = −10.07), platelet count (β = −0.47), WBC count (β = −7.85), SII (β = −0.05), NLR (β = −8.17), and PLR (β = −0.34) were all negatively associated with SαKl. In contrast, MLR was positively associated with SαKl (β = 3.94).
Table 4.
Associations between inflammation markers and serum α−klotho levels
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Neutrophil | −12.77 (−17.72, −7.82) | < 0.001 | −12.07 (−17.08, −7.06) | < 0.001 | −10.07 (−15.38, −4.76) | < 0.001 |
| Lymphocyte | −1.94 (−10.31, 6.44) | 0.652 | −8.82 (−17.33, −0.31) | 0.046 | −5.26 (−13.98, 3.47) | 0.243 |
| Platelet | −0.31 (−0.47, −0.15) | < 0.001 | −0.47 (−0.64, −0.31) | < 0.001 | −0.47 (−0.64, −0.30) | < 0.001 |
| Monocyte | −75.67 (−118.51, −32.83) | < 0.001 | −50.68 (−91.04, −10.31) | 0.016 | −36.43 (−74.41, 1.55) | 0.065 |
| WBC | −9.49 (−13.20, −5.78) | < 0.001 | −9.45 (−13.26, −5.66) | < 0.001 | −7.85 (−12.04, −3.67) | < 0.001 |
| SII | −0.06 (−0.09, −0.03) | < 0.001 | −0.06 (−0.09, −0.03) | < 0.001 | −0.05 (−0.08, −0.03) | < 0.001 |
| NLR | −14.90 (−22.18, −7.63) | < 0.001 | −10.00 (−17.07, −2.93) | 0.007 | −8.17 (−14.93, −1.41) | 0.021 |
| PLR | −0.29 (−0.45, −0.13) | < 0.001 | −0.30 (−0.46, −0.14) | < 0.001 | −0.34(−0.50, −0.18) | < 0.001 |
| LMR | 10.04 (5.92, 14.17) | < 0.001 | 3.95 (−0.03, 7.93) | 0.052 | 3.94 (0.15, 7.73) | 0.046 |
Model 1: Adjusted for none
Model 2: Adjusted for age, gender, race/ethnicity
Model 3: Adjusted for age, gender, race/ethnicity, marital status, PIR, education level, smoking status, alcohol consumption status, physical activity, diabetes, hypertension, CKD, and CVD
CI Confidence interval, WBC White blood cell, SII Systemic immune-inflammatory, NLR Neutrophil-to-lymphocyte ratio, PLR Platelet-to-lymphocyte ratio, LMR Lymphocyte-to-monocyte ratio, PIR Poverty income ratio, CKD Chronic kidney disease, CVD Cardiovascular disease
Mediation effects of inflammatory markers on BRI and SαKl levels associations
Given the observed intercorrelations among BRI, inflammatory markers, and SαKl, we performed mediation analyses (Table S2 and Fig. 2). Employing Model 3, we investigated the associations between various inflammatory markers (including neutrophil, lymphocyte, platelet, monocyte, WBC, SII, NLR, PLR, and LMR) and both BRI and SαKl levels. The mediation analyses indicated that WBC count accounted for 20.5% of the association, neutrophil count contributed 18.0%, and platelet count mediated 12.3%.
Fig. 2.
Mediation model of the effect of BRI and serum α−Klotho levels
Abbreviations: BRI, Body Roundness Index; CI, Confidence interval; WBC: White blood cell
Sensitivity analysis
To enhance transparency and address potential selection bias, sensitivity analysises were performed, with the results presented in Table S3. This table provides the characteristics of participants excluded due to missing data, with the most significant exclusions related to PIR and alcohol consumption, which accounted for 6.08% and 5.41% of the potential participant sample, respectively. Other missing data accounted for only 0.42% of the total. Comparisons between excluded and included participants showed no significant differences in key demographic or clinical variables, suggesting minimal bias in our analysis. Additionally, the relationship between BRI and SαKl levels remained consistent when BRI was transformed using the Z-score method, aligning with results from both continuous and categorical analyses (Table 2).
Additionally, after excluding 860 participants with an eGFR less than 60 mL/min, our analysis reaffirmed the robustness of our initial findings across various metrics. The inverse relationship between BRI and SαKl levels remained statistically significant, consistent with the full cohort. Similarly, the associations between BRI, inflammatory markers, and SαKl levels persisted, with inflammatory markers effectively mediating these relationships (Tables S4-S7).
Discussion
In this cross-sectional study utilizing NHANES data, we identified a significant negative association between BRI and SαKl levels. The negative relationship remained robust after stratification by age, gender, and comorbid conditions (hypertension or CKD), suggesting that these demographic and clinical factors were not fully account for the observed association. Furthermore, we found a significant interaction between gender and SαKl levels, suggesting that central adiposity may influence αKl expression through distinct mechanisms in males and females.
αKl, a protein involved in attenuating mechanisms related to aging, plays a crucial role in mineral metabolism and exerts protective effects against a range of age-related diseases, including CVD [27], osteoporosis [28], and CKD [29]. Its role as a biomarker has significant implications for clinical and public health applications, emphasizes its potential for early detection and intervention in age-related conditions, as well as for identifying individuals at risk of developing obesity and related chronic diseases. Previous research has demonstrated that increased adiposity, particularly visceral fat, is linked to reduced SαKl levels, implying that excessive body fat may inhibit αKl secretion via systemic inflammation and altered adipokine profiles [30, 31]. Given its association with both obesity and metabolic dysfunction, assessing SαKl levels in the general population could help identify individuals at higher risk of future obesity or obesity-related chronic diseases, thereby enabling earlier interventions and more targeted preventive strategies. Age-related decline SαKl levels has also been widely reported, with older individuals generally displaying lower SαKl levels, which is consistent with its role in regulation calcium and phosphate homeostasis and modulates insulin sensitivity, underscoring its pivotal role in metabolic and aging processes [19, 32].
BRI was a newly raised concept that reflect body roundness more accurately. Adiposity is more strongly associated with metabolic derangements and mortality risk than overall adiposity measured by BMI. Our study's findings reinforce these observations, showing that higher BRI values, which incorporate waist circumference and thus more accurately reflect central adiposity, were associated with lower SαKl levels, capturing the complexity of fat distribution's influence on aging and metabolic health. While many earlier studies have utilized BMI to approximate adiposity, we apply BRI to provide a more refined measure of body shape and fat distribution. The distribution of fat is important, as visceral adiposity is more strongly associated with metabolic derangements and mortality risk than overall adiposity measured by BMI. By integrating waist circumference, BRI offers a superior estimation of central adiposity which is more strongly linked to metabolic disturbances and mortality risk [4, 33].
Mechanistically, increased central adiposity fosters systemic inflammation and metabolic stress through several pathways [18, 34]. These include transcriptional regulator interacting with the plant homeodomain zinc finger and/or the bromodomain 2 mediated endoplasmic reticulum stress, non-canonical nuclear factor-kappa B (NF-κB) activation by receptor activator of NF-κB ligand, and disruptions in lipid metabolism. These pathways collectively contribute to the suppression of αKl expression, particularly through lipid metabolism disorders such as inactivation of adenosine 5 ‘-monophosphate-activated protein kinase and altered hormone-sensitive lipase signaling et al. [35–37]. Additionally, visceral adipose tissue induced oxidative stress upregulates of RPS6KB1 (evidenced by increased 8-epi-PGF2α) further exacerbate metabolic dysregulation which may diminish the protective capacity of αKl's [38, 39].
Our findings suggest that inflammatory markers, particularly neutrophils and platelets, play a key role in linking central adiposity to reduced SαKl levels. Elevated neutrophil counts release pro-inflammatory cytokines may disrupting SαKl synthesis, while increased platelets contribute to endothelial dysfunction and oxidative stress, further lowering SαKl [40, 41]. These mechanisms help explain the inverse relationship between BRI and SαKl, further elucidated by the mediating role of inflammation. Furthermore, by identifying specific inflammatory markers that correlate with increased BRI and decreased SαKl levels, our study highlights inflammation as a key mediator. This distinctive aspect of our study provides new insghts into potential therapeutic targets for aging-related conditions, underscoring the clinical relevance of targeting inflammation to modulate the effects of central adiposity on aging biomarker.
Our stratified analyses revealed a stronger inverse association between BRI and SαKl levels among females than in males, highlighting the potential for sex-specific intervention strategies. Recent studies corroborate these findings, with Carreras-Badosa et al. observing that higher SαKl levels are linked to less central obesity in girls [42], and Yin et al. reporting a pronounced inverse relationship between central obesity and SαKl levels in women [43]. These studies emphasize the need to differentiate between central and subcutaneous obesity when examining their effects on SαKl. This divergence may be attributed to sex-specific hormonal differences. Estrogen can promote subcutaneous fat deposition and enhanced αKl expression, potentially buffering females against the adverse effects of central adiposity on SαKl levels [44]. In contrast, androgen worsens visceral fat accumulation in males and may heighten inflammation and metabolic dysfunction, thereby attenuating αKl expression [45]. Moreover, as noted in recent studies, abdominal obesity in women was significantly inversely associated with SαKl levels, particularly among those who developed obesity later in life [42], while no such significant association was found in men. This further suggests that the distribution of fat—particularly visceral fat—plays a key role in influencing SαKl levels, especially in women. This sex-specific divergence underscores the importance of tailored approaches for managing central adiposity and inflammation, particularly in clinical and public health strategies aimed at mitigating age-related health declines.
The inverse association between BRI and SαKl was consistently observed across subgroups with and without hypertension or CKD, indicating that central adiposity influences SαKl levels independently of these conditions. The lack of significance in individuals with diabetes or CVD may stem from the complex interplay of metabolic dysfunction, inflammation, and altered fat distribution characteristic of these conditions. Both diabetes and CVD are associated with chronic low-grade inflammation, which may disrupt SαKl 's regulatory pathways, potentially obscuring its relationship with adiposity [46]. Furthermore, insulin resistance and vascular damage in these populations could further modulate SαKl levels, attenuating the clear inverse association seen in other groups [47].
Strengths and limitations
This study has several strengths. We leveraged a nationally representative dataset and applied appropriate sampling weights to enhance generalizability, while the comprehensive nature of NHANES facilitated adjustment for multiple confounders. However, several limitations should be considered. The cross-sectional design limits causal inferences between BRI and SαKl levels, and reliance on self-reported data for certain variables may introduce reporting bias. Although BRI provides a more nuanced measure of central adiposity than BMI, it may not encompass all aspects of body composition, and combining additional obesity indices such as body fat percentage could potentially yield a more comprehensive assessment. Additionally, the use of the ELISA technique for measuring αKl, although common, has limitations. Studies suggest that alternative methods such as immunoprecipitation immunoblot might offer more accurate results, especially for samples that have undergone freeze–thaw cycles. Furthermore, the NHANES dataset focuses primarily on serum αKl, with no data on urinary αKl. While serum αKl is widely used in research, urinary αKl may offer a non-invasive alternative, making it easier to assess in clinical practice. Our regression models accounted for key confounders, but residual confounding may still exist. Future research, including longitudinal studies and mechanistic investigations, is needed to confirm these associations, establish causality, and guide interventions aimed at preserving αKl levels and mitigating obesity-related aging phenotypes.
Conclusions
In conclusion, this study demonstrated a significant inverse association between BRI and SαKl levels, with a pronounced effect observed in females. Mediation analyses further revealed that inflammatory markers substantially influence this relationship, with WBC count, neutrophils, and platelets accounting for 20.5%, 18.0%, and 12.3% of the mediation effect, respectively. These findings suggested that increased adiposity, measured by BRI, was linked to lower SαKl levels, which may potentially contribute to metabolic dysregulation and aging-related diseases through inflammatory pathways.
Supplementary Information
Supplementary Material 1: Fig S1. Participants selection flowchart. Table S1. Univariate analysis for serum α-klotho level. Table S2. Analysis of the mediation by inflammation-related indicators of the associations of BRI and serum α−klotho levels. Table S3. Baseline characteristics of the participants in NHANES, 2007 to 2016 (including missing data). Table S4. Associations between BRI and serum α−klotho levels by multivariate linear regression (excluded 860 participants with eGFR <60 mL/min). Table S5. Associations between BRI and inflammation markers (excluded 860 participants with eGFR <60 mL/min). Table S6. Associations between inflammation markers and serum α−klotho levels (excluded 860 participants with eGFR <60 mL/min). Table S7. Analysis of the mediation by inflammation-related indicators of the associations of BRI and SαKl levels (excluded 860 participants with eGFR <60 mL/min).
Acknowledgements
We really appreciate fantastic unwavering guidance and assistance by Professor Ding Shifang.
Abbreviations
- αKl
α−Klotho
- BMI
Body Mass Index
- BRI
Body Roundness Index
- CDC
Centers for Disease Control and Prevention
- CI
Confidence interval
- CKD
Chronic kidney disease
- CVD
Cardiovascular disease
- eGFR
Estimated glomerular filtration rate
- NHANES
National Health and Nutrition Examination Survey
- PIR
Poverty income ratio
- SαKl
Serum α−Klotho
- WBC
White blood cell
- SII
Systemic immune inflammation index
- NLR
Neutrophil-to-lymphocyte ratio
- PLR
Platelet-to-lymphocyte ratio
- LMR
Lymphocyte-to-monocyte ratio
Authors’ contributions
Rui Du: Conceptualization; Methodology; Data curation; Formal analysis; Software; Project administration; Writing—original draft. Xiaoyan Tang: Conceptualization; Validation; Writing—original draft. Lei Guan: Methodology; Data curation. Yuchen Lai: Investigation; Methodology; Resources. Huijuan Xiang: Investigation; Methodology; Validation; Project administration; Resources; Supervision. Wei Huang: Conceptualization; Methodology; Resources; Supervision; Validation; Writing— review, editing.
Funding
None.
Data availability
Data used for this study are available on the NHANES website: https://www.n.cdc.gov/nchs/nhanes/.
Declarations
Ethics approval and consent to participate
The survey was administered by the National Center for Health Statistics (NCHS) and approved by the NCHS Institutional Review Board (IRB). Informed consent was obtained from the eligible subjects before initiating the data collection and NHANES health examinations. All the authors confirmed that all the methods were carried out in accordance with the relevant NHANES Analytic Guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rui Du and Xiaoyan Tang are co-first author.
Contributor Information
Huijuan Xiang, Email: xhjyxr@126.com.
Wei Huang, Email: huangwei0521@126.com.
References
- 1.Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–98. [DOI] [PubMed] [Google Scholar]
- 2.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes (Lond). 2008;32(Suppl 3):S56-59. [DOI] [PubMed] [Google Scholar]
- 3.Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, Maeda Y, McDougall A, Peterson CM, Ravussin E, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity (Silver Spring). 2013;21(11):2264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rico-Martin S, Calderon-Garcia JF, Sanchez-Rey P, Franco-Antonio C, Martinez Alvarez M, Sanchez Munoz-Torrero JF. Effectiveness of body roundness index in predicting metabolic syndrome: A systematic review and meta-analysis. Obes Rev. 2020;21(7): e13023. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Zhang L, Wu Q, Zhou Y, Jin Z, Li Z, Zhu Y. Body roundness index is a superior indicator to associate with the cardio-metabolic risk: evidence from a cross-sectional study with 17,000 Eastern-China adults. BMC Cardiovasc Disord. 2021;21(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G, Wu HK, Wu XW, Cao Z, Tu YC, Ma Y, Li BN, Peng QY, Cheng J, Wu B, et al. The feasibility of two anthropometric indices to identify metabolic syndrome, insulin resistance and inflammatory factors in obese and overweight adults. Nutrition. 2019;57:194–201. [DOI] [PubMed] [Google Scholar]
- 7.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. [DOI] [PubMed] [Google Scholar]
- 8.Castillo RF. Pathophysiologic Implications and Therapeutic Approach of Klotho in Chronic Kidney Disease: A Systematic Review. Lab Invest. 2023;103(7): 100178. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Zhao C, Zhang H, Chen M, Meng Y, Pan Y, Zhuang Q, Zhao M. Association between serum soluble alpha-klotho and bone mineral density (BMD) in middle-aged and older adults in the United States: a population-based cross-sectional study. Aging Clin Exp Res. 2023;35(10):2039–49. [DOI] [PubMed] [Google Scholar]
- 10.Bi J, Zheng M, Li K, Sun S, Zhang Z, Yan N, Li X. Relationships of serum FGF23 and alpha-klotho with atherosclerosis in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2024;23(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panczyszyn-Trzewik P, Czechowska E, Stachowicz K, Sowa-Kucma M: The Importance of alpha-Klotho in Depression and Cognitive Impairment and Its Connection to Glutamate Neurotransmission-An Up-to-Date Review. Int J Mol Sci 2023, 24(20). [DOI] [PMC free article] [PubMed]
- 12.Landry T, Shookster D, Huang H. Circulating alpha-klotho regulates metabolism via distinct central and peripheral mechanisms. Metabolism. 2021;121: 154819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125(18):2243–55. [DOI] [PubMed] [Google Scholar]
- 14.Sedighi M, Baluchnejadmojarad T, Fallah S, Moradi N, Afshin-Majd S, Roghani M. The Association Between Circulating Klotho and Dipeptidyl Peptidase-4 Activity and Inflammatory Cytokines in Elderly Patients With Alzheimer Disease. Basic Clin Neurosci. 2020;11(3):349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almroth G, Lonn J, Uhlin F, Brudin L, Andersson B, Hahn-Zoric M. Sclerostin, TNF-alpha and Interleukin-18 Correlate and are Together with Klotho Related to Other Growth Factors and Cytokines in Haemodialysis Patients. Scand J Immunol. 2016;83(1):58–63. [DOI] [PubMed] [Google Scholar]
- 16.Chihara Y, Rakugi H, Ishikawa K, Ikushima M, Maekawa Y, Ohta J, Kida I, Ogihara T. Klotho protein promotes adipocyte differentiation. Endocrinology. 2006;147(8):3835–42. [DOI] [PubMed] [Google Scholar]
- 17.Razzaque MS. The role of Klotho in energy metabolism. Nat Rev Endocrinol. 2012;8(10):579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elks CM, Francis J. Central adiposity, systemic inflammation, and the metabolic syndrome. Curr Hypertens Rep. 2010;12(2):99–104. [DOI] [PubMed] [Google Scholar]
- 19.Kuro OM. The Klotho proteins in health and disease. Nat Rev Nephrol. 2019;15(1):27–44. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey. Laboratory procedures manual. 2011.
- 21.Zhao Y, Shao W, Zhu Q, Zhang R, Sun T, Wang B, Hu X. Association between systemic immune-inflammation index and metabolic syndrome and its components: results from the National Health and Nutrition Examination Survey 2011–2016. J Transl Med. 2023;21(1):691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du R, Liu J, Tang X, Chen Z, Guan L, Gao W, Huang W. Correlation of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with serum alpha-klotho levels in US middle-aged and older individuals: Results from NHANES 2007–2016. Prev Med Rep. 2024;46: 102877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao QQ, Mo YJ, Zhu KW, Gao F, Huang B, Chen P, Jing FT, Jiang X, Xu HZ, Tang YF, et al. Platelet-to-Lymphocyte Ratio (PLR), Neutrophil-to-Lymphocyte Ratio (NLR), Monocyte-to-Lymphocyte Ratio (MLR), and Eosinophil-to-Lymphocyte Ratio (ELR) as Biomarkers in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD). Int J Chron Obstruct Pulmon Dis. 2024;19:501–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang M, Tang X, Wang P, Yang L, Du R: Association between daily alcohol consumption and serum alpha klotho levels among U.S. adults over 40 years old: a cross-sectional study. BMC Public Health 2023, 23(1):1901. [DOI] [PMC free article] [PubMed]
- 25.Du R, Tang X, Jiang M, Qian S, Yang L, Tong X, Huang W. Association between cigarette smoking and serum alpha klotho levels among US adults over 40-years-old: a cross-sectional study. Sci Rep. 2023;13(1):19519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du R, Tang X, Yang C, Shi J, Lai Y, Ding S, Huang W. Association between the duration of smoking cessation and alpha-Klotho levels in the US middle-aged and elderly population. Heliyon. 2024;10(19): e38298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bi X, Yang K, Zhang B, Zhao J. The Protective Role of Klotho in CKD-Associated Cardiovascular Disease. Kidney Dis (Basel). 2020;6(6):395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang J, Liu Q, Mao Y, Wang N, Lin W, Li L, Liang J, Chen G, Huang H, Wen J. Klotho reduces the risk of osteoporosis in postmenopausal women: a cross-sectional study of the National Health and Nutrition Examination Survey (NHANES). BMC Endocr Disord. 2023;23(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu L, Napoletano A, Provenzano M, Garofalo C, Bini C, Comai G, La Manna G: Mineral Bone Disorders in Kidney Disease Patients: The Ever-Current Topic. Int J Mol Sci 2022, 23(20). [DOI] [PMC free article] [PubMed]
- 30.Wang Z, Zhang H, Zheng G, Wang Z, Shi L. Gender-specific association between circulating serum Klotho and metabolic components in adults. BMC Endocr Disord. 2024;24(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui J, Yang Z, Wang J, Yin S, Xiao Y, Bai Y, Wang J. A cross-sectional analysis of association between visceral adiposity index and serum anti-aging protein Klotho in adults. Front Endocrinol (Lausanne). 2023;14:1082504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, Moe OW, Liang G, Li X, Mohammadi M. alpha-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature. 2018;553(7689):461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barazzoni R, Gortan Cappellari G, Semolic A, Ius M, Zanetti M, Gabrielli A, Vinci P, Guarnieri G, Simon G. Central adiposity markers, plasma lipid profile and cardiometabolic risk prediction in overweight-obese individuals. Clin Nutr. 2019;38(3):1171–9. [DOI] [PubMed] [Google Scholar]
- 34.Monteiro R, Azevedo I: Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm 2010, 2010. [DOI] [PMC free article] [PubMed]
- 35.Qiang G, Kong HW, Fang D, McCann M, Yang X, Du G, Bluher M, Zhu J, Liew CW. The obesity-induced transcriptional regulator TRIP-Br 2 mediates visceral fat endoplasmic reticulum stress-induced inflammation. Nat Commun. 2016;7:11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mori K, Mizokami A, Sano T, Mukai S, Hiura F, Ayukawa Y, Koyano K, Kanematsu T, Jimi E. RANKL elevation activates the NIK/NF-kappaB pathway, inducing obesity in ovariectomized mice. J Endocrinol. 2022;254(1):27–36. [DOI] [PubMed] [Google Scholar]
- 37.Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol. 2010;298(4):C961-971. [DOI] [PubMed] [Google Scholar]
- 38.Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Andrada P, Rotellar F, Valenti V, Moncada R, Marti P, Silva C, et al. Expression of S6K1 in human visceral adipose tissue is upregulated in obesity and related to insulin resistance and inflammation. Acta Diabetol. 2015;52(2):257–66. [DOI] [PubMed] [Google Scholar]
- 39.Fujita K, Nishizawa H, Funahashi T, Shimomura I, Shimabukuro M. Systemic oxidative stress is associated with visceral fat accumulation and the metabolic syndrome. Circ J. 2006;70(11):1437–42. [DOI] [PubMed] [Google Scholar]
- 40.Semple JW, Italiano JE Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–74. [DOI] [PubMed] [Google Scholar]
- 41.Kuro-o M. Klotho and aging. Biochim Biophys Acta. 2009;1790(10):1049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carreras-Badosa G, Puerto-Carranza E, Mas-Pares B, Gomez-Vilarrubla A, Gomez-Herrera B, Diaz-Roldan F, Riera-Perez E, de Zegher F, Ibanez L, Bassols J, et al. Higher levels of serum alpha-Klotho are longitudinally associated with less central obesity in girls experiencing weight gain. Front Endocrinol (Lausanne). 2023;14:1218949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin H, Qiu Y, Guo L, Zhu W, Li W, Zhou Y, Wei W, Liang M. Correlation of the weight-adjusted waist circumference index with Klotho in the United States: differences by sex. Sci Rep. 2024;14(1):31118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedersen SB, Kristensen K, Hermann PA, Katzenellenbogen JA, Richelsen B: Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J Clin Endocrinol Metab 2004, 89(4):1869–1878. [DOI] [PubMed]
- 45.Bjorntorp P. Hormonal control of regional fat distribution. Hum Reprod. 1997;12(Suppl 1):21–5. [DOI] [PubMed] [Google Scholar]
- 46.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaw J. Diabetes: regional variation in lower limb amputation incidence. Nat Rev Endocrinol. 2012;8(7):386–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Fig S1. Participants selection flowchart. Table S1. Univariate analysis for serum α-klotho level. Table S2. Analysis of the mediation by inflammation-related indicators of the associations of BRI and serum α−klotho levels. Table S3. Baseline characteristics of the participants in NHANES, 2007 to 2016 (including missing data). Table S4. Associations between BRI and serum α−klotho levels by multivariate linear regression (excluded 860 participants with eGFR <60 mL/min). Table S5. Associations between BRI and inflammation markers (excluded 860 participants with eGFR <60 mL/min). Table S6. Associations between inflammation markers and serum α−klotho levels (excluded 860 participants with eGFR <60 mL/min). Table S7. Analysis of the mediation by inflammation-related indicators of the associations of BRI and SαKl levels (excluded 860 participants with eGFR <60 mL/min).
Data Availability Statement
Data used for this study are available on the NHANES website: https://www.n.cdc.gov/nchs/nhanes/.