Abstract
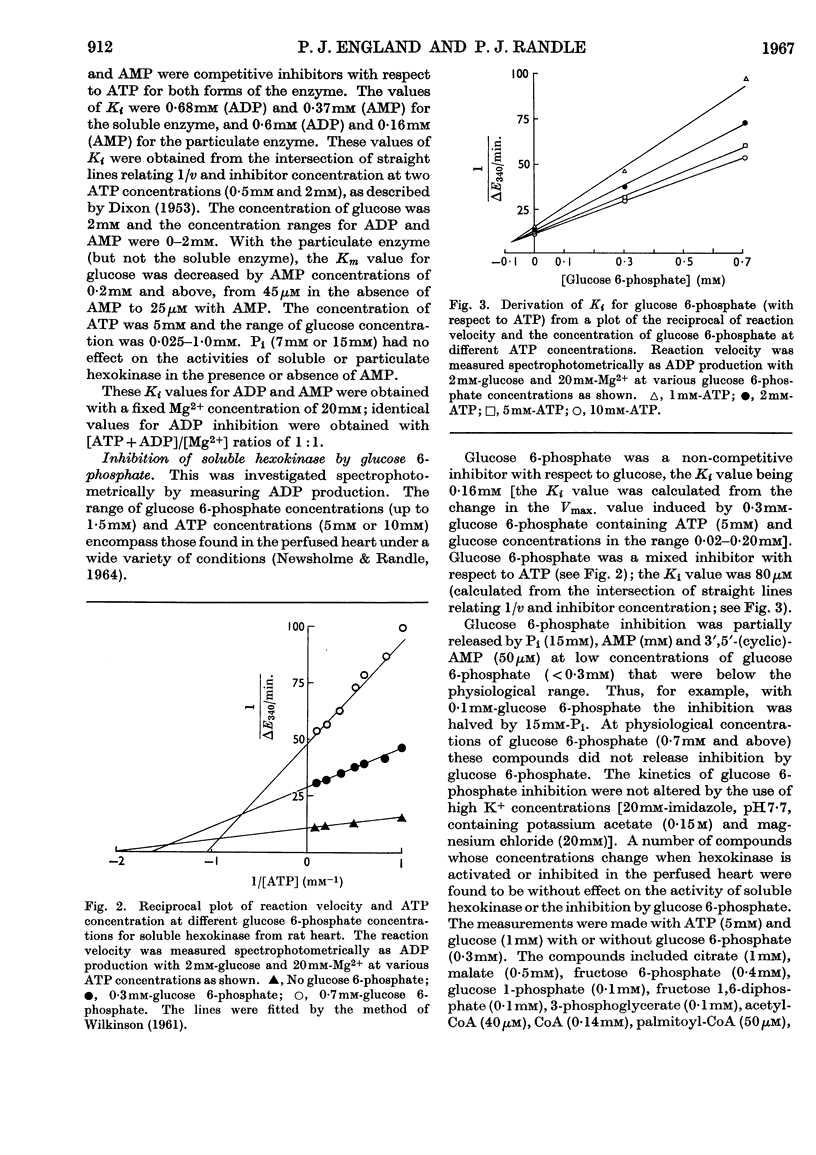
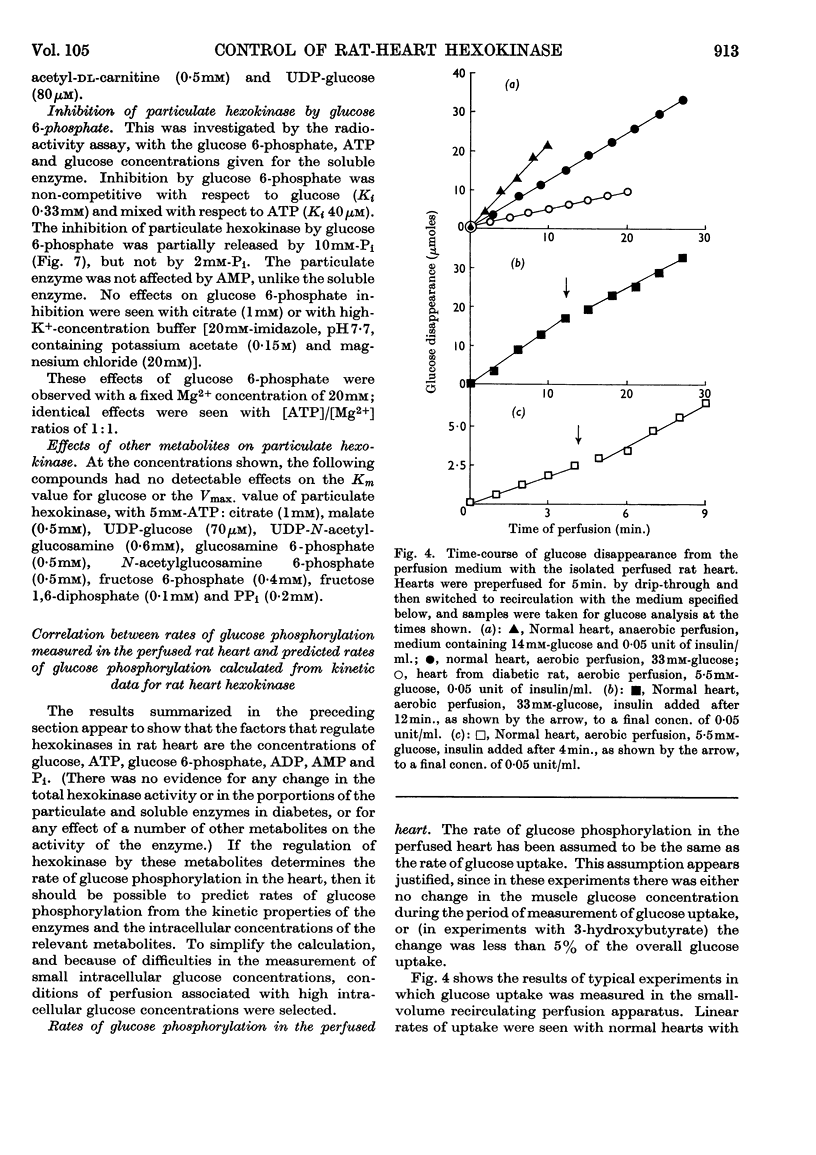
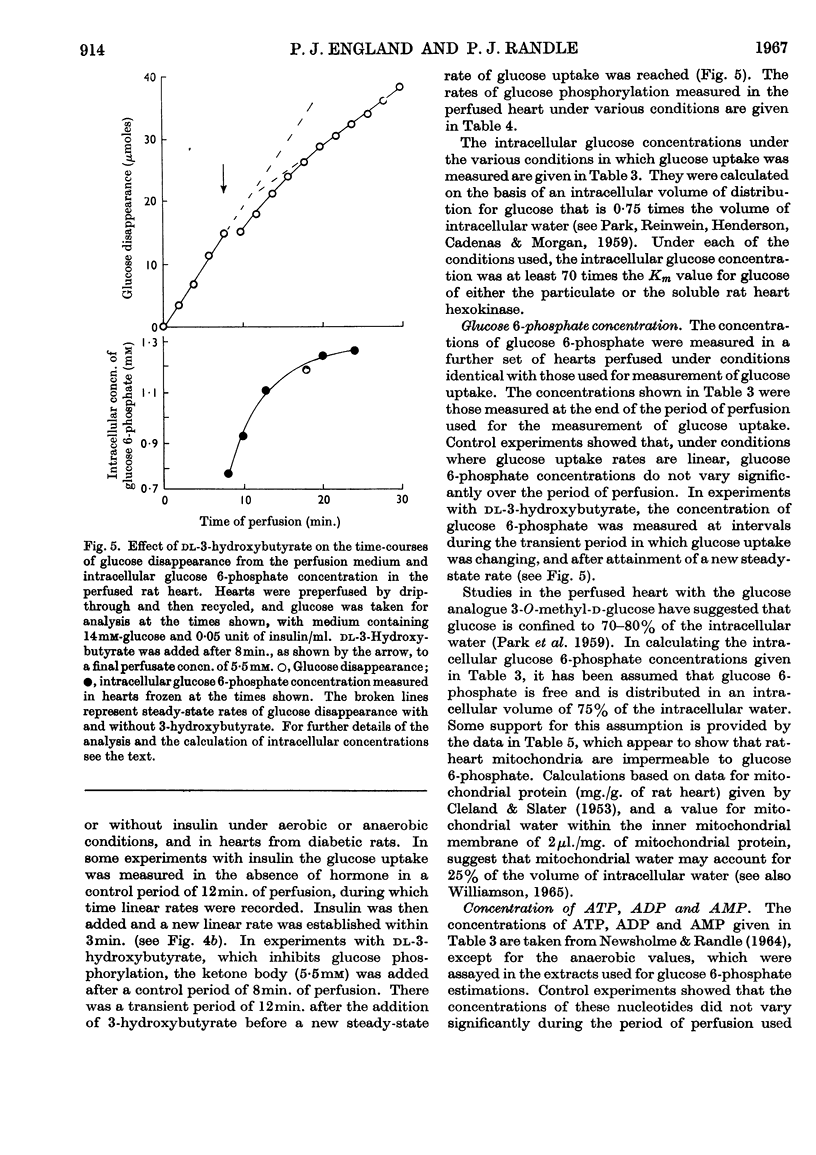
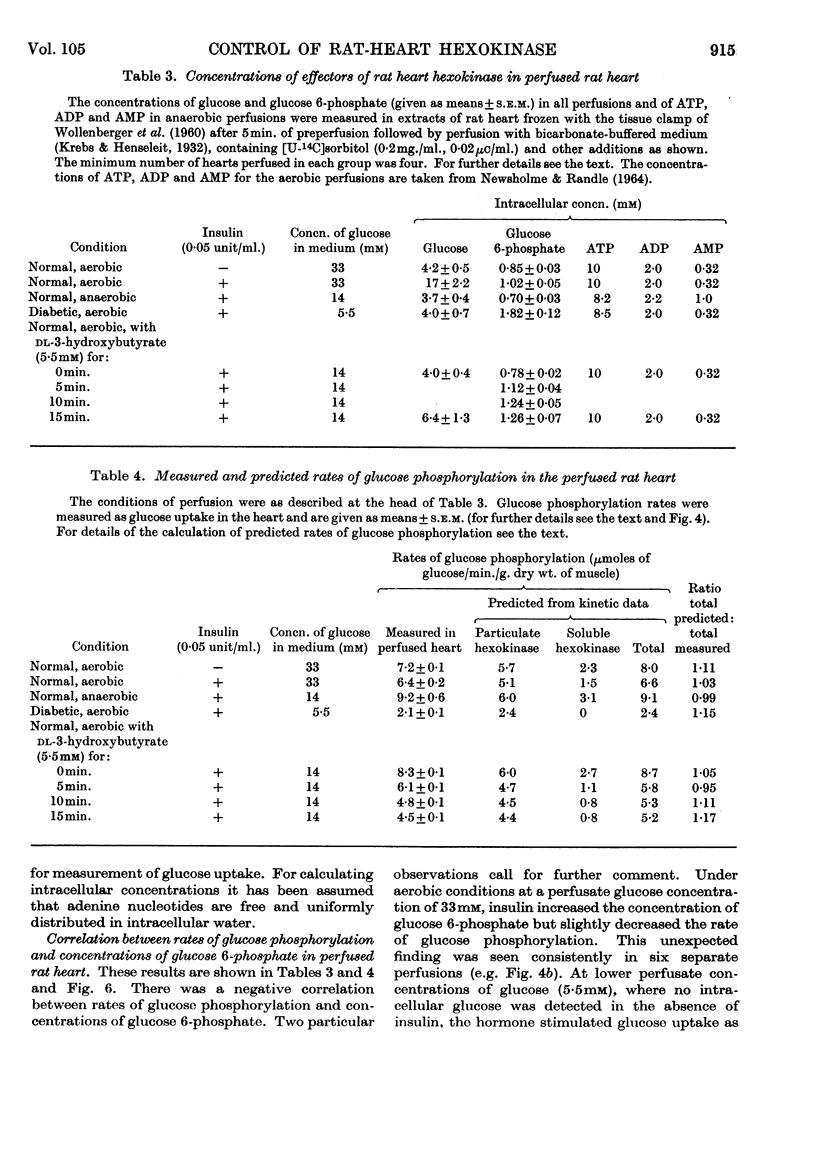
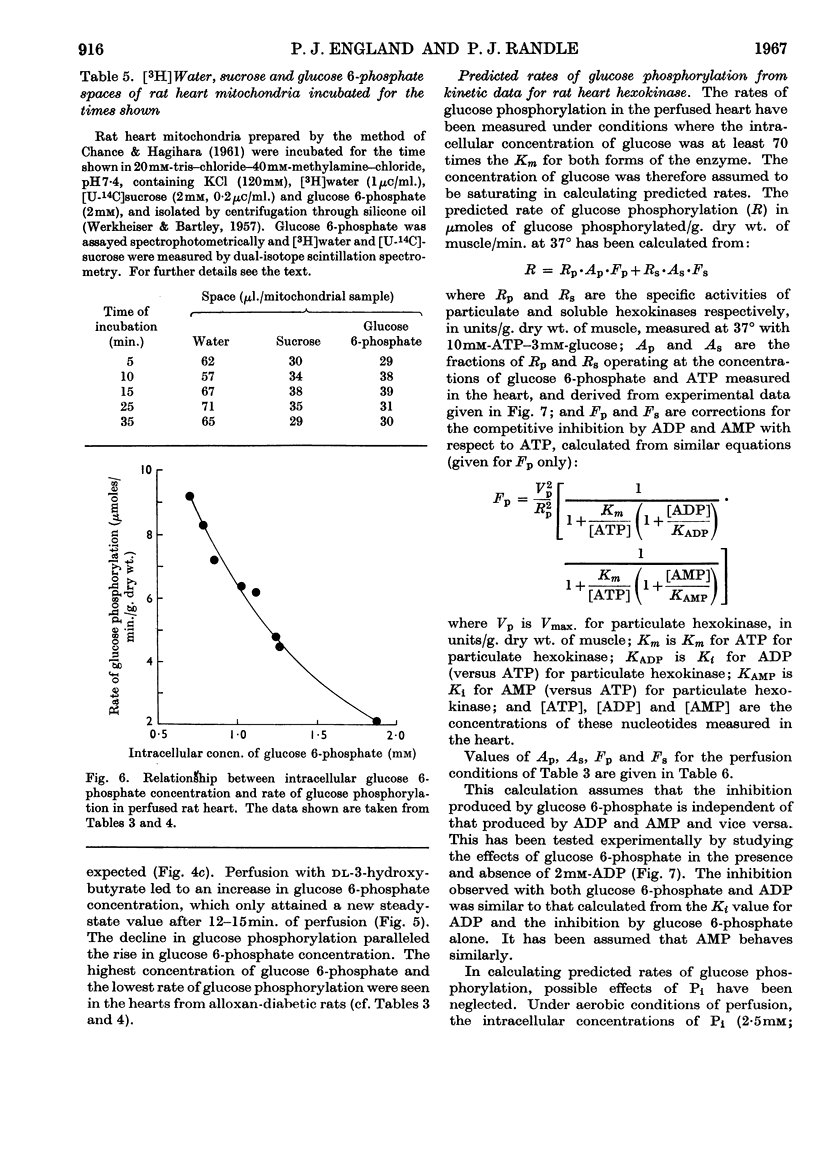
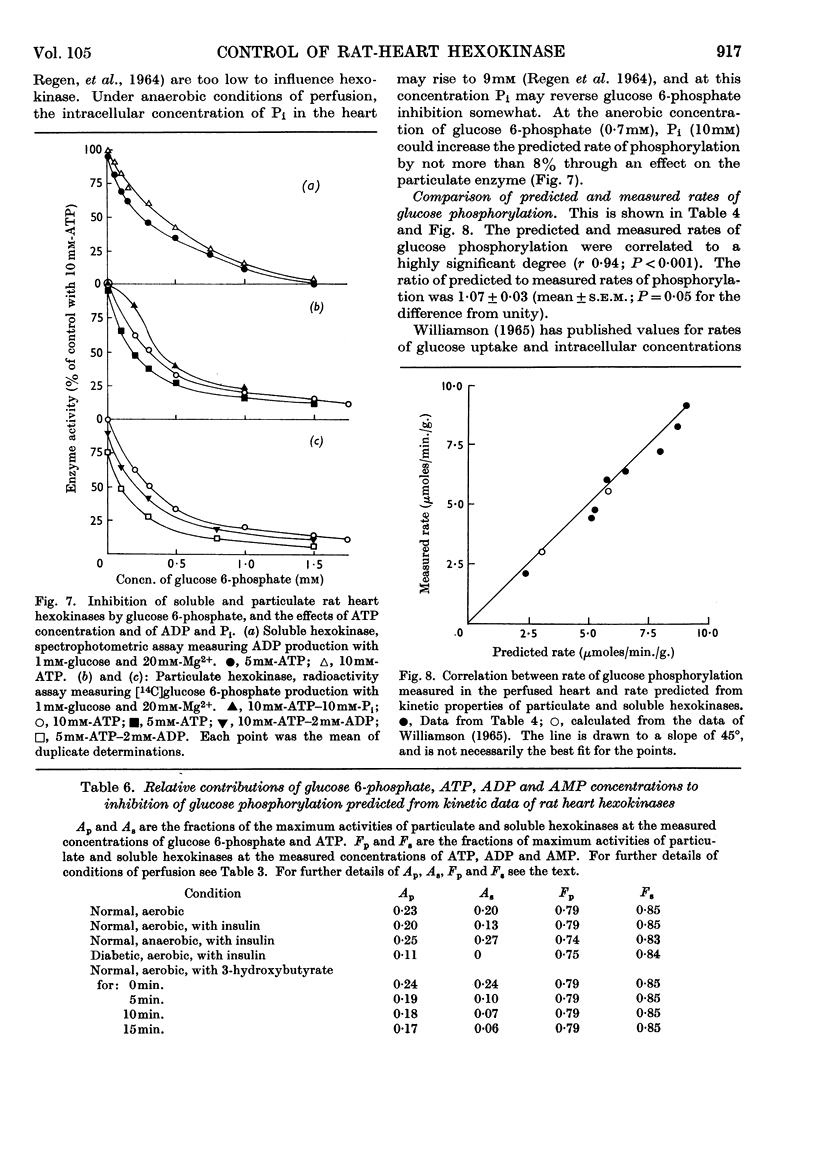
1. The kinetic properties of the soluble and particulate hexokinases from rat heart have been investigated. 2. For both forms of the enzyme, the Km for glucose was 45μm and the Km for ATP 0·5mm. Glucose 6-phosphate was a non-competitive inhibitor with respect to glucose (Ki 0·16mm for the soluble and 0·33mm for the particulate enzyme) and a mixed inhibitor with respect to ATP (Ki 80μm for the soluble and 40μm for the particulate enzyme). ADP and AMP were competitive inhibitors with respect to ATP (Ki for ADP was 0·68mm for the soluble and 0·60mm for the particulate enzyme; Ki for AMP was 0·37mm for the soluble and 0·16mm for the particulate enzyme). Pi reversed glucose 6-phosphate inhibition with both forms at 10mm but not at 2mm, with glucose 6-phosphate concentrations of 0·3mm or less for the soluble and 1mm or less for the particulate enzyme. 3. The total activity of hexokinase in normal hearts and in hearts from alloxan-diabetic rats was 21·5μmoles of glucose phosphorylated/min./g. dry wt. of ventricle at 25°. The temperature coefficient Q10 between 22° and 38·5° was 1·93; the ratio of the soluble to the particulate enzyme was 3:7. 4. The kinetic data have been used to predict rates of glucose phosphorylation in the perfused heart at saturating concentrations of glucose from measured concentrations of ATP, glucose 6-phosphate, ADP and AMP. These have been compared with the rates of glucose phosphorylation measured with precision in a small-volume recirculation perfusion apparatus, which is described. The correlation between predicted and measured rates was highly significant and their ratio was 1·07. 5. These findings are consistent with the control of glucose phosphorylation in the perfused heart by glucose 6-phosphate concentration, subject to certain assumptions that are discussed in detail.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borrebaek B. Increase in epididymal adipose tissue hexokinase activity induced by glucose and insulin. Biochim Biophys Acta. 1966 Oct 17;128(1):211–213. doi: 10.1016/0926-6593(66)90165-2. [DOI] [PubMed] [Google Scholar]
- CLELAND K. W., SLATER E. C. Respiratory granules of heart muscle. Biochem J. 1953 Mar;53(4):547–556. doi: 10.1042/bj0530547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P. Responses of acetylcholinesterase from Torpedo marmorata to salts and curarizing drugs. Mol Pharmacol. 1966 Sep;2(5):369–392. [PubMed] [Google Scholar]
- DIPIETRO D. L., WEINHOUSE S. Hepatic glucokinase in the fed, fasted, and alloxan-diabetic rat. J Biol Chem. 1960 Sep;235:2542–2545. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J. Concentrations of glycerides and phospholipids in rat heart and gastrocnemius muscles. Effects of alloxan-diabetes and perfusion. Biochem J. 1967 Aug;104(2):416–422. doi: 10.1042/bj1040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTFREUND H., HAMMOND B. R. Records of pH changes during enzyme reactions and kinetic studies with yeast hexokinase. Nature. 1963 May 18;198:667–670. doi: 10.1038/198667a0. [DOI] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- Hernandez A., Crane R. K. Association of heart hexokinase with subcellular structure. Arch Biochem Biophys. 1966 Jan;113(1):223–229. doi: 10.1016/0003-9861(66)90176-7. [DOI] [PubMed] [Google Scholar]
- Hernández A., Sols A. Transport and phosphorylation of sugars in adipose tissue. Biochem J. 1963 Jan;86(1):166–172. doi: 10.1042/bj0860166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZEN H. M., SODERMAN D. D., NITOWSKY H. M. KINETIC AND ELECTROPHORETIC EVIDENCE FOR MULTIPLE FORMS OF GLUCOSE-ATP PHOSPHOTRANSFERASE ACTIVITY FROM HUMAN CELL CULTURES AND RAT LIVER. Biochem Biophys Res Commun. 1965 Apr 23;19:377–382. doi: 10.1016/0006-291x(65)90472-9. [DOI] [PubMed] [Google Scholar]
- Katzen H. M., Schimke R. T. Multiple forms of hexokinase in the rat: tissue distribution, age dependency, and properties. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1218–1225. doi: 10.1073/pnas.54.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzen H. M. The effect of diabetes and insulin in vivo and in vitro on a low Km form of hexokinase from various rat tissues. Biochem Biophys Res Commun. 1966 Aug 23;24(4):531–536. doi: 10.1016/0006-291x(66)90352-4. [DOI] [PubMed] [Google Scholar]
- MORGAN H. E., CADENAS E., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. II. Rate-limiting steps and effects of insulin and anoxia in heart muscle from diabetic rats. J Biol Chem. 1961 Feb;236:262–268. [PubMed] [Google Scholar]
- MORGAN H. E., HENDERSON M. J., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. I. The effects of insulin and anoxia on glucose transport and phosphorylation in the isolated, perfused heart of normal rats. J Biol Chem. 1961 Feb;236:253–261. [PubMed] [Google Scholar]
- MORGAN H. E., RANDLE P. J., REGEN D. M. Regulation of glucose uptake by muscle. 3. The effects of insulin, anoxia, salicylate and 2:4-dinitrophenol on membrane transport and intracellular phosphorylation of glucose in the isolated rat heart. Biochem J. 1959 Dec;73:573–579. doi: 10.1042/bj0730573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean P., Brown J., Greenslade K., Brew K. Effect of alloxan-diabetes on the glucose-ATP phosphotransferase activity of adipose tissue. Biochem Biophys Res Commun. 1966 Apr 19;23(2):117–121. doi: 10.1016/0006-291x(66)90514-6. [DOI] [PubMed] [Google Scholar]
- NEWSHOLME E. A., RANDLE P. J., MANCHESTER K. L. Inhibition of the phosphofructokinase reaction in perfused rat heart by respiration of ketone bodies, fatty acids and pyruvate. Nature. 1962 Jan 20;193:270–271. doi: 10.1038/193270a0. [DOI] [PubMed] [Google Scholar]
- NEWSHOLME E. A., RANDLE P. J. Regulation of glucose uptake by muscle. 5. Effects of anoxia, insulin, adrenaline and prolonged starving on concentrations of hexose phosphates in isolated rat diaphragm and perfused isolated rat heart. Biochem J. 1961 Sep;80:655–662. doi: 10.1042/bj0800655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 7. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, on the concentrations of hexose phosphates, nucleotides and inorganic phosphate in perfused rat heart. Biochem J. 1964 Dec;93(3):641–651. doi: 10.1042/bj0930641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Rolleston F. S., Taylor K. Inhibition of brain hexokinase by glucose 6-phosphate. Biochem J. 1967 Sep;104(3):47P–47P. [PMC free article] [PubMed] [Google Scholar]
- PARK C. R., REINWEIN D., HENDERSON M. J., CADENAS E., MORGAN H. E. The action of insulin on the transport of glucose through the cell membrane. Am J Med. 1959 May;26(5):674–684. doi: 10.1016/0002-9343(59)90227-x. [DOI] [PubMed] [Google Scholar]
- REGEN D. M., DAVIS W. W., MORGAN H. E., PARK C. R. THE REGULATION OF HEXOKINASE AND PHOSPHOFRUCTOKINASE ACTIVITY IN HEART MUSCLE. EFFECTS OF ALLOXAN DIABETES, GROWTH HORMONE, CORTISOL, AND ANOXIA. J Biol Chem. 1964 Jan;239:43–49. [PubMed] [Google Scholar]
- SHORT D. J., PARKES A. S. Feeding and breeding of laboratory animals; a compound diet for monkeys. J Hyg (Lond) 1949 Jun;47(2):209–212. doi: 10.1017/s0022172400014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. E., Taylor P. M., Whelan W. J. Hypothesis on the mode of conversion of glucose into alpha-glucose-1-phosphate. Nature. 1967 Feb 18;213(5077):733–734. doi: 10.1038/213733b0. [DOI] [PubMed] [Google Scholar]
- TIEDEMANN H., BORN J. [Experiments on the mechanism of the Pasteur reaction. The influence of phosphates on the activity of structure-bound hexokinase]. Z Naturforsch B. 1959 Jul;14B:477–478. [PubMed] [Google Scholar]
- WEIL-MALHERBE H., BONE A. D. Studies on hexokinase. 1. The hexokinase activity of rat-brain extracts. Biochem J. 1951 Aug;49(3):339–347. doi: 10.1042/bj0490339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERKHEISER W. C., BARTLEY W. The study of steady-state concentrations of internal solutes of mitochondria by rapid centrifugal transfer to a fixation medium. Biochem J. 1957 May;66(1):79–91. doi: 10.1042/bj0660079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON J. R. GLYCOLYTIC CONTROL MECHANISMS. I. INHIBITION OF GLYCOLYSIS BY ACETATE AND PYRUVATE IN THE ISOLATED, PERFUSED RAT HEART. J Biol Chem. 1965 Jun;240:2308–2321. [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Walker D. G., Rao S. The role of glucokinase in the phosphorylation of glucose by rat liver. Biochem J. 1964 Feb;90(2):360–368. doi: 10.1042/bj0900360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watchorn E., McCance R. A. Subacute magnesium deficiency in rats. Biochem J. 1937 Aug;31(8):1379–1390. doi: 10.1042/bj0311379. [DOI] [PMC free article] [PubMed] [Google Scholar]


