Abstract
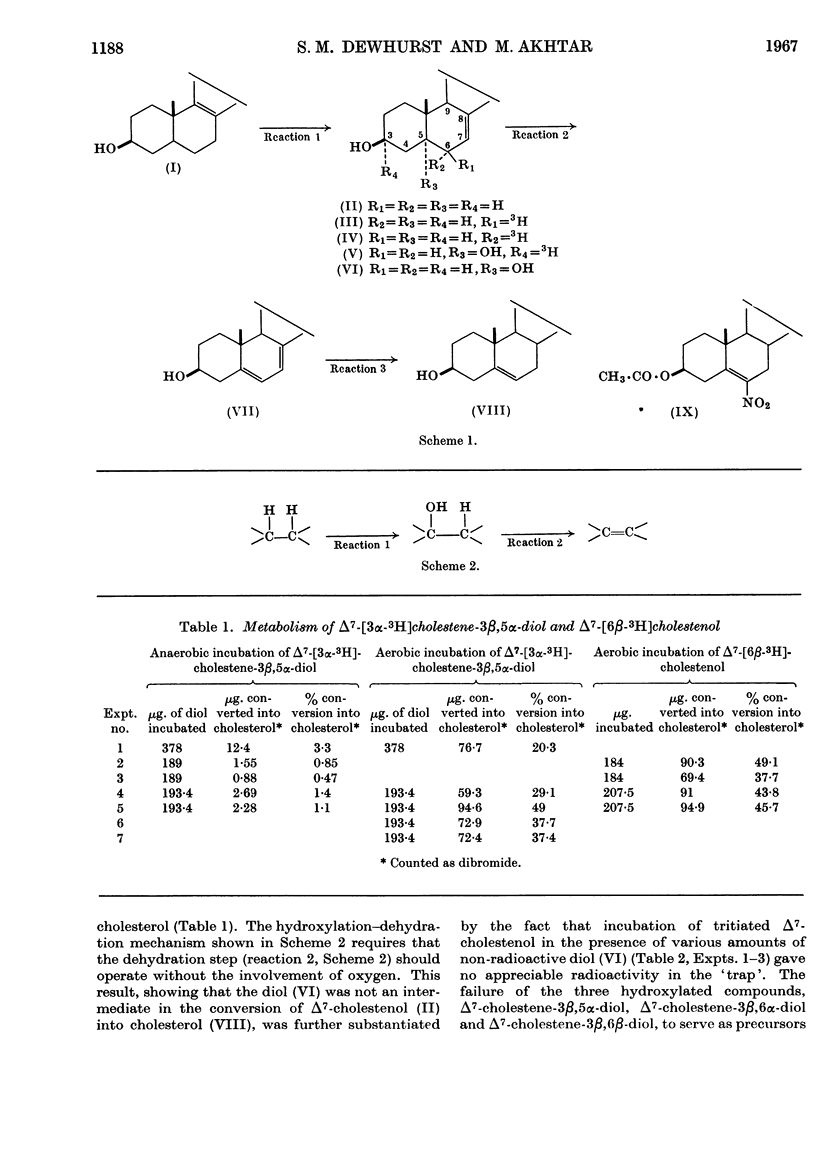
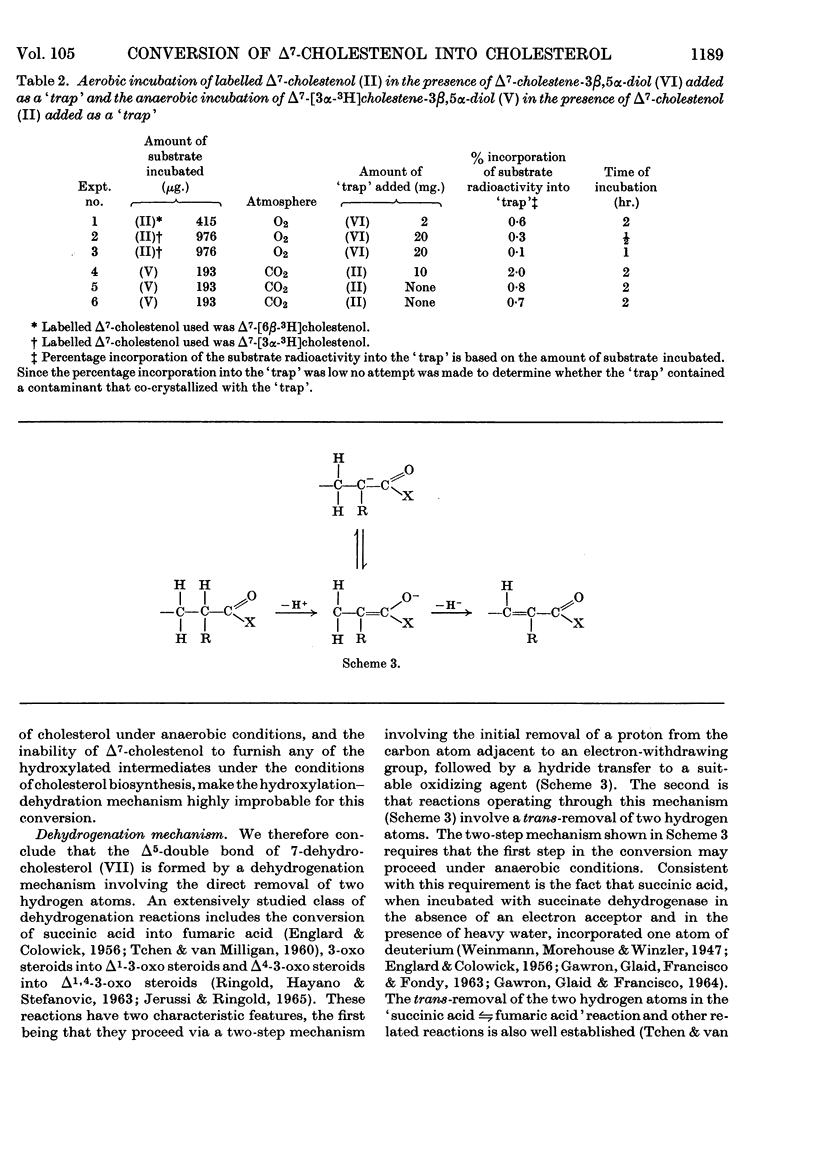
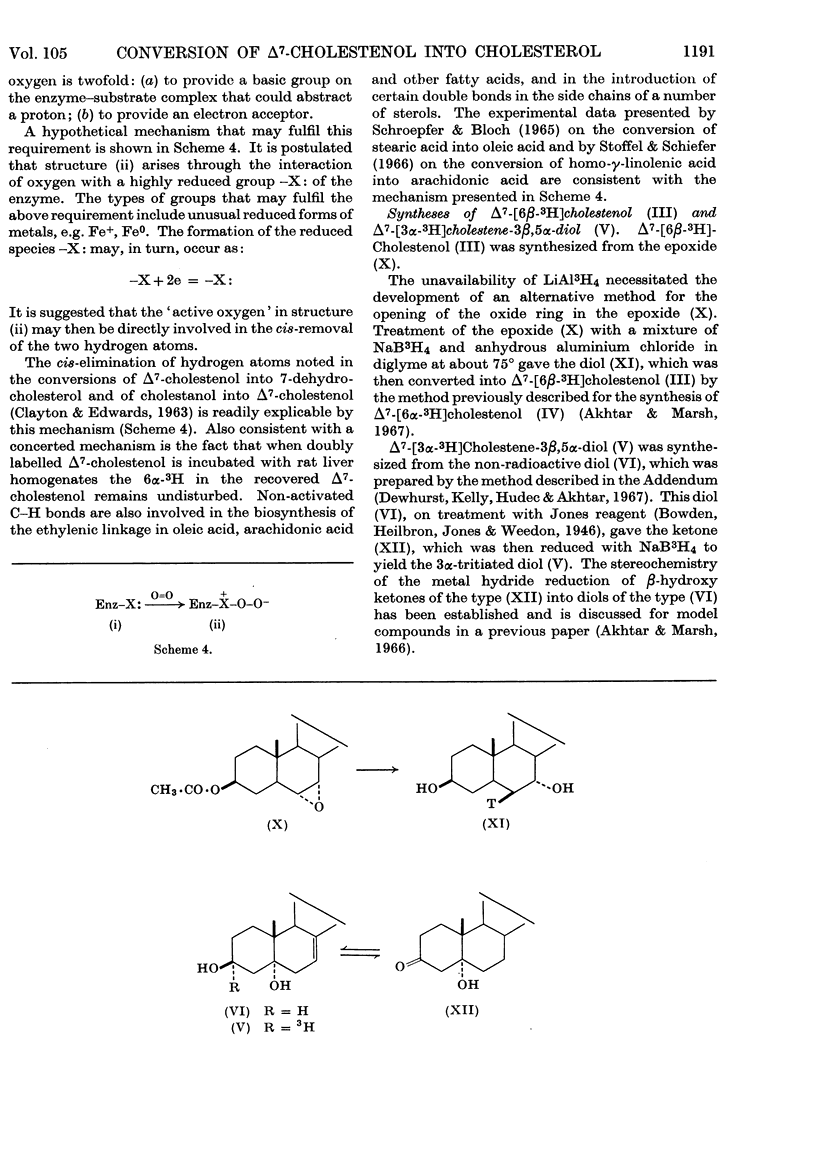
Convenient syntheses of 6β-tritiated Δ7-cholestenol and 3α-tritiated Δ7-cholestene-3β,5α-diol are described. It was shown that the conversion of 6β-tritiated Δ7-cholestenol into cholesterol is accompanied by the complete retention of label. It was unambiguously established that the overall reaction leading to the introduction of the double bond in the 5,6-position in cholesterol occurs via a cis-elimination involving the 5α- and 6α-hydrogen atoms and that during this process the 6β-hydrogen atom remains completely undisturbed. Metabolic studies with 3α-tritiated Δ7-cholestene-3β,5α-diol revealed that under anaerobic conditions the compound is not converted into cholesterol. This observation, coupled with the previous work of Slaytor & Bloch (1965), is interpreted to exclude a hydroxylation–dehydration mechanism for the origin of the 5,6-double bond in cholesterol. It was also shown that under aerobic conditions 3α-tritiated Δ7-cholestene-3β,5α-diol is efficiently converted into cholesterol and that this conversion occurs through the intermediacy of 7-dehydrocholesterol. Cumulative experimental evidence presented in this paper and elsewhere is used to suggest that the 5,6-double bond in cholesterol originates through an oxygen-dependent dehydrogenation process and a hypothetical mechanism for this and related reactions is outlined.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar M., Marsh S. The stereochemistry of the hydrogen elimination in the biological conversion of cholest-7-en-3-beta-ol into cholesterol. Biochem J. 1967 Feb;102(2):462–467. doi: 10.1042/bj1020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. B., EDWARDS A. M. Conversion of 5-alpha-cholestan-3-beta-ol to delta-7-5-alpha-cholesten-3-beta-ol in cockroaches. J Biol Chem. 1963 Jun;238:1966–1972. [PubMed] [Google Scholar]
- COLOWICK S. P., ENGLARD S. On the mechanism of an anaerobic exchange reaction catalyzed by succinic dehydrogenase preparations. J Biol Chem. 1956 Aug;221(2):1019–1035. [PubMed] [Google Scholar]
- Dempsey M. E. Pathways of enzymic synthesis and conversion to cholesterol of delta-5,7,24-cholestatrien-3 beta-ol and other naturally occurring sterols. J Biol Chem. 1965 Nov;240(11):4176–4188. [PubMed] [Google Scholar]
- GAWRON O., GLAID A. J., FONDY T. P., BECHTOLD M. M. Stereochemistry of the succinic dehydrogenase system. Nature. 1961 Mar 25;189:1004–1005. doi: 10.1038/1891004a0. [DOI] [PubMed] [Google Scholar]
- GAWRON O., GLAID A. J., FRANCISCO J., FONDY T. P. Mechanism of the succinic dehydrogenase catalysed reaction. Nature. 1963 Mar 30;197:1270–1272. doi: 10.1038/1971270a0. [DOI] [PubMed] [Google Scholar]
- Gawron O., Glaid A. J., 3rd, Francisco J. Stereochemistry of the exchange catalyzed by succinic dehydrogenase. Biochem Biophys Res Commun. 1964 Jun 1;16(2):156–161. doi: 10.1016/0006-291x(64)90354-7. [DOI] [PubMed] [Google Scholar]
- RINGOLD H. J., HAYANO M., STEFANOVIC V. Concerning the stereochemistry and mechanism of the bacterial C-1,2 dehydrogenation of steroids. J Biol Chem. 1963 Jun;238:1960–1965. [PubMed] [Google Scholar]
- Slaytor M., Bloch K. Metabolic transformation of cholestenediols. J Biol Chem. 1965 Dec;240(12):4598–4602. [PubMed] [Google Scholar]


