Abstract
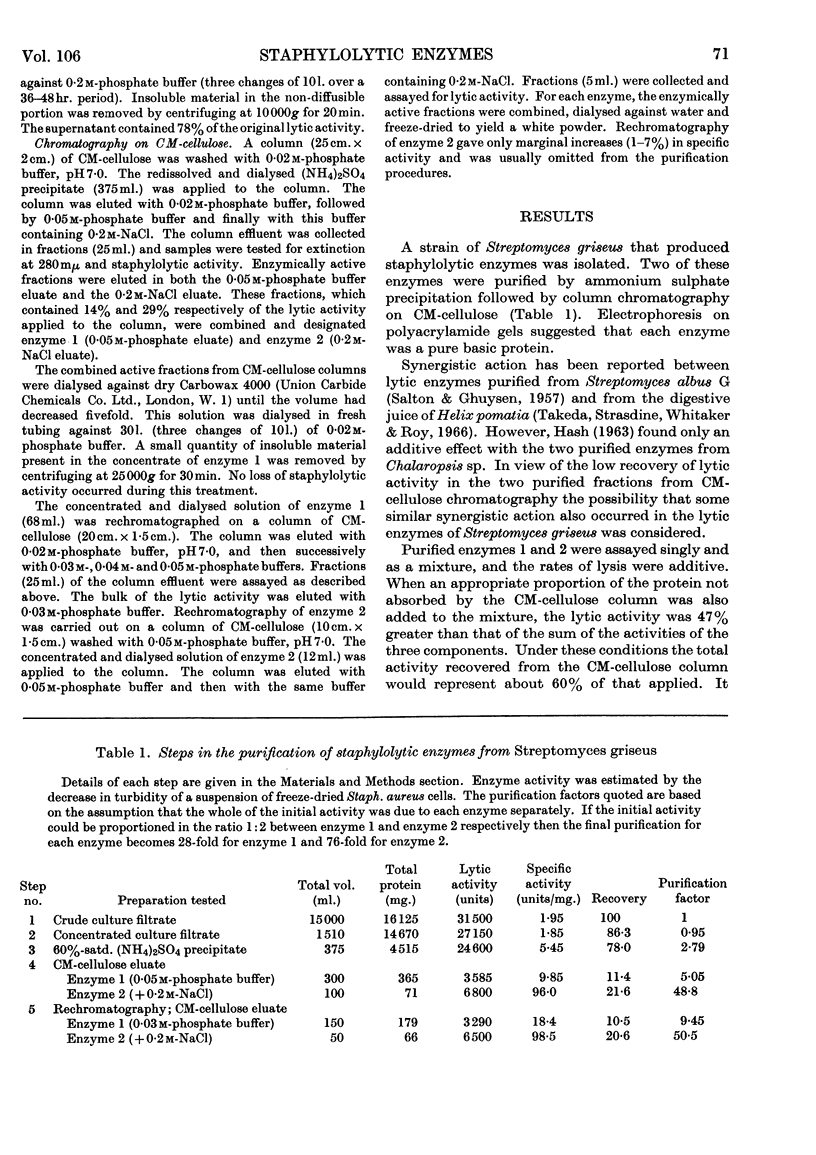
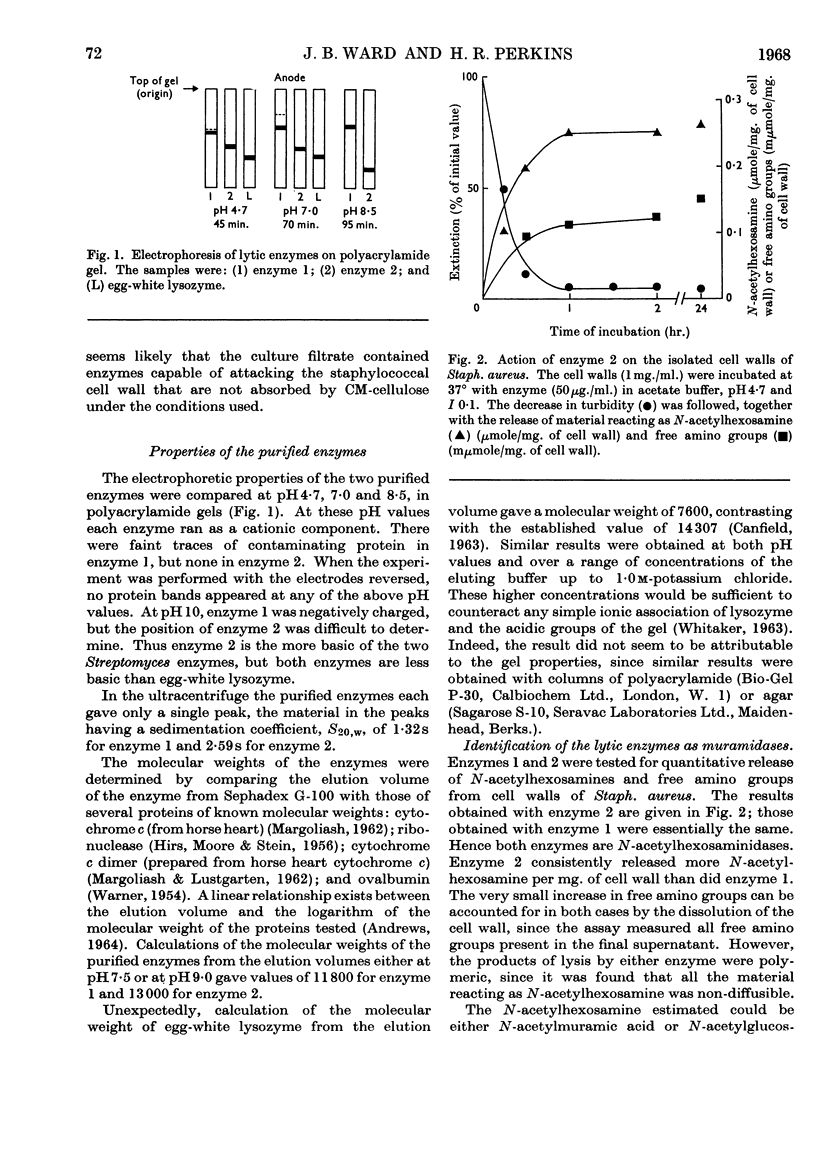
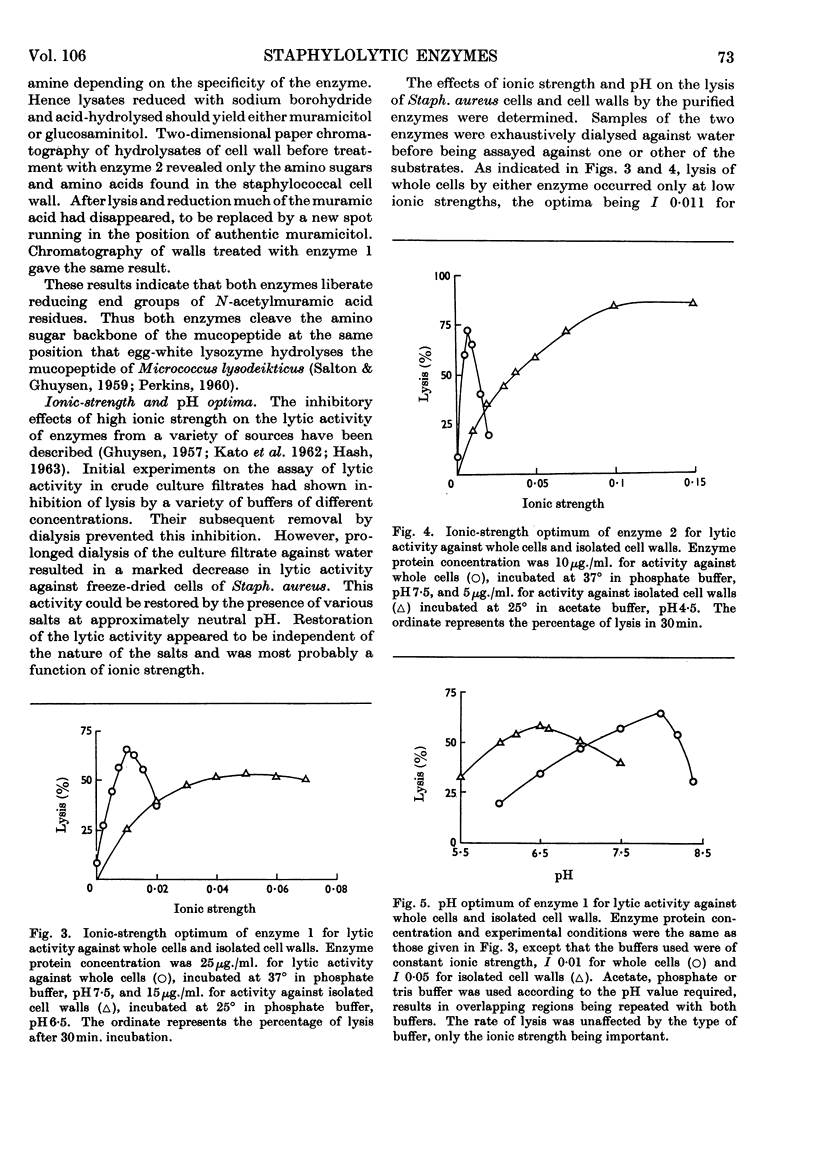
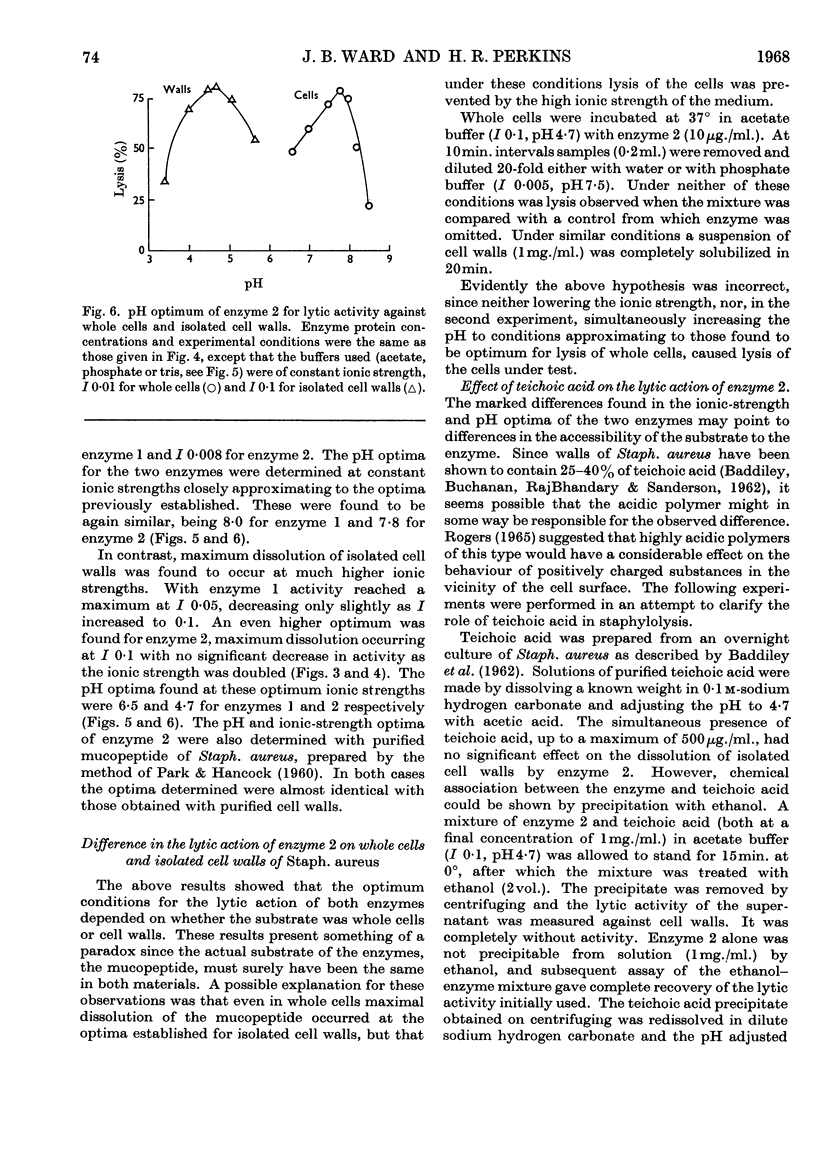
1. Two staphylolytic enzymes have been purified from cultures of a soil isolate of Streptomyces griseus. 2. The purified enzymes were shown to be basic proteins of low molecular weight. Each enzyme released N-acetylmuramic acid reducing groups from the cell walls of Staphylococcus aureus. 3. The enzymes lysed whole staphylococci best at higher pH values and lower ionic strengths than when the substrate was isolated cell walls or purified mucopeptide. 4. Added teichoic acid did not inhibit the enzymes, but it formed an ethanol-precipitable complex with them. 5. The possibility that teichoic acid on the surface of whole cells prevents the access of the enzymes to their mucopeptide substrate is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLSOP J WORK E. Cell walls of Propionibacterium species: fractionation and composition. Biochem J. 1963 Jun;87:512–519. doi: 10.1042/bj0870512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMINOFF D., MORGAN W. T. J., WATKINS W. M. Studies in immunochemistry. 11. The action of dilute alkali on the N-acetylhexosamines and the specific blood-group mucoids. Biochem J. 1952 Jun;51(3):379–389. doi: 10.1042/bj0510379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BADDILEY J., BUCHANAN J. G., RAJBHANDARY U. L., SANDERSON A. R. Teichoic acid from the walls of Staphylococcus aureus H. Structure of the N-acetylglucosaminyl-ribitol residues. Biochem J. 1962 Mar;82:439–448. doi: 10.1042/bj0820439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd W. C. A nomogram for phosphate buffers. J Biol Chem. 1965 Oct;240(10):4097–4098. [PubMed] [Google Scholar]
- CANFIELD R. E. THE AMINO ACID SEQUENCE OF EGG WHITE LYSOZYME. J Biol Chem. 1963 Aug;238:2698–2707. [PubMed] [Google Scholar]
- Ensign J. C., Wolfe R. S. Characterization of a small proteolytic enzyme which lyses bacterial cell walls. J Bacteriol. 1966 Feb;91(2):524–534. doi: 10.1128/jb.91.2.524-534.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHUYSEN J. M., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. I. PREPARATION OF FRAGMENTS BY ENZYMATIC HYDROLYSIS. Biochemistry. 1963 Sep-Oct;2:1110–1119. doi: 10.1021/bi00905a035. [DOI] [PubMed] [Google Scholar]
- GHUYSEN J. M., TIPPER D. J., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. IV. THE TEICHOIC ACID-GLYCOPEPTIDE COMPLEX. Biochemistry. 1965 Mar;4:474–485. doi: 10.1021/bi00879a016. [DOI] [PubMed] [Google Scholar]
- GLAZER A. N., WELLNER D. Adsorption of proteins on 'Sephadex'. Nature. 1962 Jun 2;194:862–863. doi: 10.1038/194862a0. [DOI] [PubMed] [Google Scholar]
- HASH J. H. PURIFICATION AND PROPERTIES OF STAPHYLOLYTIC ENZYMES FROM CHALAROPSIS SP. Arch Biochem Biophys. 1963 Sep;102:379–388. doi: 10.1016/0003-9861(63)90245-5. [DOI] [PubMed] [Google Scholar]
- HAUKENES G., ELLWOOD D. C., BADDILEY J., OEDING P. Serological cross-reactivity between polysaccharide A and teichoic acid of Staphylococcus aureus. Biochim Biophys Acta. 1961 Oct 28;53:425–426. doi: 10.1016/0006-3002(61)90461-9. [DOI] [PubMed] [Google Scholar]
- HIRS C. H., MOORE S., STEIN W. H. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [PubMed] [Google Scholar]
- Hay J. B., Archibald A. R., Baddiley J. The molecular structure of bacterial walls. The size of ribitol teichoic acids and the nature of their linkage to glycosaminopeptides. Biochem J. 1965 Dec;97(3):723–730. doi: 10.1042/bj0970723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARGOLIASH E. Amino acid sequence of chymotryptic peptides from horse heart cytochrome c. J Biol Chem. 1962 Jul;237:2161–2174. [PubMed] [Google Scholar]
- MARGOLIASH E., LUSTGARTEN J. Interconversion of horse heart cytochrome C monomer and polymers. J Biol Chem. 1962 Nov;237:3397–3405. [PubMed] [Google Scholar]
- Martin H. H. Biochemistry of bacterial cell walls. Annu Rev Biochem. 1966;35:457–484. doi: 10.1146/annurev.bi.35.070166.002325. [DOI] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- PERKINS H. R. The structure of a disaccharide liberated by lysozyme from the cell walls of Micrococcus lysodeikticus. Biochem J. 1960 Jan;74:182–186. doi: 10.1042/bj0740182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R., GHUYSEN J. M. Acetylhexosamine compounds enzymically released from Micrococcus lysodeikticus cell walls. III. The structure of DI- and tetra-saccharides released from cell walls by lysozyme and Streptomyces F1 enzyme. Biochim Biophys Acta. 1960 Dec 4;45:355–363. doi: 10.1016/0006-3002(60)91458-x. [DOI] [PubMed] [Google Scholar]
- SALTON M. R., GHUYSEN J. M. Action de l'actinomycetine sur les parois cellulaires bacteriennes. Biochim Biophys Acta. 1957 Apr;24(1):160–173. doi: 10.1016/0006-3002(57)90159-2. [DOI] [PubMed] [Google Scholar]
- SALTON M. R., GHUYSEN J. M. The structure of di- and tetrasaccharides released from cell walls by lysozyme and Streptomyces F 1 enzyme and the beta(1 to 4) N-acetylhexos-aminidase activity of these enzymes. Biochim Biophys Acta. 1959 Dec;36:552–554. doi: 10.1016/0006-3002(59)90205-7. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. Isolation of Streptomyces spp. capable of decomposing preparations of cell walls from various micro-organisms and a comparison of their lytic activities with those of certain actinomycetes and myxobacteria. J Gen Microbiol. 1955 Feb;12(1):25–30. doi: 10.1099/00221287-12-1-25. [DOI] [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. LYSOSTAPHIN: A NEW BACTERIOLYTIC AGENT FOR THE STAPHYLOCOCCUS. Proc Natl Acad Sci U S A. 1964 Mar;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrede S., Christophersen B. O. Effects of cystamine and cysteamine on the peroxidation of lipids and the release of proteins from mitochondria. Biochem J. 1966 Oct;101(1):37–41. doi: 10.1042/bj1010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIPPER D. J., STROMINGER J. L., GHUYSEN J. M. STAPHYLOLYTIC ENZYME FROM CHALAROPSIS: MECHANISM OF ACTION. Science. 1964 Nov 6;146(3645):781–782. doi: 10.1126/science.146.3645.781. [DOI] [PubMed] [Google Scholar]
- Takeda H., Strasdine G. A., Whitaker D. R., Roy C. Lytic enzymes in the digestive juice of Helix pomatia; chitinases and muramidases. Can J Biochem. 1966 May;44(5):509–518. doi: 10.1139/o66-061. [DOI] [PubMed] [Google Scholar]


